Abstract
T-bet is a critical transcription factor that regulates differentiation of Th1 cells from CD4+ precursor cells. Since T-bet directly binds to the promoter of the IFN-γ gene and activates its transcription, T-bet deficiency impairs IFN-γ production in Th1 cells. Interestingly, T-bet-deficient Th cells also display substantially augmented the production of IL-2, a T cell growth factor. Exogenous expression of T-bet in T-bet deficient Th cells rescued the IFN-γ production and suppressed IL-2 expression. IFN-γ and IL-2 reciprocally regulate Th cell proliferation following TCR stimulation. Therefore, we examined the effect of T-bet on Th cell proliferation and found that T-bet deficiency significantly enhanced Th cell proliferation under non-skewing, Th1-skewing, and Th2-skewing conditions. By using IFN-γ-null mice to eliminate the anti-proliferative effect of IFN-γ, T-bet deficiency still enhanced Th cell proliferation under both Th1- and Th2-skewing conditions. Since the anti-proliferative activity of T-bet may be influenced by IL-2 suppression in Th cells, we examined whether T-bet modulates IL-2-independent cell proliferation in a non-T cell population. We demonstrated that T-bet expression induced by ecdysone treatment in human embryonic kidney (HEK) cells increased IFN-γ promoter activity in a dose dependent manner, and sustained T-bet expression considerably decreased cell proliferation in HEK cells. Although the molecular mechanisms underlying anti-proliferative activity of T-bet remain to be elucidated, T-bet may directly suppress cell proliferation in an IFN-γ- or an IL-2-independent manner.
Keywords: T-bet, Proliferation, IFN-γ, IL-2, Ecdysone, Th cells
INTRODUCTION
CD4+ T helper (Th) cells are key regulatory immune cells that control a wide range of immune responses against pathogens. Stimulation of the Th cells with Ab against T cell receptor causes activation of Th cells and also induces Th cell proliferation in response to the exogenous IL-2. Activated CD4+ Th cells are subsequently differentiated into several subsets of effector T including Th1, Th2, and Th17, and regulatory T (Treg) cells that are distinguished by signature patterns of cytokine production (1). Th1 cells mainly produce pro-inflammatory cytokine IFN-γ and prevent against tumor development and pathogenic infection, while Th2 cells produce IL-4, IL-5, and IL-13 and mediate non-phagocytic and extracellular pathogen elimination (1,2,3). The third effector subset, Th17 cells produce IL-17 and are generated from precursor Th cells by stimulation with TGF-β and IL-6 and induction of RORγt. Treg cells are also generated from naïve CD4+ T cells by the help of TGF-β signaling and FoxP3 induction (4,5). IL-2 is a major cytokine produced by TCR-triggered Th cells and initiates cell cycle progression and proliferation in an autocrine fashion (6). IL-2 binds to its receptor complex (IL-2R), which is composed of α, β, and γ and activates STAT5-mediated gene transcription through activation of JAK3. This results in accelerated cell proliferation and induction of the IL-2/IL-2R signaling cascade (7). IL-2/IL-2R signaling is also essential for the development of Foxp3 expression in Treg cell development (8).
T-bet is a T-box-containing protein expressed in T cells and is a potent transcription factor that induces Th1 cell development from CD4+ Th precursor cells (9) through direct activation of IFN-γ gene transcription (10,11). T-bet deficiency increases the number of Th2 cells at the expense of IFN-γ-producing Th1 cells, resulting in spontaneous asthmatic phenotypes in the lung and acceleration of tumor growth (12,13,14). Restoration of T-bet into T-bet-deficient T cells restored IFN-γ production, attenuating lung inflammation and tumor progression in vitro and in vivo (15,16,17). In addition, T-bet suppresses IL-2 production in developing Th1 cells via direct interaction with NFκB/p65, causing suppression of NFκB/p65 activity on IL-2 gene transcription (18). More interestingly, T-bet modulates the development of RORγt-positive Th17 and FoxP3-positive Treg cells in a RUNX1-mediated manner; additional unknown pathways also contribute (19,20,21,22). Finally, T-bet undergoes multiple post-translational protein modifications in Th cells and plays regulatory roles in developing Th cells (23,24).
