Abstract
Flavonoids are plant pigments that have been demonstrated to exert various pharmacological effects including anti-cancer, anti-diabetic, anti-atherosclerotic, anti-bacterial, and anti-inflammatory activities. However, the molecular mechanisms in terms of exact target proteins of flavonoids are not fully elucidated yet. In this study, we aimed to evaluate the anti-inflammatory mechanism of scutellarein (SCT), a flavonoid isolated from Erigeron breviscapus, Clerodendrum phlomidis and Oroxylum indicum Vent that have been traditionally used to treat various inflammatory diseases in China and Brazil. For this purpose, a nitric oxide (NO) assay, polymerase chain reaction (PCR), nuclear fractionation, immunoblot analysis, a kinase assay, and an overexpression strategy were employed. Scutellarein significantly inhibited NO production in a dose-dependent manner and reduced the mRNA expression levels of inducible NO synthase (iNOS) and tumor necrosis factor (TNF)-α in lipopolysaccharide (LPS)-activated RAW264.7 cells. In addition, SCT also dampened nuclear factor (NF)-κB-driven expression of a luciferase reporter gene upon transfection of a TIR-domain-containing adapter-inducing interferon-β (TRIF) construct into Human embryonic kidney 293 (HEK 293) cells; similarly, NF-κ B nuclear translocation was inhibited by SCT. Moreover, the phosphorylation levels of various upstream signaling enzymes involved in NF-κB activation were decreased by SCT treatment in LPS-treated RAW264.7 cells. Finally, SCT strongly inhibited Src kinase activity and also inhibited the autophosphorylation of overexpressed Src. Therefore, our data suggest that SCT can block the inflammatory response by directly inhibiting Src kinase activity linked to NF-κB activation.
Keywords: Anti-inflammatory effect, Flavonoid, Macrophages, NF-κB, Scutellarein, Src
INTRODUCTION
Inflammation is a natural defense system to protect the body from pathogens. When immune cells such as macrophages encounter pathogens (i.e. bacteria or parasites), inflammatory reactions are spontaneously induced. These reactions include the production of cytokines, chemokines, and inflammatory mediators such as nitric oxide (NO) and tumor necrosis factor (TNF)-α [1]. However, excessively high or sustained inflammation can cause various diseases such as atherosclerosis [2], septic shock [3], and rheumatoid arthritis [4]. Macrophages, which are the major phagocytic cells that defend the body against pathogens, are known to play a critical role in inflammatory responses [5]. Macrophage activation is known to be triggered when pathogen-associated molecules like lipopolysaccharide (LPS) stimulate macrophage receptors, such as Toll-like receptor (TLR) 4. This receptor/ligand interaction activates intracellular signaling cascades mediated by various enzymes, including the non-receptor type protein kinase Src, phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), the serine/threonine-specific protein kinase AKT, and IκB kinase (IKK) [6]. Activated IKK phosphorylates IκBα, which is a suppressor of NF-κB, to stimulate translocation of NF-κB subunits into the nucleus by degradation of IκBα [7]. Eventually, the activation of inflammatory transcription factors such as NF-κB results in the expression of pro-inflammatory genes, including inducible NO synthase (iNOS), cyclooxygenase (COX)-2, and cytokines [8]. Accordingly, various types of new anti-inflammatory drugs targeting NF-κB are currently being developed [9].
Scutellarein (SCT, Fig. 1A) is the active component of Erigeron breviscapus, Clerodendrum phlomidis and Oroxylum indicum Vent and is used to treat inflammation, diabetes, nervous disorders, asthma, rheumatism, digestive disorders, and urinary disorders; it is also given as a bitter tonic [10,11]. In addition, Codariocalyx motorius with structural analogue of SCT (SCT-6-O-glucuronide) was also shown to suppress LPS-induced inflammatory responses in macrophages and a HCl/EtOH-triggered gastritis symptoms [12]. From a pharmacological perspective, SCT has been reported to exert antioxidative, anti-cancer, and neuroprotective activities [13,14]. Although SCT is known to have a flavonoid-derived backbone, the precise anti-inflammatory activity of SCT and its molecular mechanism of action are not yet completely understood. Therefore, in this study, we aimed to determine the immunopharmacological role of SCT and to identify the target of SCT in LPS-activated macrophages.
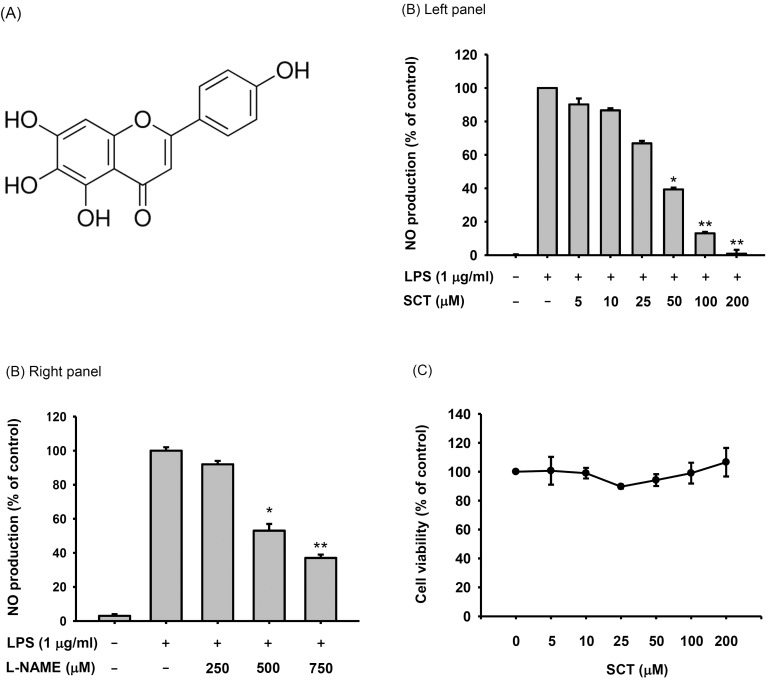
Fig. 1. Chemical structure and anti-inflammatory activity of SCT. (A) Molecular structure of SCT. (B) RAW-264.7 cells (1×106 cells/ml) were pretreated with SCT (left panel) or L-NAME (right panel) for 1 h and incubated with LPS (1 µg/ml) for 24 h. The nitric oxide levels were measured by the Griess assay. (C) Viability of RAW264.7 cells after treatment with SCT for 24 h. Viability was determined by a conventional MTT assay. *p<0.05 and **p<0.01 compared with the control group.
