Abstract
Race/sex differences are observed in cardiometabolic disease (CMD) risk and prevalence in the context of treated, chronic HIV infection, and these differences could be exacerbated by disparities in obesity prevalence. We sought to determine the effect of obesity on these disparities among people living with HIV. Prevalence of CMD (dyslipidemia, cardiovascular disorders, hypertension, diabetes, chronic kidney disease) was determined for patients seen at the University of Alabama at Birmingham HIV clinic between 7/2010 and 6/2011. Staged logistic regression was used to examine the impact of race/sex on comorbidities adjusting for key confounders including/excluding obesity (body mass index ≥30 kg/m2). Of 1,800 participants, 77% were male, 54% were black, and 25% were obese. Obesity prevalence differed by race/sex: black women 49%, black men 24%, white women 24%, white men 15% (p<0.01). Compared to white men, other groups had reduced odds for dyslipidemia and cardiovascular disorders (p<0.01). Black men had increased odds for hypertension and chronic kidney disease, while black women had a nearly 2-fold increased odds for diabetes and hypertension (all at p<0.01). The associations of black women with diabetes and hypertension were attenuated when obesity was included in the models. Other group differences remained significant. Disparities in obesity prevalence do not explain race/sex differences in all CMD among people with HIV. Obesity accounted for associations with diabetes/hypertension for black women, who may benefit from weight reduction to decrease disease risk. Further investigations into the etiology and treatment of CMD in people living with HIV should consider unique race/sex treatment issues.
Introduction
As AIDS-related mortality has declined, morbidity and mortality from chronic diseases have notably increased.1–6 People living with HIV (PLWH) in the United States are now diagnosed with a number of age-related chronic conditions including cardiovascular disease, renal disease, and diabetes at rates at least as great as and often greater than those of HIV-uninfected populations.7–12 A striking increase in obesity [body mass index (BMI) ≥30 kg/m2] prevalence among PLWH has also been observed, such that 65% of patients in some U.S. cohorts are overweight or obese.13–16 Obesity contributes to cardiometabolic disease (CMD) risk (dyslipidemia, cardiac disorders, hypertension, diabetes, chronic kidney disease) among people not infected with HIV,17 and can significantly impact the disease burden and quality of life among PLWH.15,18,19 However, little is known about the association of obesity with CMD diagnosis among PLWH, despite the risk in non-AIDS-related morbidity in this population.
The increase in non-AIDS-related morbidity has occurred concomitantly with the changing demographics of the HIV epidemic that result in higher rates of HIV infection among minority groups. In 2010, non-Hispanic black men and women represented 12% of the U.S. population, but accounted for 44% of incident HIV diagnoses in the United States.20 In the uninfected population, chronic disease prevalence and risk of obesity differ by race and sex, and higher rates of obesity are associated with greater CMD prevalence.17 Among PLWH, however, little is known regarding the associations of race, sex, and obesity with CMD risk. Therefore, in the present study we compared the prevalence of CMD and obesity by race/sex among a cross-sectional cohort of PLWH, and evaluated whether obesity impacts the associations between race/sex and CMD.
Materials and Methods
Data were obtained from the University of Alabama at Birmingham (UAB) 1917 HIV/AIDS Clinic Cohort Observational Database Project (UAB 1917 Clinic Cohort). This cohort forms a prospective clinical study that has collected detailed sociodemographic, psychosocial, and clinical data from >6,000 patients diagnosed with HIV since 1988. Over 3,000 patients currently receive primary and subspecialty HIV care at the clinic. During the study period (see below), the Clinic used a locally programmed electronic medical record (EMR) containing detailed provider encounter notes, imported all laboratory values from the central UAB laboratory, and required electronic prescriptions for all medications. The EMR and database were quality controlled, with all provider notes reviewed to ensure appropriate data capture regarding diagnoses and medications (including start and stop dates for prescriptions). New and ongoing diagnoses are recorded in patients' active problem lists, while resolved diagnoses discontinued by the provider remain part of the patient's EMR after removal from the active problem list. The UAB Institutional Review Board (IRB) approved this cross-sectional study nested in the UAB 1917 Clinic Cohort.
Participants
All patients seen at the UAB 1917 HIV Clinic between July 1, 2010 and June 30, 2011 were eligible. Inclusion criteria for this study were (1) ≥19 years of age, (2) white or African American self-reported race/ethnicity due to small numbers of other racial/ethnic patient groups, and (3) height, weight, plasma HIV viral load, and CD4 count measured within the study period. All data were acquired through electronic queries (MS SQL) of the UAB 1917 Clinic database.
