Abstract
An experiment was carried out in order to investigate fungal conidia transmission of Metarhizium anisopliae (Metschnikoff) Sorokin from vector (donor) to healthy Microcerotermes diversus Silvestri (Iso.: Termitidae) and determine the best donor/concentration ratio for transmission. After preliminary trials, concentrations of 3.1 × 104, 3.9 × 105, 3.2 × 106 and 3.5 × 108 conidia mL−1 were selected for testing. The experiment was performed at three donor : Recipient ratios of 10, 30 and 50%. The highest mortality of recipient workers was observed after 14 days at the concentration of 3.5 × 108 conidia mL−1 and donor ratio of 50%. The mortality of recipient workers was less than 20% at all concentrations at a donor ratio of 10%. Our observations indicate social behavior of M. diversus, such as grooming, can be effective in promoting epizootic outbreaks in a colony. While the current results suggest good potential for efficacy, the use of M. anisopliae as a component of integrated pest management of M. diversus still needs to be proven under field conditions.
Keywords: termites, vector, recipient, biological control
1. Introduction
Termites cause economic damage to timber, timber products, living plants and even man-made products such as foam insulation and plastics. Ecologically, termites are classified under three main groups: damp wood termites, dry wood termites and subterranean termites [1]. Subterranean termites form colonies that may contain thousands of individuals. Colonies of these termites are composed of workers, soldiers, nymphs and both primary and secondary reproductives. Workers are the most abundant caste, and they are responsible for many colony tasks, including foraging, construction and repairing the nest, feeding larva, soldiers, and other workers, and colony protection [2]. The nests of subterranean termites include simple galleries in wood or mounds which can be very complex and cryptic [3]. Microcerotermes diversus Silvestri (Iso.: Termitidae) is an extremely destructive structural wood pest and is considered to be the major species, in Iran, Iraq and Oman [4,5,6]. This species was identified as the major pest of date palms (Phoenix dactylifera L.) in Iran, Iraq and Saudi Arabia with a wide distribution [5,7]. Current management of subterranean termites in Iran mainly involves the application of a soil insecticide to reduce or isolate foraging populations [8]. Biological control is recognized as one of the alternatives to chemical pesticides [9]. Several international patents (or patent applications) involve the use of Metarhizium anisopliae (Metschnikoff)Sorokin in control of termites. Bio-BlastTM Biological Termiticide, which is based on M. anisopliae isolate ESF-1 (EcoScience Corporation, NJ, USA), has been on sale in the United States following registration in 1994 [1]. There has been increasing interest in employing fungal pathogens to combat insect pests [10]. Entomopathogenic fungi use against termites can be beneficial because of its low environmental impact [9]. M. anisopliae, a hypocrealean Ascomycete, the causal agent of green muscardine disease of insects, is an important fungus in biological control of insect pests. The fungus is a facultative pathogen, which, as an entomopathogen, can infect insects [10]. Termites are considered to be good candidates for control with pathogens because they live in an environment conducive for the pathogen, which is a humid warm with minimal diurnal temperature fluctuations and crowded environment with considerable social interactions [1,11,12]. The use of entomopathogens to control termite colonies started >40 year ago when Lund (1966) patented fungal strains as biological control agents against subterranean termites [13,14]. Most of these studies focused on Beauveria bassiana (Balsamo) Vuillemin and M. anisopliae [15]. Both fungi are known to produce cuticle-degrading enzymes that facilitate percutaneous infection of the host insect without the need for oral consumption by the target organism [16]. Fungi exhibit qualities which can make them ideal for this infection strategy including a mild-acting nature, the ability to self-replicate and the ability of fungal conidia to be spread by termite social behavior [17]. Several studies examined fungal transmission in populations of termites [9,16,17,18,19,20]. There are no published reports on the transmission of M. anisopliae in populations of M. diversus. Our study was carried out to investigate fungal conidia transmission from vector to healthy termites in a colony and to determine the best vector/concentration ratio.
2. Experimental Section
2.1. Collection of Termites
Termites were collected by a method of planting blocks of beech (Fagus orientalis Lipsky) wood, 3 × 6 × 20 cm, in infested soil areas in Ahvaz (Khuzestan, Iran). In this manner worker termites were collected for this experiment.