METHODS
Materials
SCT (isolated from Erigeron breviscapus, Purity>98%), polyethylenimine (PEI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), NG-nitro-L-arginine methyl ester (L-NAME), sodium dodecyl sulfate (SDS), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS), Phosphate buffered saline (PBS), penicillin, streptomycin, TRIzol reagent, and RPMI1640 were obtained from GIBCO (Grand Island, NY, USA). The RAW264.7 murine macrophage cell line and HEK 293 human embryonic kidney cell line were purchased from ATCC (Rockville, MD, USA). Phospho-specific and total protein antibodies raised against p65, p50, inhibitor of κBα (IκBα), IKKα/β, AKT, p85, Src, lamin A/C, and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against cyan fluorescent protein (CFP) and human influenza hemagglutinin (HA) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Expression vectors
Wild type Src (HA-Src) was used as reported previously [15,16]. The luciferase construct containing binding sites for NF-κB (NF-κB-Luc) and the plasmids encoding adaptor molecules (CFP-TRIF and Flag-hMyD88) for TLR signaling were used as reported previously [17]. All constructs were confirmed by automated DNA sequencing.
Cell culture and drug preparation
RAW264.7 cells were maintained in RPMI1640 medium supplemented with 100 U/ml of penicillin, 100 µg/ml of streptomycin, and 10% FBS. HEK 293 cells were maintained in Dulbecco's Modified Eagle Medium supplemented with 100 U/ml of penicillin, 100 µg/ml of streptomycin, and 10% FBS. Cells were grown at 37℃ and 5% CO2 in humidified air. The SCT stock solution used in the in vitro experiments was prepared in DMSO.
Measurement of NO production
After preincubation of RAW264.7 cells (1×106 cells/ml) overnight, the cells were treated with SCT (0 to 200 µM) or L-NAME (0 to 750 µM) for 1 h and then further incubated with LPS (1 µg/ml) for 24 h. The inhibitory effects of these drugs on NO production were determined by analyzing the NO levels using the Griess reagent as previously described [18,19].
mRNA analysis using polymerase chain reaction
To determine cytokine mRNA expression levels, total RNA was isolated from LPS-treated RAW264.7 cells using TRIzol according to the manufacturer's instructions. All RNA was stored at -70℃ until use. Semi-quantitative RT reactions were conducted as previously reported [20,21]. mRNA quantification was performed by real-time RT-PCR with SYBR Premix Ex Taq according to the manufacturer's instructions (Takara, Shiga, Japan) using a real-time thermal cycler (Bio-Rad, Hercules, CA, USA). Semi-quantitative RT-PCR was conducted as previously reported with minor modifications [22]. All of the primers (Bioneer, Daejeon, Korea) used are listed in Table 1.
Table 1. Real-time and semiquantitative RT-PCR primers used in this study.
Preparation of cell lysates and nuclear fractions for immunoblotting
RAW264.7 or HEK 293 cells (5×106 cells/ml) were washed 3 times in cold PBS containing 1 mM sodium orthovanadate and resuspended in lysis buffer [20 mM Tris-HCl (pH 7.4), 2 mM Ethylenediaminetetraacetic acid (EDTA), 2 mM ethyleneglycotetraacetic acid, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 µg/ml aprotinin, 10 µg/ml pepstatin, 1 mM benzimide, and 2 mM phenylmethylsulfonyl fluoride (PMSF)]. Cells were lysed by rotation for 1 h at 4℃. The lysates were clarified by centrifugation at 12,000 × g for 10 min at 4℃ and the supernatants were stored at -20℃ until needed.
Nuclear lysates were prepared using a three-step procedure [23]. After treatment, cells were collected with a rubber policeman, washed with PBS, and resuspended in 500 µl of lysis buffer containing 50 mM KCl, 0.5% Nonidet P-40, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.8), 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 20 µg/ml aprotinin, and 100 µM 1,4-dithiothreitol (DTT). Cells were lysed by incubation on ice for 4 min. Cell lysates were then spun by centrifugation at 14,000 rpm for 1 min in a microcentrifuge. During the second step, the pellet (the nuclear fraction) was washed once with wash buffer lacking Nonidet P-40. During the final step, the nuclei were resuspended in extraction buffer (lysis buffer plus 500 mM KCl and 10% glycerol). The nuclei/extraction buffer mixture was frozen at -80℃, thawed on ice, and spun by centrifugation at 14,000 rpm for 5 min. The supernatant was collected as the nuclear extract.