Study variables
Dependent variables
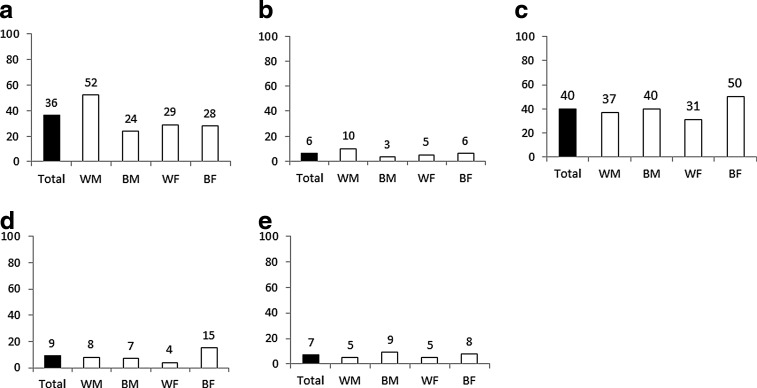
Cardiometabolic conditions are displayed in Fig. 1. Three clinical providers (M.M., J.R., and J.W.) reviewed the list of cardiometabolic diseases for accuracy and clinical significance to PLWH. Conditions were excluded from analysis if present in <2% of the study sample (e.g., stroke). Conditions that occurred in >2% of the clinic population and included for analyses were dyslipidemia, diabetes, hypertension, and chronic kidney disease. Cardiovascular disorders are composed of chronic ischemic heart disease, myocardial infarction, and cardiac insufficiency (congestive heart failure, CHF). Physician-entered diagnoses in the electronic medical record (ICD9 and/or SNOMED codes) were used to identify patients with each condition up to the end of the study period (June 30, 2011). At the 1917 Clinic, all clinical notes are reviewed prospectively to ensure that changes to diagnoses or medications noted in the clinician notes are updated in the discrete list of diagnoses and medications in the electronic medical record to ensure high-quality data. Patients are considered positive for one of the above conditions if the diagnosis has ever been entered into the medical record. As we were unable to confirm whether all patients with hypertension or dyslipidemia were taking their medications as prescribed, we included those with both treated and untreated conditions.
FIG. 1.
Prevalence (crude %) of chronic disease by race/sex for 1,800 HIV-infected patients receiving care at the UAB 1917 Clinic as of July 1, 2011. a, dyslipidemia; b, cardiovascular disorders; c, hypertension; d, diabetes; e, chronic kidney disease; WM, white male; BM, black male; WF, white female; BF, black female.
Independent variables
We examined patient demographic and laboratory information including age, sex, self-reported race/ethnicity, CD4+ T cell count, viral load, use (yes/no) and duration (years) of antiretroviral therapy, and insurance status (public, private, uninsured). If a patient had more than one value reported during the study period, the most recent data were used. Patient reported outcomes on history of tobacco use were included in the analyses. If patients had ever reported “yes” for a history of tobacco use they were dichotomously classified as positive for tobacco use in the analyses. Height and weight were used to calculate BMI [weight (kg)/height (m2)]. Patients were classified as obese if BMI was ≥30 kg/m2.
Statistical analyses
Descriptive characteristics were analyzed using chi-square tests and one-way analysis of variance. We computed prevalence, expressed as unadjusted percentages, for each cardiometabolic condition overall and by group. Because race/sex interactions were observed for each cardiometabolic disease outcome a four-level race/sex variable was created (black men, black women, white men, white women) for subsequent analyses. Each cardiometabolic condition was modeled with a staged logistic regression approach. The first model (model not shown) included race, sex, and a race×sex interaction to determine if the race/sex category was associated with cardiometabolic diseases. Adjusted Model 1 for each cardiometabolic outcome was used to evaluate whether the race/sex association remained after adjusting for age, CD4+ T cell count, health insurance status, and tobacco use. Adjusted Model 2 was used to further investigate if the race/sex association with cardiometabolic diseases remained when further adjusting the model for obesity status (categorized as yes/no). White men were used as the referent group. Consistent with prior studies,21 this staged modeling approach was employed to evaluate the putative role of differential obesity status, as a mediator on the causal pathway between race and sex with cardiometabolic disease diagnosis. We also included BMI as a continuous variable in the above models; results were not different from those reported below for BMI expressed as a dichotomous (yes/no) variable. All data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC) with a significance level of p<0.05.
