Abstract
Objectives
Phosphorylated (activated) STAT3 (pSTAT3) is a regulator of numerous genes that play an essential part in the onset, development and progression of cancer; it is involved in cell proliferation and preventing apoptosis, and in invasion, angiogenesis, and the evasion of immune surveillance. This study aimed mainly to investigate the potential prognostic role of pSTAT3 expression in oral tongue squamous cell carcinoma (SCC).
Methods
Phospho-ser727 STAT3 immunolabeling was correlated with prognostic parameters in 34 consecutive cases of pT1-T2 tongue SCCs undergoing primary surgery. Computer-based image analysis was used for the immunohistochemical reactions analysis.
Results
Statistical analysis showed a difference in disease-free survival (DFS) when patients were stratified by pN status (P=0.031). Most tumors had variable degrees (mean±SD, 80.7%±23.8%) of intense nuclear immunoreaction to pSTAT3. Our findings rule out any significant association of serine-phosphorylated nuclear STAT3 expression with tumor stage, grade, lymph node metastasis, recurrence rate, or DFS.
Conclusion
In spite of these results, it is worth further investigating the role of pSTAT3 (serine- and tyrosine-pSTAT3) in oral tongue SCC in larger series because preclinical models are increasingly showing that several anticancer strategies would benefit from STAT3 phosphorylation inhibition.
Keywords: Tongue Neoplasms, STAT3 Transcription Factor, Lymph Nodes, Prognosis
INTRODUCTION
In 2008, with an estimated 263,000 new cases, oral (including lip) squamous cell carcinoma (SCC) ranked as the 15th most common cancer worldwide when the global incidence and related mortality of 27 malignancies was estimated for 182 countries by the International Agency for Research on Cancer as part of the GLOBOCAN scheme [1]. The most common site of intraoral SCC is the tongue, which accounts for around 40% of all cases involving the oral cavity proper.
Despite advances in surgery (including reconstructive techniques) and radiotherapy and chemotherapy, the five-year survival rate for oral cancer has not improved significantly over the past several decades and it is still around 50% to 55%. There is an undeniable need for novel, more effective diagnostic and therapeutic strategies to improve the overall survival of patients with oral SCC, particularly in advanced cases. Very recent research has focused on targeted molecular therapy, immunotherapy, and latent/residual tumor cell ablation. Although many promising therapies have been tested, only anti-epidermal growth factor receptor (anti-EGFR) therapy has been approved by the U.S. Food and Drug Administration in recent years for use in SCCs of the head and neck.
STAT3 is a member of the STAT family that takes part in the normal cellular responses to cytokines and growth factors, such as transcription factors. STAT3 occurs in the cytoplasm under basal conditions and is activated as a consequence of the ligand-mediated receptor activation induced by Janus tyrosine kinases or growth factors. It seems that, while STAT2, STAT4, and STAT6 are only activated under normal conditions, STAT1, STAT3, and STAT5 would appear to have an important part to play in cancer (STAT1 as a tumor suppressor, and STAT3, and STAT5 as oncogenes) [2]. In normal cells, the ligand-dependent activation of the STATs is a transient process, lasting from a few minutes to several hours. In tumors, STAT proteins remain persistently phosphorylated, and consequently activated: phosphorylated STAT3 (pSTAT3) dimerizes and moves to the nucleus, regulating the transcription of target genes. There are reports of STAT3 being constitutively activated in lymphomas and in numerous primary cancers affecting the prostate, breast, lung, colon, liver or pancreas, and in head and neck carcinomas too [3,4,5]. The C-terminal transactivation domain of STAT3 has an important role in its activation through a tyrosine residue at position 705 and a serine residue at position 727 [2]. In particular, phosphorylation of the ser-727 site by several serine kinases, such as mammalian target of rapamycin, protein kinase C (PKC) delta, cyclin-dependent kinase 5, and PKC-ε, determines the maximal transcriptional activity of STAT3 [4]. pSTAT3 regulates many of the genes that play a part in a cancer's initiation, development, and progression, affecting cell proliferation, interfering with apoptosis, and supporting invasion, angiogenesis, and immune surveillance evasion [2,4]. It has also recently been reported that, in head and neck carcinoma, pSTAT3 is a downstream effector of EGFR, interleukin (IL) 6, and the Src family of kinases [6,7].