In this study, we investigated whether T-bet has the anti-proliferative activity in different Th cell subsets and whether the anti-proliferative activity of T-bet is dependent on modulation of IL-2 and IFN-γ.
MATERIALS AND METHODS
Mice
Wild type (WT), T-bet knockout (KO), IFN-γ KO, T-bet/IFN-γ double KO, and DTg/KO (TRE-T-bet and rtTA double transgene in T-bet KO background) mice in a C57BL/6 genetic background were housed with free access to water and food. All mice were maintained in specific pathogen-free rooms in an animal facility at Ewha Womans University. All mice handling and experiments were done in accordance with IACUC guidelines at Ewha Womans University (IACUC No. 2012-01-071, 14-030).
In vitro stimulation of CD4+ Th cells
Single cell suspensions were prepared from lymph node and spleen tissues and subjected to isolation of CD4+ Th cells using mouse CD4 micro beads (Miltenyi Biotec, Auburn, CA, USA). Isolated CD4+ Th cells were seeded onto culture plates coated with anti-CD3 Ab in the presence or absence of recombinant human IL-2 (rhIL-2, 10 U/ml). For Th1-skewing conditions, CD4+ Th cells were additionally treated with IL-12 (2 ng/ml) and anti-IL-4 (5 µg/ml). For Th2-skewing conditions, cells were treated with IL-4 (10 ng/ml) and anti-IFN-γ (5 µg/ml). Cells were then cultured for 3 days under Th1- and Th2-skewing conditions and analyzed for cell proliferation activity and cytokine levels. Separately, CD4+ Th cells were isolated from DTg/KO mice and treated with doxycycline to restore the T-bet expression in Th cells, as reported previously (16). Cell supernatants were collected for measuring cytokines, IFN-γ and IL-2 using an ELISA reader (BD Pharmingen, San Diego, CA, USA).
Thymidine incorporation assay
CD4+ Th cells were stimulated with various amounts of anti-CD3 Ab in round-bottomed 96-well plates and then treated with radiolabelled 3H-thymidine (5 mCi/5 ml) to final concentration of 1 µl/well. Cells were harvested 3 days after TCR stimulation and subjected to quantitative analysis. A scintillation beta counter was used to measure radioactivity in DNA recovered from the cells (Microbeta TopCount, Perkin Elmer, Shelton, CT, USA). Three independent experiments were performed for analyzing the results and each experiment was done in triplicate.
Ecdysone-inducible T-bet expression
T-bet cDNA was cloned into the pIND mammalian expression vector. The resulting construct was transfected into human embryonic kidney (HEK) 293 cells (EcR-HEK) that were stably transformed with the regulatory vector, pVgRXR and maintained in the selective medium containing Zeocin (1 mg/ml, Invitrogen, Carlsbad, CA, USA). Empty vector (mock) or the T-bet expression vector was transfected into EcR-HEK cells. G418 (400 µg/ml, Invitrogen) was used to select the following stable cell clones: mock (#1 and #2) and T-bet (#1, #2, #3, and #4). Subcloned cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine, G418, and Zeocin. For induction of T-bet expression, cloned cells were subcultured every 2 days and treated with the Ecdysone analog ponasterone A (PonA, Sigma-Aldrich, St Louis, MO, USA), which was replaced every alternate day.
Luciferase assay
EcR-HEK cells were transfected with mock or T-bet expression vector together with IFN-γ promoter-linked reporter gene and subsequently treated with various concentrations of PonA. Protein extracts were obtained using reporter lysis buffer (Promega, Madison, WI, USA) and used for determining relative luciferase activity using a luciferase assay kit (Promega) and luminometer (Berthold, Bad Wildbad, Germany). Relative luciferase activity was normalized by β-galactosidase activity. The relative activity was expressed as induction fold compared to that of vehicle-treated sample which was set as 1.