Results
Of 1,800 patients meeting eligibility criteria, the mean age was 44.3±10.9 years, 77% were male, 54% were black, 43% currently used tobacco, 12% had current CD4+ T cell counts <200 cells/μl, 31% were uninsured, and 25% were obese (Table 1). The prevalence of obesity varied significantly by race/ethnicity and sex: black women 49%, black men 24%, white women 24%, and white men 15% (p<0.01). For participants classified as obese, there was a difference in the median (interquartile range, IQR) BMI by race but not sex: black women 36.5 (32.8–42.4), white women 36.3 (33.4–42.6), black men 32.9 (31.3–36.7), and white men 33.3 (31.3–36.5). When we evaluated the proportion of obese participants with a BMI >40 (class III obesity), we again observed differences by race but not sex: black women 31.1%, white women 29.6%, black men 9.4%, and white men 11.1%.
Table 1.
Demographic Characteristics [Mean±SD or n (%)] Stratified by Race/Sex for 1,800 HIV-Infected Patients Receiving Care at the UAB 1917 HIV/AIDS ClinicBetween 7/2010 and 6/2011
| Variable | Total n=1,800 | Black men n=655 | Black women n=308 | White men n=725 | White women n=112 | p valuea |
|---|---|---|---|---|---|---|
| Age (years) | 44.3±10.9 | 41.5±11.4 | 46.1±10.8 | 46.0±10.1 | 45.5±10.3 | <0.01 |
| Height (cm) | 175.2±9.6 | 177.8±7.8 | 164.5±8.3 | 179.0±7.2 | 165.2±7.4 | <0.01 |
| Weight (kg) | 83.4±20.2 | 84.8±19.4 | 84.6±26.0 | 83.1±17.0 | 73.8±23.4 | <0.01 |
| Body mass index | 27.2±6.6 | 26.7±5.7 | 31.2±9.3 | 25.8±4.9 | 27.0±8.4 | <0.01 |
| % Obese (BMI ≥30) | 445 (24.7%) | 159 (24.3%) | 151 (49.0%) | 108 (14.9%) | 27 (24.1%) | <0.01 |
| Health insurance | <0.01 | |||||
| Uninsured | 558 (31.0%) | 268 (40.9%) | 75 (24.4%) | 189 (26.1%) | 26 (23.2%) | |
| Public | 675 (37.5%) | 230 (35.1%) | 155 (50.3%) | 232 (32.0%) | 58 (51.8%) | |
| Private | 567 (31.5%) | 157 (24.0%) | 78 (25.3%) | 304 (41.9%) | 28 (25.0%) | |
| Sexual risk factor (men; n=1,380) | <0.01 | |||||
| Heterosexual | 282 (22.4%) | 194 (33.5%) | — | 88 (13.0%) | — | |
| MSM | 976 (77.6%) | 386 (66.5%) | — | 590 (87.0%) | — | |
| % started ART | 1,695 (94.2%) | 621 (94.8%) | 281 (91.2%) | 690 (95.2%) | 103 (92.0%) | 0.05 |
| Time on ART (yrs) | 6.5±5.9 | 5.2±5.2 | 6.5±5.7 | 7.6±6.1 | 7.1±6.5 | <0.01 |
| Tobacco use | 778 (43.2%) | 263 (40.2%) | 90 (29.2%) | 371 (51.2%) | 54 (48.2%) | <0.01 |
| CD4+ T cell count (cells/μl) | 533.4±295.0 | 463.6±266.8 | 570.1±333.3 | 570.2±280.9 | 603.0±351.0 | <0.01 |
| log10 plasma HIV RNA (copies/ml) | 2.1±1.0 | 2.3±1.1 | 2.3±1.2 | 1.9±0.8 | 2.1±0.9 | <0.01 |
| % plasma HIV RNA <200 copies/ml | 1,409 (78.3%) | 476 (72.7%) | 227 (73.7%) | 621 (85.7%) | 85 (75.9%) | <0.01 |
One-way ANOVA for continuous measures and chi-square for categorical measures.
BMI, body mass index; MSM, men who have sex with men; ART, antiretroviral therapy.
Group differences were observed when the prevalence of cardiometabolic conditions was compared by race/gender. White men had a higher prevalence of dyslipidemia and cardiovascular disorders than other participants (Fig. 1a and 1). Black women were more likely to be diagnosed with hypertension and diabetes compared to other groups (Fig. 1c and d; all at p<0.01). Black men had a higher prevalence of chronic kidney disease (CKD) than all groups (p=0.04), and a higher hypertension prevalence compared to white men and women (p<0.05 for both).
