Abstract
Elucidation of the accurate subunit stoichiometry of oligomeric membrane proteins is fraught with complexities. The interpretations of chemical cross-linking, analytical ultracentrifugation, gel filtration, and low-resolution electron microscopy studies are often ambiguous. Staphylococcal alpha-hemolysin (alpha HL), a homooligomeric toxin that forms channels in cell membranes, was believed to possess six subunits arranged around a sixfold axis of symmetry. Here, we report that analysis of x-ray diffraction data and chemical modification experiments indicate that the alpha HL oligomer is a heptamer. Self-rotation functions calculated using x-ray diffraction data from single crystals of alpha HL oligomers show a sevenfold axis of rotational symmetry. The alpha HL pore formed on rabbit erythrocyte membranes was determined to be a heptamer by electrophoretic separation of alpha HL heteromers formed from subunits with the charge of wild-type alpha HL and subunits with additional negative charge generated by targeted chemical modification of a single-cysteine mutant. These data establish the heptameric oligomerization state of the alpha HL transmembrane pore both in three-dimensional crystals and on a biological membrane.
Full text
PDF

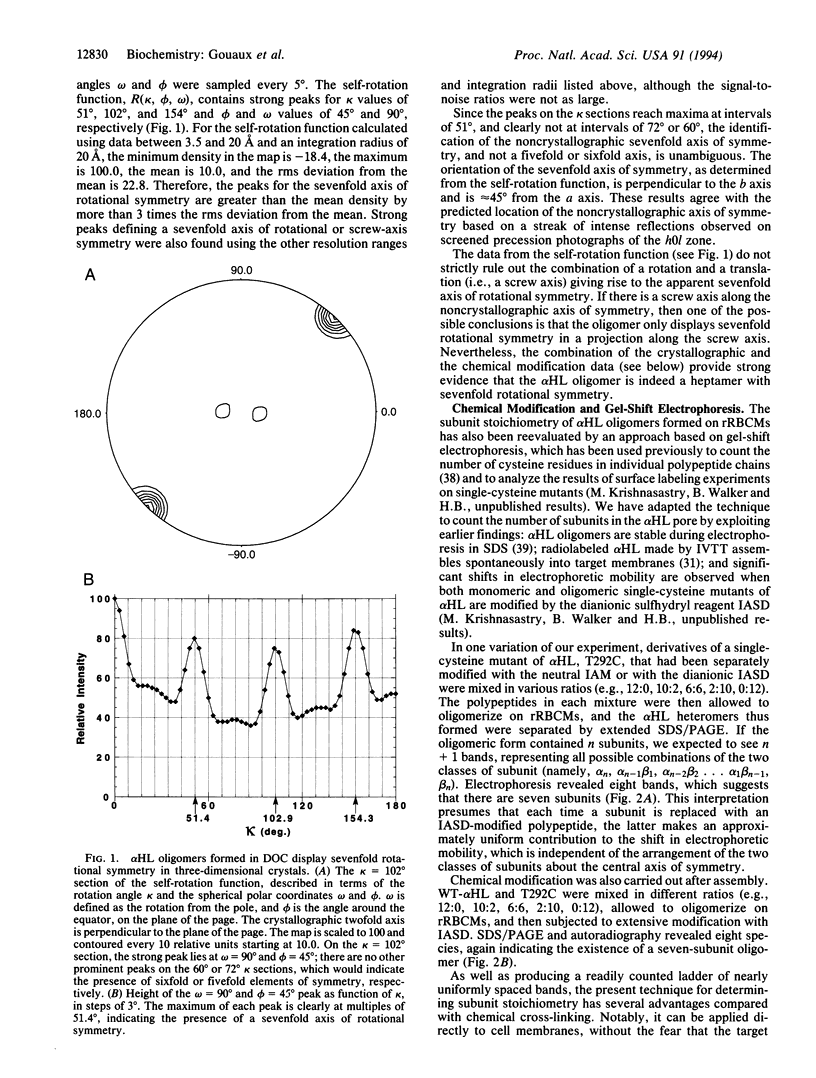
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
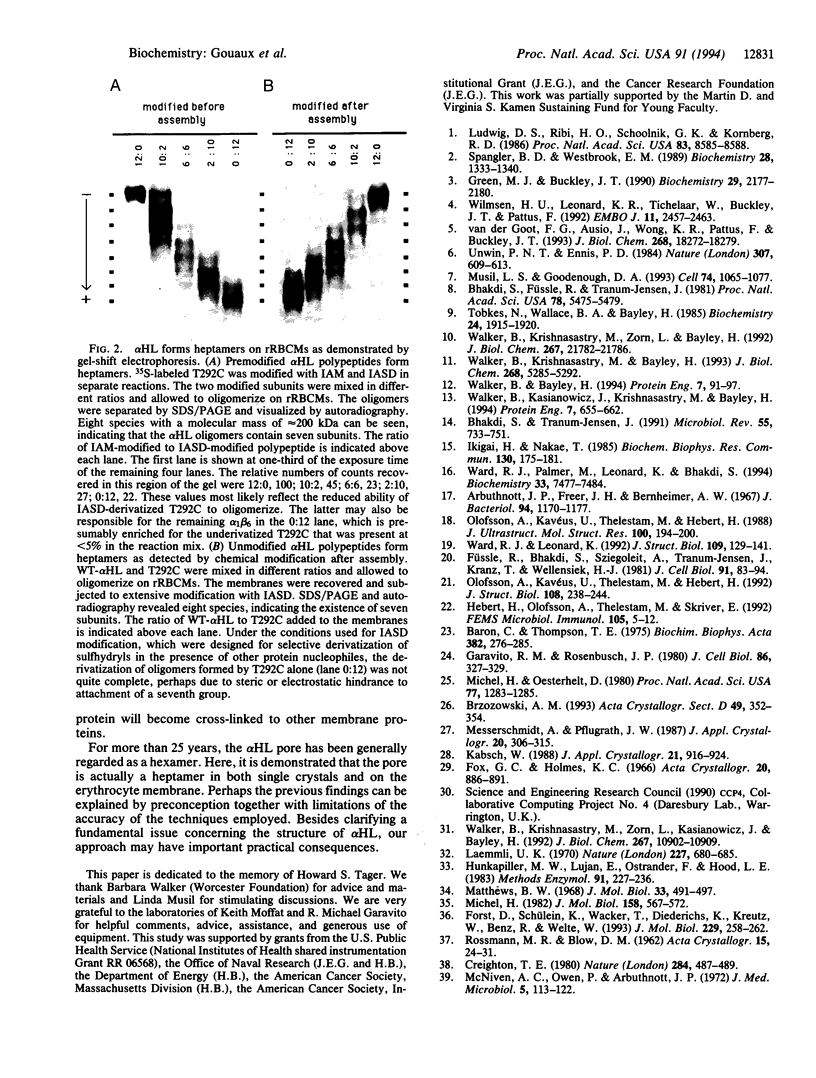
- Arbuthnott J. P., Freer J. H., Bernheimer A. W. Physical states of staphylococcal alpha-toxin. J Bacteriol. 1967 Oct;94(4):1170–1177. doi: 10.1128/jb.94.4.1170-1177.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski A. M. Crystallization of a Humicola lanuginosa lipase-inhibitor complex with the use of polyethylene glycol monomethyl ether. Acta Crystallogr D Biol Crystallogr. 1993 May 1;49(Pt 3):352–354. doi: 10.1107/S0907444993000952. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Counting integral numbers of amino acid residues per polypeptide chain. Nature. 1980 Apr 3;284(5755):487–489. doi: 10.1038/284487a0. [DOI] [PubMed] [Google Scholar]