Whole cell and nuclear lysates were then analyzed by immunoblotting. Proteins were separated on 10% SDS-polyacrylamide gels and transferred by electroblotting to polyvinylidenedifluoride membranes. Nonspecific binding sites on the membranes were blocked for 60 min at room temperature in Tris-buffered saline containing 3% bovine serum albumin (BSA) and 0.1% Tween 20. The membranes were then incubated for 60 min with the appropriate primary antibodies at 4℃, washed 3 times with the same buffer, and incubated for an additional 60 min with HRP-conjugated secondary antibodies. The total and phosphorylated levels of p65, p50, c-Fos, c-Jun, IκBα, IKKα, IKKβ, AKT, Src, hemagglutinin (HA), cyan fluorescent protein (CFP), lamin A/C, and β-actin were visualized using an ECL system (Amersham, Little Chalfont, Buckinghamshire, UK) as reported previously [24].
DNA transfection and luciferase reporter gene activity assay
HEK 293 cells were seeded in 6 cm petri dishes (5×105 cells/ml) and transfected with plasmids driving the expression of HA-Src, Flag-hMyD88 or CFP-TRIF (1 µg/ml each). Transfections were performed according to the PEI method as reported previously [25,26]. All experiments were performed 24 h post-transfection. SCT was added to the cells 1 h before termination. For reporter gene assays, HEK 293 cells (5×105 cells/ml) were seeded in 24-well plates and transfected with 1 µg each of plasmids expressing NF-κB-Luc and TRIF, as well as β-galactosidase. Transfections were carried out using the PEI method according to the procedures outlined in previous reports [25,26]. Luciferase assays were performed using the Luciferase Assay System (Promega, Madison, WI, USA), as previously reported [27].
In vitro kinase assay with purified enzymes and immunoprecipitated enzymes
To evaluate the abilities of purified enzymes to inhibit Src kinase activity, a kinase profiler service using radioactive ATP from Millipore (Billerica, MA, USA) was used. Purified Src (1~5 mU) was incubated in reaction buffer in a final volume of 25 µl. The reaction was initiated by the addition of Mg-ATP. After incubation for 40 min at room temperature, the reaction was stopped by the addition of 5 ml of a 3% phosphoric acid solution. Ten microliters of the reaction was then spotted onto a P30 Filtermat, which was then washed three times for 5 min in 75 mM phosphoric acid and once in methanol prior to drying and scintillation counting.
Statistical analyses
All data (Figs. 1B, 1C, 2B, 2C, 3A right panel, 3B right panel, 4A right panel, 4B right panel, 5A, 5B lower panel, and 5C lower panel) are expressed as means±SDs. For statistical comparisons, results were analyzed using ANOVA/Scheffe's post-hoc test and the Kruskal-Wallis/Mann-Whitney test. A p-value<0.05 was considered to indicate a statistically significant difference. All statistical tests were carried out using SPSS software (SPSS Inc., Chicago, IL, USA, v.20). All results are representative of at least two biological replicates conducted using the same numbers of samples or mice.
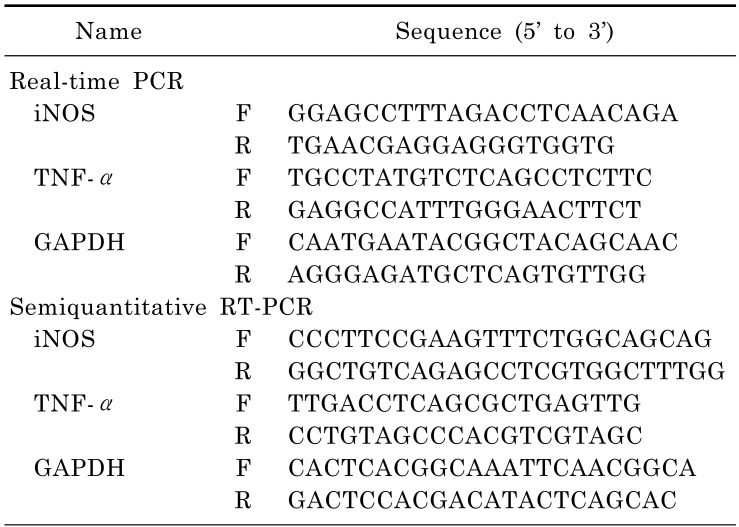
Fig. 2. Effect of SCT on inflammatory gene expression in LPS-treated RAW264.7 cells. (A) RAW264.7 cells (1×106 cells/ml) were incubated with LPS (1 µg/ml) for 6 h after treatment with SCT. The mRNA expression levels of iNOS and TNF-α were determined by RT-PCR. (B and C) iNOS and TNF-α mRNA expression levels were measured by real-time PCR. **p<0.01 compared with the control group.