The aim of this study was to conduct a preliminary (because of the retrospective setting and the limited number of considered cases) investigation into the potentially prognostic role of pSTAT3 expression in a series of consecutive cases of pT1-T2 of primary oral tongue SCC. This is the first time anti-phospho-ser727 STAT3 antibody has been used in oral tongue SCC.
MATERIALS AND METHODS
Patients
The study was approved by the Padova University Otolaryngology Section's Ethical Committee. It involved 34 consecutive cases (25 male patients, 9 female ones; mean age, 66.2±12.2 years) of primary pT1-2 tongue SCC. Twenty-eight out of 34 cases had been previously evaluated for angiogenin, endoglin, and Mib-1 expressions [8]. Upper aero-digestive tract endoscopy and neck ultrasonography (with or without fine needle aspiration cytology), contrast-enhanced computed tomography (CT) or/and magnetic resonance imaging of the head and neck, chest X-rays, and liver ultrasonography were performed in all patients.
After primary partial glossectomy with unilateral or bilateral cervical lymph node dissection (at the Otolaryngology Section of Padova University), the final histopathology report confirmed that all patients had negative surgical margins. According to the TNM classification of the International Union Against Cancer (Union Internationale Contre le Cancer) and the American Joint Committee on Cancer (7th edition) [9], the pathological stage of the primary tongue lesions (pT) was T1 in 16 cases, and T2 in 18; the regional lymph nodes were pathologically staged (pN) as N0 in 26 cases and N+ in eight. Twenty-nine patients underwent elective neck dissection, five therapeutic neck dissection. There were no cases of distant metastases. The pathological grade was G1 in 12 cases, G2 in 13, and G3 in nine. Median hospitalization time was 9 days. Eight patients underwent postoperative radiotherapy (PORT) according to current guidelines [10]. The PORT total dose was 60 Gy (conventional fractionation 2 Gy/fraction, once a day). The treatment time was 30 days.
The clinical follow-up schedule (adjustable to patients' individual characteristics) was as follows: (1) once a month for the 1st year after treatment; (2) every 2 months in the 2nd year; (3) every 3 months in the 3rd year; (4) every 4 months in the 4th year; (5) every 6 months in the 5th year; and (6) every 12 months thereafter. Neck ultrasonography and chest X-rays were also performed at least yearly. Contrast-enhanced CT of the neck, total body positron emission tomography, chest CT, and liver ultrasonography were repeated as necessary.
Immunohistochemistry
Immunohistochemical reactions were obtained on sections 4- to 5-µm-thick obtained from the 34 formalin-fixed and paraffin-embedded tumor samples. No cases were excluded because of the lack of representative carcinoma tissue. All immunohistochemical stains were handled automatically (Bond maX, Menarini, Florence, Italy), as described elsewhere [11], using an anti-phospho-ser727 STAT3 primary antibody (rabbit polyclonal, SAB Signalway Antibody, Pearland, TX, USA; working dilution 1:200, 30 minutes, citrate buffer), according to the manufacturer's instructions. The prepared sections were lightly counterstained with hematoxylin. Since their constitutive STAT3 activation [12], breast carcinoma samples served as positive controls, according to producer's suggestions, and sections incubated with no primary antibody as negative controls. For each slide, nuclear immunoreaction in tumor cells was scored separately by two pathologists (FM and RC) on a four-tired scale based on the staining intensity: 0, absent staining; 1+, weak; 2+, moderate; and 3+, strong; as reported elsewhere [6]; the pathologists were unaware of the patients' prognosis.
Image analysis
An image analysis (IA) workstation was used to assess nuclear pSTAT3 expression in all samples. The system comprised a conventional Zeiss Axioskop light microscope (Zeiss, Jena, Germany) with a color digital, Peltier-cooled video camera (MicroPublisher 5.0 RTV, QImaging, Surrey, BC, Canada) that was connected to a PC with Image-Pro Plus software, Version 7 for Windows (Media Cybernetics Inc., Bethesda, MD, USA). Five areas of tumor tissue or morphologically normal mucosa 1,378 µ × 954 µ in size were always examined blindly with the aid of a 495-point sampling grid by that the program superimposed on images acquired with a 50× field of view; the analysis relied on a tailored subroutine (Winrec, Immagini & Computer, Milano, Italy) that enabled the points intercepting the positive and negative areas to be identified and counted interactively. In compliance with stereological best practice, the proportion of the area found positive was calculated as a percentage (%).
