Abstract
Reticulitermes nelsonae, a new species of Rhinotermitidae (Isoptera) is described based on specimens from Sapelo Island, GA, Thomasville, GA, Havelock, NC, and Branford, FL. Adult (alate) and soldier forms are described. Diagnostic characters are provided and incorporated into a supplemental couplet of a dichotomous key to the known species of Reticulitermes found in Georgia, USA.
Keywords: new species, taxonomy, phylogenetics, morphometrics, dichotomous key, Isoptera, Termitoidea
1. Introduction
Members of the family Rhinotermitidae, commonly known as “subterranean termites”, have a cryptic lifestyle making them difficult to study [1,2]. In the United States, the genus Reticulitermes includes several economically notorious species that cause billions of dollars in structural damage every year [3,4,5,6,7]. Proper identification is critical to understanding the economic and ecological importance of these insects [8,9], and although there are four described species of Reticulitermes endemic to the southeastern United States, the current keys only address three [10,11,12]. Proper identification is complicated by intraspecific morphological variation which is characteristic of this genus as demonstrated by the quantitative measures that provide overlapping ranges for a number of characters for species collected in the United States [13,14,15,16]. The greatest diversity of Reticulitermes in the USA is in the eastern states where authors have suggested the presence of additional species [16,17,18,19,20,21,22].
Kollar (1837) [23] described the first extant member of the genus, R. flavipes from specimens found in Vienna, Austria, and this species was later found to be endemic to the eastern United States [10]. Three additional species were subsequently described from the eastern USA, including Reticulitermes (Termes) virginicus Banks 1907 [24], R. hageni Banks 1920 [10] and R. malletei Clément et al. 1986 [11]. Scheffrahn et al. (2001) [25] proposed that R. malletei was a nomen nudum yet Austin et al. (2007) [12] provided 16SrRNA sequence data and morphometrics supporting species status for R. malletei and showed that all nomenclatural requirements for the designation were met. Reticulitermes santonensis Feytaud 1924 [26] has been synonymized with R. flavipes [27,28]. Table 1 summarizes the taxonomic literature on Reticulitermes that mention the species endemic to the southeastern USA.
Table 1.
Summary of taxonomic literature that provide morphology and life history information on Reticulitermes spp. found in the southeastern United States, parenthetical letters are defined as follows: a = alate, s = soldier, species abbreviations are defined as follows: Rf = R. flavipes, Rv = R. virginicus, Rh = R. hageni, Rm = R. malletei, Ra = R. arenincola, Rhp = R. hesperus, Rt = R. tibialis.
| Reference citation | Caste, notes | Pages | Species from USA |
|---|---|---|---|
| Banks, N. & Snyder, T.E. (1920) | (a, s) descriptions, illustrations, key, flight times | 42–47, 148–164 | Rf, Rv, Rh, Rhp, Rt |
