Abstract
Cytomegalovirus (CMV) is associated with poor outcomes, including physical function impairment, in older HIV-uninfected adults. Whether CMV is associated with physical functional impairment in HIV-infected adults is unknown. The primary objective of this study was to determine the relationship between CMV-specific humoral and cell-mediated immune responses with functional impairment in well-controlled HIV infection. In a case-control study, low-function cases were matched by age, gender, and time from HIV diagnosis to high-function controls. Quantitative CMV IgG and %CMV-specific CD8+ and CD4+ T cells (interferon-γ expression following CMV pp65 stimulation) were used to estimate physical function. Among 30 low-function cases and 48 high-function matched controls, CMV IgG ranged from <10 to 8,830 EU/ml, including four controls with results <10 EU/ml. Each log10 increase in CMV IgG was associated with 5-fold greater odds of low function (p=0.01); these findings were robust to adjustment for concomitant CD4+ count, tobacco use, and age; to exclusion of subjects with CMV IgG <10 EU/ml; and to adjustment for hepatitis C viremia. %CMV-specific CD4+ or CD8+ T cells were not associated with low function. In bivariable models, the relationship between CMV IgG and physical function was attenuated and was no longer significant when including IL-6, CD4/CD8 ratio, or the Veterans Aging Cohort Study Index score. High levels of CMV-specific IgG were associated with impaired physical function. Attenuation of the strength of this association in bivariable models suggests an indirect relationship mediated by systemic inflammation and immune suppression.
Introduction
Cytomegalovirus (CMV) infection is ubiquitous among adults with seroprevalence of up to 95% in HIV-uninfected elderly populations,1,2 and as high as 99% in HIV-infected populations.3 After primary infection, CMV typically establishes latency, but periodically reactivates, particularly in the presence of host immune suppression. In contrast to other infections such as influenza or pneumococcus, the immune response to CMV is greater in older age, with increases in both humoral [CMV-specific immunoglobulin G (IgG)] and in the cell-mediated (proportions of CMV-specific T cells) immune responses, possibly reflecting repeated reactivations or reinfections. In healthy middle-aged persons, 4–5% of the total CD4+ or CD8+ T cells produce interferon-γ in response to in vitro CMV antigenic stimulation.4 In elderly persons, CMV-specific T cells can comprise up to 40% of CD8+ T cells,5,6 have shortened telomeres, have little to no proliferative capacity to new antigens, and are resistant to apoptosis.7
CMV seropositivity and high proportions of terminally differentiated CMV-specific T cells characterize an “immune risk profile” that is associated with increased mortality risk among elderly populations.8 An association between an increased proportion of CMV-specific CD4+ T cells or CMV IgG and neurofibrillary tangles in Alzheimer's disease provides evidence of a relationship between immune responses to CMV and cognitive changes.9 Among HIV-uninfected elderly populations, CMV-specific IgG levels are also associated with institutionalization,7 poor functional status,1,10 frailty,2,11,12 and mortality.12–15 Whether CMV directly contributes to complications of aging or whether CMV leads to immune senescence, or immune activation and inflammation, which subsequently lead to physical function impairment and frailty, is unclear. Prior studies have demonstrated associations between CMV IgG and mortality, and CMV IgG and interleukin (IL)-6 and tumor necrosis factor (TNF)-α12,14 suggesting that chronic CMV infection contributes to age-related complications through heightened inflammation.
Both CMV IgG and the percentage of CMV-specific T cells are higher in persons with HIV infection compared to HIV-uninfected controls,3,16 and CMV-specific T cells are higher among HIV-infected persons on antiretroviral therapy (ART) compared to ART-naive persons.3,17 CMV-specific cell-mediated immune responses increase with age such that the highest percentages of CMV-specific CD4+ or CD8+ T cells are seen among HIV-infected older adults on ART.18 These CMV-specific humoral and cell-mediated immune responses are associated with comorbid disease markers among virologically suppressed HIV-infected persons.19–21 Furthermore, reductions in immune activation, as measured by the percentage of CD38+HLA-DR+CD8+ T cells, among HIV-infected, CMV-seropositive participants after treatment with valganciclovir, an inhibitor of CMV replication, provide direct evidence that CMV drives immune activation during chronic HIV infection.22
We have previously demonstrated a strong association between markers of inflammation and immune activation with physical function impairment among middle-aged HIV-infected persons on effective ART.23 The goals of the present study were to (1) determine the relationship between CMV-specific humoral and cell-mediated immune responses and functional impairment in well-controlled HIV infection and (2) explore the impact of clinical characteristics, inflammation, and immune activation on those relationships.