Statistical analysis
Fisher exact test and the Mann-Whitney U-test were applied, as appropriate. For disease-free survival (DFS), we considered the months elapsing from when the treatment was completed up until there was a loco-regional recurrence. We used the log-rank test and Cox regression modeling to establish the DFS after stratifying patients in the light of the variables considered and the associated hazards. Considering the low number of considered cases, the median pSTAT3 nuclear expression value among SCCs was used as the threshold for the analyses. Statistical significance was assumed for a P-value of <0.05, while values in the range of 0.05≤ P<0.10 were considered as indicating a statistical trend. The STATA 8.1 (Stata Corp., College Station, TX, USA) was used for all analyses.
RESULTS
Patients' clinical outcome
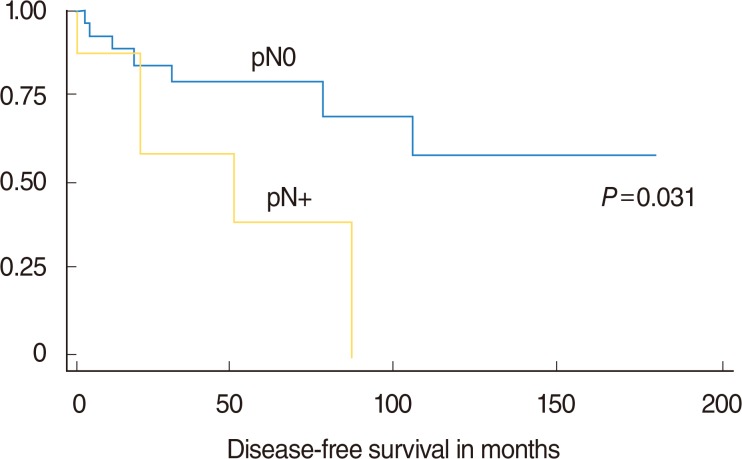
The average follow-up was 61.7±42.3 months (median, 56.0 months). Twelve patients developed SCC recurrences (9 local and 3 loco-regional) after a mean 34.6±33.6 months (median, 19 months). The Mann-Whitney U-test ruled out any significant difference in the mean duration of the follow-up between patients with and without recurrent disease (P=0.88). In accordance with follow-up protocol, 22 patients showed no evidence of recurrent disease at last clinical control; on the other hand, 3 patients died of disease, and 9 of other causes. After grouping patients by whether or not their disease recurred after treatment, Fisher exact test showed a difference in the distribution by lymph node status (pN0 vs. pN+, P=0.09, indicating a trend towards significance), but not for pT (pT1 vs. pT2, P=0.29), or grade (G1 vs. G2-3, P<0.999). The hazard ratio for recurrence during the follow-up considered was 4.5 times higher for pN+ patients, and 2.4 times higher for pT2 cases. The log-rank test found a difference in DFS (in months) when patients were stratified by nodal status ([pN0 vs. pN+, P=0.031]; One patient was staged as cN0 but resulted pN+ [occult metastasis]) (Fig. 1) and pT (pT1 vs. pT2) (P=0.09, trend towards significance), but the same did not apply to grade (G1 vs. G2-3, P=0.86).
Fig. 1. Disease-free survival in oral tongue squamous cell carcinoma patients stratified by pN-status (pN0 vs. pN+).