Dyslipidemia
In unadjusted analyses, the prevalence of diagnosed dyslipidemia was lower in black men [odds ratio (OR) 0.31; 95% confidence interval (CI) 0.25–0.39], black women (OR 0.37; 95% CI 0.27–0.49), and white women (OR 0.39; 95% CI 0.25–0.60) compared to white men. Table 2 shows the results of staged logistic regression modeling for the presence of dyslipidemia after adjustment for covariates. In Model 1, white men presented with a significantly greater prevalence of dyslipidemia compared to all other groups even when adjusting for covariates. In Model 2, obesity was significantly associated with risk for dyslipidemia (OR 1.72; 95% CI 1.31–2.27) but did not explain race/sex differences in dyslipidemia prevalence.
Table 2.
Odds Ratios for Dyslipidemia, Cardiovascular Disorders, and Hypertension Diagnoses by Race/Sex for 1,800 HIV-Infected Patients Receiving Care at the UAB 1917 HIV/AIDS Clinic as of July 1, 2011
| Dyslipidemia | Cardiovascular disorders | Hypertension | Diabetes | Chronic kidney disease | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Racea | ||||||||||
| WM | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| BF | 0.37 (0.27–0.52) | 0.31 (0.22–0.44) | 0.61 (0.36–1.06) | 0.55 (0.31–0.97) | 1.85 (1.37–2.49) | 1.28 (0.94–1.76) | 1.82 (1.18–2.80) | 1.36 (0.86–2.14) | 1.34 (0.77–2.33) | 1.19 (0.67–2.11) |
| BM | 0.46 (0.36–0.60) | 0.43 (0.33–0.57) | 0.33 (0.19–0.57) | 0.31 (0.18–0.55) | 1.80 (1.40–2.31) | 1.65 (1.28–2.13) | 1.10 (0.72–1.67) | 1.00 (0.65–1.52) | 2.36 (1.51–3.70) | 2.28 (1.45–3.58) |
| WF | 0.39 (0.23–0.63) | 0.37 (0.22–0.60) | 0.49 (0.20–1.19) | 0.48 (0.20–1.17) | 0.81 (0.51–1.28) | 0.72 (0.45–1.15) | 0.47 (0.18–1.22) | 0.44 (0.17–1.15) | 0.87 (0.35–2.17) | 0.86 (0.35–2.14) |
| Age (per 10 years) | 2.00 (1.75–2.27) | 2.03 (1.78–2.31) | 1.90 (1.51–2.38) | 1.92 (1.53–2.42) | 1.88 (1.67–2.11) | 1.94 (1.72–2.19) | 1.77 (1.47–2.12) | 1.84 (1.53–2.23) | 1.47 (1.20–1.79) | 1.48 (1.21–1.81) |
| CD4+ <200 | 0.41 (0.27–0.62) | 0.44 (0.29–0.66) | 0.85 (0.42–1.70) | 0.88 (0.44–1.77) | 0.47 (0.33–0.66) | 0.53 (0.37–0.75) | 0.71 (0.39–1.28) | 0.81 (0.45–1.48) | 1.15 (0.67–1.97) | 1.21 (0.71–2.09) |
| Insurance status | ||||||||||
| Private | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Public | 0.52 (0.39–0.68) | 0.52 (0.40–0.69) | 1.90 (1.18–3.06) | 1.92 (1.19–3.09) | 0.91 (0.71–1.17) | 0.94 (0.73–1.22) | 1.52 (1.02–2.26) | 1.57 (1.05–2.35) | 2.08 (1.31–3.30) | 2.12 (1.33–3.37) |
| Uninsured | 0.60 (0.44–0.81) | 0.61 (0.45–0.82) | 1.29 (0.69–2.45) | 1.30 (0.69–2.45) | 0.80 (0.61–1.05) | 0.84 (0.63–1.11) | 0.85 (0.50–1.44) | 0.87 (0.51–1.49) | 1.18 (0.65–2.15) | 1.20 (0.66–2.18) |
| History tobacco use | 1.10 (0.87–1.40) | 1.15 (0.91–1.46) | 1.17 (0.78–1.76) | 1.21 (0.80–1.82) | 0.65 (0.53–0.81) | 0.69 (0.55–0.85) | 0.70 (0.49–1.00) | 0.76 (0.53–1.09) | 0.61 (0.41–0.90) | 0.63 (0.42–0.94) |
| Time on ART | 1.67 (1.50–1.85) | 1.69 (1.52–1.87) | 1.29 (1.09–1.52) | 1.30 (1.09–1.54) | 1.20 (1.09–1.32) | 1.24 (1.12–1.37) | 1.16 (1.01–1.35 | 1.19 (1.03–1.38) | 1.52 (1.29–1.78) | 1.53 (1.30–1.80) |
| Obesity | 1.72 (1.31–2.27) | 1.48 (0.92–2.40) | 2.98 (2.32–3.84) | 2.52 (1.74–3.66) | 1.49 (0.97–2.28) | |||||
WM, white male; BF, black female; BM, black male; WF, white female; significant results bolded (p<0.05). Model 1 covariates=race/sex, age, CD4+ T cell count, insurance status, history of tobacco use, and time on ART. Model 2 includes covariates of Model 1 plus obesity status (yes/no).
