Abstract
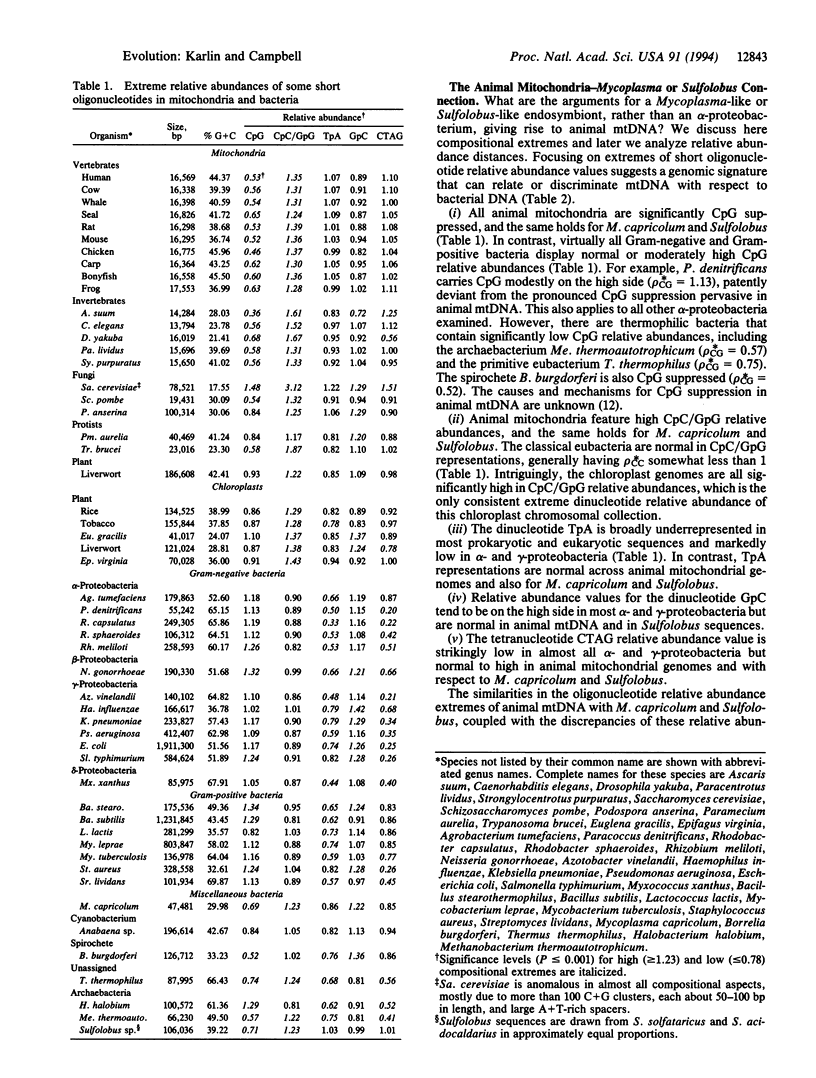
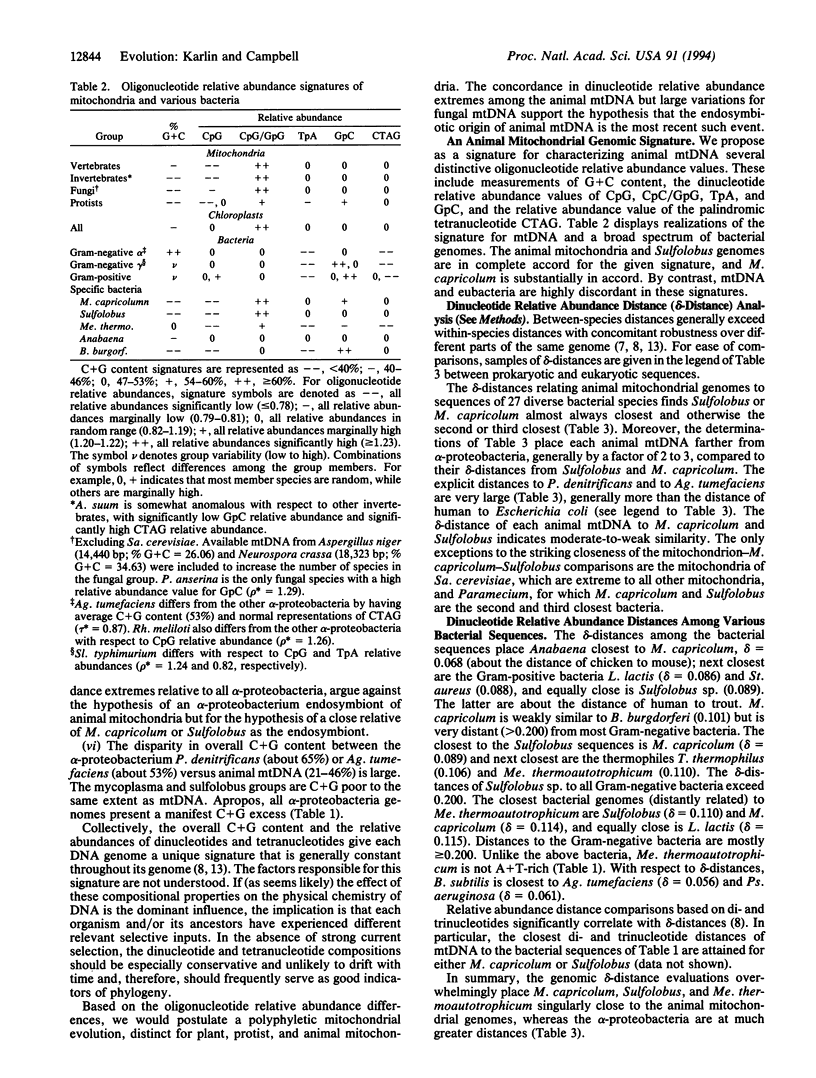
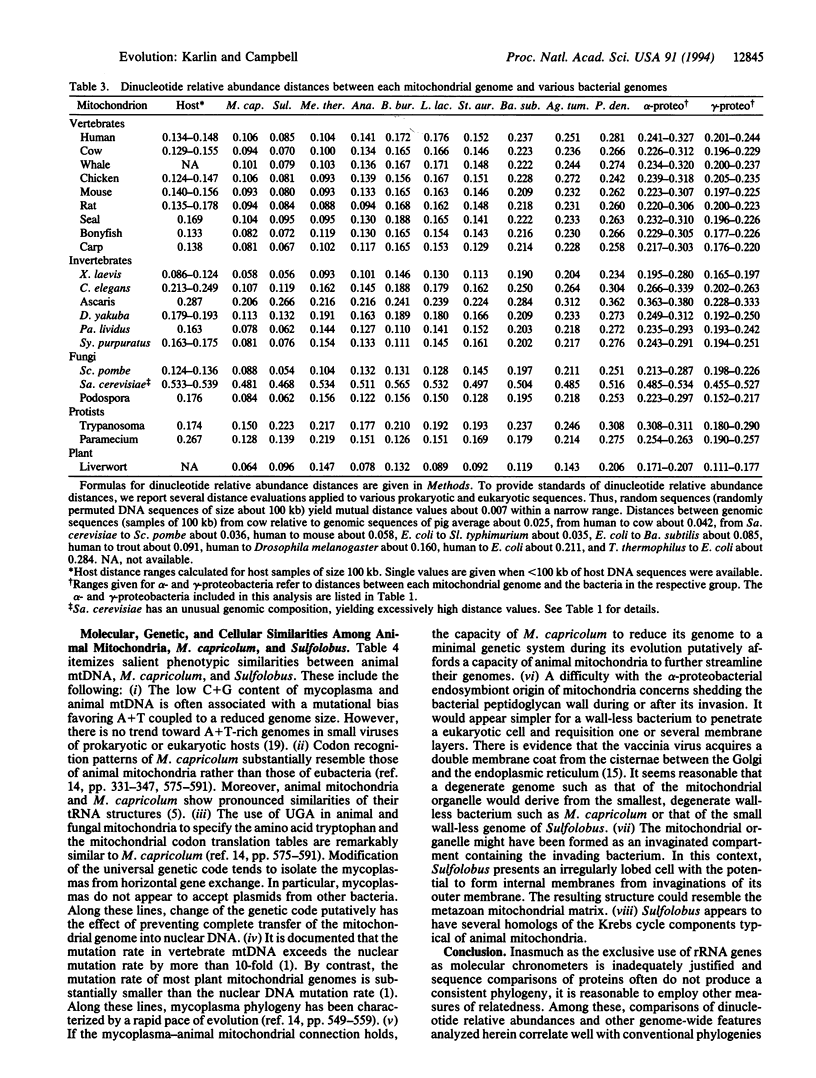
We present considerable data supporting the hypothesis that a Sulfolobus- or Mycoplasma-like endosymbiont, rather than an alpha-proteobacterium, is the ancestor of animal mitochondrial genomes. This hypothesis is based on pronounced similarities in oligonucleotide relative abundance extremes common to animal mtDNA, Sulfolobus, and Mycoplasma capricolum and pronounced discrepancies of these relative abundance values with respect to alpha-proteobacteria. In addition, genomic dinucleotide relative abundance measures place Sulfolobus and M. capricolum among the closest to animal mitochondrial genomes, whereas the classical eubacteria, especially the alpha-proteobacteria, are at excessive distances. There are also considerable molecular and cellular phenotypic analogies among mtDNA, Sulfolobus, and M. capricolum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Benachenhou-Lahfa N., Forterre P., Labedan B. Evolution of glutamate dehydrogenase genes: evidence for two paralogous protein families and unusual branching patterns of the archaebacteria in the universal tree of life. J Mol Evol. 1993 Apr;36(4):335–346. doi: 10.1007/BF00182181. [DOI] [PubMed] [Google Scholar]