pSTAT3 expression, nuclear staining intensity, and clinicopathological features of oral tongue SCC
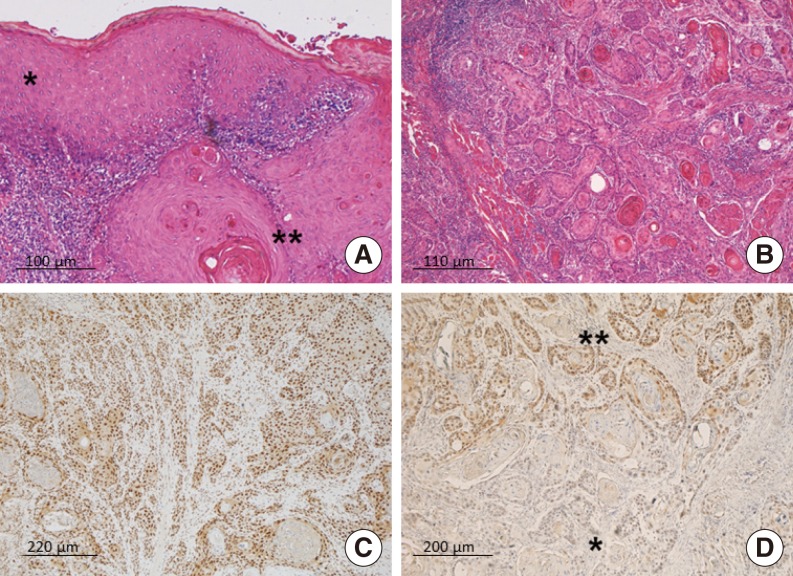
The tongue SCCs covered the whole range from well to poorly differentiated neoplasms (Fig. 2A, B). The well differentiated tumors featured keratinizing squamous epithelium with an abundance of keratin pearls. In cases with a moderate to poor differentiation, neoplastic cells with pronounced cytonuclear atypia were arranged in large anastomosing areas with little or no sign of keratin pearls, or occasionally with formed cords.
Fig. 2. Representative images of the series considered. (A) The micrograph shows normal tongue epithelium (asterisk) adjacent to squamous cell carcinoma (double asterisk) (H&E). (B) In this moderately-differentiated squamous cell carcinoma of the tongue, neoplastic cells infiltrating the muscle are arranged in large anastomosing areas and form keratin pearls (H&E). (C) A squamous cell carcinoma showing a strong, diffuse nuclear immunoreaction to pSTAT3 (pSTAT3 immunohistochemistry). (D) This micrograph shows the absence of pSTAT3 expression in the well-differentiated tumor areas (asterisk), and strong, diffuse immunolabeling in the less differentiated areas (double asterisk) (pSTAT3 immunohistochemistry).
pSTAT3 immunostaining in normal tongue mucosa was considered first with a view to determine its usual pattern of expression. pSTAT3 showed a basal to surface gradient in this stratified squamous epithelium: there was ample, strong nuclear labeling in the basal and spinous layers, while staining in the granular layer was weak and scarce, and there was no immunoreaction in the keratin layer. In normal mucosa, IA measured a mean of 30.4%±1.1% pSTAT3 immunohistochemically positive nuclei. Most of tumors extensively showed an intense nuclear immunoreaction to pSTAT3 (Fig. 2C). Overall, pSTAT3 expression was strong and diffuse in moderately and poorly differentiated tumor, but weak or absent in well differentiated areas, especially if keratin pearls were abundant (Fig. 2D).
All 34 oral tongue SCCs revealed some degree of pSTAT3 immunoreactivity: the intensity of this pSTAT3 nuclear reaction was classed as 1+ in six cases, 2+ in 12, and 3+ in 16. IA indicated a mean nuclear pSTAT3 expression of 80.7%±23.8%, in tongue carcinoma that was remarkably higher compared with of normal mucosa's one (30.4%); the median expression value in carcinoma (90.0%) was used as threshold for the following analyses.
Statistical analysis excluded any significant difference in pSTAT3 expression between pT1 SCCs and pT2 cases (Mann-Whitney U-test, P=0.36), between those with pN0 and pN+ (Mann-Whitney U-test, P=0.40), and between patients with G1 and G2-3 disease (Mann-Whitney U-test, P=0.23). Table 1 shows the mean pSTAT3 expression emerging from IA in the various clinicopathological groups of our sample of oral tongue SCC patients. The Mann-Whitney U-test found no significant relationships between the intensity of the pSTAT3 nuclear reaction and pT (P=0.23), lymph node status (P=0.25), or pathological grade (P=0.49).
Table 1. Image analysis-measured pSTAT3 expression and disease-free survival (DFS) associated with classical pathological variables in oral tongue squamous cell carcinoma.
