Abstract
This study aimed to investigate the effect of pituitary adenylate cyclase-activating peptide (PACAP) on the pacemaker activity of interstitial cells of Cajal (ICC) in mouse colon and to identify the underlying mechanisms of PACAP action. Spontaneous pacemaker activity of colonic ICC and the effects of PACAP were studied using electrophysiological recordings. Exogenously applied PACAP induced hyperpolarization of the cell membrane and inhibited pacemaker frequency in a dose-dependent manner (from 0.1 nM to 100 nM). To investigate cyclic AMP (cAMP) involvement in the effects of PACAP on ICC, SQ-22536 (an inhibitor of adenylate cyclase) and cell-permeable 8-bromo-cAMP were used. SQ-22536 decreased the frequency of pacemaker potentials, and cell-permeable 8-bromo-cAMP increased the frequency of pacemaker potentials. The effects of SQ-22536 on pacemaker potential frequency and membrane hyperpolarization were rescued by co-treatment with glibenclamide (an ATP-sensitive K+ channel blocker). However, neither NG-nitro-L-arginine methyl ester (L-NAME, a competitive inhibitor of NO synthase) nor 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ, an inhibitor of guanylate cyclase) had any effect on PACAP-induced activity. In conclusion, this study describes the effects of PACAP on ICC in the mouse colon. PACAP inhibited the pacemaker activity of ICC by acting through ATP-sensitive K+ channels. These results provide evidence of a physiological role for PACAP in regulating gastrointestinal (GI) motility through the modulation of ICC activity.
Keywords: ATP-sensitive K+ (KATP) channels, Colon, Interstitial cells of Cajal (ICC), Pituitary adenylate cyclase-activating polypeptide (PACAP)
INTRODUCTION
Pituitary adenylate cyclase-activating polypeptide (PACAP) was first identified in ovine hypothalamic extracts and has the ability to stimulate cyclic AMP (cAMP) formation in anterior pituitary cells [1]. PACAP belongs to the vasoactive intestinal polypeptide (VIP)/glucagon/growth hormone-releasing factor/secretin superfamily and is involved in the regulation of important biological functions [2].
Many reports have identified roles for PACAP in gut function. Usually, PACAP is found in enteric nerves, particularly in myenteric nerves [3], giving it the ability to influence neuronal and muscular activity in the gastrointestinal (GI) tract. PACAP stimulates longitudinal muscle contractions, utilising acetylcholine and tachykinins as neurotransmitters [4]. PACAP has been found to function as an inhibitory neurotransmitter in the circular muscle of the mouse antrum [5,6,7]. In addition, some reports suggest that PACAP can act directly on myocytes in the GI tract. For example, PACAP can relax intestinal smooth muscle [8,9,10,11,12] and has an important role in intestinal motor coordination in the rat colon [13].
It is well known that the interstitial cells of Cajal (ICC) are pacemaker cells for GI motility and generate the pacemaker currents that underlie slow waves. ICC are coupled electrically to each other and to neighboring smooth muscle cells via gap junctions [14]. Slow waves generated from ICC are propagated in ICC networks and conducted passively to smooth muscle cells [15]. Many neurotransmitters (e.g., acetylcholine, 5-hydroxytryptamine, nitric oxide) have an excitatory or inhibitory effect on the pacemaker activity of ICC [16,17,18], supporting the idea that ICC have a critical role in controlling smooth muscle motility in the GI tract.
Although the effects of PACAP on GI motility have been extensively characterized, the exact mechanism of PACAP action on GI motility or on ICC in the GI tract has not yet been explored. Here, we found that PACAP inhibits the pacemaker activity of colonic ICC and identified a role for ATP-sensitive K+ (KATP) channels in this process.
METHODS
Animal and tissue preparation
All experiments were performed according to the Guiding Principles for the Care and Use of Animals approved by the Ethics Committee of Chosun University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize both the number of animals used and their suffering.
Balb/C mice (3~7 days old) of either sex were anesthetized with diethyl ether and sacrificed by cervical dislocation. The small intestine was excised 1 cm below the pyloric ring to the cecum and opened along the mesenteric border. Luminal contents were washed away with Krebs-Ringer bicarbonate solution.
Cell Culture
The isolated tissue was pinned to the base of a Slygard dish, and the mucosa was removed by sharp dissection. Small strips of intestinal muscle were equilibrated in calcium-free Hank's solution with the following constituents: 5.36 mM KCl, 125 mM NaCl, 0.336 mM NaOH, 0.44 mM Na2HCO3, 10 mM glucose, 2.9 mM sucrose, and 11 mM HEPES. The pH was adjusted to 7.4 with Tris for 30 min. Cells were dispersed by incubating for 15 min at 37℃ in an enzymatic solution containing collagenase (1.3 mg/ml; Worthington Biochemicals, Lakewood, NJ, USA) bovine serum albumin (2 mg/ml; Sigma, St. Louis, MO, USA), trypsin inhibitor (2 mg/ml; Sigma), and ATP (0.27 mg/ml). The cells were finely chopped and placed onto sterile glass coverslips coated with poly-L-lysine in 35 mm culture dishes and incubated at 37℃ in a 95% O2/5% CO2 incubator in smooth muscle growth medium (SMGM; Cambrex Bio Science, Walkersville, MD, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (5 ng/ml; Sigma).
Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from the cultured ICC. Currents or potentials were amplified using an Axopatch 1-D (Axon Instruments, Foster City, CA, USA). Command pulse was applied using an IBM-compatible personal computer and pClamp software (version 9.2; Axon Instruments). The data were filtered at 5 kHz. All experiments were carried out at 30℃.
Solutions
The cells were bathed in a standard solution containing the following: 5 mM KCl, 135 mM NaCl, 2 mM CaCl2, 10 mM glucose, 1.2 mM MgCl2, and 10 mM HEPES. The standard solution was adjusted to pH 7.4 with Tris. The pipette solution contained the following: 120 mM K-aspartate, 20 mM KCl, 5 mM MgCl2, 2.7 mM K2ATP, 0.1 mM Na2GTP, 2.5 mM creatine phosphate disodium, 0.1 mM EGTA, and 5 mM HEPES. The solution was adjusted to pH 7.4 with Tris.
Drugs and chemicals
Drugs used for the experiments were purchased from Sigma-Aldrich. Drugs were dissolved in appropriate solvents (DMSO or distilled water) and stock solutions (10 mM or 100 mM) were prepared and stored in aliquots at the designated temperatures. All light-sensitive drugs were protected from light by wrapping their containers in aluminum foil. The required concentrations of the drugs were prepared at the time of the experiments and added to the bath solution. All drugs were applied to the whole-cell preparation by superfusion. The final concentration of DMSO in all the drug preparations was <0.5%.
Statistical analysis
Data are expressed as mean±standard error. Differences between groups were evaluated by a Student's t-test. A p value <0.05 was considered to be statistically significant. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
RESULTS
Effect of PACAP on pacemaker currents generated by colonic ICC
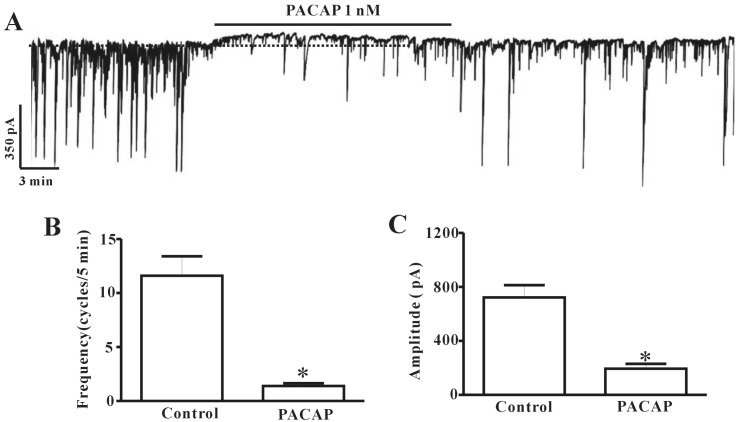
ICC cultured from mouse colon were identified with Kit immunofluorescence. Kit-positive cells had a distinctive morphology that was easily recognized in cultures. We used electrophysiological assays to determine the effect of PACAP on ICC. Electrophysiological recordings were performed from cultured colonic ICC under voltage-clamp mode at -70 mV. Under control conditions, ICC showed spontaneous pacemaker currents. The frequency and amplitude recorded from colonic ICC were measured as 11.6±1.8 cycles/5 min and 722.4±91.09 pA (n=5), respectively, under physiological conditions (Fig. 1B and C). Administration of PACAP (1 nM) to the bath solution slightly showed outward currents and inhibited pacemaker activity (Fig. 1A). The frequency and amplitude values of pacemaker currents under voltage-clamp mode in the presence of PACAP (1 nM) were significantly different from control values (n=5, Fig. 1B and C).
Fig. 1. Effects of pituitary adenylate cyclase-activating peptide (PACAP) on pacemaker currents recorded in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) shows the pacemaker currents of ICC exposed to PACAP (1 nM) in voltage-clamp mode at -70 mV holding potentials. PACAP inhibited the amplitude and frequency of pacemaker currents in ICC. The dotted lines indicate the control resting current levels. Responses to PACAP are summarized in (B) and (C). Bars represent the means±SE. *Asterisks indicate a statistically significant difference from controls (p<0.05).