2.2. Fungal Isolate
M. anisopliae strain DEMI 001 (Iranian Research Institute of Plant Protection collection), isolated from Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) in Saravan (Iran), was used. The fungus was cultured on Sabouraud Dextrose Agar with 1% yeast extract. Petri dish cultures were incubated at 28 ± 1 °C and 85 ± 5% relative humidity. Two-week-old, sporulated cultures were used for testing.
2.3. Preparation of Fungal Suspension
Two-week-old conidial suspensions were prepared by lightly scraping the surface of fungal cultures with a sterile scalpel and suspending the conidia in 100 mL distilled sterile Tween 80® 1% solution. The conidial concentration of the suspensions was determined with a haemocytometer.
2.4. Experiment
The pathogenicity and viability of M. anisopliae was demonstrated on M. diversus through the Koch test. According to the preliminary trials ‘determination of concentration test’, concentrations of 3.1 × 104, 3.9 × 105, 3.2 × 106 and 3.5 × 108 conidia mL−1 were selected for testing. The experiment was performed in three vector (donor) : Recipient (target) ratios of 10, 30 and 50%. Each treatment included four replications of 50 workers each. Termites that had been designated as vectors were dyed blue by being allowed to feed on filter paper treated with 1 mL of 0.25% Nile Blue solution. Next, the termites were immersed in each concentration suspension. They were then placed into the population as vectors (Figure 1). Control vector termites were immersed in distilled water containing Tween 80. Each test unit consisted of a plastic Petri dish (9 cm diameter) containing No. 1 grade Whatman filter paper that was sprayed with 1 mL distilled water. Mortality was recorded for 14 days after introduction of the vector termites. Termite cadavers that were blue were not removed from Petri dishes to allow the fungus to spread in the group.
Figure 1.
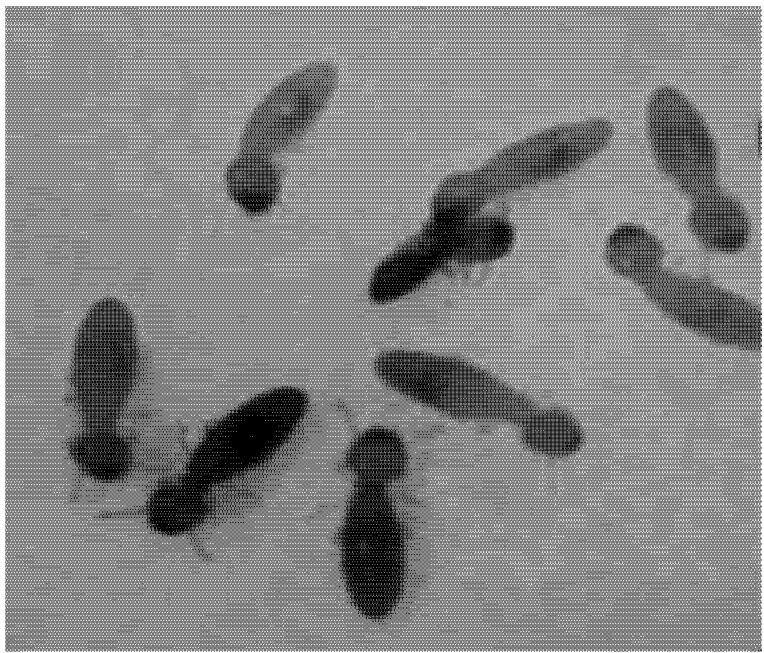
Placing blue vector termites (dark termites) among healthy termites (white termites).
2.5. Statistical Analysis
Mortality data were subjected to angular transformation and analyzed using analysis of variance (ANOVA). PROC MIXED was used in the SAS software (SAS Institute, 2000). Means were compared by using the least significant difference (LSD) at α = 0.05 after ANOVA (SAS Institute, 2000). Corrected mortality from fungal treatments was calculated using Abbott’s formula (Abbott, 1925).