RESULTS
Increased proliferation activity in T-bet-deficient Th cells
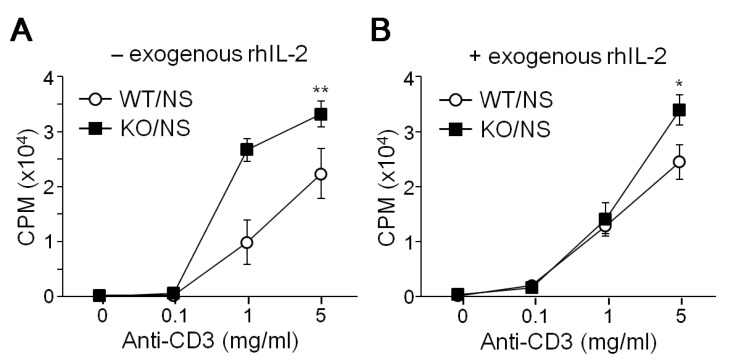
We examined the proliferation activity of CD4+ Th cells from WT and T-bet KO mice following TCR stimulation. Under non-skewing conditions, CD4+ Th cells proliferated in response to the anti-CD3 stimulus in a dose dependent manner, while T-bet-deficient Th cells showed hyper-proliferative activity in comparison (Fig. 1A). Treatment with excess amount of rhIL-2 had no additional effect on Th cell proliferation in WT or KO mice (Fig. 1B). TCR-induced Th cell proliferation was more prominent in T-bet KO cells regardless of exogenous IL-2 treatment.
Figure 1. Effect of T-bet on Th cell proliferation. CD4+ Th cells were isolated from WT and T-bet KO mice in the absence (-, A) or presence (+, B) of exogenous rhIL-2 and stimulated with different amounts of anti-CD3 under non-skewing (NS) conditions for 3 days. Cells were seubsequently incubated with 3H-thymidine and subjected to liquid scintillation counting using Micro Beta and TopCount equipment (Perkin Elmer). Y-axes represent count per minute (CPM). Three independent experiments were performed using 6 mice for each experiment. Data are expressed as the average ±SD of 6 mice. *p<0.05, **p<0.005.
Hyper-proliferation of T-bet-null Th cells under Th1- and Th2-skewing conditions
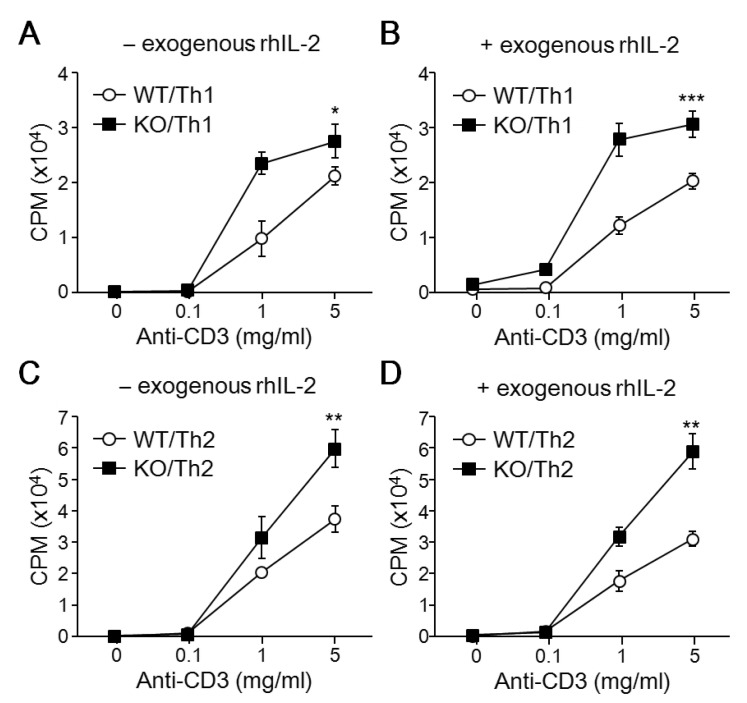
Since T-bet expression is increased by treatment with IL-12 during Th cell differentiation, CD4+ Th cells from WT and T-bet KO mice were stimulated with anti-CD3 Ab and then cultured for 2 days with Th1-skewing cytokines. Like in non-skewing conditions, developing T-bet-null Th1 cells are more proliferative than WT Th cells (Fig. 2A). Exogenous rhIL-2 addition caused no change in cell proliferation of developing Th1 cells (Fig. 2B). Because T-bet deficiency induces Th2 cytokine production, we skewed the cells with IL-4 cytokine to eliminate the developmental difference between WT and T-bet KO Th cells. In response to anti-CD3 stimulation, developing Th2 cells were much more proliferative compared to cells in either non-skewing or Th1-skewing conditions (Fig. 2C). Additionally, T-bet KO Th2 cells are more proliferative than WT cells regardless of IL-2 presence (Fig. 2C and D). These results suggest that T-bet deficiency promotes Th cell proliferation in response to TCR stimulation during Th cell development into both Th1 and Th2 cells.