Effect of PACAP on pacemaker potentials generated by colonic ICC
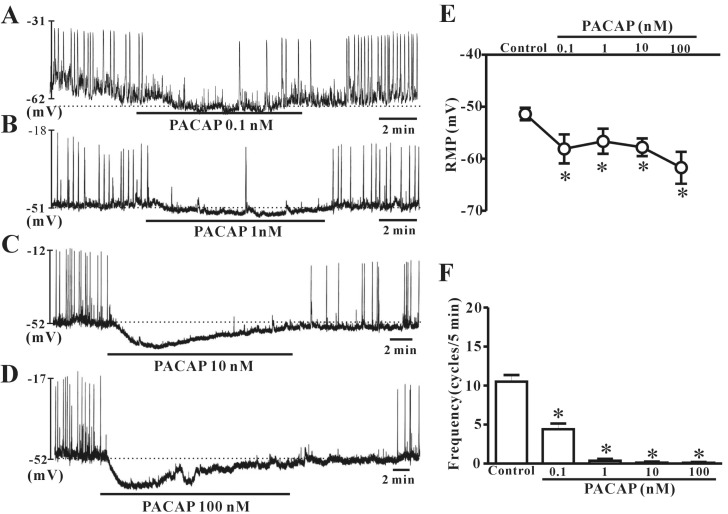
We examined whether exogenous exposure to PACAP triggered dose-dependent effects on ICC pacemaker potentials in current-clamp mode (I=0). Under a current clamp, ICC generated spontaneous pacemaker potentials. The application of 0.1 nM or 1 nM PACAP induced slight hyperpolarization with reduction of frequency (Fig. 2A and B). Next, we found that pacemaker potentials were markedly inhibited and strong hyperpolarization was induced by the presence of 10 nM or 100 nM PACAP (Fig. 2C and D). Under control conditions, the resting membrane potential and frequency generated by colonic ICC were recorded as -51.4±1.2 mV and 10.6±0.9 cycles/5 min, respectively (n=18, Fig. 2E and F). When ICC were treated with high concentrations of PACAP, the resting membrane potentials were measured as -57.8±1.7 mV at 10 nM (n=4) and -61.7±3.02 mV at 100 nM (n=5). The mean values of pacemaker potential frequencies were changed to 0.2±0.1 cycles/5 min at 10 nM and 0.1±0.19 cycles/5 min at 100 nM by addition of high dose PACAP (Fig. 2E and F).
Fig. 2. Effects of various concentrations of pituitary adenylate cyclase-activating peptide (PACAP) on pacemaker potentials recorded in cultured interstitial cells of Cajal (ICC) from mouse colon. (A), (B), (C), and (D) show pacemaker potentials of ICC exposed to PACAP (0.1 nM, 1 nM, 10 nM, or 100 nM) under current-clamp mode (I=0). Vertical solid lines represent the amplitude of pacemaker potentials and horizontal solid lines represent the duration of recording (s) of pacemaker potentials. The dotted lines indicate the resting membrane potential. (E) and (F) summarize the effects of PACAP on pacemaker potentials in ICC. Bars represent the means±SE. *Asterisks indicate a statistically significant difference from controls (p<0.05). Con, control.
Confirmation of cAMP action on colonic ICC
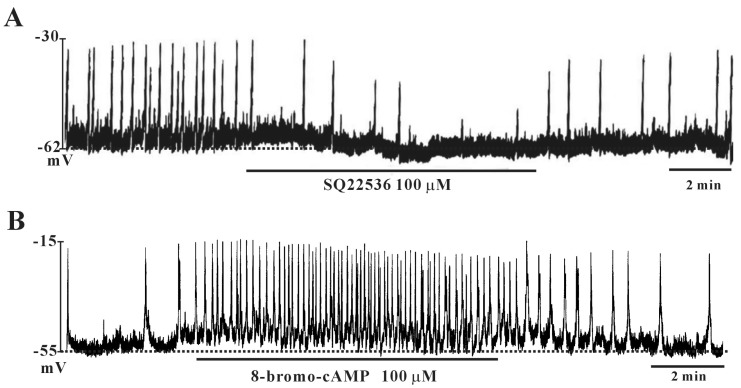
To investigate whether PACAP stimulates adenylate cyclase with a subsequent increase in cAMP levels, we tested the effects of SQ-22536 (adenylate cyclase inhibitor) and cell-permeable 8-bromo-cAMP. SQ-22536 (100 µM) inhibited the frequency of pacemaker potentials and induced slight hyperpolarization of the membrane (Fig. 3A). In contrast, 8-bromo-cAMP (100 µM) increased the frequency of pacemaker potentials and slightly depolarized the membrane (Fig. 3B).