3. Results and Discussion
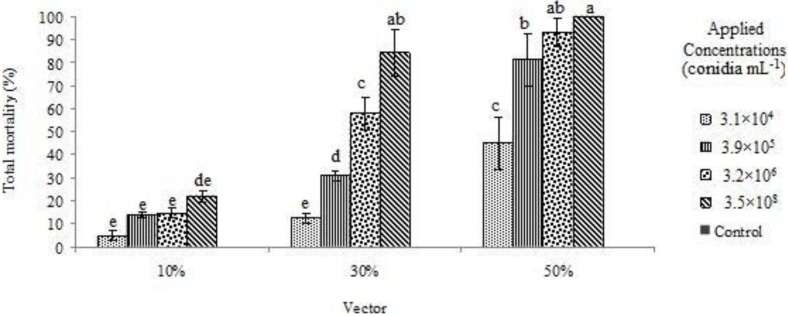
Figure 2 shows total mortality (vector and recipient) for the different conidial concentrations and proportions of vectors to recipients. The highest mortality was observed after 14 days at a concentration of 3.5 × 108 conidia mL−1, with a 50% vector ratio. Total mortality was less than 30% at all concentrations, when the percentage of vectors was 10%. When the conidial concentrations were 3.5 × 108, 3.2 × 106 and 3.9 × 105 conidia mL−1 with a vector ratio of 50% or when the concentration was 3.5 × 108 conidia mL−1 with a 30% vector ratio, the total mortality was larger than 80%. There was no significant difference between a concentration of 3.5 × 108 conidia mL−1 with 50% and 30% vector ratios, and a concentration of 3.2 × 106 conidia mL−1with a 50% vector ratio. Total mortality indicated no significant difference between the concentration of 3.9 × 105 conidia mL−1 at 50% vector ratio and the 3.5 × 108 conidia mL−1 concentration at 30% vector ratio and the 3.2 × 106 conidia mL−1 concentration with the 50% vector ratio. In general, total mortality was significantly different at all concentration levels between the 50, 10 and 30% vector ratios at each conidial concentration. Total mortality data also indicated significant differences at all levels between the 30 and 10% vector ratios, with mortalities at the 30% vector ratio being consistently higher than at the 10% vector ratio.
Figure 2.
The level of total mortality for different treatment combinations after 14 days.
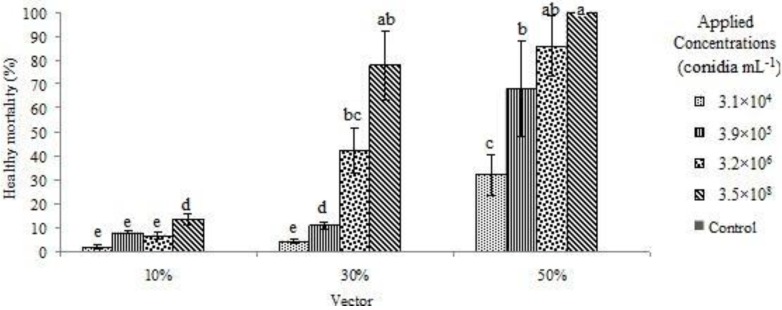
Figure 3 shows the mortality of the recipient termites only at the different concentrations and proportions of vectors to recipients. The highest mortality level was observed at 14 days post-treatment at a concentration of 3.5 × 108 conidia mL−1 and with a 50% vector ratio. At all concentrations, the mortality of recipient workers was less than 20% when the vector ratio was 10%. When the conidia concentrations were 3.5 × 108 and 3.2 × 106 conidia mL−1 with a 50% vector : Recipient ratio, mortality of the target termites was more than 80%. When the concentration was 3.9 × 105 conidia mL−1 with a 50% vector: Recipient ratio, or when the concentration was 3.5 × 108 conidia mL−1 with a 30% vector ratio, mortality of workers was more than 65%. There were no significant differences among mortalities when the concentration was 3.5 × 108 conidia mL−1 with 50 and 30% vector ratios and the concentration of 3.2×106 conidia mL−1 with 50% vector ratio. The recipient mortality showed no significant differences among the treatments of 3.9 × 105 conidia mL−1 with 50% vector ratio, 3.5 × 108 conidia mL−1 with 30% vector ratio and 3.2 × 106 conidia mL−1 with a 50% vector ratio. In general, the mortality of recipient termites showed significant difference between the 50% vector ratio and the 10 and 30% vector ratios at the same conidia concentrations. In addition, the mortality of recipient termites was significantly larger at all concentrations with the 30% vector ratio than with the 10% vector ratio.