A sensitivity analysis of patients with dyslipidemia excluding those diagnosed with cardiovascular disorders was also conducted. White men again presented with a greater prevalence of dyslipidemia even when obesity was added to the model, and obesity remained independently associated with dyslipidemia diagnosis (OR 1.58; 95% CI 1.20–2.07).
Cardiovascular disorders
Black men and women present with a significantly lower diagnosed prevalence of cardiovascular disorders compared to white men in unadjusted models and in adjusted Model 1. There was no difference in prevalence between white men and women (OR 0.49; 95% CI 0.20–1.19) in Model 1. Current obesity was not significantly associated with a diagnosis of a cardiac disorder in Model 2 (OR 1.48; 95% CI 0.92–2.40), and did not impact the association of race/sex with cardiovascular disorders. These results are summarized in Table 2.
Hypertension
Black women were almost twice as likely as white men to have a diagnosis of hypertension (OR 1.72; 95% CI 1.21–2.05) in unadjusted models (data not shown). When adjusting for covariates (Table 2; Model 1), both black women and men had a higher prevalence of hypertension compared to white men. When included in the model, obese individuals were three times more likely to be diagnosed with hypertension than other BMI groups (OR 2.98; 95% CI 2.32–3.84). The inclusion of obesity as a covariate also attenuated the relationship with hypertension risk for black women, but not for black men.
Diabetes
A nearly 2-fold increase in diabetes risk (OR 1.82; 95% CI 1.18–2.80) was observed for black women compared to white men in Model 1 (Table 2). However, the relationship between diabetes diagnosis and black women was no longer significant when obesity was added to the model, and obesity was significantly associated with the presence of a diabetes diagnosis (OR 2.52; 95% CI 1.74–3.66).
Chronic kidney disease
Black men had a significantly greater risk for chronic kidney disease compared to white men in the unadjusted model and after adjusting for all covariates in Model 1 and Model 2 (OR 2.28; 95% CI 1.45–3.58), Table 2). There was no significant difference when white and black women were compared to white men. Obesity classification was not associated with a greater prevalence of chronic kidney disease in this cohort. As hypertension can be a significant driver of kidney disease, an additional model was analyzed that included hypertension diagnosis as a covariate. Hypertension was significantly associated with chronic kidney disease diagnosis, but did not impact race/sex differences (data not shown).
Discussion
People living with HIV are increasingly burdened with chronic diseases that have a tremendous impact on clinical care and quality of life.15,22,23 This cross-sectional investigation reveals significant disparities by sex and race in the prevalence of obesity and CMD among our cohort of HIV-infected patients. It is the first, to our knowledge, to investigate the contribution of obesity to race/sex disparities in CMD among HIV-infected patients, and suggests that obesity has a differential impact on this association depending on the disease diagnosis.
Obesity, as defined by BMI, was more prevalent among women than men and among black patients compared to white men and women. In particular, 49% of black women presented with a BMI >30, and this population may represent a high-priority group for weight management interventions. This is consistent with prior research indicating an increased risk for obesity among HIV-infected and -uninfected women and minority individuals, which was associated with higher rates of CMD and multimorbidity.7,15,24–26
Diagnoses of diabetes and hypertension were more prevalent among black women, and black men also had higher prevalence of hypertension compared to white men and women. Obesity was associated with a 2.5- to 3-fold increase in risk for these diseases, and significantly attenuated risk for disease diagnosis among black women. Previous investigations have confirmed a higher risk for type 2 diabetes and hypertension among minority versus white uninfected and HIV-infected cohorts, with black men and women having up to a 77% higher risk for diabetes than whites. 7,27,28
Researchers report a significant and similar degree of association between weight gain and diabetes diagnosis among all groups, particularly white and black women, and Hu et al. estimated that over 90% of diabetes diagnoses in the Nurses' Health Study were attributable to poor diet, low physical activity, and smoking.27,29 Overall diet quality is reported to be poorer among black compared to white populations, and black women may be more sensitive to the effects of dietary factors on blood pressure and glycemic control,27,30 though not all studies report these findings.31 Research on lifestyle interventions and diabetes risk among HIV-infected groups is limited, though HIV-infected patients may experience less improvement in insulin sensitivity with weight loss and less responsiveness to metformin compared to HIV-uninfected patients.32,33 Similarly, the impact of weight management on hypertension risk in HIV-infected individuals is not well studied, despite evidence that interventions that prevent weight gain are associated with lower blood pressure and reduced use of antihypertensive medications in HIV-uninfected cohorts.34–36 However, research among HIV-uninfected populations combined with our study findings suggests that weight maintenance or planned weight loss could play a crucial role in diabetes and blood pressure control and prevention among HIV-infected black women who present with high obesity prevalence. More work to determine the most effective interventions to achieve this goal is warranted.