| Goellner, E. J. (1931) | (a, s) descriptions, illustrations, ecology | 227–234 | Rf, Rt, Ra |
| Kofoid, C.A. (1934) | (a), descriptions, flight times | 193–194 | Rf, Rv, Rh, Ra, Rhp, Rt |
| Miller, E.M. & Miller, D.B. (1943) | (a, s) descriptions, illustrations, flight times | 101–107 | Rf, Rv, Rh |
| Banks, F.A. (1946) | (a, s) descriptions, illustrations, key, flight times | 1–29 | Rf, Rv, Ra, Rt |
| Miller E.M. (1949) | (a, s) descriptions, illustrations, key, flight times | 6–7, 14–15, 20–22, 26 | Rf, Rv, Rh |
| Snyder, T.E. (1954) | (a, s) descriptions, illustrations, key, flight times | 26, 51–56 | Rf, Rv, Rh, Ra |
| Miller E. M. (1964) | (a) flight times | 5, 16 | Rf, Rv, Rh |
| Weesner, F.M. (1965) | (a) descriptions, illustrations, key, flight times | 36–44, 51 | Rf, Rv, Rh, Ra, Rhp, Rt |
| Clément, J.L., Howard, R., Blum, M. & Lloyd, H. (1986) | (a, s) descriptions, life history | 67–70 | Rf, Rv, Rh, Rm |
| Nutting, W.L. (1990) | (a, s) descriptions, illustrations, key, flight times | 997–1030 | Rf, Rv, Rh, Ra, Rhp, Rt |
| Scheffrahn RH, Su N-Y. (1994) | (a, s) descriptions, illustrations, key, flight times | 465–473 | Rf, Rv, Rh |
| Hostettler, N.C., Hall, D.W. & Scheffrahn, R.H. (1995) | (s) descriptions, photographs | 119–129 | Rf, Rv, Rh |
| Ye, W., Lee, C.-Y., Scheffrahn, R.H., Aleong, J.M., Su, N.-Y., Bennett, G.W., Scharf, M.E., (2004) | (s) descriptions, photographs | 815–822 | Rf, Rv, Rh, Ra |
| Brown, K., Kard, B. & Payton, M. (2005) | (a, s) descriptions, photograph | 277–284 | Rf, Rv, Rh |
| Austin, J.W., Bagnères, A.-G., Szalanski, A.L., Scheffrahn, R.H., Heintschel, B.P., Messenger, M.T., Clement, J.-L. & Gold, R.E. (2007) | (a, s) descriptions, illustrations, photographs, flight times | 1–26 | Rf, Rv, Rh, Rm, Ra, Rhp, Rt |
| Wang, C., Zhou, X., Li, S., Schwinghammer, M., Scharf, M., Buczkowski, G. & Bennett, G. (2009) | (a, s) descriptions, key, photographs | 1029–1036 | Rf, Rv, Rh, Ra, Rt |
Herewith we provide a formal description for a new species collected in the southeastern USA, with diagnostic morphological characters and genetic corroboration. We also identify specific quantifiable morphological characters in combination with selected qualitative characters that are included in dichotomous keys to the soldier and alate of Reticulitermes species found in Georgia, USA.
2. Materials and Methods
2.1. Specimens
The morphological data for the new species were obtained from 96 soldiers, 141 alates, and 20 soldier mandible pairs. The number of soldier and alate specimens examined for the four previously described species ranged from 32 to 431 [29]. Genetic data for the new species were obtained from 156 specimens collected from Sapelo Island, GA (McIntosh Co.), Thomasville, GA (Thomas Co.), Havelock, NC (Craven Co.) and Branford, FL (Suwannee Co.).
All specimens were preserved in 70–100% ethanol. The number of specimens examined and collection information for the new species description are listed in Table 2.