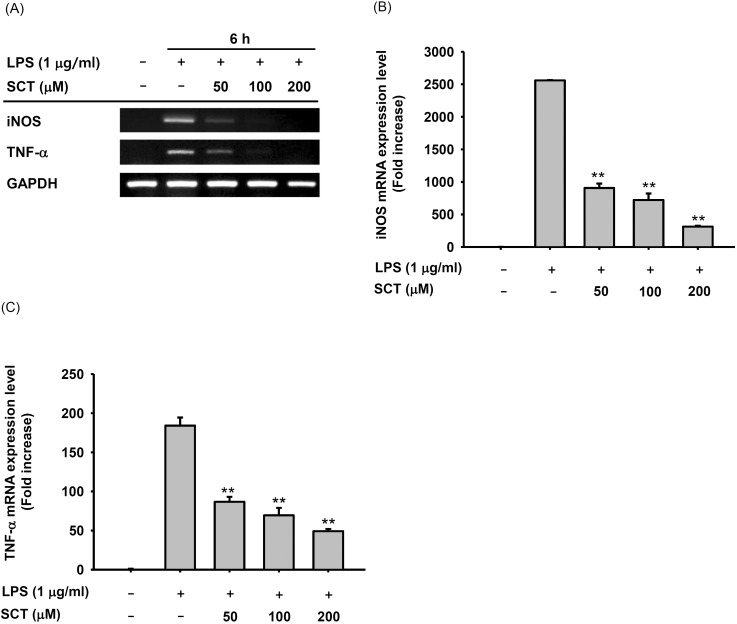
Fig. 3. Effect of SCT on the transcriptional activation of inflammatory gene expression. (A, left panel) HEK 293 cells (5×105 cells/ml) were incubated with SCT for 24 h. Cell viability was measured by the MTT assay. (A, right panel) HEK 293 cells were treated with SCT for 24 h after cotransfection with NF-κB-Luc, TRIF, or pcDNA for 24 h. Luciferase activity was determined using a luminometer. (B left panel) Nuclear translocation of NF-κB subunits was detected by immunoblot analysis of nuclear fractions. Relative intensity (B right panel) was calculated using total levels by the DNR Bio-Imaging system. *p<0.05 and **p<0.01 compared with the control group.
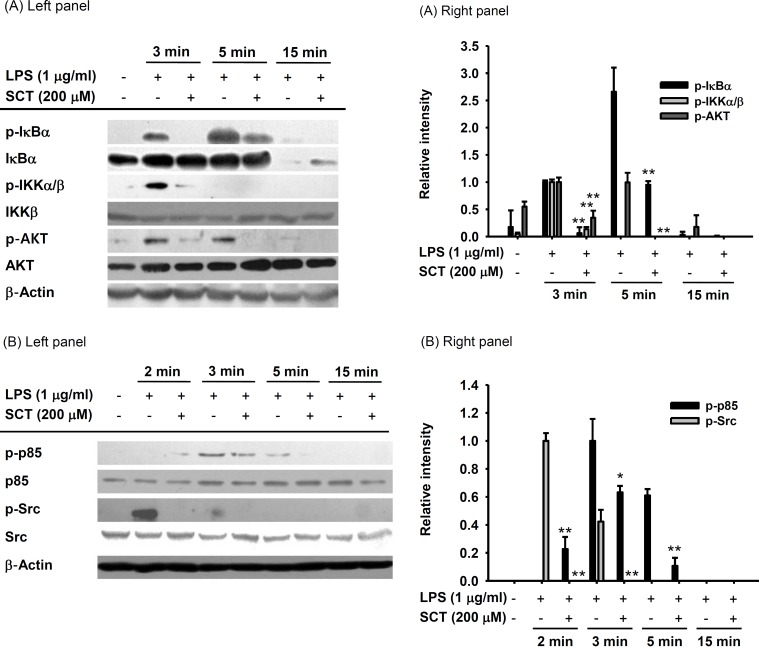
Fig. 4. Effect of SCT on signaling events upstream of NF-κB activation. (Left panels of A and B) RAW264.7 cells (1×106 cells/ml) were incubated with LPS (1 µg/ml) for the indicated times after treatment with SCT (200 µM). The levels of phosphorylated IκBα, IKKα/β, AKT, p85/PI3K, and Src were detected by immunoblot analysis. Relative intensity (Right panels of A and B) was calculated using total levels by the DNR Bio-Imaging system. *p<0.05 and **p<0.01 compared with the control group.
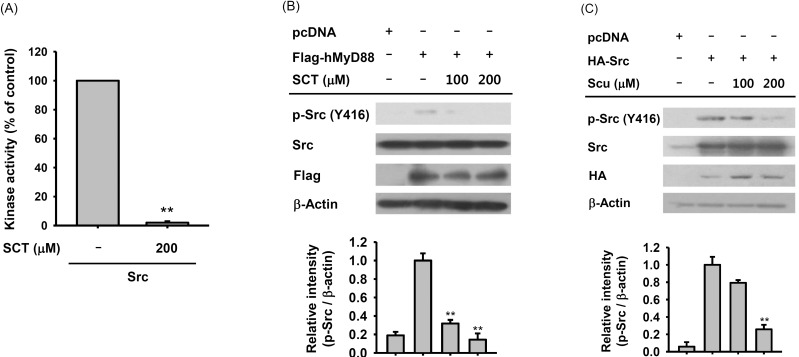
Fig. 5. Effect of SCT on the regulation of Src kinase. (A) The inhibitory effect of SCT on Src kinase activity was determined using an established kinase assay with purified Src enzyme. (B and C) HEK 293 cells (5×105 cells/ml) were transfected with Flag-hMyD88 or HA-Src plasmids and then incubated for 24 h. The transfected cells were then treated with SCT for 12 h. The level of phosphorylated Src was measured by immunoblotting. Relative intensity (Bottom panels of B and C) was calculated using β-actin levels by the DNR Bio-Imaging system. **p<0.01 compared with the control group.