HIV-infected white men in this study presented with a significantly higher prevalence of dyslipidemia and cardiovascular disorders, even after adjusting for age, compared to all other groups and despite having a lower average BMI and less obesity. Obesity was associated with a 1.5 times greater risk for dyslipidemia overall, but did not attenuate race/sex associations with disease diagnosis. This is consistent with literature among HIV-infected and HIV-uninfected populations suggesting that men have more dyslipidemia and greater cardiovascular disease (CVD) risk than age-matched premenopausal women.7,37 As the average age of women in our study was 45 years, more information is needed to determine whether postmenopausal HIV-infected women would have comparable rates of dyslipidemia and cardiac disorders.
A combination of viral/host biology and environmental factors may contribute to this notable disparity in CVD risk. White men may have been infected with HIV for longer periods of time, 9 which we were unable to control for. HIV infection is an independent risk factor for cardiovascular disease, and is also associated with chronic inflammation.38–40 This chronic inflammatory phenotype is characterized by elevated biomarkers of cardiovascular disease,41–43 and duration of host infection could contribute to the elevated risk observed among white men in this study. Host genetics could further play a role in observed disparities. Kilpelainen et al. identified a genetic mutation of the insulin receptor substrate 1 (IRS-1) gene that is simultaneously associated with a decreased accumulation of subcutaneous fat (i.e., lower overall body fat percentage) and decreased adiponectin levels with an abnormal lipid profile. This variant was present in 64% of European men and men of Asian North-Indian descent and is associated with coronary artery disease.44,45 Precisely how host genetics interact with the impact of HIV infection on host immunity and inflammation is largely unexplored yet could provide vital information regarding effective treatments for CVD in HIV-infected groups, particularly white men with an observed increased risk.
Our study did not identify a significant association of current obesity prevalence with chronic kidney disease, although obesity is considered an independent contributor to kidney disease among HIV-uninfected individuals.46 Future studies could also include those with CKD defined by estimated glomerular filtration (eGFR) rates that might capture a broader range of CKD disease severity and impact the associations with obesity. We did observe a significantly higher prevalence of chronic kidney disease among black men, which was somewhat attenuated when obesity was included in the model. As weight loss is one side effect of kidney disease, it is possible that our use of current obesity prevalence, which fails to account for lifetime obesity status, limited our ability to detect an association between obesity and chronic kidney disease.
This study investigates the prevalence of current obesity and CMD diagnosis, and is thus subject to certain limitations. The lack of lifetime BMI records on participants limits our ability to account for changes in weight status that may have resulted after CMD diagnosis. Additional research on not just the associations of obesity, but also more specific body morphology disorders such as lipoatrophy and lipohypertrophy, is needed and is not addressed here. The use of BMI also limits our ability to investigate the contribution of specific adipose tissue depots to disease risk. We were further limited by lack of objective data on dietary intake and physical activity, factors that both contribute to and interact with obesity's impact on metabolism and CMD risk. Also, our use of a sample from the Southeastern United States with a historically higher incidence of obesity and CMD may limit the generalizability of study results to other geographic regions. However, recent studies have observed increasing obesity prevalence in HIV-infected individuals across the United States, suggesting that the role of obesity in health and quality of life is a pressing issue for many patient groups.