Materials and Methods
Study population
Details of the study population, clinical assessments, and measurement of markers of inflammation, immune activation, and immune senescence have been previously published.23–25 Briefly, individuals with HIV-1 infection on antiretroviral therapy for a minimum of 6 months and no plasma HIV-1 RNA >200 copies/ml within the prior 6 months underwent physical function testing by the Short Physical Performance Battery and Fried's frailty assessment. Cases with low physical function, defined by a combination of deficits on both functional assessments,23 were matched by age, gender, and time since HIV diagnosis to controls with high physical function (no deficits on either functional assessment). Stored samples and existing data were used for the current study. Veterans Aging Cohort Study (VACS) Index scores were calculated as previously described.26 Approval was obtained by the Colorado Multiple Institutional Review Board and informed consent was obtained from all participants.
Quantitative immunoglobulins
Plasma IgG antibodies against CMV, varicella zoster virus (VZV), and combined herpes simplex virus (HSV) 1 and 2 were measured in stored specimens using enzyme immunoassay kits (Diamedix Corp., Miami, FL). All assays were performed according to the manufacturer's instructions, with the following exception: the VZV IgG kit was modified to include a standard curve consisting of the World Health Organization Biological Standard #90/690 (National Institute for Biological Standards and Control, NIBSC, Hertfordshire, UK) diluted from 20 milli-international units per milliliter (mIU) to 0.625 mIU/ml.
CMV-specific T cell responses
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and resuspended in RPMI 1640 plus 10% human Ab serum with the addition of anti-CD28 and -CD49d monoclonal antibodies (mAbs) (1 μg/ml; BD Biosciences, San Jose, CA). Samples with >12% nonviable cells were excluded (one sample). Cells were stimulated with a pool of 15 overlapping CMV pp65 peptides (2 μg/ml of each peptide; NIH AIDS Reference and Reagent Program), staphylococcal enterotoxin B (SEB, 1 μg/ml; Toxin Technologies), or medium control. Cells were incubated at a 45° slant for a total of 6 h at 37°C in a humidified 5% CO2 atmosphere with the addition of 1 μg/ml brefeldin A (BD Biosciences, San Jose, CA) after 2 h of stimulation. Stimulated PBMCs were washed with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and stained for viability 30 min at 4°C (Live/Dead Fixable Aqua Dead Cell Stain for 405 nm; Invitrogen, Life Technologies, Grand Island, NY). Cells were then washed and surface stained with fluorochrome-conjugated mAbs anti-CD4 (Cy5.5), anti-CD3 (Brilliant Violet 605), and anti-CD8 (Alexa 405, all antibodies purchased from BD Biosciences) for 30 min at 4°C. Cells were washed, fixed, permeabilized (Invitrogen), and intracellularly stained with anti-interferon (IFN)-γ (PE Cy7) and anti-TNF-α (Alexa 700) for 30 min at 4°C. Finally, cells were again washed and fixed with 1% formaldehyde.
Fluorescence minus one (FMO) controls were used in all staining. Cells were analyzed using an LSR-II flow cytometer (BD Immunocytometry Systems, San Jose, CA). Electronic compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. The data files were analyzed using Diva software (BD Biosciences) and FlowJo Software (Tree Star, Ashland, OR). Lymphocytes were gated by their forward and side scatter profile. CD3+ cells were selected, and expression of CD4 and CD8 was analyzed in a bivariate dot plot with CD8 to exclude CD4/CD8 double-positive T cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid).
Study design and data analysis
This study used a matched case-control design as previously described,23 except that one case and the matched control were excluded from the current study because adequate specimens were not available. Data were collected and managed with Research Electronic Data Capture (REDCap) hosted at the University of Colorado.27 Comparisons were considered significant if p≤0.05 and there was no correction for multiple comparisons. Demographics were summarized by low/high-function group using frequency with percent for categorical and mean with standard error (or geometric mean and geometric 95% confidence interval) for continuous variables; differences were tested with chi-square tests or two-sample t-tests, respectively. Odds ratios (OR) and 95% confidence intervals (CI) are presented from conditional logistic regression for the primary analysis where conditional odds of low function was estimated for each measure, with adjustment for most recent CD4+ T cell count, age, and smoking status. Skewed measures were log transformed prior to analysis.
