Abstract
Objectives
Adenotonsillar hypertrophy is the most common etiology in pediatric obstructive sleep apnea syndrome (OSAS), and adenotonsillectomy is the mainstay of treatment modalities. This study evaluates the long-term effectiveness of adenotonsillectomy in children with OSAS.
Methods
Subjective symptoms evaluated with a 7-point Likert scale and objective respiratory disturbances evaluated by polysomnography were compared before and after adenotonsillectomy.
Results
A total of 17 children with OSAS aged 4-15 years (mean age, 6.65±3.02 years; male:female, 13:4) completed the study. The mean follow-up period was 57 months (range, 30 to 98 months). Significant changes were found in apnea-hypopnea index (from 12.49±12.96 to 1.96±2.01, P<0.001), apnea index (from 5.64±7.57 to 0.53±0.78, P=0.006), minimum SaO2 (from 81.88±14.36 to 92.76±4.31, P=0.003), snoring (from 43.28±70.63 to 10.70±13.72, P=0.042), and arousal index (from 19.58±7.57 to 11.36±3.99, P=0.006) after adenotonsillectomy. Significant changes were also found after surgery in most of symptoms including snoring, witnessed apnea, morning headache, mouth breathing, gasping during sleep, restless sleep, nasal obstruction, and difficulty with morning arousal. Long-term surgical cure rate and response rate were 47.1% (8/17) and 70.6% (12/17), respectively.
Conclusion
Most of subjective OSAS symptoms and objective respiratory disturbances improved continuously about 5 years after adenotonsillectomy in children with OSAS. However, close follow-up and a sufficient observation period are necessary because of the risk for long-term incomplete resolution.
Keywords: Child, Obstructive Sleep Apnea, Tonsillectomy, Adenoidectomy, Treatment
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a common disease in childhood, with a prevalence of 1% to 3% [1,2,3,4]. Pediatric OSAS is characterized by repetitive partial or complete collapse and/or prolonged narrowing of the upper airway during sleep [5]. The most common cause of upper airway obstruction in pediatric OSAS is adenotonsillar hypertrophy [6]. Narrowing of the upper airway because the adenoids and tonsils occupy most of the pharyngeal space is common in children aged 3 to 8 years. Therefore pediatric OSAS incidence peaks in preschool children [7].
Diverse symptoms can be found in children with OSAS, such as snoring, mouth breathing, restless sleep, nasal obstruction, witnessed apnea, and difficulty with morning arousal [6]. If undiagnosed or untreated, OSAS can increase the risk of growth problems (failure to thrive), neurocognitive consequences, and cardiovascular complications [8,9,10]. Early diagnosis and adequate treatment are required to treat or prevent these symptoms and morbidities.
Adenotonsillectomy is the primary treatment in the majority of children with OSAS [1]. Numerous studies have evaluated the effectiveness of adenotonsillectomy in pediatric OSAS, and earlier investigations reported that the surgical success rate appears to vary from 52.9% to 90.5% [11,12]. Although adenotonsillectomy is a highly effective therapeutic method, recent studies suggest that it is not a perfect one [13,14]. Factors related to incomplete resolution after surgery are thought to include obesity, severe OSAS, and asthma [13]. Therefore, a sufficient observational period to monitor residual or recurrent cases is very important to determine the exact effect of adenotonsillectomy on pediatric OSAS.
Despite the volume of clinical research on the efficacy of adenotonsillectomy in children with OSAS [11,12,13], long-term follow-up studies comparing symptoms and polysomnography before and after adenotonsillectomy are relatively rare. Furthermore, the literature contains little information about the long-term effectiveness of adenotonsillectomy in Asian children with OSAS. Therefore, this study evaluated the long-term subjective and objective outcomes of adenotonsillectomy in Korean children with OSAS.
MATERIALS AND METHODS
Subjects
This study was approved by the Institutional Review Board of Korea University Ansan Hospital (AS11184). The inclusion criteria were as follows: children who (1) complained of diverse symptoms and/or signs associated with OSAS; (2) underwent standard, in-laboratory polysomnography preoperatively; (3) were diagnosed with pediatric OSAS based on the International Classification of Sleep Disorders, 2nd edition [5]; (4) were treated with adenotonsillectomy between January 2004 and December 2009; and (5) completed a questionnaire and standard polysomnography at least 30 months after surgery. The exclusion criteria were as follows: children (1) with craniofacial abnormality or neuromuscular disease; (2) with developmental delay or psychiatric disorder; or (3) who refused postoperative standard polysomnography or participation in the current study.