Fig. 3. Effects of SQ-22536 and cell-permeable 8-bromo-cyclic AMP (8-bromo-cAMP) on pacemaker potentials in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) Treatment with SQ-22536 (100 µM) showed inhibitory effects on pacemaker potentials. (B) Treatment with 8-bromo-cAMP (100 µM) increased the frequency of pacemaker potentials in colonic ICC. Vertical solid lines show the amplitude of pacemaker potentials and horizontal solid lines show the duration of recording (s) of pacemaker potentials.
Effect of various potassium channels inhibitors on PACAP-induced action
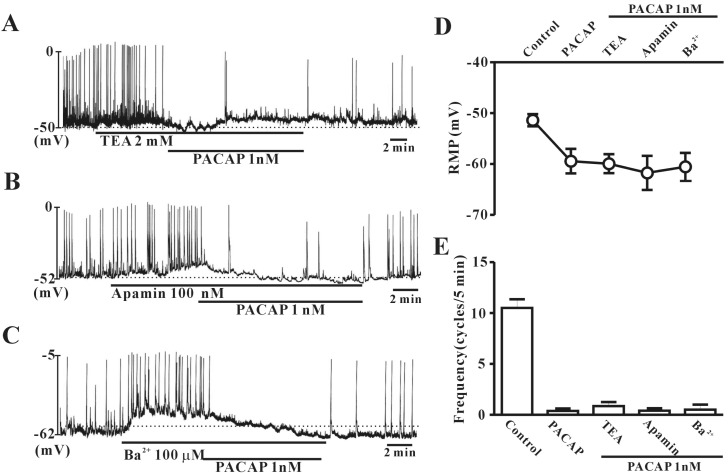
To rule out the possible involvement of potassium channels, colonic ICC were exposed to tetraethylammonium chloride (TEA) (a voltage-dependent K+ channel blocker), apamin (a Ca2+-dependent K+ channel blocker), or (C) Ba2+ (an inward rectifier K+ channel blocker). In the presence of TEA (2 mM), apamin (100 nM), or Ba2+ (100 µM), PACAP (1 nM) still inhibited the frequency of pacemaker potentials and generated hyperpolarization of the ICC membrane in colonic ICC (Fig. 4A, B and C). Also, the values for frequency or resting membrane potentials with TEA+PACAP, apamin+PACAP, or Ba2++PACAP were not significantly different from PACAP alone (n=5~6, Fig. 4D and E).
Fig. 4. Effects of various K+ channel blockers on pituitary adenylate cyclase-activating peptide (PACAP)-mediated inhibition of pacemaker potentials recorded in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) Tetraethylammonium chloride (TEA, a voltage-dependent K+ channel blocker; 2 mM), (B) apamin (a Ca2+-dependent K+ channel blocker; 100 nM), or (C) Ba2+ (an inward rectifier K+ channel blocker; 100 µM) did not have any effect on pacemaker potentials in colonic ICC. Vertical solid lines show the amplitude of pacemaker potentials and horizontal solid lines show the duration of recording (s) of pacemaker potentials. (D) and (E) summarize the effects of PACAP on pacemaker potentials in colonic ICC with K+ channel blockers. Bars represent the means±SE. *Asterisks indicate a statistically significant difference from controls (p<0.05). Con, control.
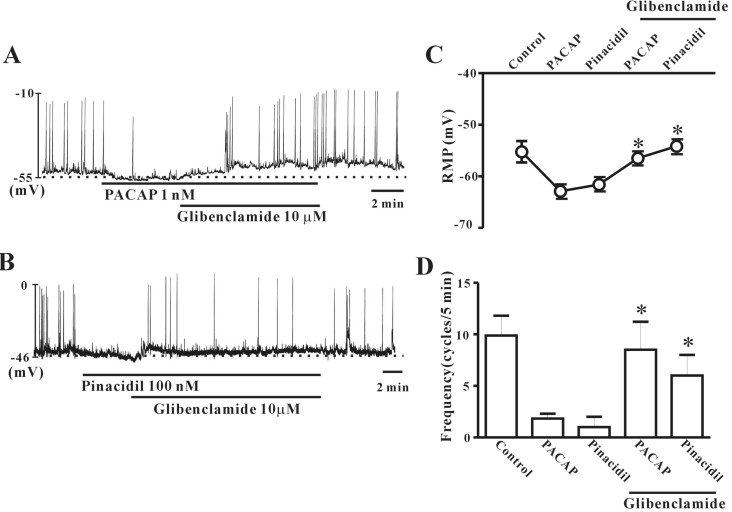
Effect of ATP-sensitive K+ channel blocker on PACAP-induced activity
To investigate the role of ATP-sensitive K+ (KATP) channels in PACAP action on colonic ICC, we tested the effects of PACAP when combined with glibenclamide (KATP channel blocker). First, we checked the PACAP (1 nM)-induced inhibitory action on pacemaker potentials in colonic ICC, and then co-treated with glibenclamide (10 µM). Glibenclamide inhibited the effects of PACAP, and ICC returned to normal pacemaker potential conditions (Fig. 5A). To confirm this result, we tested the effects of pinacidil, a KATP channel opener. Pinacidil (100 nM) had an inhibitory action on pacemaker potentials in colonic ICC, similar to PACAP. This effect was rescued by co-treatment with glibenclamide (Fig. 5B). Fig. 5C and D show that no significant difference was observed between PACAP alone and PACAP with glibenclamide (n=3~4).