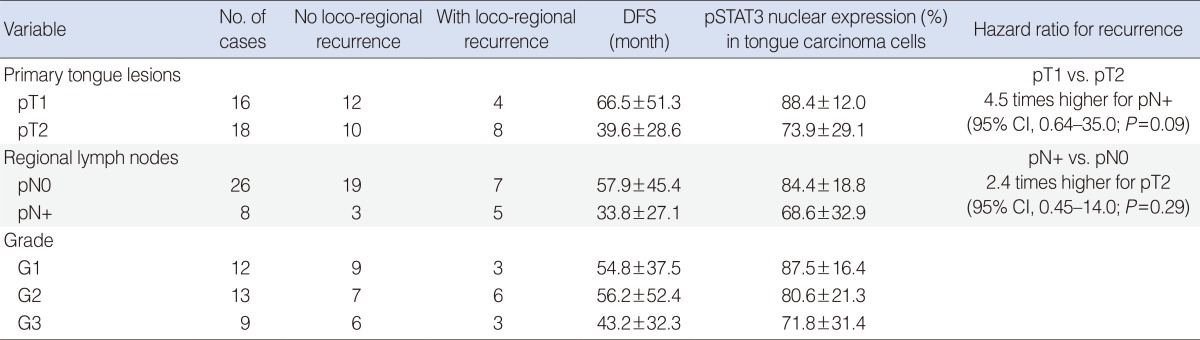
Values are presented as mean±standard deviation.
pSTAT3 expression and prognosis in oral tongue SCC
The Mann-Whitney U-test ruled out any significant difference in pSTAT3 expression between patients with and without recurrent disease (P=0.31). Cox regression model also excluded any significant difference in DFS (in months) for different pSTAT3 expression levels in the oral tongue carcinoma cells (P=0.50). Our statistical analysis also confirmed that there was no significant association between the intensity of the pSTAT3 nuclear reaction and disease recurrence rate (Mann-Whitney U-test, P=0.90) or DFS (log-rank, P=0.98).
DISCUSSION
Given that this was a preliminary investigation, the main strength of the study lies in the homogeneity of the series of patients considered, i.e., (1) they all had primary pT1-T2 oral tongue SCCs; (2) they underwent surgery performed consecutively by the same team; (3) pathological T and N staging was available for all cases; (4) only surgical specimens (not biopsies) of oral tongue SCC were assessed. In addition, the present study focused on the expression of serine-pSTAT3, which has been considerably less thoroughly investigated than tyrosine-pSTAT3 in head and neck SCC, and a computer-based IA system was used to ensure a highly accurate, precise, and reproducible analysis of the immunostained slides. The main weaknesses of our investigation, on the other hand, concern the retrospective setting and the limited number of cases considered.
From a conventional pathological viewpoint, pN stage correlated significantly with prognosis (DFS) in the present series, as already reported extensively for oral tongue SCC. Local invasion and spread to regional lymph nodes in the neck are common in oral SCC, facilitated by the carcinoma's highly-invasive behavior and by the lymphatic drainage in the cervical region [13]. The tongue has a rich blood supply and lymphatic drainage, and this explains why up to 65 percent of patients with primary tongue lesions already have neck disease by the time they are diagnosed.
STAT3 is a transcription factor with important roles in cancer formation and progression; it is involved in several cellular mechanisms, such as proliferation, inhibition of apoptosis, immune escape, epithelial-mesenchymal transition, invasion, and angiogenesis [14,15]. In normal cells, STAT3 is transiently phosphorylated in response to several extracellular signals [16], but in a variety of cancers (including squamous carcinomas of the head and neck) it is constitutively activated, causing neoplastic cell transformation (i.e., acting as an oncogene) [4,7,15,17,18,19]. Although STAT3 activation is not the only, nor even a specific molecular change in head and neck carcinogenesis, Grandis and colleagues demonstrated that it is very common and it occurs early, so it seems to be crucial to maintaining the neoplastic phenotype in this group of cancers [18,19,20,21,22]. Consistently with the above literature and with our previous findings in temporal bone SCCs [6], the tongue SCCs in our sample showed strong (intensity ≥2+ in 28/34 cases) and diffuse nuclear pSTAT3 immunolabeling (mean±SD, 80.7%±23.8%; median, 90.0%).