Symptoms and signs
The children and their caregivers were asked to describe the children's OSAS symptoms using a questionnaire before and after surgery. The questionnaire asked about common symptoms and signs, including snoring, witnessed apnea, daytime sleepiness, morning headache, mouth breathing, memory reduction, daytime fatigue, gasping during sleep, restless sleep, nasal obstruction, rhinorrhea, sneezing, itching, postnasal drip, and difficulty with morning arousal [6]. These symptoms and signs were evaluated using a 7-point Likert scale ranging from 0 (none of the time) to 6 (all of the time).
Physical findings
Height and weight were measured for each subject. Body mass index (BMI), defined as bodyweight (kg) divided by height squared (m2), was calculated from each subject's weight and height. To evaluate each child's overall physical growth on a chart of the general population, we also calculated the z-score of BMI and height that adjusted the value by age and sex based on the 2007 Korean National Growth Charts [15,16].
Tonsil size was determined by visual inspection of the otorhinolaryngologist. Palatine tonsils were graded from 1 to 4 (grade 1, 0% to 24%; grade 2, 25% to 49%; grade 3, 50% to 74%; grade 4, above 75% in relation to the oropharynx), according to the degree of oropharyngeal space occupied [17].
To assess adenoid size, all patients underwent radiologic evaluation preoperatively [18]. Adenoidal hypertrophy was diagnosed when the adenoid-nasopharynx ratio was higher than 0.67 [19]. In most children, tonsil size exceeded 50% (grade 3) and adenoid size exceeded 75%.
Polysomnographic data
Preoperative and postoperative standard overnight polysomnography were carried out for all subjects using a computerized polysomnographic device (Alice 4, Respironics, Atlanta, GA, USA). The sleep stage and respiratory events before and after surgery were scored according to the manual of the American Academy of Sleep Medicine [20]. The following polysomnographic parameters were checked: total sleep time (TST, minutes), sleep efficiency (%), arousal index (events/hour of TST), sleep stages (N1, N2, N3, and R; % of TST), apnea-hypopnea index (AHI, events/hour of TST), apnea index (events/hour of TST), minimum SaO2 (arterial oxygen saturation, %), and snoring (% of TST).
Statistical analysis
Statistical analysis was carried out using the IBM SPSS 20.0 (IBM Co., Armonk, NY, USA). The paired t-test was used for parametric comparisons before and after surgery. The Wilcoxon signed-rank test was used for nonparametric comparisons before and after surgery. A P-value <0.05 was considered statistically significant.
RESULTS
Subjects
A total of 17 subjects, thirteen male and four female children (mean age, 6.65±3.02 years; mean BMI, 18.30±2.93 kg/m2) completed this study. The mean follow-up period was 57 months (range, 30 to 98 months). Changes in BMI before and after surgery are shown in Table 1. There were significant changes after adenotonsillectomy in BMI and BMI z-score.
Table 1. Changes in body mass index (BMI) before and after adenotonsillectomy (n=17).
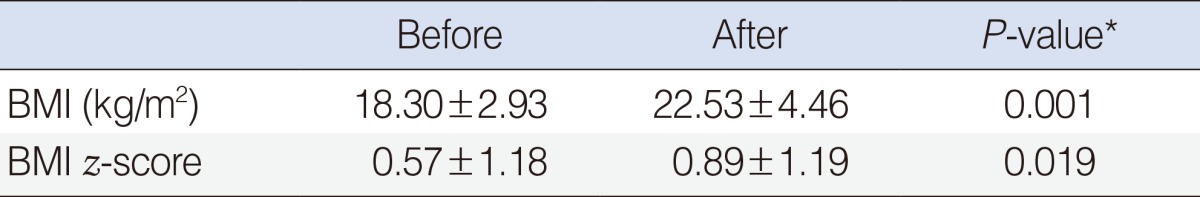
Values are presented as mean±SD.
*P<0.05, statistically significant difference.