Fig. 5. Effects of KATP channel blockers or a channel opener on pituitary adenylate cyclase-activating peptide (PACAP)-mediated inhibition of pacemaker potentials recorded in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) Glibenclamide (an ATP-sensitive K+ channel blocker; 10 µM) blocked PACAP-induced membrane hyperpolarization and reduced the frequency of pacemaker potentials. (B) Pinacidil (100 nM) reduced the frequency of pacemaker potentials and induced hyperpolarization of the cell membrane in colonic ICC. Glibenclamide (10 µM) blocked the pinacidil-induced action. Vertical solid lines show the amplitude of the pacemaker potentials and horizontal solid lines show the duration of recording (s) of pacemaker potentials. (C) and (D) summarize the effects of PACAP on pacemaker potentials in colonic ICC with KATP channel blockers. Bars represent the means±SE. *Asterisks indicate a statistically significantly difference from controls (p<0.05). Con, control.
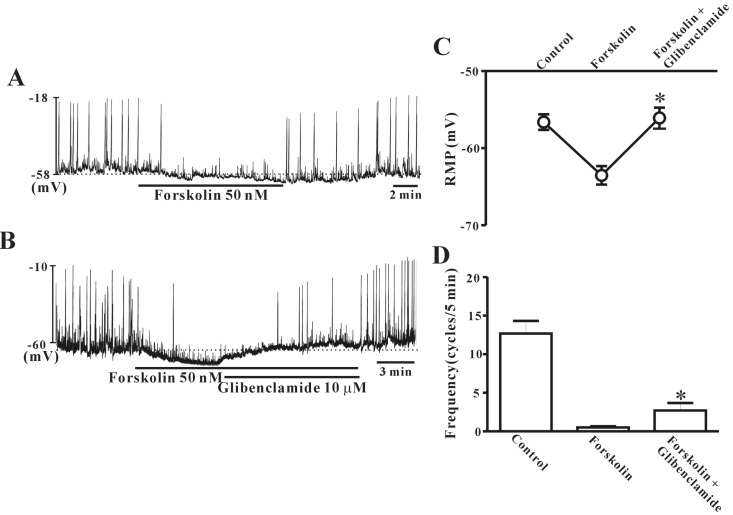
Forskolin mimics PACAP action on colonic ICC
To verify the role of cAMP in ICC regulation, we tested the effects of forskolin on pacemaker potentials. Under a current clamp, ICC generated spontaneous pacemaker potentials. The application of 50 nM forskolin induced hyperpolarization of the cell membrane with reduction of frequency (Fig. 6A). Glibenclamide (10 µM) rescued the forskolin-induced hyperpolarization of the ICC membrane and the decreased pacemaker potential frequency (Fig. 6B). Fig. 6C and D show that no significant difference was observed between forskolin alone and forskolin with glibenclamide in ICC (n=10).
Fig. 6. Effects of forskolin on pacemaker potentials in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) Treatment with forskolin (50 nM) had an inhibitory effect on pacemaker potentials, similar to that of pituitary adenylate cyclase-activating peptide (PACAP). (B) Co-treatment with glibenclamde (10 µM) inhibited forsklin-induced action on colonic ICC. (C) and (D) summarize the effects of forskolin on pacemaker potentials in colonic ICC with glibenclamide. Bars represent the means±SE. *Asterisks indicate a statistically significantly difference from controls (p<0.05). Con, control.