Table 2.
Collection information by site number, county (McIntosh Co. = Sapelo Island, Thomas Co. = Thomasville), sample size, and collection date by caste (s = soldier, a = alate) for specimens of R. nelsonae used for morphological measurements.
| Soldier | |||
| Site ID | County | Sample Size | Collection Date |
| Site 1s | McIntosh Co. | 7 | Nov 2007 |
| Site 2s | McIntosh Co. | 16 | Feb 2007 |
| Site 3s | McIntosh Co. | 9 | Feb 2007 |
| Site 4s | McIntosh Co. | 2 | Feb 2007 |
| Site 5s | McIntosh Co. | 3 | Feb 2007 |
| Site 6s | McIntosh Co. | 4 | Feb 2007 |
| Site 7s | McIntosh Co. | 11 | Feb 2007 |
| Site 8s | McIntosh Co. | 2 | Jul 2009 |
| Site 9s | McIntosh Co. | 1 | Jul 2009 |
| Site 10s | McIntosh Co. | 2 | Jul 2009 |
| Site 11s | McIntosh Co. | 2 | Jul 2009 |
| Site 12s | Thomas Co. | 1 | Nov 2009 |
| Site 13s | Thomas Co. | 2 | Nov 2009 |
| Site 14s | Thomas Co. | 4 | Nov 2009 |
| Site 15s | Thomas Co. | 1 | Nov 2009 |
| Site 16s | Thomas Co. | 2 | Nov 2009 |
| Site 17s | Thomas Co. | 1 | Nov 2009 |
| Site 18s | Thomas Co. | 2 | Nov 2009 |
| Site 19s | Thomas Co. | 1 | Nov 2009 |
| Site 20s | Thomas Co. | 2 | Nov 2009 |
| Site 21s | Thomas Co. | 2 | Nov 2009 |
| Site 22s | Thomas Co. | 1 | Nov 2009 |
| Site 23s | Thomas Co. | 1 | Nov 2009 |
| Site 24s | Thomas Co. | 1 | Nov 2009 |
| Site 25s | Thomas Co. | 1 | Nov 2009 |
| Site 26s | Thomas Co. | 1 | Nov 2009 |
| Site 27s | Thomas Co. | 2 | Nov 2009 |
| Site 28s | Thomas Co. | 3 | Nov 2009 |
| Site 29s | Thomas Co. | 2 | Nov 2009 |
| Site 30s | Thomas Co. | 2 | Nov 2009 |
| Site 31s | Thomas Co. | 1 | Nov 2009 |
| Site 32s | Thomas Co. | 1 | Nov 2009 |
| Site 33s | Thomas Co. | 1 | Jan 2010 |
| Site 34s | Thomas Co. | 1 | Jan 2010 |
| Site 35s | Thomas Co. | 2 | Mar 2010 |
| Total = 35 | N = 96 | ||
| Alate | |||
| Site ID | County | Sample Size | Collection Date |
| Site 4a | McIntosh Co. | 14 | Feb 2007 |
| Site 6a | McIntosh Co. | 6 | Feb 2007 |
| Site 4a | McIntosh Co. | 19 | Mar 2007 |
| Site 36a | McIntosh Co. | 19 | Mar 2007 |
| Site 3a | McIntosh Co. | 5 | Feb 2007 |
| Site 2a | McIntosh Co. | 14 | Feb 2007 |
| Site 5a | McIntosh Co. | 1 | Feb 2007 |
| Site 7a | McIntosh Co. | 13 | Feb 2007 |
| Site 37a | McIntosh Co. | 50 | May 2005 |
| Total = 9 | N = 141 | ||
Accurate Reticulitermes species attributions are best determined using both soldiers and alates from the same collection site supported by genetic and behavioral information. The specific measurement points used for the quantitative dataset are shown in Figure 1 and Figure 2.
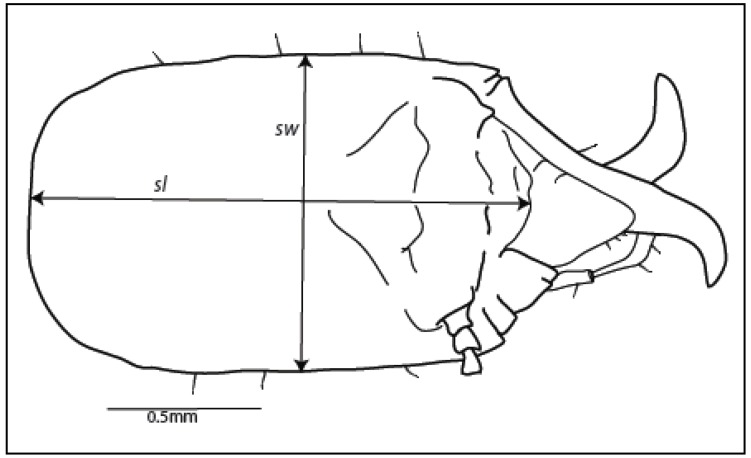
Figure 1.
Standard measurements for soldier head capsule length (sl) and width (sw). Note that the length (sl) measurement does not include mandibles. Scale bar = 0.5 mm.
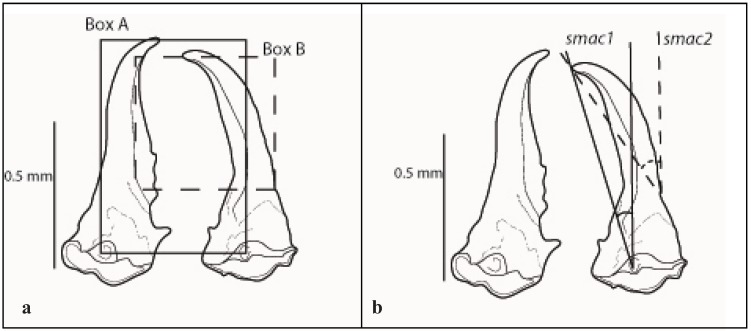
Figure 2.
Diagram of soldier mandibles, dorsal view. Illustrating (a) the box method used to determine the 90° for measurement, (b) angles measured for smac1 (Box A) and smac2 (Box B). Scale bar = 0.5 mm.