Figure 2. Effect of T-bet on Th1 and Th2 cell proliferation. CD4+ Th cells isolated from WT and T-bet KO mice were activated using anti-CD3 and differentiated into Th1 and Th2 cells in the presence (+) or absence (-) of exogenous rhIL-2. Developing Th1 and Th2 cells were incubated with 3H-thymidine to determine cell proliferation rate. Y-axes represent count per minute (CPM). A total of 6 mice per group were analyzed and data are presented as the average ±SD of 3 experiments. *p<0.05, **p<0.005, ***p<0.0005.
IFN-γ-independent anti-proliferative activity of T-bet
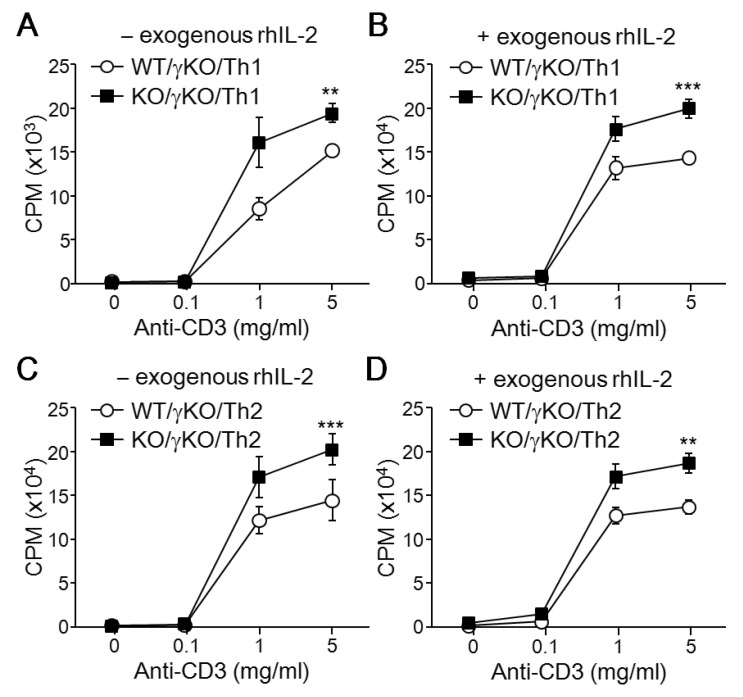
IFN-γ plays an inhibitory role in regulation of proliferation in immune cells; its production was impaired in T-bet deficient CD4+ Th cells, suggesting that the hyper-proliferative activity of T-bet deficient CD4+ Th cells may be due to diminished production levels of IFN-γ. To assess the IFN-γ dependency in the anti-proliferative activity of T-bet, we examined the effect of T-bet on Th proliferation in an IFN-γ-deficient genetic background. Interestingly, T-bet/IFN-γ double KO mice showed increased proliferation in response to TCR stimulation when compared to IFN-γ single KO mice under both Th1-skewing conditions (Fig. 3A) and Th2-skewing conditions (Fig. 3C). This result was unaffected by exogenous rhIL-2 (Fig. 3B and D). These results imply that the anti-proliferative activity of T-bet is independent of IFN-γ production.