Symptoms and signs
Changes in subjective symptoms before and after surgery are presented in Table 2. After adenotonsillectomy, most of subjective OSAS symptoms changed significantly, including snoring (from 5.53±1.07 to 2.53±2.00, P=0.001), witnessed apnea (from 2.94±2.11 to 0.82±1.47, P=0.005), morning headache (from 1.82±1.62 to 0.59±1.12, P=0.015), mouth breathing (from 5.41±1.33 to 2.71±1.83, P=0.001), gasping during sleep (from 2.00±2.10 to 0.50±1.03, P=0.042), restless sleep (from 4.38±1.82 to 2.25±1.69, P=0.003), nasal obstruction (from 4.53±1.50 to 2.53±1.97, P=0.004), and difficulty with morning arousal (from 3.56±2.13 to 1.65±2.00, P=0.011). However, no significant changes were found after surgery in other OSAS symptoms (daytime sleepiness, memory reduction, daytime fatigue) or nasal symptoms (rhinorrhea, sneezing, itching, postnasal drip).
Table 2. Changes in subjective symptoms before and after adenotonsillectomy (n=17).
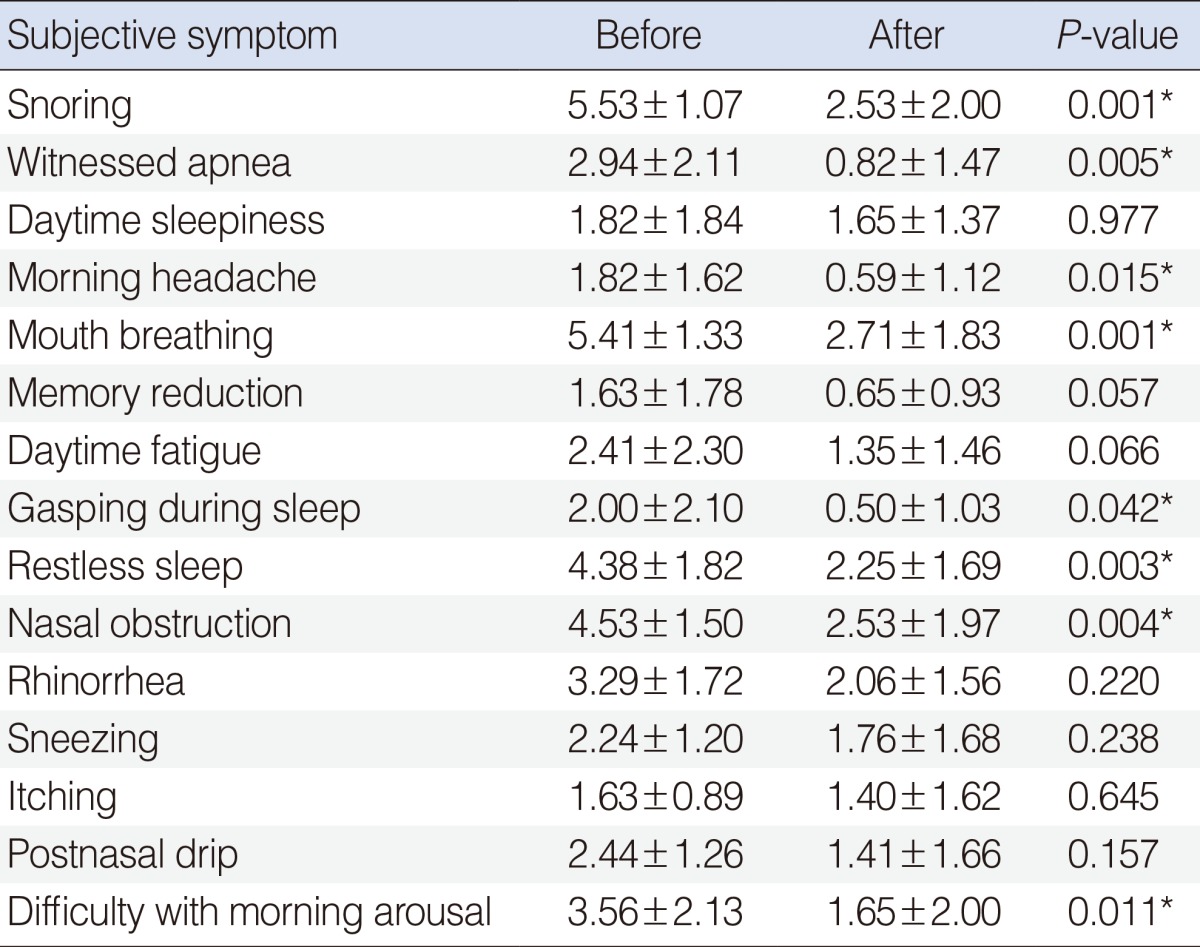
Values are presented as mean±SD.