The monotonic relationships between immune responses to CMV and markers of inflammation, immune activation, or senescence were assessed with the Spearman correlation coefficient. We chose IL-6 for model inclusion among the inflammatory markers, consistent with known relationships between IL-6 and CMV.12,28 The outcome of low function was modeled using bivariable conditional logistic regression models with CMV IgG and one additional effect variable (smoking, IL-6, %CD38+HLA-DR+CD8+ T cells, VACS Index scores, age, CD4+ T cells, and CD4/CD8 ratio) for each model. Analyses were performed in SAS v.9.3 (Cary, NC).
Results
Study population
Among 30 low-function cases and 48 high-function age- and gender-matched controls,23 77% were male and 76% were white. The mean age was 53 years and 96% had an HIV-1 RNA below the limits of detection of 48 copies/ml. No subjects were taking corticosteroids, ganciclovir, or valganciclovir; one case and no controls had a history of CMV end-organ disease. The low- and high-function groups were similar by baseline demographic characteristics with the exception that low-function cases were more likely to be current tobacco users, have a lower CD4/CD8 ratio, and have a higher VACS Index Score (Table 1).
Table 1.
Baseline Characteristics of the Case-Control Population
| Characteristic | Low-function cases N=30 | High-function controls N=48 | p value |
|---|---|---|---|
| Age (years)a,b | 52.8 (0.8) | 52.9 (0.7) | 0.94 |
| Femaleb,c | 8 (27) | 10 (21) | 0.56 |
| Whitec | 22 (73) | 37 (77) | 0.71 |
| Hispanicc | 7 (23) | 6 (13) | 0.22 |
| Current tobacco usec | 15 (50) | 10 (21) | 0.009 |
| Current CD4 count (cells/μl)d | 474 (369, 609) | 598 (531, 673) | 0.096 |
| Nadir CD4 count (cells/μl)d | 55 (33, 93) | 84 (52, 136) | 0.23 |
| Ratio CD4:CD8 T lymphocytesd | 0.6 (0.5, 0.8) | 1.0 (0.8, 1.1) | 0.001 |
| Detectable HIV-1 RNAc | 1 (3) | 2 (4) | 0.85 |
| Time since HIV diagnosis (years)b,d | 11.6 (8.3, 16.3) | 13.1 (10.6, 16.1) | 0.55 |
| Detectable plasma HCV RNAc | 6 (20) | 4 (9) | 0.15 |
| VACS Index Scoreb | 23 (19, 28) | 17 (15, 19) | 0.007 |
Mean (standard error).
Case-control matching criteria.
Frequency (percentage).
Geometric mean and 95% confidence interval.
VACS, Veterans Aging Cohort Study; HCV, hepatitis C virus.
Humoral response to CMV and association with physical function
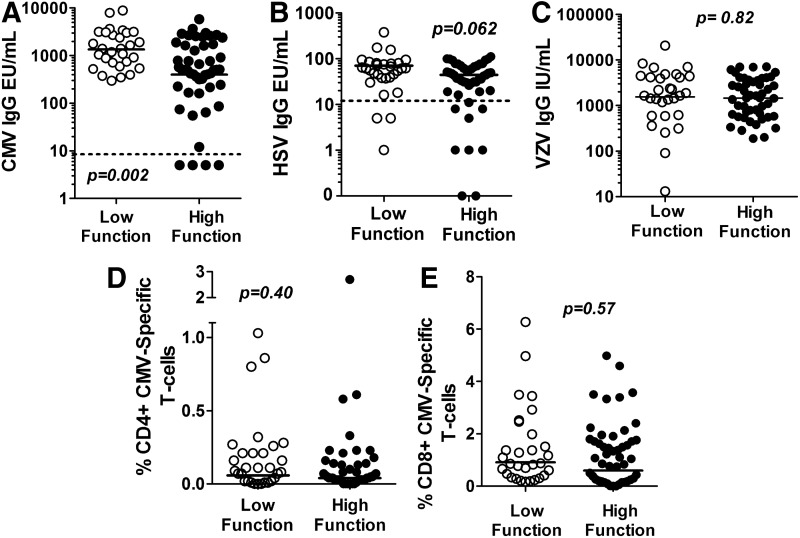
CMV IgG ranged from <10 to 8,830 EU/ml, including four participants (5%), all controls, with concentrations below the manufacturer's cutoff for positive results of 10 EU/ml. CMV IgG was significantly greater among the low-function than the high-function group (p=0.002; Fig. 1A), with a nearly 5-fold greater odds of low function (OR 4.8; 95% CI 1.4, 16.7) per log10 increase in CMV IgG EU/ml (p=0.015). These findings were robust to adjustment for concomitant CD4+ count, tobacco use, and age (OR 4.0; 95% CI 1.2, 12.8; p=0.021) or to exclusion of subjects with CMV IgG <10 EU/ml (OR 4.8; 95% CI 1.3, 17.2; p=0.016). Similarly, the relationship between physical function and CMV IgG remained robust after adjusting for hepatitis C viremia (OR 5.1; 95% CI 1.4, 18.4; p=0.001).