Figure 3. IFN-γ independence of T-bet's anti-proliferation activity. CD4+ Th cells were isolated from IFN-γ KO mice and IFN-γ/T-bet double KO mice and stimulated with anti-CD3 in the either presence (+) or absence (-) of exogenous rhIL-2. Cells were cultured for an additional 2 to 3 days under Th1-skewing (A) or Th2-skewing (B) conditions. Cell proliferation rates of developing Th cells from either T-bet WT or T-bet KO mice established IFN-γ-deficient background were determined using a thymidine incorporation assay. A total of 5 mice per group were used for analysis and data are expressed as the average ±SD. **p<0.005, ***p<0.0005.
T-bet expression is closely associated with IFN-γ induction and IL-2 suppression in Th cells
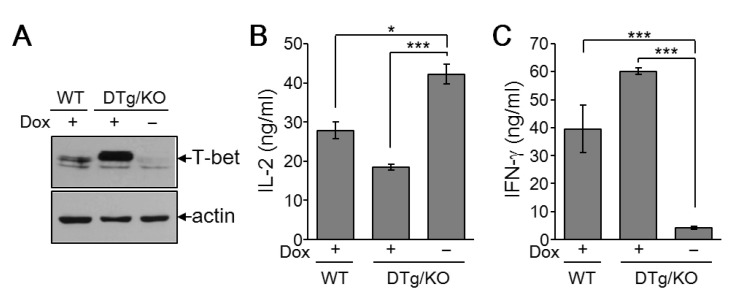
To assess the effect of exogenous T-bet on Th cell proliferation in T-bet KO cells, we generated a Th cell-specific, tetracycline-inducible T-bet transgenic mouse line in a T-bet deficient background (DTg/KO). Treatment of CD4+ Th cells with the tetracycline derivative doxycycline substantially increased T-bet expression in DTg/KO mice (Fig. 4A). Exogenous T-bet expression in T-bet-null cells rescued T-bet function, as assayed by levels of IL-2 and IFN-γ (Fig. 4B). The increased IL-2 expression in T-bet KO cells was suppressed by the induction of T-bet (Fig. 4B). T-bet induction also rescued IFN-γ levels (Fig. 4C). These results confirm that T-bet suppresses IL-2 production in Th cells and that the change in proliferation in T-bet deficient mice is due to loss of T-bet function.
Figure 4. IL-2 suppression and IFN-γ induction by T-bet in Th cells. Spleens and lymph node tissues were collected from WT and DTg/KO mice and CD4+ Th cells were isolated. Isolated Th cells were stimulated with anti-CD3 (2 µg/mL) in the either presence (+) or absence (-) of doxycycline (0.5 µg/mL) for 3 days. (A) Cell pellets were harvested and subjected to protein analysis by immunoblotting with Ab against T-bet or actin. (B) Cell supernatants were harvested for IL-2 and IFN-γ analysis by ELISA. Five independent experiments were performed and data are expressed as the average ±SD for 5 mice per group. *p<0.05; ***p<0.0005.