*P<0.05, statistically significant difference.
Polysomnographic data
Changes in polysomnographic data before and after surgery are summarized in Table 3. After surgery, all respiratory parameters changed significantly, including AHI (from 12.49±12.96 to 1.96±2.01, P<0.001), apnea index (from 5.64±7.57 to 0.53±0.78, P=0.006), minimum SaO2 (from 81.88±14.36 to 92.76±4.31, P=0.003), and snoring (from 43.28±70.63 to 10.70±13.72, P=0.042). In sleep parameters, significant changes were found in arousal index (from 19.58±7.57 to 11.36±3.99, P=0.006), stage N2 (from 41.01±6.70 to 50.26±6.36, P=0.001), and stage N3 (from 28.69±6.90 to 23.85±8.23, P=0.007), whereas significant postoperative changes were not observed in TST, sleep efficiency, sleep stage N1, and sleep stage R.
Table 3. Change in objective polysomnographic data before and after adenotonsillectomy (n=17).
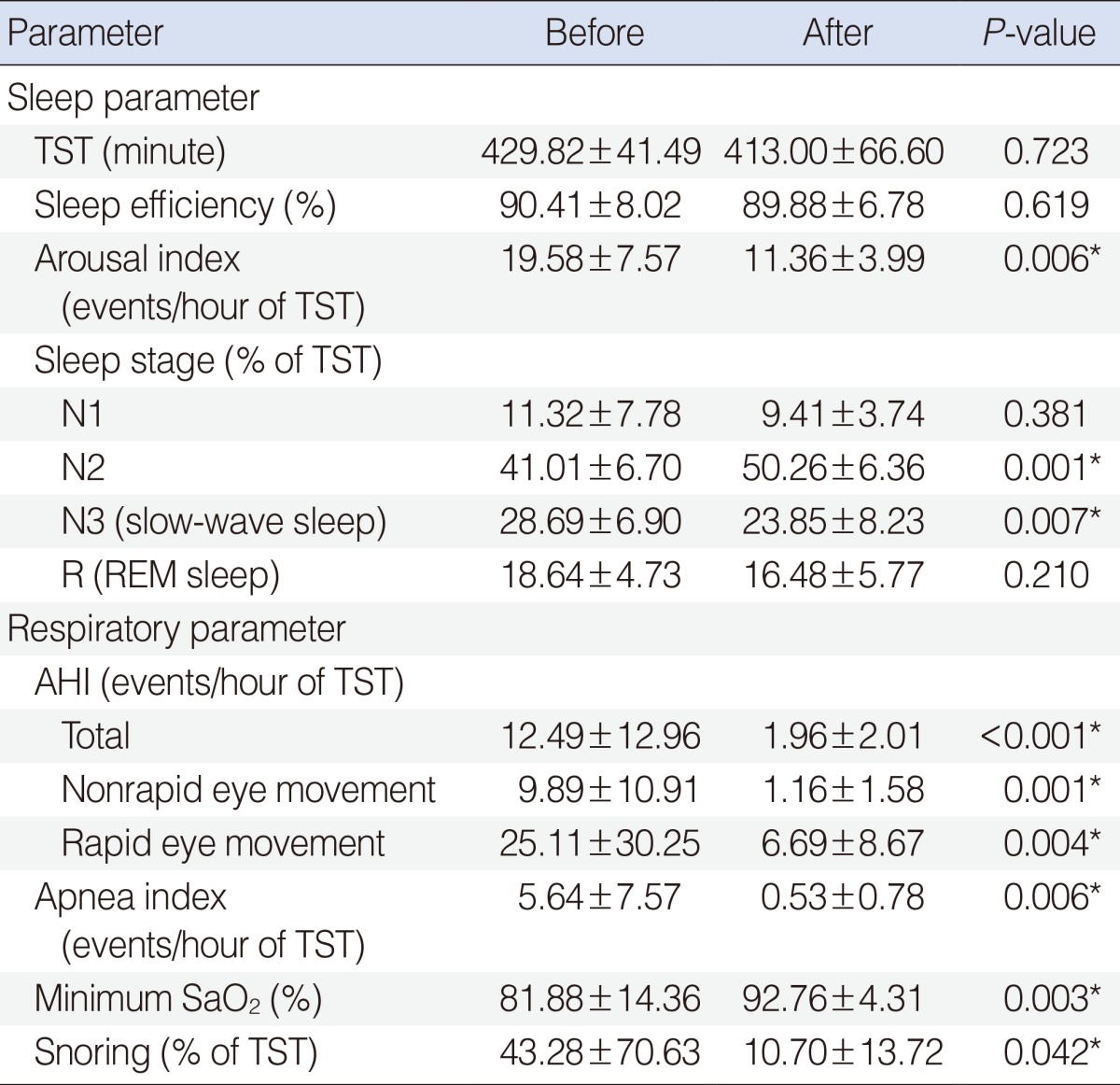
Values are presented as mean±SD.