2.2. Soldier
Four characters were measured on soldier head capsules: length without mandibles (sl), width (sw), and two separate angles of curvature for the right mandible (smac1, smac2). A fifth character, ratio of length:width (sl:sw), was calculated to determine its usefulness as a character for species separation.
Soldier head capsules were removed from the body and mounted by placing a minuten pin into the occipital foramen. The opposite end of the minuten was positioned into a cube of foam mounted on a standard size # 2 insect pin. Soldier head capsule length (sl) was measured from the clypeal sulcus to the posterior edge as seen from a dorsal view (near the occipital foramen), and width (sw) was measured at a 90° angle from the mid-point of sl (Figure 1).
Soldier mandibles were dissected from head capsules and mounted on two-sided tape positioned inside a 2 mm × 2 mm grid box. Mandibles were positioned dorsal side up and parallel to the bottom line of the grid box to establish a 90° vertical line for the right mandible angle of curvature measurement (Figure 2a). The soldier right mandible angle of curvature (smac) was measured from two positions: the dorsal condyle (smac1) and external curvature inflexion point (smac2) (Figure 2b).
2.3. Alate
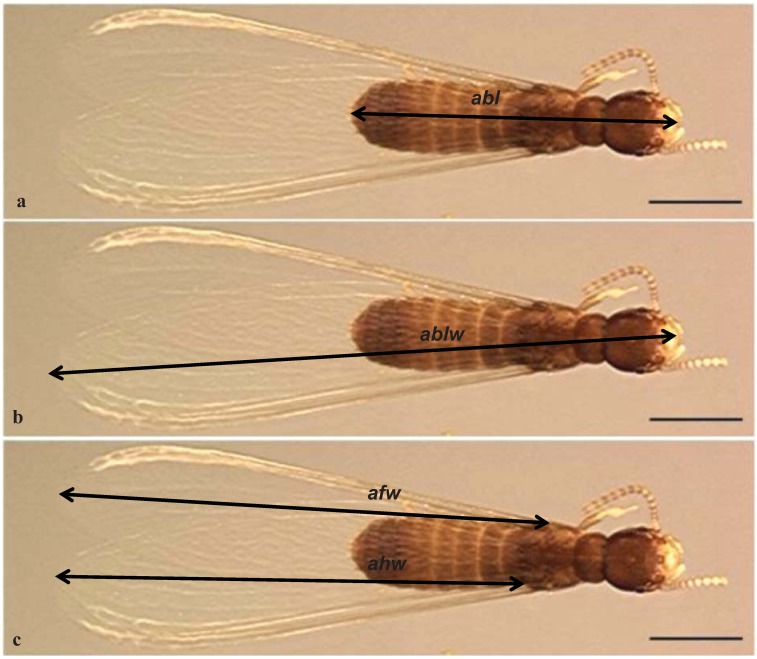
Alates were mounted between a glass slide and cover slip in 100% ethanol [29]. Qualitative characters included body color (abc) and wing pigmentation (awp). Quantitative characters included body length (abl), body length including wings (ablw), average forewing length (afw) and average hind wing length (ahw) (Figure 3a–c).
Figure 3.
Standard measurements for alate characters: length of body only (abl) body-wing (ablw), wings (afw and ahw) measuremements. Scale bar = 1.0 mm.
2.4. Morphometrics, Imaging and Statistics
Soldier and alate specimen were prepared under a binocular dissecting microscope (CIT-OVAL2, Carl Zeiss aus Jena, Jena, Germany and Leica WILD M10, Wetzlar, Germany). Images were taken with a Sony DKC-5000 camera attached to a Leica WILD M10 stereomicroscope (Wetzlar, Germany) using Adobe Photoshop v.8.0 (Adobe Systems, San Jose, CA, USA). All soldier and alate images were taken at 25× and 20× magnification respectively, and calibrated with a micrometer using the internal preset calibration setting in AutoMontage Pro, v.5.0.1 (Cambridge, UK). Morphometric measurements were recorded using the AutoMontage Pro, v.5.0.1 and exported to Microsoft Office Excel (Redmond, Washington, USA). All statistical analyses of mean, standard deviation and simulation of sample size were performed using SAS v.9.2 (SAS Institute Inc., Cary, NC, USA), one-way analyses of variance (ANOVA) performed on each character state to determine if it contributed significantly to species separation. Sequential t-tests with LSD (protected least square deviation) pair-wise comparison were used to determine species differences for all characters measured. Step-Wise Discriminant Analysis (SWDA) was used to determine which morphological characters were most useful in species separation. The reliability and accuracy of each character state was determined using multiple Discriminant Function Analyses (DFA) [29].