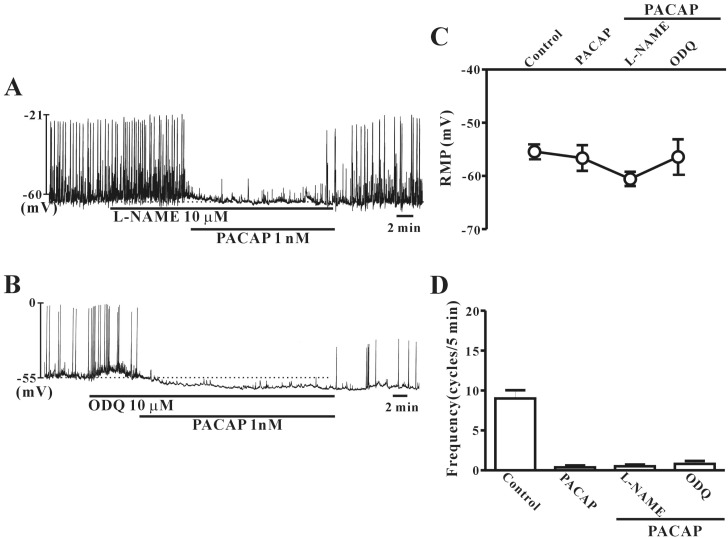
No role for cGMP in PACAP-induced activity in colonic ICC
To study whether PACAP-induced action on colonic ICC is regulated by nitric oxide (NO) signal pathways, L-NAME (a competitive inhibitor of NO synthase) or ODQ (an inhibitor of guanylate cyclase) were tested in combination with PACAP. Pretreatment with L-NAME (10 µM) or ODQ (10 µM) did not affect PACAP-induced inhibitory action or hyperpolarization of the membrane in colonic ICC (Fig. 7A and B). Fig. 7C and D show that no significant differences in frequency or resting membrane potentials were observed between PACAP alone and PACAP with L-NAME or ODQ (n=4~5).
Fig. 7. Effects of NG-nitro-L-arginine methyl ester (L-NAME) or 1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) on pituitary adenylate cyclase-activating peptide (PACAP)-induced responses of pacemaker potentials in cultured interstitial cells of Cajal (ICC) from mouse colon. (A) L-NAME (a competitive inhibitor of NO synthase; 10 µM) did not have any effect on PACAP-induced action in colonic ICC. (B) ODQ (an inhibitor of guanylate cyclase; 10 µM) also did not show any effect on PACAP-induced action in colonic ICC. (C) and (D) summarize the effects of PACAP on pacemaker potentials in colonic ICC with L-NAME or ODQ. Bars represent the means±SE. Con, control.
DISCUSSION
In the present study, we have shown that PACAP inhibits the frequency of pacemaker activity and hyperpolarizes the membrane in colonic ICC. This suggests that PACAP is an inhibitory neurotransmitter that targets colonic ICC. In support of this, many reports have suggested that PACAP has an inhibitory action on the motility of several areas of the GI tract. In vitro studies have shown that PACAP relaxes the rat colon [19,20] and ileum [11,21]. PACAP may participate in the descending relaxation phase of the peristaltic reflex [13]. Electrical-field stimulation and stretching of colon segments induce PACAP release and descending relaxation of the rat colon smooth muscle [13]. Furthermore, previous report showed VIP that is inhibitory neuropeptide like as PACAP inhibited pacemaker activity in small intestinal ICCs [22]. We predicted that PACAP inhibits GI motility not only by controlling smooth muscle directly through its release from enteric neurons, but also by regulation of ICC.
NO is the representative inhibitory neurotransmitter regulating GI motility released from enteric neurons. However, its mechanism of action is different from PACAP. While NO diffuses into target cells and activates soluble guanylate cyclase leading to cGMP production [23], PACAP usually binds to receptors in target cells leading to increased cAMP levels [24]. Our results suggest that ICC may also be targets for inhibitory neurotransmitters, including PACAP, in the modulation of GI motility. We have previously shown that ICC are target cells for NO [18]. Although it is under debate, many reports suggest NO can be produced within enteric neurons, smooth muscle cells, or ICC. Although we did not test whether PACAP is released from enteric neurons in this study, it is possible that ICC may control or relay PACAP action released from enteric neurons that can then act on muscle cells. Further studies are needed to investigate this.
Stimulation of cells by PACAP characteristically evokes an increase in cytoplasmic cAMP [25]. With this in mind, we examined the role of the cAMP pathway in the PACAP-induced effects on colonic ICC, and found that the cAMP pathway does not appear to be involved. Cell-permeable 8-bromo-cAMP did not inhibit the pacemaker activity of colonic ICC, and our results suggest that production of cAMP in colonic ICC has an excitatory effect. Furthermore, our previous report showed there is no effect of cAMP on pacemaker activity in small intestinal ICC [26]. Also, pretreatment with an adenylate cyclase inhibitor does not influence prostaglandin E2 (PGE2) action on pacemaker currents, and PGE2 does not stimulate the production of cAMP [27]. This suggests that inhibition of pacemaker activity by PACAP does not use the common pathway (cAMP-mediated) in colonic ICC. Next, we focused on the possible role of cGMP in mediating PACAP action on colonic ICC. As several studies have suggested that NO facilitates the release of VIP or PACAP [13], we investigated whether cGMP is involved in mediating PACAP-induced activity in colonic ICC. However, PACAP-induced action in colonic ICC was not affected by either an NO synthase inhibitor or a guanylate cyclase inhibitor.