RESULTS
Effect of scutellarein on NO production and cell viability
To determine whether SCT has an anti-inflammatory activity in macrophages during inflammatory conditions induced by LPS, we first checked NO production level in LPS-stimulated RAW 264.7 cells in the presence or absence of SCT or L-NAME (as a control drug). As shown in Fig. 1B, SCT strongly suppressed the production of NO in a dose-dependent manner up to 99% at a dose of 200 µM. Dose-dependent NO inhibitory profile was also observed in L-NAME-treated condition (Fig. 1B right panel). Importantly, SCT did not significantly affect the viability of RAW264.7 cells up to 200 µM (Fig. 1C).
Effect of scutellarein on the mRNA expression of inflammatory genes
We next examined the mRNA expression levels of inflammatory genes in LPS-treated RAW264.7 cells. As shown in Fig. 2, LPS alone dramatically triggered the mRNA expression of iNOS and TNF-α, while SCT strongly suppressed the upregulated levels of the inflammatory genes, according to both RT-PCR (Fig. 2A) and Real-time PCR (Fig. 2B to C) analyses.
Effect of scutellarein on the transcriptional activation
In order to investigate how SCT modulated inflammatory gene expression, we employed a reporter gene assay with luciferase constructs containing NF-κB promoter using HEK 293 cells. In fact, NF-κB mediated luciferase activity was revealed to be highly increased under the overexpression of TRIF, a TLR4 adaptor molecule up to 50 folds. Interestingly, however, SCT strongly suppressed this activity by 55% at 200 µM of SCT (Fig. 3A right panel), although the viability of HEK 293 cells was rather enhanced at 200 µM (Fig. 3A left panel). To confirm the possibility that NF-κB pathway can be blocked by SCT treatment, we determined the translocation levels of NF-κB subunits (p65 and p50) in the nucleus of LPS-treated RAW264.7 cells. Expectedly, SCT (200 µM) clearly diminished the translocation levels of p65 and p50 at 15, 30, and 60 min during LPS stimulation (Fig. 3B).
Effect of scutellarein on the activation of NF-κ B translocation signaling
Since the phosphorylation of IκBα is a critical step involved in the translocation of NF-κB, we next investigated whether SCT is able to modulate the LPS-induced phosphorylation of IκBα by using immunoblotting analysis. As shown in Fig. 4A, the phosphorylation level of IκBα was highly reduced by SCT at 3 to 15 min. Moreover, incubation of SCT (200 µM) also inhibited enhanced phosphorylation of IKK, AKT, p85, and Src, which participate in increasing IκBα phosphorylation, at 3 to 5 min after LPS treatment (Fig. 4A and 4B).
Effect of scutellarein on the activation of Src kinase
Since SCT blocked the phosphorylation of Src (Fig. 4B), we attempted to identify whether there is downregulation of Src autophosphorylation by this compound. To do this, in vitro Src kinase activity was measured. As Fig. 5A shows, 200 µM of SCT significantly inhibited the kinase activity of Src, indicating that Src can directly be a target of SCT. Similarly, the phosphorylation level of Src induced by MyD88 overexpression was also reduced in HEK293 cell (Fig. 5B). In addition, Src phosphorylation mediated by HA-Src was also decreased by 200 µM of SCT (Fig. 5C).
DISCUSSION
Based on the reported findings that SCT tetramethyl ether and SCT 7-O-β-d-glucuronide have anti-inflammatory activity [28,29,30], we explored whether SCT suppresses inflammatory responses. We first investigated the effect of SCT on NO production in LPS-treated macrophages. As shown in Fig. 1B, SCT inhibited NO secretion by LPS-stimulated RAW264.7 cells in a dose-dependent manner. Importantly, SCT did not affect the viability of RAW264.7 cells at concentrations up to 200 µM (Fig. 1C). Since NO is known to be a key molecule in the inflammatory response [31,32], this finding suggests that SCT could inhibit other inflammatory activities of macrophages. To test this hypothesis, we next determined whether SCT affects inflammatory gene expression in LPS-activated RAW264.7 cells. Testing the profile of inflammatory gene expression patterns is one way to identify the anti-inflammatory spectrum of a compound. As shown in Fig. 2, LPS-induced upregulation of iNOS and TNF-α mRNA was significantly suppressed by SCT (Fig. 2A), as predicted. In addition, quantitative real-time PCR showed that SCT strongly inhibited iNOS (Fig. 2B) and TNF-α (Fig. 2C) expression in a dose-dependent manner. This result implies that SCT inhibits NO and cytokine production with a broad-spectrum mode of action at the transcriptional level.