Figure 3.
The level of healthy (recipient) workers mortality for different treatment combinations.
The time to 50 and 90% mortality (LT50 and LT90, respectively) reflected the overall mortality data. Table 1 shows LT50 and LT90 values for the different vector ratios at different conidia concentrations. The lowest LT50 value was 2.33 days and the lowest LT90 value was 12.86 days; both at a concentration of 3.5 × 108 conidia mL−1 and 50% vector ratio (df = 12, F = 28.69, p < 0.0001). The largest LT50 and LT90 values were obtained with a 10% vector ratio. Our results show that the LT50 and LT90 were reduced as the vector ratios increased. They were also reduced by increasing the number of vectors at each concentration.
Table 1.
The level of LT50 and LT90 for different treatment combinations.
| Concentration | Vector (%) | LT50(day) | LT90(day) |
|---|---|---|---|
| (Conidia mL−1) | (95% Fiducial limits) | (95% Fiducial limits) | |
| 3.1 × 104 | 10 | -* | - |
| 30 | - | - | |
| 50 | 19.5 (17–23) | - | |
| 3.9 × 105 | 10 | - | - |
| 30 | 30 (21–55) | - | |
| 50 | 7.27 (6.4–8.29) | 27.81(21.22–41.63) | |
| 3.2 × 106 | 10 | - | - |
| 30 | 12.12 (10.32–15.04) | - | |
| 50 | 4.68 (3.51–5.85) | 23.49(15.96–48.05) | |
| 3.5 × 108 | 10 | - | - |
| 30 | 6.23 (4.51–8.58) | 47.32(24.77–215.03) | |
| 50 | 2.33 (1.20–3.32) | 12.86(8.82–26.99) |
* The high values of LT50 and LT90 are not reported.
Mechanical transmission of the infectious conidia by workers exposed to the fungi outside of the nest is very important for these fungi to be effective against termites. Our research offers a better understanding of the contamination process and the conidia transfer between termites.
Our study showed that vectors are able to transfer fungi successfully to recipient workers at high concentrations of conidia suspensions and a high ratio of vectors in M. diversus. Vector : Recipient ratios of 30 and 50% were more effective than the 10% vector ratio, especially with high concentrations of conidia. Due to lower and slower mortality, treatment of vector workers with low concentrations of conidia suspension and a vector : Recipient ratio of 10% were not promising. Results from our present research confirmed results reported by Grace and Zoberi (1992), Bayon et al. (2000), Myles (2002) and Wright et al. (2002) with other termite species [9,16,17,21].
Desyanti (2010) applied the entomopathogenic fungus Myrothecium roridum Tode ex Steudel and Metarhizium sp. with vector ratios of 5, 10 and 15% using Coptotermes gestroi Wasman (Blattodea: Rhinotermitidae) [20]. Desyanti et al. (2009) also reported on the transmission of entomopathogenic fungi Metarhizium brunneum Petch and M. roridum in Cryptotermes sp. (Blattodea: Kalotermitidae) [19]. They used vector ratios of 10, 20, 30, 40 and 50% and an inoculum concentration of 107 conidia mL−1. They showed that there was a correlation between the proportion of vector to recipient termites and application period with mortality. Our findings are consistent with those results. Overall, concentrations should be used that cause higher mortality in a shorter timeframe. These results may be beneficial in field applications because social behaviors of termites, such as grooming and trophallaxis, may be effective in creating epizootic conditions. At the same time, the conidia transmission may also reduced by the cleaning behavior of nestmates in large colonies under field conditions.
Because termites live at high densities, horizontal transmission is extremely important for the fungal application in the field. Horizontal transmission between individuals of the same species (autodissemination) can occur through direct contact between contaminated and uncontaminated individuals or indirectly via conidia that have been deposited on the substrate [22,23,24]. In addition, a positive relationship between insect movement and the transmission of entomopathogenic fungi has been observed in a number of systems [25]. Cory and Hoover (2006) stated that insects and entomopathogenic fungi were under opposing selection pressures. Insects gain a selective advantage from detecting and avoiding fungal pathogens, while the successful infection to an insect by an entomopathogen requires contact between the host and the pathogen. The behavior of insects can influence whether contact is made, with change in activity increasing or decreasing the likelihood of infection [26].