- Burge C., Campbell A. M., Karlin S. Over- and under-representation of short oligonucleotides in DNA sequences. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1358–1362. doi: 10.1073/pnas.89.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon L. R., Burge C., Clayton D. A., Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3799–3803. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. Origin and evolution of organelle genomes. Curr Opin Genet Dev. 1993 Dec;3(6):884–890. doi: 10.1016/0959-437x(93)90009-e. [DOI] [PubMed] [Google Scholar]
- Gray M. W. The endosymbiont hypothesis revisited. Int Rev Cytol. 1992;141:233–357. doi: 10.1016/s0074-7696(08)62068-9. [DOI] [PubMed] [Google Scholar]
- Heibel G. E., Anzenbacher P., Hildebrandt P., Schäfer G. Unusual heme structure in cytochrome aa3 from Sulfolobus acidocaldarius: a resonance Raman investigation. Biochemistry. 1993 Oct 12;32(40):10878–10884. doi: 10.1021/bi00091a043. [DOI] [PubMed] [Google Scholar]
- Karlin S., Cardon L. R. Computational DNA sequence analysis. Annu Rev Microbiol. 1994;48:619–654. doi: 10.1146/annurev.mi.48.100194.003155. [DOI] [PubMed] [Google Scholar]
- Karlin S., Doerfler W., Cardon L. R. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not in those of large eukaryotic viruses? J Virol. 1994 May;68(5):2889–2897. doi: 10.1128/jvi.68.5.2889-2897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Ladunga I., Blaisdell B. E. Heterogeneity of genomes: measures and values. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12837–12841. doi: 10.1073/pnas.91.26.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Ladunga I. Comparisons of eukaryotic genomic sequences. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12832–12836. doi: 10.1073/pnas.91.26.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinov R. Nearest neighbor nucleotide patterns. Structural and biological implications. J Biol Chem. 1981 Aug 25;256(16):8458–8462. [PubMed] [Google Scholar]
- Sodeik B., Doms R. W., Ericsson M., Hiller G., Machamer C. E., van 't Hof W., van Meer G., Moss B., Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993 May;121(3):521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]