To further investigate the mechanism by which SCT inhibits the expression of proinflammatory genes, we sought the target transcription factors involved. Interestingly, SCT tetramethyl ether has been reported to suppress NF-κB activation [30]. Moreover, the expression levels of phosphory-lated EGFR, ERK, and NF-κB in proliferative lung cancer cells have been found to be reduced during SCT treatment [33]. Therefore, we next examined whether SCT suppresses NF-κB activation. To this end, we tested the effect of SCT on the activity of an NF-κB-driven luciferase reporter construct in HEK 293 cells. Since HEK 293 cells do not express TLR4, one of its adaptor molecules, TRIF, was also introduced to activate TLR4-induced signaling events leading to NF-κB activation. Indeed, TRIF overexpression strongly induced NF-κB-driven luciferase activity [34]. Under these conditions, TRIF-transfected HEK 293 cells displayed up to 50-fold increased levels of NF-κB-driven luciferase activity (Fig. 3A, right panel). Treatment with SCT clearly decreased this NF-κB-driven luciferase activity (Fig. 3A, right panel). Importantly, SCT did not affect cell viability at this concentration (Fig. 3A, left panel), implying that NF-κB could be targeted by SCT. To test this possibility, we next prepared nuclear fractions to determine the levels of translocated NF-κB by immunoblot analysis. As depicted in Fig. 3B, SCT treatment reduced the nuclear levels of p65 and p50, which are components of the NF-κB transcription factor [35], suggesting that the NF-κB translocation pathway could be targeted by SCT. So far, numerous naturally occurring compounds with anti-inflammatory properties such as quercetin, luteolin, kaempferol, resveratrol, and ginsenoside Rd have been reported to inhibit NF-κB activation [18,36,37,38,39]. The active forms of NF-κB have also been identified in many different inflammatory diseases such as colitis, pancreatitis, and gastritis [9,40]. Therefore, our results are consistent with previous results and strongly imply that SCT may exert its anti-inflammatory actions by inhibiting the NF-κB pathway.
Since SCT suppressed the nuclear translocation of NF-κB, we investigated further upstream signaling events, including IκBα, IKK, AKT, p85/PI3K, and Src phosphorylation. These molecules all regulate NF-κB translocation [41,42]. As shown in Fig. 4A, the level of phosphorylated IκBα was greatly reduced by treatment with SCT at 3, 5, and 15 min, although both total- and phospho-IκBα levels were markedly decreased at 15 min, due to increased degradation pathway of IκBα linked to phosphorylation without altering mRNA level (data not shown). In addition, the levels of phosphorylated IKK and AKT, two kinases upstream of IκBα, were also decreased by SCT treatment (Fig. 4B). Similarly, phosphorylation levels of p85, the regulatory subunit of PI3K [43], and Src were also reduced under the same conditions (Fig. 4B). Since Src kinase is known to undergo autophosphorylation and to act upstream of PI3K [44], this result implies that SCT suppresses Src kinase activity. This finding is important because Src plays an important role in the upregulation of NF-κB [41].
To determine whether SCT directly targets Src kinase, we employed an established kinase activity assay with purified Src as reported previously [45,46]. As shown in Fig. 5A, SCT greatly reduced the kinase activity of Src, indicating that SCT is capable of directly interfering with Src activity. To test this finding by another strategy, we overexpressed Src and TRIF and looked at the level of phospho-Src upon treatment with SCT. As expected, the enhanced level of phospho-Src observed under MyD88 or Src expression conditions was significantly decreased by SCT treatment (Fig. 5B and 5C), providing further evidence that SCT directly targets Src. The mechanisms by which SCT directly blocks Src kinase activity are not yet understood. Recent studies with flavonoids have indicated that 6,7,4'-trihydroxyisoflavone suppresses PI3K activity by directly competing for ATP [47]. Additionally, the finding that kaempferol competes with ATP for direct binding to Src [48] raises the possibility that SCT, which has a flavonoid backbone, might similarly bind to the ATP binding site of Src, thereby inhibiting its kinase activity. Future studies will aim to test this hypothesis.
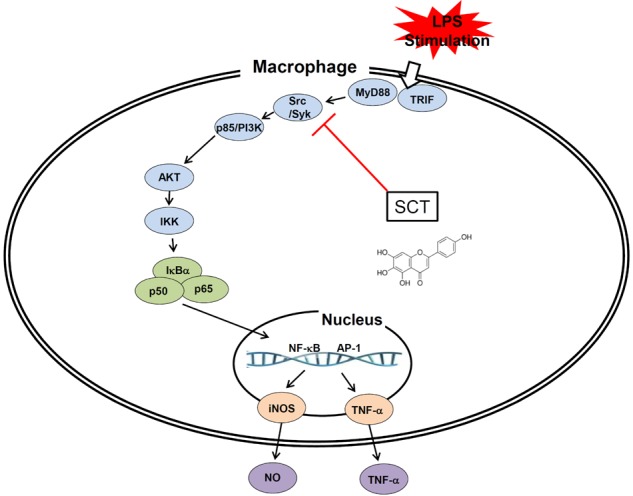
In summary, here we showed that SCT blocks NO production and reduces inflammatory gene expression in LPS-stimulated macrophages without decreasing cell viability. These anti-inflammatory activities of SCT appear to result from suppressed NF-κB activation, including inhibition of upstream signaling proteins such as IκBα, IKK, AKT, p85/PI3K, and Src (Fig. 6). Therefore, in agreement with previous reports that SCT is a beneficial pharmacological remedy [49], we propose that SCT can be developed as a novel anti-inflammatory drug.
Fig. 6. Schematic diagram of the inhibitory action of SCT on LPS-induced inflammatory responses in macrophage cells.
ACKNOWLEDGMENTS
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project no. PJ009241), Rural Development Administration, Korea.
ABBREVIATIONS
- SCT
scutellarein
- NO
nitric oxide
- TNF-α
tumor necrosis factor-α
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- TLR
Toll-like receptor
- MyD88
Myeloid differentiation primary response gene 88
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PEI
polyethylenimine
- IKK
IκB kinase
- COX-2
cyclooxygenase-2
Footnotes
DISCLAIMER: The authors alone are responsible for the content and writing of the paper.