On the other hand Chouvenc et al. (2008) stated that termites might gain a selective advantage in detecting the risk of attack from entomopathogenic fungi and by responding via behavioral avoidance or through post-contact responses such as grooming. These responses may reduce the efficiency of the fungal infection in the field. In contrast, fungal pathogens could gain an advantage by attracting or remaining invisible to host insects [27]. The ability of insects to detect and respond to entomopathogenic fungi within the order Hypocreales has been widely assessed, with reports of avoidance of fungi by species within the Coleoptera, Isoptera, Hemiptera and Orthoptera [1,18,27,28,29,30,31]. Yanagawa etal. (2010) noted that C. formosanus protects itself from entomopathogenic fungus by mutual grooming behavior. These termites remove foreign organisms, such as fungal conidia, from the body surfaces of their nestmates. In addition, they found that C. formosanus could detect fungus species via odor [32].
Generally, social interactions such as allogrooming, proctodeal and stomodeal trophallaxis, coprophagy and cannibalism of diseased or injured nestmates increase the dissemination of pathogens. These behaviors may actually also decrease infection risk. Termites avoid contaminated areas or infected nestmates, and may move to a new nest after an encounter with pathogenic or parasitic microbes.
The effect of the hygiene behaviors of termites towards entomopathogenic fungi should be considered in future research. It is important to find a way of weakening such defensive mechanisms. If these protective mechanisms were made less effective, occurrence of epizootics should increase. The use of insecticides, such as imidacloprid, in combination with an entomopathogen can be an effective strategy. These insecticides should be compatible with disease agents and applied in the form of sublethal concentrations to weaken behavioral responses of termites and thus increase the possibility of disease transmission [33]. The use of sub-lethal concentrations of imidacloprid in R. flavipes, affected hygiene function (e.g., grooming), resulting in increased infection with B. bassiana [34].
4. Conclusion
Direct inoculation of fungi onto the body surface of vector termites was used in this study. Further work is recommended to examine the effect of fungus transmission from mycotic termite cadavers within the rest of the colony. Because termite contact with pathogens is important for creating a fungal epizootic among the population, processes associated with this phenomenon should be examined closely. It seems that the successful microbial control of termites requires consideration of not only the infectivity and pathogenicity of the agent, but also the termite protective behaviors. If solutions can be found to overcome the termite activities which reduce the fungal epizootics in a colony, the probability of successful biological control will be greater. In subsequent research, virulence of this pathogen can be compared with other isolates. In addition, the optimum condition for occurrence of maximum pathogenicity of entomopathogenic fungus can be investigated. Using M. anisopliae as a component of integrated pest management of M. diversus still needs to be proven in field application.
Acknowledgments
We thank Stefan T. Jaronski for suggestions regarding this manuscript. We also thank the anonymous referees for reviewing earlier drafts of this paper. This research was supported by Shahid Chamran University of Ahvaz, Iran.
References and Notes
- 1.Rath A.C. The use of entomopathogenic fungi for control of termites. Biocontrol. Sci. Techn. 2000;10:563–581. doi: 10.1080/095831500750016370. [DOI] [Google Scholar]
- 2.Arab A., Costa-Leonardo A.M., Casarin F.E., Guaraldo A.C., Chaves R.C. Foraging activity and demographic patterns of two termite species (Isoptera: Rhinotermitidae) living in urban landscapes in southeastern Brazil. Eur. J. Entomol. 2005;102:691–697. [Google Scholar]