Investigating the human oral SCC cell line MIKS81-5, Naher et al. [23] found that IL-22 induced a transient tyrosine phosphorylation of STAT3 (the change in serine phosphorylation was subtle); and IL-22 prompted a pSTAT3 translocation into the nucleus. Tyrosine-pSTAT3 immune expression was examined by Seethala et al. [24] in two separate groups of patients with head and neck SCC (involving 61 and 69 cases), and they found pSTAT3 unrelated to clinical outcome in either group. When Macha et al. [25] studied 94 cases of oral SCC, however, they reported that the DFS was significantly shorter for those with higher tyrosine-pSTAT3 levels in the nucleus than for cases not expressing nuclear pSTAT3; and judging from their statistical analysis, nuclear pSTAT3 was the most significant predictor of a poor prognosis. Our findings ruled out any association of serine-phosphorylated nuclear STAT3 expression with tumor stage, grade, lymph node involvement, recurrence rate, or DFS. Zhao et al. [26] analyzed the baseline clinical characteristics of 80 lingual SCC patients vis-à-vis their STAT3 expression, finding that its expression correlated significantly with lymph node metastasis and disease stage. They also correlated STAT3 expression with prognosis in 39 patients, finding its expression significantly related to carcinoma recurrence and death. There are notable differences between our own and the Zhao et al. [26] study, however, that can explain these conflicting results. Our series of tongue SCCs included only locally confined tumors (pT1 and pT2) and only a few cases (8/34) with lymph node disease, and this could have affected our results. We also analyzed a phosphorylated form of STAT3 (in serine 727, reported to be the more active form), ignoring the other variant (phospho-tyrosine 705 STAT3) evaluated by other authors. Since both kinds of phosphorylation appear to activate STAT3 (whether they do so alternately or concurrently remains to be seen), leading to its dimerization and translocation in the nucleus, and consequently to the transcription of its target genes, we cannot definitely rule out any pSTAT3 involvement in tongue SCC. Finally, we considered only nuclear pSTAT3 immunolabeling as evidence of positivity (since pSTAT3 acts as a transcription factor), and a very precise quantification method was used (IA). Rather than the limited number and the loco-regionally homogeneously extended tongue SCCs, the major factor limiting our attempt to correlate pSTAT3 expression with the clinic-pathological variables was pSTAT3 uniformly intense and widespread diffused immunostaining among the series. This indicates that probably other markers are involved in pSTAT3 activity, such as its coactivators CREB-binding Protein/p300, APE1/Ref-1, and NCOA/Src1a, and should be analyzed together [4].
Although our results suggest that pSTAT3 has no prognostic value, its strong and diffuse expression in tongue SCC remains intriguing. Different pathways (transforming growth factor-α/EGFR, IL-6/gp130/Jak, Src, and nicotine) converge on pSTAT3, which might ultimately have a pivotal role in cell proliferation [18,19,27,28,29,30]. This abundance of activators could point to STAT3 having a complementing oncogenic activity, and this would make it an ideal target to investigate for novel therapies.