To further understand the mechanism of PACAP action on colonic ICC, we investigated the role of KATP channels. There are reports that KATP channels are involved in PACAP-induced action in some tissues. For example, glibenclamide inhibits PACAP-induced relaxation of human pulmonary arteries and coronary arteries and stimulates KATP channels in smooth muscle cells [28,29]. Interestingly, a KATP channel blocker inhibited the PACAP-induced effect on pacemaker activity in colonic ICC. In particular, the membrane hyperpolarization induced by PACAP was markedly inhibited by the KATP channel blocker. We also examined the effects of forskolin, as previous reports have shown that forskolin inhibits the pacemaker activity of small intestinal ICC through KATP channels [30]. In this study, we found a similar effect of forskolin treatment. We have previously reported that ICC express KATP channels in the small intestine and ICC are modulated by bile salts and antidepressants via KATP channel modulation [31,32].
This study provides the first evidence that the inhibitory action of PACAP on colon motility may occur through ICC. The inhibition of electrical pacemaker activity in colonic ICC by PACAP appears to be closely related to KATP channels but not to the cAMP pathway. Further studies should address how PACAP stimulates the KATP channels and aim to identify the components involved in this regulatory mechanism.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Clinical Research Center of the Chosun University Hospital (2010).
ABBREVIATIONS
- PACAP
pituitary adenylate cyclase-activating peptide
- ICC
interstitial cells of Cajal
- cAMP
cyclic AMP
- L-NAME
NG-nitro-L-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α] quinoxalin-1-one
- GI
gastrointestinal
- VIP
vasoactive intestinal polypeptide
- TEA
tetraethylammonium chloride
- NO
nitric oxide
- PGE2
prostaglandin E2
References
- 1.Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 2.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 3.Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res. 1995;279:385–392. doi: 10.1007/BF00318496. [DOI] [PubMed] [Google Scholar]
- 4.Katsoulis S, Clemens A, Schwörer H, Creutzfeldt W, Schmidt WE. PACAP is a stimulator of neurogenic contraction in guinea pig ileum. Am J Physiol. 1993;265:G295–G302. doi: 10.1152/ajpgi.1993.265.2.G295. [DOI] [PubMed] [Google Scholar]
- 5.Toyoshima M, Takeuchi T, Goto H, Mukai K, Shintani N, Hashimoto H, Baba A, Hata F. Roles of PACAP and PHI as inhibitory neurotransmitters in the circular muscle of mouse antrum. Pflugers Arch. 2006;451:559–568. doi: 10.1007/s00424-005-1491-6. [DOI] [PubMed] [Google Scholar]
- 6.Mukai K, Takeuchi T, Toyoshima M, Satoh Y, Fujita A, Shintani N, Hashimoto H, Baba A, Hata F. PACAP- and PHI-mediated sustained relaxation in circular muscle of gastric fundus: findings obtained in PACAP knockout mice. Regul Pept. 2006;133:54–61. doi: 10.1016/j.regpep.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Hagi K, Azuma YT, Nakajima H, Shintani N, Hashimoto H, Baba A, Takeuchi T. Involvements of PHI-nitric oxide and PACAP-BK channel in the sustained relaxation of mouse gastric fundus. Eur J Pharmacol. 2008;590:80–86. doi: 10.1016/j.ejphar.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Jin JG, Katsoulis S, Schmidt WE, Grider JR. Inhibitory transmission in tenia coli mediated by distinct vasoactive intestinal peptide and apamin-sensitive pituitary adenylate cyclase activating peptide receptors. J Pharmacol Exp Ther. 1994;270:433–439. [PubMed] [Google Scholar]
- 9.McConalogue K, Lyster DJ, Furness JB. Electrophysiological analysis of the actions of pituitary adenylyl cyclase activating peptide in the taenia of the guinea-pig caecum. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:538–544. doi: 10.1007/BF00169388. [DOI] [PubMed] [Google Scholar]
- 10.Katsoulis S, Schmidt WE, Schwarzhoff R, Folsch UR, Jin JG, Grider JR, Makhlouf GM. Inhibitory transmission in guinea pig stomach mediated by distinct receptors for pituitary adenylate cyclase-activating peptide. J Pharmacol Exp Ther. 1996;278:199–204. [PubMed] [Google Scholar]
- 11.Ekblad E, Sundler F. Distinct receptors mediate pituitary adenylate cyclase-activating peptide- and vasoactive intestinal peptide-induced relaxation of rat ileal longitudinal muscle. Eur J Pharmacol. 1997;334:61–66. doi: 10.1016/s0014-2999(97)01144-8. [DOI] [PubMed] [Google Scholar]