Development of a T-bet expression system in non-T cells
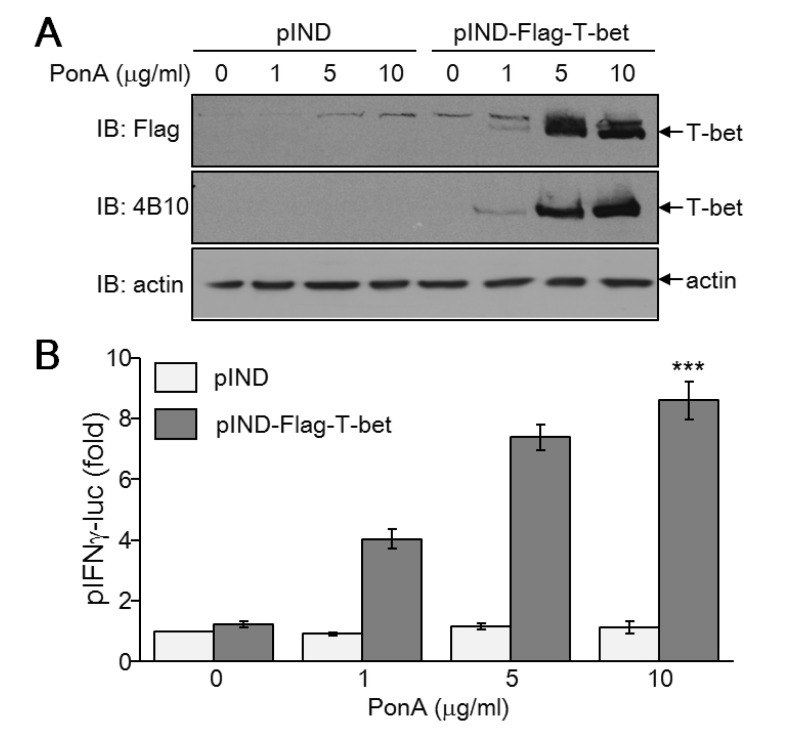
In contrast to IFN-γ, which has no regulatory effect on T-bet-mediated inhibition of Th cell proliferation, IL-2 is a critical factor for regulating Th cell proliferation (18). Additionally, we found that T-bet suppresses IL-2 levels. To rule out a T-bet-mediated effect on IL-2 suppression that influences Th cell proliferation, we established an inducible T-bet expression system in IL-2-independent EcR-HEK cells that were transfected with ecdysone receptor. Subsequent transfection of ecdysone receptor-binding elementlinked T-bet expression vector into EcR-HEK cells induced T-bet expression proportionate to the concentration of PonA (Fig. 5A). IFN-γ promoter activity was increased in a dose dependent manner, mirroring T-bet expression levels (Fig. 5B). Thus, we confirmed functionality of the ecdysone-inducible expression system of T-bet in a non-T cell population that proliferates in an IL-2-independent manner.
Figure 5. Ecdysone-inducible expression of functional T-bet in non-T cells. EcR-HEK cells were transiently transfected with T-bet expression vector and subsequently treated with PonA for 24 hours. (A) Whole cell protein extracts were resolved by SDS-PAGE and subjected to immunoblotting with Ab against Flag, T-bet (4B10), or actin. (B) EcR-HEK cells were transiently transfected with T-bet expression vector together with pIFN-γ-luciferase reporter gene and pCMVβ as an internal control. After treatment with different concentrations of PonA, cells were harvested for determination of luciferase activity. At least three independent reporter assays were performed and data are given as the average ±SD. ***p<0.0005.
Attenuation of cell growth by T-bet induction
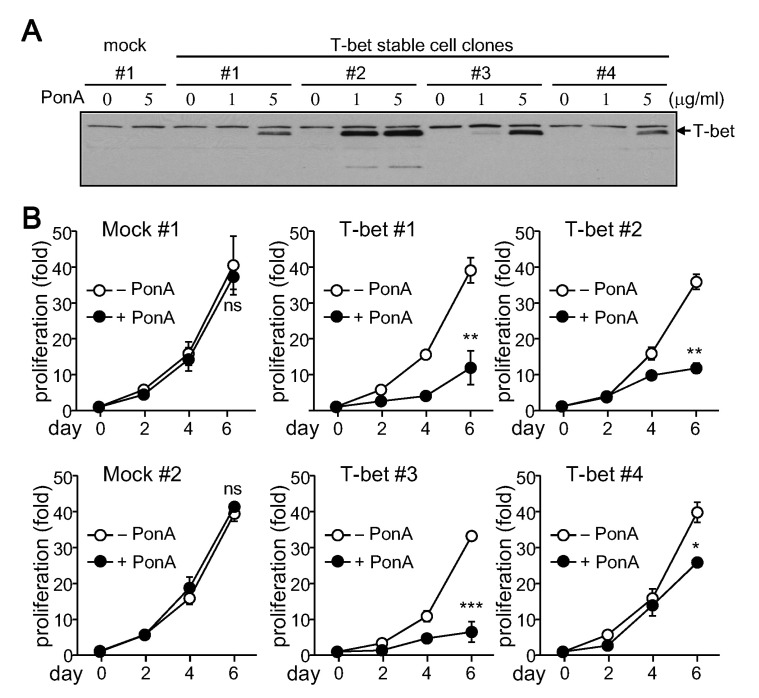
To study T-bet's direct role in cell proliferation, EcR-HEK cell clones stably expressing T-bet were selected and cultured for analysis of cell growth rate. Two mock clones and four different T-bet cell clones were treated with PonA. While T-bet expression was not induced in mock clones after treatment with PonA, an increase in T-bet expression was observed to varying degrees in T-bet stable cell clones (Fig. 6A). Cell growth rates of different stable cell clones were calculated by counting total cell numbers following each passage. While PonA had no effect on cell growth in mock clones, cell proliferation rate was substantially decreased by T-bet expression (Fig. 6B). These results suggest that T-bet has an inhibitory role in cytokine independent cell proliferation.