TST, total sleep time; AHI, apnea-hypopnea index; SaO2, arterial oxygen saturation.
*P<0.05, statistically significant difference.
Surgical cure and response rate
When surgical cure was defined as reduction of AHI ≥75% and postoperative AHI <1, the long-term cure rate of adenotonsillectomy was 47.1% (8/17) in the present study. If surgical response rate was defined as reduction of AHI ≥75% and postoperative AHI <5, the long-term response rate was 70.6% (12/17).
DISCUSSION
We conducted this study to assess the long-term effectiveness of adenotonsillectomy on subjective symptoms and objective respiratory disturbances among Korean pediatric patients with OSAS. The key findings of our study are as follows: (1) Most of subjective OSAS symptoms improved significantly with at least five years after adenotonsillectomy; (2) Significant alleviations were found postoperatively in polysomnographic parameters associated with respiratory disturbances; and (3) The five-year cure rate and response rate of adenotonsillectomy in Korean children with OSAS were 47.1% (8/17) and 70.6% (12/17), respectively.
Adenotonsillectomy is regarded as the most effective treatment method in most OSAS children with adenotonsillar hypertrophy. Many investigations have demonstrated relatively good outcomes for adenotonsillectomy [12,21,22]. According to the meta-analysis by Brietzke and Gallagher [21] evaluating the effect of adenotonsillectomy on pediatric OSAS, the mean change in AHI was a decrease of 13.92 events per hour (95% confidence interval [CI], 10.05 to 17.79; P<0.001). They also reported that the overall success rate with adenotonsillectomy was 82.9% (95% CI, 76.2 to 89.5; P<0.001) based on a comparison between preoperative and postoperative polysomnographic data from 14 studies [21]. There is a possibility that the high success rate is related to the inclusion of several studies using generous success criteria (e.g., postoperative AHI<5). In our previous study regarding the effect of adenotonsillectomy in Korean children with OSAS, the overall cure rate was 90.9% (20/22) 3 months after surgery [22].
However, recent clinical research has shown surgical results that were not as good as expected in pediatric OSAS [13,14]. To determine the efficacy of adenotonsillectomy in treating OSAS in children, Bhattacharjee et al. [13] performed a retrospective multicenter study in which adenotonsillectomy led to significant alleviation of AHI (from 18.2±21.4 to 4.1±6.4, P<0.001) in most children with OSAS, whereas the overall cure (complete resolution) rate was relatively low at 27.2% (157/578). The low cure rate is presumably associated with the high proportion of obese children (50.6%) in the study population. In our study, we also saw that BMI z-score significantly increased after adenotonsillectomy. Significant elevation of BMI z-score might lead to long-term incomplete resolution (residual or recurrent disease) after surgery.
In the current study, significant improvements were postoperatively observed in most of subjective OSAS symptoms, including snoring, witnessed apnea, restless sleep, and difficulty with morning arousal. However, after surgery, there were no significant changes in a few OSAS symptoms, such as daytime sleepiness. These results are thought to be primarily related to well-sustained stage N3 (slow-wave or deep sleep) caused by the increased arousal threshold of childhood [6,7]. Also, daytime sleepiness is a relatively uncommon complaint in pediatric OSAS [6]. Therefore, the chance for alleviation after surgery may be small because the preoperative symptomatic level of daytime sleepiness is low.
Although the literature contains a great deal of research on the efficacy of pediatric sleep apnea surgery, most studies identified only short-term outcomes. The aforementioned meta-analysis of 14 articles reported that the mean period between preoperative and postoperative polysomnographic evaluations was 98 days [21]. One of the strengths of this study was the relatively long mean follow-up duration of 57 months (range, 30 to 98 months).
Interestingly, we found that stage N3 significantly reduced after surgery in long-term follow-up, despite the improvement of OSAS. These results might be associated with the natural course of sleep stage development in children. Generally, the proportion of stage N2 increased with age in children and adolescents, while the proportion of stage N3 decreased [23].