- 3.Roisin Y. Queen replacement in the termite Microcerotermes papuanus. Entomol. Exp. Appl. 1990;5:83–90. doi: 10.1111/j.1570-7458.1990.tb01383.x. [DOI] [Google Scholar]
- 4.Edwards R., Mill A.E. Termites in buildings: Their biology and control. Rentokil Limited; London, UK: 1986. [Google Scholar]
- 5.Habibpour B., Mossadegh M.S., Henderson G., Moharramipour S. Laboratory evaluation of two insect growth regulators (IGRs) on Microcerotermes diversus (Silvestri) (Isoptera: Termitidae) in southwest Iran. Sociobiology. 2007;50:1199–1209. [Google Scholar]
- 6.Habibpour B. Laboratory of flurox, a chitin synthesis inhibitor, on the termite, Microcerotermes divers. J. Insect Sci. 2010;10:1–8. doi: 10.1673/031.010.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan J.W.M., El Bakri A. Termite damage to date palms (Phoenix dactylifera L.) in Northern Sudan with particular reference to the Dongle District. Trop. Sci. 1990;30:95–108. [Google Scholar]
- 8.Habibpour B. PhD Thesis. Department of Plant Protection, College of Agriculture, Shahid Chamran University of Ahvaz; Ahvaz, Iran: 2006. Laboratory and field evaluation of bait-toxicants for suppression subterranean termite populations in Ahvaz (Iran) [Google Scholar]
- 9.Bayon I.L., Ansard D., Brunet C., Girardi S., Paulmier I. Biocontrol of Reticulitermes santonensis by entomopathogenic fungi improvement of the contamination Process; Presented at the International Research Group on Wood Protection (IRG/WP/DOC 00–10359); Kona, Hawaii, USA. 14-19 May 2000. [Google Scholar]
- 10.Tajick Ghanbalani M.A., Asgharzadeh A., Hadizadeh A.R., Mohammadi Sharif M. A quick method for Metarhizium anisopliae isolation from cultural soils. Am. J. Agr. Biol. Sci. 2009;4:152–155. doi: 10.3844/ajabssp.2009.152.155. [DOI] [Google Scholar]
- 11.Kramm K.R., West D.F., Rockenbach P.G. Termite pathogens: Transfer of the entomopathogen Metarhizium anisopliae between Reticulitermes sp. Termites. J. Invertebr. Pathol. 1982;39:1–5. doi: 10.1016/0022-2011(82)90150-1. [DOI] [Google Scholar]
- 12.Ignoffo C.M. Environmental factors affecting persistence of entomopathogens. Fla. Entomol. 1992;75:516–525. doi: 10.2307/3496133. [DOI] [Google Scholar]
- 13.Wang C., Powell J.E. Cellulose bait improves the effectiveness of Metarhizium anisopliae as a microbial control of termites (Isoptera: Rhinotermitidae) Biol. Control. 2004;30:523–529. doi: 10.1016/j.biocontrol.2004.02.007. [DOI] [Google Scholar]
- 14.Chouvenc T., Su N.Y. Apparent synergy among defense mechanisms in subterranean termites (Rhinotermitidae) against epizootic events: Limits and potential for biological control. J. Econ. Entomol. 2010;103:1327–1337. doi: 10.1603/EC09407. [DOI] [PubMed] [Google Scholar]
- 15.Culliney T.W., Grace J.K. Prospects for the biological control of subterranean termites (Iso.: Rhinotermitidae) with special reference to Coptotermes formosanus. B Entomol. Res. 2000;90:9–21. doi: 10.1017/S0007485300000663. [DOI] [PubMed] [Google Scholar]
- 16.Wright M.S., Osbrink W.L.A., Lax A.R. Transfer of entomopathogenic fungi among Formosan subterranean termites and subsequence mortality. J. Appl. Entomol. 2002;126:20–23. doi: 10.1046/j.1439-0418.2002.00604.x. [DOI] [Google Scholar]
- 17.Grace J.K., Zoberi M.H. Experimental evidence for transmission of Beauveria bassiana by Reticulitermes flavipes workers (Isoptera: Rhinotermitidae) Sociobiology. 1992;20:23–28. [Google Scholar]
- 18.Myles T.G. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisoplia. Sociobiology. 2002;40:243–255. [Google Scholar]
- 19.Desyanti W.H., Zulyusri Y., Yumarni A., Jasni H. Transmission of entomopathogenic fungus Metarhizium anisopliae Petch and Myrothecium roridum Tode ex Steudel in colony of dry wood termites Coptotermes sp. (Blattodea: Kalotermitidae) using vector; Proceeding of the Sixth Conference of the Pacific Rim Termite Research Group; Kyoto, Japan. 2–3 March 2009; pp. 16–19. [Google Scholar]