2.5. Behavior
Fully developed, not-yet flown, winged alates of the new species were collected from infested wood on Sapelo Island on three separate dates.
2.6. Dichotomous Key
A dichotomous key for the Reticulitermes species of Georgia was constructed using morphological and behavioral (flight phenology) data.
2.7. Molecular Data
Sequence data for two mtDNA genes obtained from workers, soldiers and alates were employed to provide additional support for the new species. Genomic DNA was extracted from selected specimens using either Promega’s Wizard Genomic DNA Purification Kit or Qiagen’s DNeasy Extraction Kit, following a modified protocol [22]. Primers used for amplification of the entire length of the mitochondrial COII and partial COI genes are listed in Table 3. Amplified PCR products were sequenced at Molecular Cloning Laboratories (South San Francisco, CA, USA) or Eurofins MWG Operon (Huntsville, AL, USA).
Table 3.
Primer sequences used for amplification and gene sequencing.
| Gene of interest | Primer pair (forward and reverse) | Sequences (5’→3’) |
|---|---|---|
| COII (685 bp) | TL2-J-3037 | ATG GCA GAT TAG TGC AAT GG |
| TK-N-3785 | GTT TAA GAG ACC AGT ACT TG | |
| COI (~800 bp) (partial sequence) | C1-J-2195 | TTG ATT CTT TTG GTC ACT CCA TGA AGT |
| TL2-N-3014 | TCC TAA TTG CAC TTA ATC TGC CAT ATT |
Sequences were curated with Sequencher 4.5 (Gene Codes Corp., Ann Arbor, MI, USA) and aligned with MUSCLE (MEGA 5 [30], or Phylogeny.fr: Robust Phylogenetic Analysis For The Non-Specialist [31]) using the default settings. Gaps were coded as missing. An estimate of net evolutionary divergence was calculated between the five Southeastern Reticulitermes using MEGA 5 by determining the number of base substitutions per site. The sequence data were used to infer optimal phylogenetic trees employing the following tree estimation methods as implemented by the listed software package: Maximum Likelihood (PHYML) and Maximum Parsimony (MEGA 5) [26,27,28,29,30,31,32]. ML (Maximum Likelihood) analysis for COI and COII was performed with PHYML 3.0 on the Phylogeny.fr: Robust Phylogenetic Analysis For The Non-Specialist [31]web server using the GTR+G+I model. The MP (Maximum Parsimony) analysis of COI and COII sequence used MEGA 5 with Close-Neighbor-Interchange (CNI) search and 1000 bootstrap replicates. Graphical representations of the resulting trees were improved using FigTree [32].
Phylogenetic relationships of Reticulitermes from the southeastern USA were compared to GenBank sequences from other regions of the world to support the hypothesis that the new species was not previously described. Primary molecular voucher specimens were deposited at the University of Georgia Collection of Arthropods (UGCA), Georgia Museum of Natural History, Athens, Georgia, USA. DNA extraction vouchers were deposited in HSERP Laboratory, University of Georgia, Athens, GA, USA.
3. Results and Discussion
3.1. Morphological Characters
The new species had the smallest range of measurements for both alate and soldier samples for all morphological characters examined with the exception of soldier head capsule ratio (sl:sw), and soldier right mandible angles of curvature (smac1 and smac2) (Table 4a). The range of sl:sw was similar to R. virginicus, and smac1 and smac2 were similar to R. flavipes (Table 4a).
Table 4a.