- 12.Parkman HP, Pagano AP, Ryan JP. PACAP and VIP inhibit pyloric muscle through VIP/PACAP-preferring receptors. Regul Pept. 1997;71:185–190. doi: 10.1016/s0167-0115(97)01031-8. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR, Katsoulis S, Schmidt WE, Jin JG. Regulation of the descending relaxation phase of intestinal peristalsis by PACAP. J Auton Nerv Syst. 1994;50:151–159. doi: 10.1016/0165-1838(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 14.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 15.Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 16.So KY, Kim SH, Sohn HM, Choi SJ, Parajuli SP, Choi S, Yeum CH, Yoon PJ, Jun JY. Carbachol regulates pacemaker activities in cultured interstitial cells of Cajal from the mouse small intestine. Mol Cells. 2009;27:525–531. doi: 10.1007/s10059-009-0076-1. [DOI] [PubMed] [Google Scholar]
- 17.Shahi PK, Choi S, Zuo DC, Yeum CH, Yoon PJ, Lee J, Kim YD, Park CG, Kim MY, Shin HR, Oh HJ, Jun JY. 5-hydroxytryptamine generates tonic inward currents on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2011;15:129–135. doi: 10.4196/kjpp.2011.15.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CG, Kim YD, Kim MY, Kim JS, Choi S, Yeum CH, Parajuli SP, Park JS, Jeong HS, So I, Kim KW, Jun JY. Inhibition of pacemaker currents by nitric oxide via activation of ATP-sensitive K+ channels in cultured interstitial cells of Cajal from the mouse small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007;376:175–184. doi: 10.1007/s00210-007-0187-1. [DOI] [PubMed] [Google Scholar]
- 19.Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, Takewaki T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mungun Z, Rossowski WJ, Coy DH. Pituitary adenylate cyclase activating polypeptide relxed gastrointestinal smooth muscles in rat. Clin Res. 1991;39:236A. doi: 10.3109/00365529209000091. [DOI] [PubMed] [Google Scholar]
- 21.Katsoulis S, Clemens A, Schwörer H, Creutzfeldt W, Schmidt WE. Pituitary adenylate cyclase activating polypeptide (PACAP) is a potent relaxant of the rat ileum. Peptides. 1993;14:587–592. doi: 10.1016/0196-9781(93)90149-b. [DOI] [PubMed] [Google Scholar]
- 22.Kim BJ, Lee JH, Jun JY, Chang IY, So I, Kim KW. Vasoactive intestinal polypeptide inhibits pacemaker activity via the nitric oxide-cGMP-protein kinase G pathway in the interstitial cells of Cajal of the murine small intestine. Mol Cells. 2006;21:337–342. [PubMed] [Google Scholar]
- 23.Shuttleworth CW, Sanders KM. Involvement of nitric oxide in neuromuscular transmission in canine proximal colon. Proc Soc Exp Biol Med. 1996;211:16–23. doi: 10.3181/00379727-211-43950c. [DOI] [PubMed] [Google Scholar]
- 24.Jin JG, Murthy KS, Grider JR, Makhlouf GM. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am J Physiol. 1993;264:G470–G477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- 25.McCulloch DA, MacKenzie CJ, Johnson MS, Robertson DN, Holland PJ, Ronaldson E, Lutz EM, Mitchell R. Additional signals from VPAC/PAC family receptors. Biochem Soc Trans. 2002;30:441–446. doi: 10.1042/bst0300441. [DOI] [PubMed] [Google Scholar]
- 26.Jun JY, Choi S, Yeum CH, Chang IY, Park CK, Kim MY, Kong ID, So I, Kim KW, You HJ. Noradrenaline inhibits pacemaker currents through stimulation of β1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br J Pharmacol. 2004;141:670–677. doi: 10.1038/sj.bjp.0705665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006;18:187–198. doi: 10.1159/000097516. [DOI] [PubMed] [Google Scholar]
- 28.Bruch L, Bychkov R, Kästner A, Bülow T, Ried C, Gollasch M, Baumann G, Luft FC, Haller H. Pituitary adenylatecyclase-activating peptides relax human coronary arteries by activating K(ATP) and K(Ca) channels in smooth muscle cells. J Vasc Res. 1997;34:11–18. doi: 10.1159/000159197. [DOI] [PubMed] [Google Scholar]
- 29.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 30.Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, Sanders KM. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000;527 Pt 1:149–162. doi: 10.1111/j.1469-7793.2000.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, So I, Kim KW, You HJ. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005;144:242–251. doi: 10.1038/sj.bjp.0706074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi S, Park CG, Kim MY, Lim GH, Kim JH, Yeum CH, Yoon PJ, So I, Kim KW, Jun JY. Action of imipramine on activated ATP-sensitive K+ channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006;78:2322–2328. doi: 10.1016/j.lfs.2005.09.032. [DOI] [PubMed] [Google Scholar]