- Forst D., Schülein K., Wacker T., Diederichs K., Kreutz W., Benz R., Welte W. Crystallization and preliminary X-ray diffraction analysis of ScrY, a specific bacterial outer membrane porin. J Mol Biol. 1993 Jan 5;229(1):258–262. doi: 10.1006/jmbi.1993.1028. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito R. M., Rosenbusch J. P. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol. 1980 Jul;86(1):327–329. doi: 10.1083/jcb.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. J., Buckley J. T. Site-directed mutagenesis of the hole-forming toxin aerolysin: studies on the roles of histidines in receptor binding and oligomerization of the monomer. Biochemistry. 1990 Feb 27;29(8):2177–2180. doi: 10.1021/bi00460a031. [DOI] [PubMed] [Google Scholar]
- Hebert H., Olofsson A., Thelestam M., Skriver E. Oligomer formation of staphylococcal alpha-toxin analyzed by electron microscopy and image processing. FEMS Microbiol Immunol. 1992 Sep;5(1-3):5–12. doi: 10.1111/j.1574-6968.1992.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Conformational alteration in alpha-toxin from Staphylococcus aureus concomitant with the transformation of the water-soluble monomer to the membrane oligomer. Biochem Biophys Res Commun. 1985 Jul 16;130(1):175–181. doi: 10.1016/0006-291x(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Ribi H. O., Schoolnik G. K., Kornberg R. D. Two-dimensional crystals of cholera toxin B-subunit-receptor complexes: projected structure at 17-A resolution. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8585–8588. doi: 10.1073/pnas.83.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McNiven A. C., Owen P., Arbuthnott J. P. Multiple forms of staphylococcal alpha-toxin. J Med Microbiol. 1972 Feb;5(1):113–122. doi: 10.1099/00222615-5-1-113. [DOI] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Three-dimensional crystals of membrane proteins: bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1283–1285. doi: 10.1073/pnas.77.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993 Sep 24;74(6):1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Olofsson A., Kavéus U., Thelestam M., Hebert H. The projection structure of alpha-toxin from Staphylococcus aureus in human platelet membranes as analyzed by electron microscopy and image processing. J Ultrastruct Mol Struct Res. 1988 Aug;100(2):194–200. doi: 10.1016/0889-1605(88)90026-2. [DOI] [PubMed] [Google Scholar]
- Olofsson A., Kavéus U., Thelestam M., Hebert H. The three-dimensional structure of trypsin-treated Staphylococcus aureus alpha-toxin. J Struct Biol. 1992 May-Jun;108(3):238–244. doi: 10.1016/1047-8477(92)90024-5. [DOI] [PubMed] [Google Scholar]