List of soldier characters measured by species showing mean, standard deviation, range of values and range of mean ± one std. dev.
| Soldier head capsule | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Length (sl), mm | Width (sw), mm | Ratio of length: width (sl:sw) | ||||||||||||
| Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | |
| R. flavipes | 1.693 | 0.119 | 1.21 | 1.91 | 1.57–1.81 | 1.044 | 0.074 | 0.73 | 1.17 | 0.97–1.12 | 1.625 | 0.084 | 1.43 | 1.83 | 1.54–1.71 |
| R. virginicus | 1.625 | 0.068 | 1.37 | 1.84 | 1.56–1.69 | 0.920 | 0.039 | 0.76 | 1.01 | 0.88–0.96 | 1.767 | 0.063 | 1.56 | 1.95 | 1.70–1.83 |
| R. hageni | 1.434 | 0.092 | 1.16 | 1.59 | 1.34–1.53 | 0.862 | 0.030 | 0.75 | 0.91 | 0.82–0.90 | 1.656 | 0.073 | 1.44 | 1.78 | 1.58–1.73 |
| R. malletei | 1.490 | 0.058 | 1.33 | 1.64 | 1.43–1.55 | 0.879 | 0.029 | 0.78 | 0.95 | 0.85–0.91 | 1.695 | 0.066 | 1.52 | 1.87 | 1.63–1.76 |
| R. nelsonae | 1.407 | 0.127 | 1.14 | 1.72 | 1.28–1.42 | 0.784 | 0.054 | 0.70 | 0.99 | 0.73–0.84 | 1.793 | 0.092 | 1.52 | 1.98 | 1.70–1.89 |
| Soldier mandible angle of curvature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | smac1, ° | smac2, ° | ||||||||
| Mean | Std. dev. | Min. a | Max. b | Range of Mean ± 1 | Mean | Std. dev. | Min. a | Max. b | Range of Mean ± 1 | |
| R. flavipes | 10.61 | 2.089 | 7.3 | 15.1 | 8.0–13.0 | 27.29 | 2.730 | 22.1 | 31.4 | 25.0–30.0 |
| R. virginicus | 13.62 | 1.719 | 11.7 | 18.1 | 12.0–15.0 | 32.60 | 2.003 | 29.4 | 36.7 | 31.0–35.0 |
| R. hageni | 8.39 | 1.199 | 6.9 | 11.2 | 7.0–10.0 | 23.39 | 1.307 | 21.5 | 27.3 | 22.0–25.0 |
| R. malletei | 10.51 | 1.366 | 8.1 | 13.2 | 9.0–12.0 | 25.85 | 2.282 | 22.4 | 32.9 | 24.0–28.0 |
| R. nelsonae | 10.66 | 2.206 | 7.2 | 14.6 | 8.0–13.0 | 27.27 | 2.654 | 24.1 | 34.0 | 25.0–30.0 |
Note: Shaded boxes represent values that were used in building the dichotomous key for soldiers and alates.
The range of soldier head capsule measurements for the new species was 1.14–1.72 mm for sl and 0.70–0.99 mm for sw (Table 4a). The sl:sw for soldier head capsules ranged from 1.52–1.98 (Table 4a). The range for the soldier right mandible angle of curvature was 7.2–14.6° for smac1 and 24.1–34.0° for smac2 (Table 4a). Alate body length without wing (abl) ranged from 3.26–4.63 mm, and alate body length including wing (ablw) ranged from 6.53–7.88 mm (Table 4b). The length of average forewings (afw) was slightly longer than the average hind wings (ahw), ranging from 4.94–5.98 mm, and 4.81–6.21 mm, respectively (Table 4b).
Table 4b.
List of alate characters measured by species showing mean, standard deviation, range of values and range of mean ± one std. dev.