ACKNOWLEDGMENTS
This study was supported in part by grant No. 60A07-1341/12 (G. Marioni) from the University of Padova. The authors thank Frances Coburn for correcting the English version of the paper.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Dec;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012 Oct;41(4):1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 3.Manni S, Brancalion A, Mandato E, Tubi LQ, Colpo A, Pizzi M, et al. Protein kinase CK2 inhibition down modulates the NF-κB and STAT3 survival pathways, enhances the cellular proteotoxic stress and synergistically boosts the cytotoxic effect of bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS One. 2013 Sep;8(9):e75280. doi: 10.1371/journal.pone.0075280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Dobi E, Monnien F, Kim S, Ivanaj A, N'Guyen T, Demarchi M, et al. Impact of STAT3 phosphorylation on the clinical effectiveness of anti-EGFR-based therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2013 Mar;12(1):28–36. doi: 10.1016/j.clcc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Marioni G, Nucci R, Marino F, Cappellesso R, Pillon M, Zanoletti E, et al. Evaluation of the prognostic role of pSTAT3 expression in temporal bone squamous cell carcinoma. Otol Neurotol. 2013 Oct;34(8):1476–1482. doi: 10.1097/MAO.0b013e3182a036c9. [DOI] [PubMed] [Google Scholar]
- 7.Tsien CI, Nyati MK, Ahsan A, Ramanand SG, Chepeha DB, Worden FP, et al. Effect of erlotinib on epidermal growth factor receptor and downstream signaling in oral cavity squamous cell carcinoma. Head Neck. 2013 Sep;35(9):1323–1330. doi: 10.1002/hed.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marioni G, Staffieri A, Fasanaro E, Stramare R, Giacomelli L, Bernardi E, et al. The role of angiogenin in pT1-T2 tongue carcinoma neo-angiogenesis and cell proliferation: an exploratory study. J Oral Pathol Med. 2013 Sep;42(8):606–611. doi: 10.1111/jop.12053. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 10.National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines) Head and neck cancers. ver. 2. 2013 [Internet] Fort Wathington: National Comprehensive Cancer Network; c2015. [cited 2015 Jun 10]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 11.Rosato A, Menin C, Boldrin D, Dalla Santa S, Bonaldi L, Scaini MC, et al. Survivin expression impacts prognostically on NSCLC but not SCLC. Lung Cancer. 2013 Feb;79(2):180–186. doi: 10.1016/j.lungcan.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001 May;20(20):2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 13.de Sousa SF, Gleber-Netto FO, de Oliveira-Neto HH, Batista AC, Nogueira Guimarães, de Aguiar MC. Lymphangiogenesis and podoplanin expression in oral squamous cell carcinoma and the associated lymph nodes. Appl Immunohistochem Mol Morphol. 2012 Dec;20(6):588–594. doi: 10.1097/PAI.0b013e31824bb3ea. [DOI] [PubMed] [Google Scholar]
- 14.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000 May;19(21):2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004 Feb;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 16.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002 Apr;8(4):945–954. [PubMed] [Google Scholar]
- 17.Li L, Shaw PE. Autocrine-mediated activation of STAT3 correlates with cell proliferation in breast carcinoma lines. J Biol Chem. 2002 May;277(20):17397–17405. doi: 10.1074/jbc.M109962200. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest. 1998 Oct;102(7):1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000 Apr;97(8):4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993 Aug;53(15):3579–3584. [PubMed] [Google Scholar]
- 21.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998 Jun;90(11):824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 22.Rubin Grandis J, Melhem MF, Barnes EL, Tweardy DJ. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer. 1996 Sep;78(6):1284–1292. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1284::AID-CNCR17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Naher L, Kiyoshima T, Kobayashi I, Wada H, Nagata K, Fujiwara H, et al. STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma. Int J Oncol. 2012 Nov;41(5):1577–1586. doi: 10.3892/ijo.2012.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seethala RR, Gooding WE, Handler PN, Collins B, Zhang Q, Siegfried JM, et al. Immunohistochemical analysis of phosphotyrosine signal transducer and activator of transcription 3 and epidermal growth factor receptor autocrine signaling pathways in head and neck cancers and metastatic lymph nodes. Clin Cancer Res. 2008 Mar;14(5):1303–1309. doi: 10.1158/1078-0432.CCR-07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macha MA, Matta A, Kaur J, Chauhan SS, Thakar A, Shukla NK, et al. Prognostic significance of nuclear pSTAT3 in oral cancer. Head Neck. 2011 Apr;33(4):482–489. doi: 10.1002/hed.21468. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Zhang J, Xia H, Zhang B, Jiang T, Wang J, et al. Stat3 is involved in the motility, metastasis and prognosis in lingual squamous cell carcinoma. Cell Biochem Funct. 2012 Jun;30(4):340–346. doi: 10.1002/cbf.2810. [DOI] [PubMed] [Google Scholar]
- 27.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003 Jun;63(11):2948–2956. [PubMed] [Google Scholar]
- 28.Squarize CH, Castilho RM, Sriuranpong V, Pinto DS, Jr, Gutkind JS. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006 Sep;8(9):733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003 Aug;278(34):31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 30.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006 Oct;20(12):2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]