- Spangler B. D., Westbrook E. M. Crystallization of isoelectrically homogeneous cholera toxin. Biochemistry. 1989 Feb 7;28(3):1333–1340. doi: 10.1021/bi00429a059. [DOI] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Two configurations of a channel-forming membrane protein. Nature. 1984 Feb 16;307(5952):609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Walker B., Bayley H. A pore-forming protein with a protease-activated trigger. Protein Eng. 1994 Jan;7(1):91–97. doi: 10.1093/protein/7.1.91. [DOI] [PubMed] [Google Scholar]
- Walker B., Kasianowicz J., Krishnasastry M., Bayley H. A pore-forming protein with a metal-actuated switch. Protein Eng. 1994 May;7(5):655–662. doi: 10.1093/protein/7.5.655. [DOI] [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Bayley H. Functional complementation of staphylococcal alpha-hemolysin fragments. Overlaps, nicks, and gaps in the glycine-rich loop. J Biol Chem. 1993 Mar 5;268(7):5285–5292. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Bayley H. Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J Biol Chem. 1992 Oct 25;267(30):21782–21786. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Kasianowicz J., Bayley H. Functional expression of the alpha-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. Deletion of five C-terminal amino acids selectively impairs hemolytic activity. J Biol Chem. 1992 May 25;267(15):10902–10909. [PubMed] [Google Scholar]
- Ward R. J., Leonard K. The Staphylococcus aureus alpha-toxin channel complex and the effect of Ca2+ ions on its interaction with lipid layers. J Struct Biol. 1992 Sep-Oct;109(2):129–141. doi: 10.1016/1047-8477(92)90044-b. [DOI] [PubMed] [Google Scholar]
- Ward R. J., Palmer M., Leonard K., Bhakdi S. Identification of a putative membrane-inserted segment in the alpha-toxin of Staphylococcus aureus. Biochemistry. 1994 Jun 14;33(23):7477–7484. doi: 10.1021/bi00189a056. [DOI] [PubMed] [Google Scholar]
- Wilmsen H. U., Leonard K. R., Tichelaar W., Buckley J. T., Pattus F. The aerolysin membrane channel is formed by heptamerization of the monomer. EMBO J. 1992 Jul;11(7):2457–2463. doi: 10.1002/j.1460-2075.1992.tb05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot F. G., Ausio J., Wong K. R., Pattus F., Buckley J. T. Dimerization stabilizes the pore-forming toxin aerolysin in solution. J Biol Chem. 1993 Aug 25;268(24):18272–18279. [PubMed] [Google Scholar]