| Species | Body (abl), mm | Body-wing (ablw), mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | |
| R. flavipes | 4.783 | 0.383 | 3.77 | 5.83 | 4.40–5.17 | 8.973 | 0.402 | 8.05 | 9.94 | 8.57–9.38 |
| R. virginicus | 4.021 | 0.214 | 3.56 | 4.44 | 3.81–4.24 | 7.414 | 0.213 | 6.89 | 7.90 | 7.20–7.63 |
| R. hageni | 4.083 | 0.323 | 3.41 | 5.35 | 3.76–4.41 | 7.810 | 0.318 | 7.25 | 8.64 | 7.49–8.13 |
| R. malletei | 4.023 | 0.302 | 3.53 | 4.99 | 3.72–4.33 | 8.238 | 0.394 | 6.91 | 9.28 | 7.84–8.63 |
| R. nelsonae | 3.928 | 0.236 | 3.26 | 4.63 | 3.69–4.16 | 7.080 | 0.291 | 6.53 | 7.88 | 6.79–7.37 |
| Species | Forewing (afw), mm | Hind wing (ahw), mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | Mean | Std. dev. | Min. a | Max. b | Range of mean ± 1 | |
| R. flavipes | 6.810 | 0.331 | 5.97 | 7.74 | 6.48–7.14 | 6.550 | 0.332 | 5.70 | 7.44 | 1.29–1.35 |
| R. virginicus | 5.532 | 0.193 | 5.15 | 6.05 | 5.34–5.73 | 5.418 | 0.193 | 4.78 | 5.78 | 1.32–1.37 |
| R. hageni | 5.965 | 0.263 | 5.47 | 6.52 | 5.70–6.23 | 5.739 | 0.257 | 5.24 | 6.19 | 1.28–1.34 |
| R. malletei | 6.375 | 0.328 | 5.13 | 7.31 | 6.04–6.70 | 6.100 | 0.339 | 4.92 | 6.95 | 1.27–1.32 |
| R. nelsonae | 5.430 | 0.212 | 4.94 | 5.98 | 5.22–5.64 | 5.315 | 0.297 | 4.81 | 6.21 | 1.27–1.31 |