FIG. 1.
Distribution of cytomegalovirus IgG (A), herpes simplex virus 1 and 2 IgG (B), varicella zoster virus IgG (C), % CMV-specific CD4+ T cells (D), and % CMV-specific CD8+ T cells (E) between low and high physical function groups. Group geometric means are indicated by the solid line. The assay limits of detection for the immunoglobulin assays are indicated by the dotted line, with the exception of varicella zoster virus where all participants were positive.
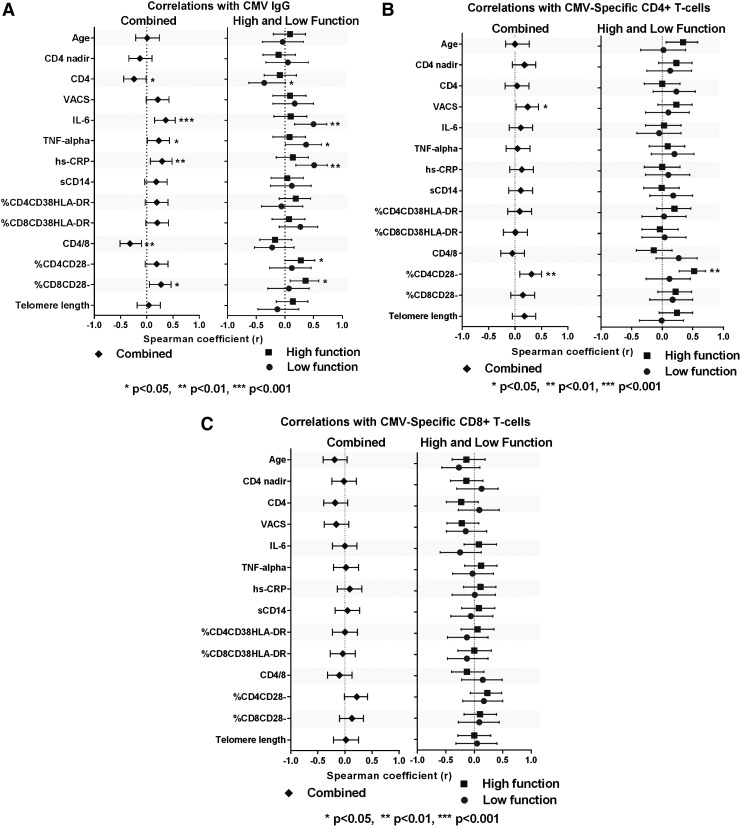
A weak, negative correlation between CMV IgG and the concomitant CD4+ lymphocyte count (r=−0.24; p=0.04) and a trend toward a positive correlation with the VACS Index (r=0.21; p=0.06) were detected. No correlations between CMV IgG and age or CD4+ nadir were detected (Fig. 2A). Weak to moderate correlations that reached statistical significance were detected between CMV IgG and IL-6, TNF-α, and hs-CRP (r≥0.23; p<0.05). Correlations between CMV IgG and markers of T cell or monocyte activation were not significant, although a trend toward a positive correlation with the %CD8+CD38+HLA-DR+ was detected (r=0.20; p=0.07). CMV IgG correlated negatively with the CD4/CD8 ratio (r=−0.32; p=0.004) and positively with the %CD8+CD28– T cells (r=0.27, p=0.02) and %CD4+CD28– T cells (r=0.19; p=0.09).
FIG. 2.
Correlations between cytomegalovirus (CMV) humoral or cell-mediated immune response and baseline characteristics, inflammation, activation, and senescence markers. (A–C) Correlations including all subjects (diamond) are shown in the left panels; correlations separated by high (square) and low (circle) physical function are shown in the right panels.