We included both treated and untreated hypertension and dyslipidemia in the current analysis, as we were unable to verify that patients prescribed medications for those conditions took them as prescribed. Future investigations into the association of body weight with these conditions in treated versus untreated hypertension and dyslipidemia could provide interesting insights into the interplay of adiposity and pharmaceutical effects. This study also relied on the use of EMR diagnosis history entered by HIV providers to classify participants as having a cardiometabolic disease. While these are the patients that would receive treatment and control efforts within the clinic setting, the lack of fasting laboratory values or potentially missing data due to underreporting by providers may have resulted in an underestimation of CMD prevalence among the study sample.
The strengths of this study include the comprehensive clinical data and large sample size with significant numbers of African Americans and women to facilitate a thorough comparison of white men and women with African American men and women. Evaluating the effect of sex and race separately can limit the ability to detect interactions in these variables, and our study demonstrates the need to consider disease risk within these subpopulations to identify specific areas of risk for potential intervention.
In conclusion, we have identified a possible differential impact of obesity on CMD risk among our cohort of HIV-infected patients, with race and sex playing an important role in health disparities. Our initial findings suggest that obesity prevention and weight maintenance may have a greater impact within the context of certain cardiometabolic diagnoses, and that individuals at risk of diabetes or hypertension in particular may benefit from these efforts. Additional research is necessary to confirm these findings and evaluate the etiology of body fat accumulation and CMD in the context of HIV infection, as well as to determine whether the increasing prevalence of obesity in HIV-infected individuals and its association with some cardiometabolic conditions among this group translates into increased mortality risk. Additionally, a longer follow-up to assess the association of BMI and BMI changes over time with cardiometabolic disease risk is needed to determine whether obesity is associated with disease risk across all time points or only at certain ages and stages of infection.
As the HIV-infected population continues to experience increased chronic disease morbidity, our ability to elucidate the mechanisms of disease occurrence and identify which patient groups would most benefit from specific lifestyle and pharmaceutical interventions will greatly impact the overall care and quality of life for patients living with HIV.
Acknowledgments
This study was supported by the UAB Center for AIDS Research (grant P30-AI27767) and CNICS Research Network (R24-AI067039) and the Mary Fisher CARE Fund. A.L.W. received financial support from a National Institutes of Health Ruth L. Kirschstein National Research Service Award (grant 5T32AI52069-08SI) and the UAB-VA Health Services Research/Comparative Effectiveness Research Training Program. The views expressed are those of the authors.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.French AL, Gawel SH, Hershow R, et al. : Trends in mortality and causes of death among women with HIV in the United States: A 10-year study. J Acquir Immune Defic Syndr 2009;51(4):399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, et al. : Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007;146(2):87–95 [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Sabin CA, Youle M, et al. : Changes in AIDS-defining illnesses in a London Clinic, 1987–1998. J Acquir Immune Defic Syndr 1999;21(5):401–407 [PubMed] [Google Scholar]
- 4.Currier JS, Taylor A, Boyd F, et al. : Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003;33(4):506–512 [DOI] [PubMed] [Google Scholar]
- 5.Magoni M, Scarcella C, Vassallo F, et al. : The evolving burden of HIV infection compared with other chronic diseases in northern Italy. HIV Med 2011;12(3):129–137 [DOI] [PubMed] [Google Scholar]
- 6.Smit E, Skolasky RL, Dobs AS, et al. : Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol 2002;156(3):211–218 [DOI] [PubMed] [Google Scholar]
- 7.Buchacz K, Baker RK, Palella FJ Jr, et al. : Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013;18(1):65–75 [DOI] [PubMed] [Google Scholar]
- 8.Crum-Cianflone N, Ganesan A, Teneza-Mora N, et al. : Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS 2010;24(6):353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsley LA, Cuervo-Rojas J, Munoz A, et al. : Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS 2008;22(13):1589–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas GM, Mehta SH, Atta MG, et al. : End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS 2007;21(18):2435–2443 [DOI] [PubMed] [Google Scholar]
- 11.Worm SW, De Wit S, Weber R, et al. : Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation 2009;119(6):805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinikoor MJ, Napravnik S, Floris-Moore M, et al. : Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses 2013;29(7):1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakey W, Yang LY, Yancy W, et al. : Short communication: From wasting to obesity: Initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses 2013;29(3):435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crum-Cianflone N, Tejidor R, Medina S, et al. : Obesity among patients with HIV: The latest epidemic. AIDS Patient Care STDS 2008;22(12):925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DJ, Westfall AO, Chamot E, et al. : Multimorbidity patterns in HIV-infected patients: The role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr 2012;61(5):600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tate T, Willig AL, Willig JH, et al. : HIV infection and obesity: Where did all the wasting go? Antivir Ther 2012;17(7):1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PW, Morrato EH, Ghushchyan V, et al. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care 2005;28(7):1599–1603 [DOI] [PubMed] [Google Scholar]