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
References
- 1.Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 4.Stuhlmüller B, Ungethüm U, Scholze S, Martinez L, Backhaus M, Kraetsch HG, Kinne RW, Burmester GR. Identification of known and novel genes in activated monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:775–790. doi: 10.1002/1529-0131(200004)43:4<775::AID-ANR8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 6.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 7.Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999;26:717–719. [PubMed] [Google Scholar]
- 9.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Mohan Maruga Raja MK, Mishra SH. Comprehensive review of Clerodendrum phlomidis: a traditionally used bitter. Zhong Xi Yi Jie He Xue Bao. 2010;8:510–524. doi: 10.3736/jcim20100602. [DOI] [PubMed] [Google Scholar]
- 11.Harminder, Singh V, Chaudhary AK. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum vent. Indian J Pharm Sci. 2011;73:483–490. doi: 10.4103/0250-474X.98981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E, Yoon KD, Lee WS, Yang WS, Kim SH, Sung NY, Baek KS, Kim Y, Htwe KM, Kim YD, Hong S, Kim JH, Cho JY. Syk/Src-targeted anti-inflammatory activity of Codariocalyx motorius ethanolic extract. J Ethnopharmacol. 2014;155:185–193. doi: 10.1016/j.jep.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Tang H, Tang Y, Li N, Shi Q, Guo J, Shang E, Duan JA. Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol Biochem Behav. 2014;118:51–59. doi: 10.1016/j.pbb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Siraichi JT, Felipe DF, Brambilla LZ, Gatto MJ, Terra VA, Cecchini AL, Cortez LE, Rodrigues-Filho E, Cortez DA. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in Southern Brazil. PLoS One. 2013;8:e72733. doi: 10.1371/journal.pone.0072733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang KJ, Shin S, Piao L, Shin E, Li Y, Park KA, Byun HS, Won M, Hong J, Kweon GR, Hur GM, Seok JH, Chun T, Brazil DP, Hemmings BA, Park J. Regulation of 3-phosphoinositidedependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem. 2008;283:1480–1491. doi: 10.1074/jbc.M706361200. [DOI] [PubMed] [Google Scholar]
- 16.Byeon SE, Yu T, Yang Y, Lee YG, Kim JH, Oh J, Jeong HY, Hong S, Yoo BC, Cho WJ, Hong S, Cho JY. Hydroquinone regulates hemeoxygenase-1 expression via modulation of Src kinase activity through thiolation of cysteine residues. Free Radic Biol Med. 2013;57:105–118. doi: 10.1016/j.freeradbiomed.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Shen T, Yang WS, Yi YS, Sung GH, Rhee MH, Poo H, Kim MY, Kim KW, Kim JH, Cho JY. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid Based Complement Alternat Med. 2013;2013:210736. doi: 10.1155/2013/210736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Chung JH, Yoon JS, Ha YM, Bae S, Lee EK, Jung KJ, Kim MS, Kim YJ, Kim MK, Chung HY. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res. 2013;37:54–63. doi: 10.5142/jgr.2013.37.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn CK, Park SJ, Lee MY, Cha MJ, Kim OH, You HJ, Chang IY, Yoon SP, Jeon YJ. Silibinin inhibits LPS-induced macrophage activation by blocking p38 MAPK in RAW 264.7 Cells. Biomol Ther (Seoul) 2013;21:258–263. doi: 10.4062/biomolther.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YG, Chain BM, Cho JY. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int J Biochem Cell Biol. 2009;41:811–821. doi: 10.1016/j.biocel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Zhu J, Cao HZ, Xie XL, Huang JJ, Chen XH, Luo ZY. Isolation and characterization of LHT-type plant amino acid transporter gene from Panax ginseng Meyer. J Ginseng Res. 2013;37:361–370. doi: 10.5142/jgr.2013.37.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn SH, Kim SK, Kim YO, Kim HD, Shin YS, Yang SO, Kim SY, Lee SW. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J Ginseng Res. 2013;37:442–450. doi: 10.5142/jgr.2013.37.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byeon SE, Lee YG, Kim BH, Shen T, Lee SY, Park HJ, Park SC, Rhee MH, Cho JY. Surfactin blocks NO production in lipopolysaccharide-activated macrophages by inhibiting NFkappaB activation. J Microbiol Biotechnol. 2008;18:1984–1989. [PubMed] [Google Scholar]
- 24.Lee JA, Lee MY, Shin IS, Seo CS, Ha H, Shin HK. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch Pharm Res. 2012;35:739–746. doi: 10.1007/s12272-012-0419-x. [DOI] [PubMed] [Google Scholar]
- 25.Shen T, Lee J, Park MH, Lee YG, Rho HS, Kwak YS, Rhee MH, Park YC, Cho JY. Ginsenoside Rp1, a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKβ-mediated NF-κB pathway in HEK293 cells. J Ginseng Res. 2011;35:200–208. doi: 10.5142/jgr.2011.35.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by Dammarane-type Saponins from Panax ginseng leaves. J Ginseng Res. 2012;36:146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung KK, Lee HS, Cho JY, Shin WC, Rhee MH, Kim TG, Kang JH, Kim SH, Hong S, Kang SY. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006;79:2022–2031. doi: 10.1016/j.lfs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 28.Tan ZH, Yu LH, Wei HL, Liu GT. The protective action of scutellarin against immunological liver injury induced by concanavalin A and its effect on pro-inflammatory cytokines in mice. J Pharm Pharmacol. 2007;59:115–121. doi: 10.1211/jpp.59.1.0015. [DOI] [PubMed] [Google Scholar]