Figure 6. Suppression of cell proliferation by T-bet. Stable T-bet expressing cell clones were established in EcR-HEK cells and maintained in DMEM containing G418 and Zeocin. (A) Stable cells were treated with PonA for 24 h. Protein extracts were prepared from each stable cell clone and analyzed by immunoblotting with the T-bet (4B10) Ab. (B) Stable cell clones were cultured with or without PonA at the indicated concentrations. Cells were counted by Trypan blue exclusion assay every 2 days in triplicate. Data are expressed as the average±SD from 3 separate experiments. ns, not significant; *p<0.05; **p<0.005; ***p<0.0005.
DISCUSSION
This study shows that T-bet induces IFN-γ production and suppresses IL-2 expression in developing Th1 cells. Additionally, the data demonstrate that T-bet-positive Th1 cells are less proliferative than developing Th2 cells. This suggests that T-bet-mediated cytokine regulation may cause delayed cell proliferation during Th1 cell development. However, our results indicate that T-bet plays an inhibitory role in cell proliferation independent of the cytokines IL-2 and IFN-γ. The observed increase in Th cell proliferation caused by T-bet deficiency was also observed in T-bet-deficient IFN-γ KO cells, implying that the anti-proliferative function of T-bet is IFN-γ-independent. In addition, T-bet expression suppressed cell proliferation in non-T cell population whose proliferation is independent of IL-2 and IFN-γ. Collectively, these data show that T-bet has an anti-proliferative activity that is independent of cytokine regulation.
Although it is clear that T-bet suppresses cell growth rate in non-T cell lines and Th cells, the molecular mechanisms remain unclear. Previous research showed that T-bet functions as a transcriptional activator in a direct way (9). Additionally, T-bet undergoes multiple post-translational protein modifications such as phosphorylation at serine, threonine, or tyrosine residues (24) and inhibits gene transcription through interaction with many other transcription factors including GATA-3 (15), NF-κB/p65 (18), NFATp (23), and RUNX1 (19). Thus, expression and post-translational protein modification of T-bet may influence interactions with regulatory proteins involved in cell cycle progression. T-bet may also affect apoptotic cell death, resulting in decreased cell number. The specific effects of T-bet on cell cycle progression and apoptotic cell death remain an interesting area of future research.
IL-2 is a critical factor in early activation of naïve CD4+ Th cells upon TCR stimulation. IL-2 positively regulates its own production through activation of IL-2R signaling and induces cell proliferation (8,25). As a result, IL-2 influences specification of Th cell development by cooperatively activating several transcription factors and producing signature cytokines (26,27). We compensated for the altered levels of IL-2 between WT and T-bet KO cells by treating Th cells with exogenous rhIL-2 and evaluating its effects on Th cell proliferation. Exogenous rhIL-2 addition caused no significant change in proliferation; however, it is possible that endogenous IL-2 production in response to anti-CD3 stimulation could be sufficient to activate Th cell proliferation. In order to confirm whether the anti-proliferation function of T-bet is independent of IL-2 suppression in Th cells, functional assessment of T-bet needs to be performed in the absence of IL-2. However, IL-2 deficiency reduces T cell survival, so it is difficult to identify the specific role of T-bet on Th cell proliferation. Instead, we showed that enforced T-bet expression caused suppression of cell growth rate in non-IL-2-producing cells. How T-bet suppresses cell growth rate in non-IL-2-producing cells is not yet understood. Finally, it still remains unclear whether IFN-γ is induced by T-bet and therefore affects cell proliferation.