Humoral response to other herpesviruses and association with physical function
Twelve participants had negative responses for HSV-1/2 antibody and five were equivocal. The mean HSV-1/2 antibody was not significantly different between the low-function and high-function groups (Fig. 1B; p=0.062). The odds of being low function per 1 log increase in HSV IgG did not reach statistical significance (OR 3.72; 95% CI 0.98, 14.16; p=0.054), and was further attenuated after adjustment for CD4+ count, age, and tobacco use (OR 3.09; 95% CI 0.79, 12.06; p=0.10). All participants were seropositive for VZV. The mean VZV IgG was not significantly different in the low-function compared to the high-function group (Fig. 1C; p=0.82). Similarly, the odds of having low physical function did not change with a change in VZV IgG (OR 1.02; 95% CI 0.43, 2.44; p=0.97).
Cell-mediated response to CMV and association with physical function
The percentages of CMV-specific IFN-γ-producing CD4+ or CD8+ T cells were not significantly different between the low-function and high-function groups (Fig. 1D and E). Similarly, low function was not associated with the % of CD4+ or CD8+ CMV-specific T cells producing IFN-γ, TNF-α, or both (Supplementary Table S1).
CMV-specific CD4+ or CD8+ T cells did not correlate with age, CD4+ nadir, or current CD4+ count (Fig. 2B and C). CMV-specific CD4+ and CD8+ T cells were not correlated with markers of inflammation or lymphocyte or monocyte activation (Fig. 2). The CMV-specific CD4+ T cells correlated moderately with the %CD4+CD28– T cells (r=0.37; p<0.001) and weakly with %CD4+CD28– T cells (r=0.22; p=0.058). No significant associations were seen between CMV cell-mediated immune responses and other markers of immune senescence (Fig. 2). CMV-specific CD8+ T cells weakly correlated with CMV IgG (r=0.24; 95% CI 0.02, 0.44; p=0.033), but CMV-specific CD4+ T cells did not (r=0.13; 95% CI –0.10, 0.35; p=0.26).
Effect of clinical characteristics, inflammation, and immune activation on the relationship between CMV and low physical function
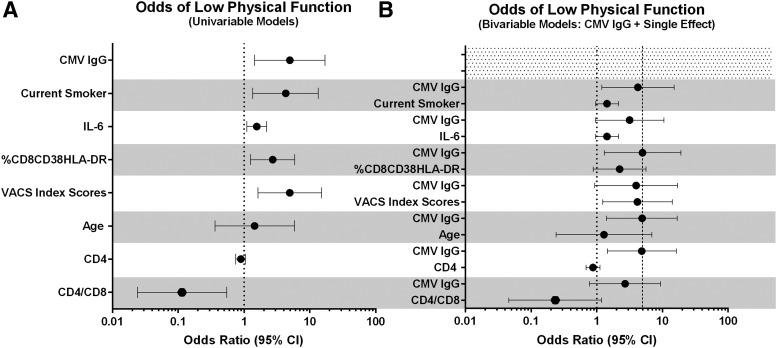
Results of univariable and bivariable conditional logistic regression models are shown in Fig. 3A and B. The relationship between CMV IgG and low physical function remained robust to inclusion of smoking, %CD8+CD38+HLA-DR+, age, and current CD4+ T cells, but was attenuated and no longer significant when IL-6, VACS index, or the CD4/CD8 ratio was included in the model.
FIG. 3.
(A) The odds of having low physical function by each variable listed on the y-axis (univariable models). The dot indicates the increase in the odds of low function per 1 unit increase in CMV IgG and the CD4/CD8 ratio per 0.2 pg/ml increase in interleukin (IL)-6, per 10% increase in %CD8CD38HLA-DR, per 10 point increase in the VACS Index score, per 5 year increase in age, and per 100 cells/μl increase in CD4+ count. The dotted line marks an odds ratio of 1. (B) The odds of low physical function per 1 unit increase in CMV IgG, with each bivariable model including CMV and the single corresponding variable. The dotted line marks an odds ratio of 1 and the dashed line marks the unadjusted odds of low function per increase in CMV IgG, for visual comparison.
Discussion
Among HIV-infected middle-aged adults on effective ART, we found that higher CMV humoral immune responses were associated with greater odds of functional impairment. The absence of an association between physical function and VZV or HSV-1/2 antibodies provided evidence that the relationship was specific to CMV, and not a heightened generalized humoral response to and/or a higher rate of reactivations of herpesviruses. In contrast, the odds of physical function impairment were not significantly associated with greater CMV cell-mediated responses. In bivariable regression models, however, the association of CMV IgG with physical function did not remain significant when other factors including inflammation, the VACS index, or CD4/CD8 ratio were included, suggesting that one or more of these markers might mediate the effect of CMV on physical function.