- 20.Desyanti W.H. Contagious test of the entomopathogenic fungus originated from west Sumatera Indonesia between individual in colony of subterranean termites Coptotermes gestroi Wasman (Blattodea: Rhinotermitidae); In Proceeding of the Seventh Conference of the Pacific Rim Termite Research Group; Singapore. 1–2 March 2010; pp. 16–19. [Google Scholar]
- 21.Myles T.G. Laboratory studies on the transmission of Metarhizium anisopliae in the Eastern subterranean termite, Reticulitermes flavipes (Isoptera: Rhinotermitidae), with a method for applying appropriate doses of conidia to trapped termites for release. Sociobiology. 2002;40:265–276. [Google Scholar]
- 22.Quesada-Moraga E., Martin-Carballo I., Garrido-Jurado I., Santiago-Alvarez C. Horizontal transmission of Metarhizium anisopliae among laboratory populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) Biol. Control. 2008;47:115–124. doi: 10.1016/j.biocontrol.2008.07.002. [DOI] [Google Scholar]
- 23.Roy H.E., Pell J.K. Interactions between entomopathogenic fungi and other natural enemies: Implications for biological control. Biocontrol. Sci. Technol. 2000;10:737–752. doi: 10.1080/09583150020011708. [DOI] [Google Scholar]
- 24.Vega F.E., Dowd P.F., Lacey L.A., Pell J.K., Jackson D.M., Klein M.G. Dissemination of beneficial microbial agents by insects. In: Lacey L.A., Kaya H.K., editors. Field Manual of Techniques in Invertebrate Pathology. Springer; London, UK: 2000. pp. 153–177. [Google Scholar]
- 25.Baverstock J., Roy H.E., Pell J.K. Entomopathogenic fungi and insect behavior: From unsuspecting hosts to targeted vectors. Biocontrol. 2010;55:89–102. doi: 10.1007/s10526-009-9238-5. [DOI] [Google Scholar]
- 26.Cory J.S., Hoover K. Plant-mediated effects in insect-pathogen interactions. Trends Ecol. Evol. 2006;21:278–286. doi: 10.1016/j.tree.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Chouvenc T., Su N.Y., Elliott M.L. Interaction between the subterranean termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) and the entomopathogenic fungus Metarhizium anisopliae in foraging arenas. J. Econ. Entomol. 2008;101:885–893. doi: 10.1603/0022-0493(2008)101[885:IBTSTR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Staples J.A., Milner R.J. A laboratory evaluation of the repellency of Metarhizium anisopliae conidia to Coptotermes lacteus (Isoptera: Rhinotermitidae) Sociobiology. 2000;36:133–148. [Google Scholar]
- 29.Meyling N.V., Pell J.K. Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol. Entomol. 2006;31:162–171. doi: 10.1111/j.0307-6946.2006.00781.x. [DOI] [Google Scholar]
- 30.Thompson S.R., Brandenburg R.L. Tunneling responses of mole crickets (Orthoptera: Gryllotalpidae) to the entomopathogenic fungus, Beauveria bassiana. Environl. Entomol. 2005;34:140–147. doi: 10.1603/0046-225X-34.1.140. [DOI] [Google Scholar]
- 31.Villani M.G., Krueger S.R., Schroeder P.C., Consolie F., Consolie N.H., Preston-Wilsey L.M., Roberts D.W. Soil application effects of Metarhizium anisopliae on Japanese-beetle (Coleoptera: Scarabaeidae) behavior and survival in turfgrass microcosms. Environ. Entomol. 1994;23:502–513. [Google Scholar]
- 32.Yanagawa A., Yokohari F., Shimizu S. Influence of fungal odor on grooming behavior of the termite, Coptotermes formosanus. J. Insect Sci. 2010;10:141–155. doi: 10.1673/031.010.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorne B.L., Breisch N.L. Effects of sublethal exposure to imidacloprid on subsequent behavior of subterranean termite Reticulitermes virginicus (Isoptera: Rhinotermitidae) J. Econ. Entomol. 2001;94:492–498. doi: 10.1603/0022-0493-94.2.492. [DOI] [PubMed] [Google Scholar]
- 34.Boucias D.G., Stokes C., Storey G., Pendland J.C. The effects of imidacloprid on the termite Reticulitermes flavipes and its interaction with the mycopathogen Beauveria bassiana. Pflanzenschutz-Nachrichten Bayer. 1996;49:103–144. [Google Scholar]