- 18.Boodram B, Plankey MW, Cox C, et al. : Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the Women's Interagency HIV Study. AIDS Patient Care STDS 2009;23(12):1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koethe JR, Dee K, Bian A, et al. : Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and nonobese HIV-infected adults on antiretroviral therapy. AIDS Res Hum Retroviruses 2013;29(7):1019–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC: Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012;17(No. 4) [Google Scholar]
- 21.Mugavero MJ, Lin HY, Allison JJ, et al. : Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr 2009;50(1):100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palella FJ, Jr, Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic 2006;43(1):27–34 [DOI] [PubMed] [Google Scholar]
- 23.Vance DE, Mugavero M, Willig J, et al. : Aging with HIV: A cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 2011;22(1):17–25 [DOI] [PubMed] [Google Scholar]
- 24.Amorosa V, Synnestvedt M, Gross R, et al. : A tale of 2 epidemics: The intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr 2005;39(5):557–561 [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Kit BK, and Ogden CL: Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307(5):491–497 [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Kit BK, and Flegal KM: Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 2013(131):1–8 [PubMed] [Google Scholar]
- 27.Shai I, Jiang R, Manson JE, et al. : Ethnicity, obesity, and risk of type 2 diabetes in women: A 20-year follow-up study. Diabetes Care 2006;29(7):1585–1590 [DOI] [PubMed] [Google Scholar]
- 28.Prevention CfDCa: National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA, 2011 [Google Scholar]
- 29.Hu FB, Manson JE, Stampfer MJ, et al. : Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345(11):790–797 [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Hebert JR, Manson JE, et al. :. Determinants of racial/ethnic disparities in incidence of diabetes in postmenopausal women in the U.S.: The Women's Health Initiative 1993–2009. Diabetes Care 2012;35(11):2226–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao Y, Tinker L, Olendzki BC, et al. : Racial/ethnic disparities in association between dietary quality and incident diabetes in postmenopausal women in the United States: The Women's Health Initiative 1993–2005. Ethn Health 2014;19(3):328–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeds DC, Patterson BW, Overton T, et al. : Metabolic benefits of weight loss are blunted in obese, HIV-infected women. Obesity (Silver Spring) 2011;19(S1):S112 [Google Scholar]
- 33.Han JH, Crane HM, Bellamy SL, et al. : HIV infection and glycemic response to newly initiated diabetic medical therapy. AIDS 2012;26(16):2087–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aucott L, Poobalan A, Smith WC, et al. : Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: A systematic review. Hypertension 2005;45(6):1035–1041 [DOI] [PubMed] [Google Scholar]
- 35.Siebenhofer A, Jeitler K, Berghold A, et al. : Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database Syst Rev 2011(9):CD008274 [DOI] [PubMed] [Google Scholar]
- 36.Bonfils PK, Taskiran M, Damgaard M, et al. : Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase in mid-regional pro-atrial natriuretic peptide in morbid obese patients. J Hypertens 2015;33(6):1215–1225 [DOI] [PubMed] [Google Scholar]
- 37.Rosamond W, Flegal K, Furie K, et al. : Heart disease and stroke statistic–2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117(4):e25–146 [DOI] [PubMed] [Google Scholar]
- 38.Durand M, Sheehy O, Baril JG, et al. : Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr 2011;57(3):245–253 [DOI] [PubMed] [Google Scholar]
- 39.Triant VA, Lee H, Hadigan C, and Grinspoon SK: Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92(7):2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff JL, Starfield B, and Anderson G: Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162(20):2269–2276 [DOI] [PubMed] [Google Scholar]
- 41.Kitagawa T, Yamamoto H, Horiguchi J, et al. : Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging 2009;2(2):153–160 [DOI] [PubMed] [Google Scholar]
- 42.Rominger A, Saam T, Wolpers S, et al. : 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50(10):1611–1620 [DOI] [PubMed] [Google Scholar]
- 43.van der Velde AE: Reverse cholesterol transport: From classical view to new insights. World J Gastroenterol 2010;16(47):5908–5915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilpelainen TO, Qi L, Brage S, et al. : Physical activity attenuates the influence of FTO variants on obesity risk: A meta-analysis of 218,166 adults and 19,268 children. PLoS Med 2011;8(11):e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samani NJ, Erdmann J, Hall AS, et al. : Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357(5):443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ejerblad E, Fored CM, Lindblad P, et al. : Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006;17(6):1695–1702 [DOI] [PubMed] [Google Scholar]