The association between CMV humoral immune responses and impaired physical function has been previously described in HIV-uninfected cohorts: among 724 women (87% CMV-seropositive) between ages 70 and 79 years in the Women's Health and Aging Studies I and II, CMV seropositivity was independently associated with prevalent frailty after adjusting for additional comorbid and demographic characteristics.2 Of the 635 women from this cohort with longitudinal data, those in the highest quartile of CMV IgG had a greater incidence of frailty compared to CMV-seronegative participants (hazard ratio 3.46).12 Similarly, among 295 older (mean age 78 years), community dwelling men and women, both CMV seropositivity (77%) and higher CMV IgG titers were associated with increased relative risk of frailty.11 In contrast, in a cohort of 567 men and women aged 80 years and older (74% CMV seropositive), CMV seropositivity was associated with a lower prevalence of frailty in adjusted analyses, and higher CMV IgG titers were not associated with frailty or physical function impairment, after adjustments for age, sex, and education.29
These findings suggest that the relationship between CMV seropositivity and frailty or physical function impairment may differ by gender or by age, with a survival effect among the oldest populations. To the best of our knowledge, the results we have presented herein are the first description of the association between impaired physical function and CMV in either a middle-aged or an HIV-infected cohort, suggesting that the relationship between CMV and impaired physical function may be apparent many years earlier among HIV-infected adults with a high prevalence of CMV seropositivity.
CMV humoral responses were associated with heightened markers of inflammation suggesting a role of active CMV infection in the ongoing inflammation of HIV-infected adults despite long-term effective ART and virologic suppression. Although not statistically significant, we detected a similar trend toward an association between CMV IgG and markers of T cell activation (%CD8+CD38+HLA-DR+). A recently published transcriptome analysis exploring pathways associated with high CMV IgG titers found that multiple metabolic (androgens, cholesterol, inositol processing) and immune response-associated pathways were increased, thus providing potential direct or indirect mechanistic links between CMV IgG and physical function.30 The association between CMV IgG and the “immune risk profile” was apparent even in our middle-aged population, with a strong association between lower CD4/CD8 ratio and higher CMV IgG.
The results of our bivariable model suggest a complex relationship between CMV IgG, inflammation or T cell activation, and physical function. Hunt et al. previously demonstrated that administration of the anti-CMV therapy vanganciclovir led to a significant reduction in T cell activation but not IL-6 or other markers of inflammation, supporting a key role of CMV as a driver of immune activation, but not inflammation, in HIV-1 infection.22 We detected only a weak correlation between CMV IgG and immune activation that suggested CMV contributed but was not the only driver of immune activation in our population. Similarly, the strong association between CMV IgG and physical function impairment was attenuated when adjusted for IL-6 but not for T cell activation, suggesting that IL-6, but not %CD8+CD38+HLA-DR+, partially explained the association between CMV IgG and impaired physical function.31
We found discordance between the cell-mediated and humoral CMV responses in persons with HIV-1. This discordance was also previously demonstrated in healthy blood donors.32 The most likely explanation is that while CMV antibodies increase with each reactivation, the relationship between cell-mediated responses is more complex: strong cell-mediated immune responses limit CMV reactivations, but when the virus escapes immune control and reactivates it also boosts cell-mediated responses. Several lines of evidence support this model: (1) CMV antibody titers are higher in older people, suggesting multiple and/or prolonged reactivations33 and (2) in individuals with AIDS, robust CMV-specific cell-mediated responses are associated with a lower risk of developing CMV retinitis or end-organ disease.34,35
It should also be noted that a small study of HIV-infected persons found that detectable CMV DNA was negatively correlated with CMV IgG17 and among 12 HIV-uninfected CMV-seropositive women from the Women's Health and Aging Study, CMV IgG titers were minimally different between two time points 12 years apart, despite detectable CMV DNA at both time points in seven participants.36 Furthermore, CMV cell-mediated immune responses to the pp65 peptide pool may underestimate total T cell responses against CMV.37 Thus, the reason for discordance between cell-mediated and humoral responses in our HIV-infected participants cannot be entirely explained and further investigation is needed.