- 29.Rao YK, Fang SH, Hsieh SC, Yeh TH, Tzeng YM. The constituents of Anisomeles indica and their anti-inflammatory activities. J Ethnopharmacol. 2009;121:292–296. doi: 10.1016/j.jep.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Pandith H, Zhang X, Thongpraditchote S, Wongkrajang Y, Gritsanapan W, Baek SJ. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J Ethnopharmacol. 2013;147:434–441. doi: 10.1016/j.jep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 31.Clancy RM, Abramson SB. Nitric oxide: a novel mediator of inflammation. Proc Soc Exp Biol Med. 1995;210:93–101. doi: 10.3181/00379727-210-43927aa. [DOI] [PubMed] [Google Scholar]
- 32.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 33.Cheng CY, Hu CC, Yang HJ, Lee MC, Kao ES. Inhibitory effects of scutellarein on proliferation of human lung cancer A549 cells through ERK and NFκB mediated by the EGFR pathway. Chin J Physiol. 2014;57:182–187. doi: 10.4077/CJP.2014.BAC200. [DOI] [PubMed] [Google Scholar]
- 34.Kim MH, Yoo DS, Lee SY, Byeon SE, Lee YG, Min T, Rho HS, Rhee MH, Lee J, Cho JY. The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects. Pharmazie. 2011;66:293–300. [PubMed] [Google Scholar]
- 35.O'Shea JM, Perkins ND. Regulation of the RelA (p65) transactivation domain. Biochem Soc Trans. 2008;36:603–608. doi: 10.1042/BST0360603. [DOI] [PubMed] [Google Scholar]
- 36.Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218:1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Chen KC, Chen CY, Lin CR, Yang TY, Chen TH, Wu LC, Wu CC. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013;93:924–933. doi: 10.1016/j.lfs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Yang X, Liu T, Guan M, Feng X, Dong W, Chu X, Liu J, Tian X, Ci X, Li H, Wei J, Deng Y, Deng X, Chi G, Sun Z. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int Immunopharmacol. 2012;14:209–216. doi: 10.1016/j.intimp.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Eo SH, Cho H, Kim SJ. Resveratrol inhibits nitric oxideinduced apoptosis via the NF-Kappa B pathway in rabbit articular chondrocytes. Biomol Ther (Seoul) 2013;21:364–370. doi: 10.4062/biomolther.2013.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batra S, Balamayooran G, Sahoo MK. Nuclear factor-κB: a key regulator in health and disease of lungs. Arch Immunol Ther Exp (Warsz) 2011;59:335–351. doi: 10.1007/s00005-011-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, Cho JY. Functional role of Akt in macrophage-mediated innate immunity. Front Biosci (Landmark Ed) 2011;16:517–530. doi: 10.2741/3702. [DOI] [PubMed] [Google Scholar]
- 43.Huang CH, Mandelker D, Gabelli SB, Amzel LM. Insights into the oncogenic effects of PIK3CA mutations from the structure of p110alpha/p85alpha. Cell Cycle. 2008;7:1151–1156. doi: 10.4161/cc.7.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osusky M, Taylor SJ, Shalloway D. Autophosphorylation of purified c-Src at its primary negative regulation site. J Biol Chem. 1995;270:25729–25732. doi: 10.1074/jbc.270.43.25729. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Yu T, Lee YG, Yang WS, Oh J, Jeong D, Lee S, Kim TW, Park YC, Sung GH, Cho JY. Methanol extract of Hopea odorata suppresses inflammatory responses via the direct inhibition of multiple kinases. J Ethnopharmacol. 2013;145:598–607. doi: 10.1016/j.jep.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Jeong D, Yang WS, Yang Y, Nam G, Kim JH, Yoon DH, Noh HJ, Lee S, Kim TW, Sung GH, Cho JY. In vitro and in vivo anti-inflammatory effect of Rhodomyrtus tomentosa methanol extract. J Ethnopharmacol. 2013;146:205–213. doi: 10.1016/j.jep.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Seo SG, Yang H, Shin SH, Min S, Kim YA, Yu JG, Lee DE, Chung MY, Heo YS, Kwon JY, Yue S, Kim KH, Cheng JX, Lee KW, Lee HJ. A metabolite of daidzein, 6,7,4'-trihydroxyisoflavone, suppresses adipogenesis in 3T3-L1 preadipocytes via ATP-competitive inhibition of PI3K. Mol Nutr Food Res. 2013;57:1446–1455. doi: 10.1002/mnfr.201200593. [DOI] [PubMed] [Google Scholar]
- 48.Lee KM, Lee KW, Jung SK, Lee EJ, Heo YS, Bode AM, Lubet RA, Lee HJ, Dong Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem Pharmacol. 2010;80:2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian LH, Li NG, Tang YP, Zhang L, Tang H, Wang ZJ, Liu L, Song SL, Guo JM, Ding AW. Synthesis and bio-activity evaluation of scutellarein as a potent agent for the therapy of ischemic cerebrovascular disease. Int J Mol Sci. 2011;12:8208–8216. doi: 10.3390/ijms12118208. [DOI] [PMC free article] [PubMed] [Google Scholar]