The present study had several limitations. First, it was not a multi-institutional randomized controlled trial (RCT). In particular, our study had no control group. The multicenter, single-blind RCT by Marcus et al. [24] reported a cure rate (normalization of OSAS) significantly higher in the early adenotonsillectomy group than in the watchful waiting group (79% vs. 46%, P<0.001). Second, our study sample size was relatively small. Third, the subjects of our study were not representative of the general pediatric population with OSAS. After consideration of these limitations, future studies will be needed to confirm the long-term efficacy of adenotonsillectomy in pediatric OSAS.
In conclusion, this study found that most of subjective OSAS symptoms and objective respiratory parameters improved significantly at least 5 years after adenotonsillectomy in Korean children with OSAS. However, close follow-up and a sufficient observation period are important in pediatric OSAS because of the risk for long-term incomplete resolution (residual or recurrent disease).
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090084).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008 Feb;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009 Jun;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003 Apr;142(4):383–389. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic & coding manual. 2nd ed. Westchester (NY): American Academy of Sleep Medicine; 2005. [Google Scholar]
- 6.Choi JH, Kim EJ, Choi J, Kwon SY, Kim TH, Lee SH, et al. Obstructive sleep apnea syndrome: a child is not just a small adult. Ann Otol Rhinol Laryngol. 2010 Oct;119(10):656–661. doi: 10.1177/000348941011901002. [DOI] [PubMed] [Google Scholar]
- 7.Nixon GM, Brouillette RT. Sleep. 8: paediatric obstructive sleep apnoea. Thorax. 2005 Jun;60(6):511–516. doi: 10.1136/thx.2003.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieminen P, Lopponen T, Tolonen U, Lanning P, Knip M, Lopponen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002 Apr;109(4):e55. doi: 10.1542/peds.109.4.e55. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010 Nov;126(5):e1161–e1167. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 10.Li AM, Au CT, Sung RY, Ho C, Ng PC, Fok TF, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008 Sep;63(9):803–809. doi: 10.1136/thx.2007.091132. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Li KK, Khramtsov A, Pelayo R, Martinez S. Sleep disordered breathing: surgical outcomes in prepubertal children. Laryngoscope. 2004 Jan;114(1):132–137. doi: 10.1097/00005537-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Nieminen P, Tolonen U, Lopponen H. Snoring and obstructive sleep apnea in children: a 6-month follow-up study. Arch Otolaryngol Head Neck Surg. 2000 Apr;126(4):481–486. doi: 10.1001/archotol.126.4.481. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010 Sep;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 14.Huang YS, Guilleminault C, Lee LA, Lin CH, Hwang FM. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014 Jan;37(1):71–76. doi: 10.5665/sleep.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chae HW, Suh I, Kwon AR, Kim YJ, Kim YH, Kang DR, et al. Longitudinal standards for height and height velocity in Korean children and adolescents: the Kangwha study [corrected] J Korean Med Sci. 2013 Oct;28(10):1512–1517. doi: 10.3346/jkms.2013.28.10.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo JY, Cho YG, Kang JH, Hur YI, Park HA, Kim KW, et al. New diagnostic criteria for obesity and overweight in Korean children and adolescents using 2007 Korean National Growth Charts. Obes Res Clin Pract. 2013 May-Jun;7(3):e182–e189. doi: 10.1016/j.orcp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987 Aug;13(2):149–156. doi: 10.1016/0165-5876(87)90091-7. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979 Sep;133(3):401–404. doi: 10.2214/ajr.133.3.401. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Jiaqing A, Yuchuan L, Shen K. A case-control study of obstructive sleep apnea-hypopnea syndrome in obese and nonobese chinese children. Chest. 2008 Mar;133(3):684–689. doi: 10.1378/chest.07-1611. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester (NY): American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 21.Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg. 2006 Jun;134(6):979–984. doi: 10.1016/j.otohns.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Choi JH, Kim EJ, Choi J, Kim TH, Kwon SY, Lee SH, et al. The effect of adenotonsillectomy on changes of position during sleep in pediatric obstructive sleep apnea syndrome. Am J Rhinol Allergy. 2009 Nov-Dec;23(6):e56–e58. doi: 10.2500/ajra.2009.23.3363. [DOI] [PubMed] [Google Scholar]
- 23.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004 Nov;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013 Jun;368(25):2366–2376. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]