Our study has several limitations, including the cross-sectional design. Because of the lack of longitudinal samples and limitations of CMV detection at a single time point, we chose not to include a measure of CMV DNA. pp65 is an immunodominant CMV antigen and the majority of CMV-infected individuals would be expected to mount a CD4 and CD8 T cell response; however, other epitopes not included in our studies may have elicited a greater immune response among some individuals.38–40 The age range of our study population is relatively narrow, and findings at higher and lower extremes of age may contrast the findings we have presented herein. The small sample size may have also limited (1) the ability to find significant associations of CMV cell-mediated responses with activation and/or inflammation; (2) the number of variables that could be considered in the regression models; and (3) the ability to detect associations within low- or high-function groups. Lastly, no corrections for multiple comparisons were done and associations should be considered exploratory.
Associations between CMV and complications of aging have been demonstrated in multiple cohorts of both HIV-infected and -uninfected persons.15,36,41–44 Our study provides the first demonstration of a strong relationship between CMV IgG and impaired physical function in HIV infection. Whether CMV alone plays a causative role in the deterioration of physical function or is a marker of another variable that is associated with decreased physical function could not be determined in this study. In the absence of an adequate animal model for human CMV infection, additional studies in human subjects are needed to further clarify the significance of these associations. Longitudinal studies including close monitoring of CMV reactivation and perhaps therapeutic interventions that suppress CMV reactivation may clarify the relationships between immune activation, inflammation, and CMV with physical function impairment in HIV-infected persons.
Supplementary Material
Acknowledgments
K.M.E., A.A.A., B.E.P., C.C.W., S.M., and T.B.C. contributed to the study design. K.M.E., A.A.A., E.R., B.E.P., C.C.W., A.W., S.M., and T.B.C. contributed to data collection, data interpretation, and manuscript preparation. A.A.A., S.M., and K.M.E. led the data analysis. K.M.E. prepared the first draft of the manuscript. The findings of this study were presented in part at the 2014 American Geriatrics Society Annual Meeting (Orlando, FL) and the 2014 and 2015 Conference on Retroviruses and Opportunistic Infections (Atlanta, GA and Boston, MA, respectively).
This work was supported by the National Institutes of Health [NIH/NCATS Colorado CTSI UL1 TR001082, and NIH/NIA K23AG050260 and R03AG040594 to K.M.E.] and the John A. Hartford Foundation Center of Excellence in Geriatric Medicine (K.M.E.). The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aiello AE, Haan MN, Pierce CM, et al. : Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci 2008;63:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmaltz HN, Fried LP, Xue QL, et al. : Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc 2005;53:747–754 [DOI] [PubMed] [Google Scholar]
- 3.Naeger DM, Martin JN, Sinclair E, et al. : Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 2010;5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylwester AW, Mitchell BL, Edgar JB, et al. : Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005;202:673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almanzar G, Schwaiger S, Jenewein B, et al. : Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol 2005;79:3675–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N, Hislop A, Gudgeon N, et al. : Herpesvirus-specific CD8 T cell immunity in old age: Cytomegalovirus impairs the response to a coresident EBV infection. J Immunol 2004;173:7481–7489 [DOI] [PubMed] [Google Scholar]
- 7.Vescovini R, Biasini C, Telera AR, et al. : Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 2010;184:3242–3249 [DOI] [PubMed] [Google Scholar]
- 8.Hadrup SR, Strindhall J, Kollgaard T, et al. : Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol 2006;176:2645–2653 [DOI] [PubMed] [Google Scholar]
- 9.Lurain NS, Hanson BA, Martinson J, et al. : Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 2013;208:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, et al. : Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age 2012;34:479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haeseker MB, Pijpers E, Dukers-Muijrers NH, et al. : Association of cytomegalovirus and other pathogens with frailty and diabetes mellitus, but not with cardiovascular disease and mortality in psycho-geriatric patients; a prospective cohort study. Immun Ageing 2013;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GC, Kao WH, Murakami P, et al. : Cytomegalovirus infection and the risk of mortality and frailty in older women: A prospective observational cohort study. Am J Epidemiol 2010;171:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savva GM, Pachnio A, Kaul B, et al. : Cytomegalovirus infection is associated with increased mortality in the older population. Aging cell 2013;12:381–387 [DOI] [PubMed] [Google Scholar]