T-bet is a critical transcription factor for not only Th1 cell differentiation but also Th2, Th17, or Treg cell development. T-bet undergoes multiple post-translational modification and associates with transcription factor like GATA-3, NFATp, NF-kB, and RUNX1, thus resulting in the suppression of other cell lineage commitment (15,18,24). When compared to the proliferation activity of Th1 and Th2 cells, Th17 and Treg cells are less proliferative. Suppression of Th cell proliferation and suppression of IL-2 production by T-bet may intrinsically affect Th cell differentiation program. It would be interesting to discover the effects and their molecular mechanisms of anti-proliferative activity of T-bet during Th cell lineage commitment.
ACKNOWLEDGEMENTS
This work was supported by Mid-career Researcher Program through NRF grant funded by the MEST (NRF-2013R1A2 A2A01068302).
Abbreviations
- T-bet
T-box-containing protein expressed in T cells
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyler T, Connor CA, Kiss EA, Diefenbach A. T-bet and Gata3 in controlling type 1 and type 2 immunity mediated by innate lymphoid cells. Curr Opin Immunol. 2013;25:139–147. doi: 10.1016/j.coi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Miller SA, Weinmann AS. Molecular mechanisms by which T-bet regulates T-helper cell commitment. Immunol Rev. 2010;238:233–246. doi: 10.1111/j.1600-065X.2010.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11:536–542. doi: 10.1016/j.intimp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009;11:594–598. doi: 10.1016/j.micinf.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2) J Autoimmun. 2008;31:7–12. doi: 10.1016/j.jaut.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Letourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009;123:758–762. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 10.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 12.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 13.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 14.Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008;180:8004–8010. doi: 10.4049/jimmunol.180.12.8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 16.Park JW, Min HJ, Sohn JH, Kim JY, Hong JH, Sigrist KS, Glimcher LH, Hwang ES. Restoration of T-box-containing protein expressed in T cells protects against allergen-induced asthma. J Allergy Clin Immunol. 2009;123:479–485. doi: 10.1016/j.jaci.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Lee K, Min HJ, Jang EJ, Hong JH, Hwang ES. In vivo tumor suppression activity by T cell-specific T-bet restoration. Int J Cancer. 2010;127:2129–2137. doi: 10.1002/ijc.25238. [DOI] [PubMed] [Google Scholar]
- 18.Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson RC, Turner DG, Mair I, O'Connor RA, Anderton SM. T-bet Expression by Foxp3(+) T Regulatory Cells is Not Essential for Their Suppressive Function in CNS Autoimmune Disease or Colitis. Front Immunol. 2015;6:69. doi: 10.3389/fimmu.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang ES. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med J. 2010;51:484–491. doi: 10.3349/ymj.2010.51.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J Immunol. 2013;190:5764–5770. doi: 10.4049/jimmunol.1203403. [DOI] [PubMed] [Google Scholar]
- 24.Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamorano J, Wang HY, Wang R, Shi Y, Longmore GD, Keegan AD. Regulation of cell growth by IL-2: role of STAT5 in protection from apoptosis but not in cell cycle progression. J Immunol. 1998;160:3502–3512. [PubMed] [Google Scholar]
- 26.Ben-Sasson SZ, Le GG, Conrad DH, Finkelman FD, Paul WE. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145:1127–1136. [PubMed] [Google Scholar]
- 27.Hwang ES, White IA, Ho IC. An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proc Natl Acad Sci U S A. 2002;99:13026–13030. doi: 10.1073/pnas.202474499. [DOI] [PMC free article] [PubMed] [Google Scholar]