- 14.Roberts ET, Haan MN, Dowd JB, et al. : Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010;172:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gkrania-Klotsas E, Langenberg C, Sharp SJ, et al. : Seropositivity and higher immunoglobulin G antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis 2013;56:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burbelo PD, Ching KH, Morse CG, et al. : Altered antibody profiles against common infectious agents in chronic disease. PLoS One 2013;8:e81635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianella S, Morris SR, Tatro E, et al. : Virologic correlates of anti-CMV IgG levels in HIV-1-infected men. J Infect Dis 2014;209:452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appay V, Fastenackels S, Katlama C, et al. : Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS 2011;25:1813–1822 [DOI] [PubMed] [Google Scholar]
- 19.Hsue PY, Hunt PW, Sinclair E, et al. : Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006;20:2275–2283 [DOI] [PubMed] [Google Scholar]
- 20.Masia M, Robledano C, Ortiz de la Tabla V, et al. : Increased carotid intima-media thickness associated with antibody responses to varicella-zoster virus and cytomegalovirus in HIV-infected patients. PLoS One 2013;8:e64327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrinello CM, Sinclair E, Landay AL, et al. : Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis 2012;205:1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt PW, Martin JN, Sinclair E, et al. : Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011;203:1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlandson KM, Allshouse AA, Jankowski CM, et al. : Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013;208:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandson KM, Allshouse AA, Jankowski CM, et al. : Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012;13:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erlandson KM, Allshouse AA, Jankowski CM, et al. : Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013;63:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Justice AC, Modur SP, Tate JP, et al. : Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: A North American cross cohort analysis. J Acquir Immune Defic Syndr 2013;62:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geist LJ. and Dai LY: Cytomegalovirus modulates interleukin-6 gene expression. Transplantation 1996;62:653–658 [DOI] [PubMed] [Google Scholar]
- 29.Mathei C, Vaes B, Wallemacq P, et al. : Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc 2011;59:2201–2208 [DOI] [PubMed] [Google Scholar]
- 30.Marttila S, Jylhava J, Kananen L, et al. : Molecular mechanisms associated with the strength of the anti-CMV response in nonagenarians. Immun Ageing 2014;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ. and Greenland S: Modern Epidemiology (2nd ed.). Lippincott Williams & Wilkins, Philadelphia, 1998 [Google Scholar]
- 32.Zhu J, Shearer GM, Marincola FM, et al. : Discordant cellular and humoral immune responses to cytomegalovirus infection in healthy blood donors: Existence of a Th1-type dominant response. Int Immunol 2001;13:785–790 [DOI] [PubMed] [Google Scholar]
- 33.McVoy MA. and Adler SP: Immunologic evidence for frequent age-related cytomegalovirus reactivation in seropositive immunocompetent individuals. J Infect Dis 1989;160:1–10 [DOI] [PubMed] [Google Scholar]
- 34.Sinclair E, Tan QX, Sharp M, et al. : Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon-gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis 2006;194:1537–1546 [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Tierney C, Kendall MA, et al. : Cytomegalovirus-specific immunity and protection against viremia and disease in HIV-infected patients in the era of highly active antiretroviral therapy. J Infect Dis 2006;193:488–493 [DOI] [PubMed] [Google Scholar]
- 36.Li H, Weng P, Najarro K, et al. : Chronic CMV infection in older women: Longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp65 (NLV)-specific CD8 T-cell frequencies with twelve year follow-up. Exp Gerontol 2014;54:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Margolick JB, Bream JH, et al. : Heterogeneity of CD4+ and CD8+ T-cell responses to cytomegalovirus in HIV-infected and HIV-uninfected men who have sex with men. J Infect Dis 2014;210:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern F, Bunde T, Faulhaber N, et al. : Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis 2002;185:1709–1716 [DOI] [PubMed] [Google Scholar]
- 39.Wills MR, Carmichael AJ, Mynard K, et al. : The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: Frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol 1996;70:7569–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beninga J, Kropff B, and Mach M: Comparative analysis of fourteen individual human cytomegalovirus proteins for helper T cell response. J Gen Virol 1995;76(Pt 1):153–160 [DOI] [PubMed] [Google Scholar]
- 41.Trzonkowski P, Mysliwska J, Szmit E, et al. : Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine 2003;21:3826–3836 [DOI] [PubMed] [Google Scholar]
- 42.Bartlett DB, Firth CM, Phillips AC, et al. : The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 2012;11:912–915 [DOI] [PubMed] [Google Scholar]
- 43.Bennett JM, Glaser R, Malarkey WB, et al. : Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav Immun 2012;26:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtner M, Cicconi P, Vita S, et al. : Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J Infect Dis 2015;211:178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.