Note: Shaded boxes represent values that were used in building the dichotomous key for soldiers and alates.
3.2. Behavior
Alate samples were collected from Sapelo Island on 12 May 2005, 6 February 2007 and 6 March 2007. All samples of fully developed, sclerotized and winged, alates were collected directly from sampling devices prior to flight. We predict that flights of the new species would occur during the same time frame as the alates were fully sclerotized and winged.
3.3. Dichotomous Key
Keys to the soldiers and alates of the Reticulitermes species of Georgia, USA, were constructed (Appendix 1, Appendix 2, respectively) based on values shown in Table 4a-Table 4b in shaded boxes representing the mean ± 1 std. dev. values for each character, with the exception of sl:sw. A minimum of 9 soldier head capsules are recommended to obtain a 95% confidence level for correct identification of R. flavipes, R. virginicus, and the new species, and 29 specimens for separating R. malletei from R. hageni. The minimum number of alate specimens recommended to obtain a 95% confidence level for discriminating all five species is 6. A more detailed discussion of the statistical analyses and generation of minimum sample size can be found in Lim (2011) [29].
3.4. Molecular Data
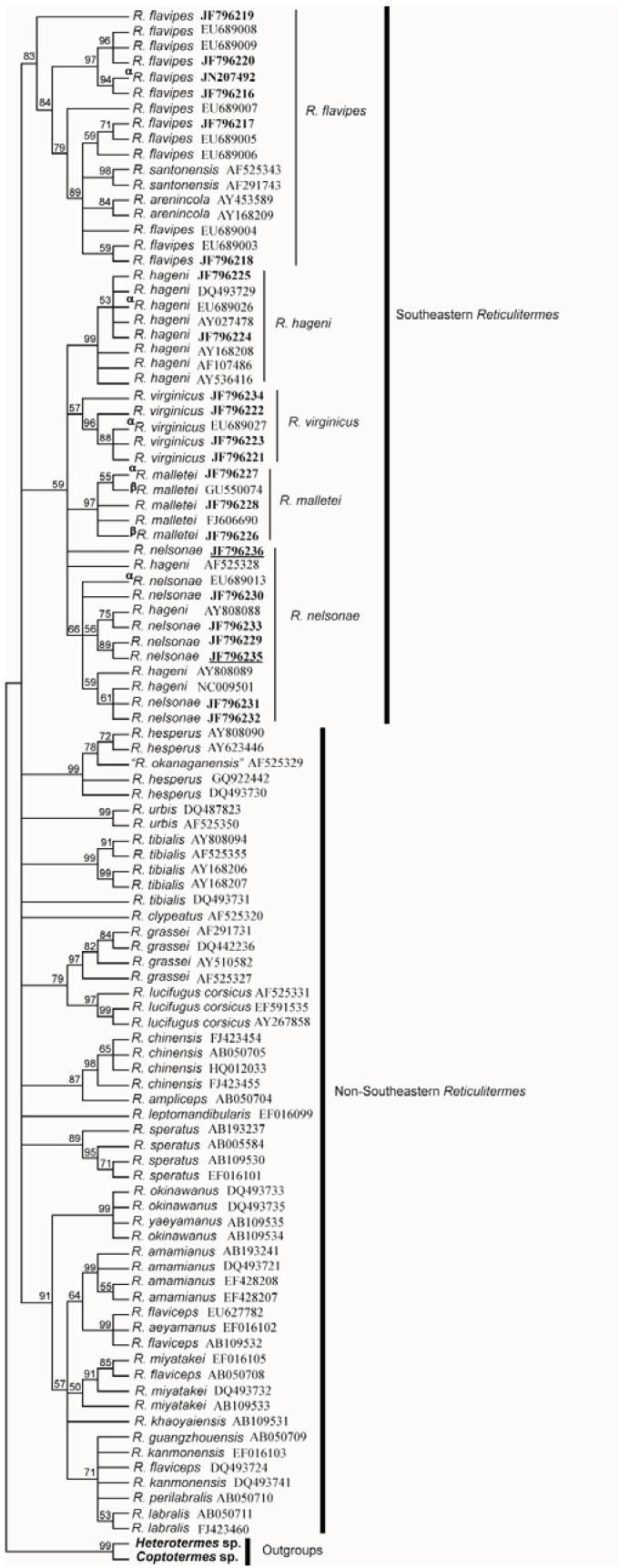
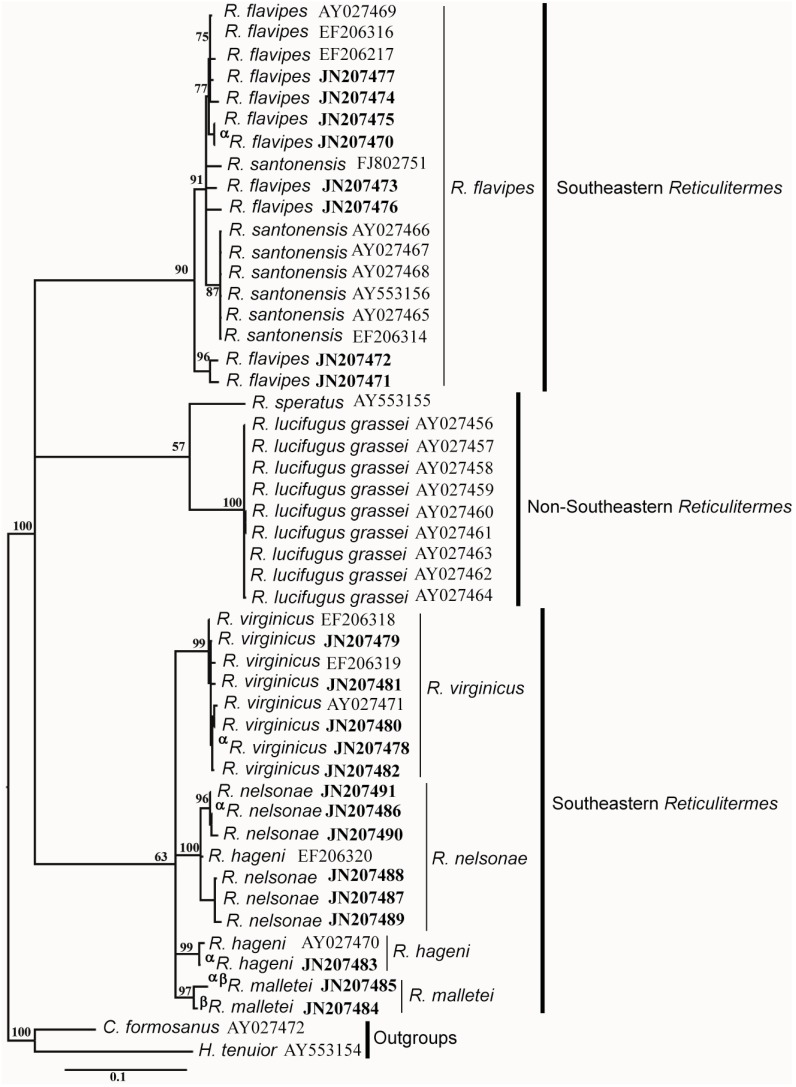
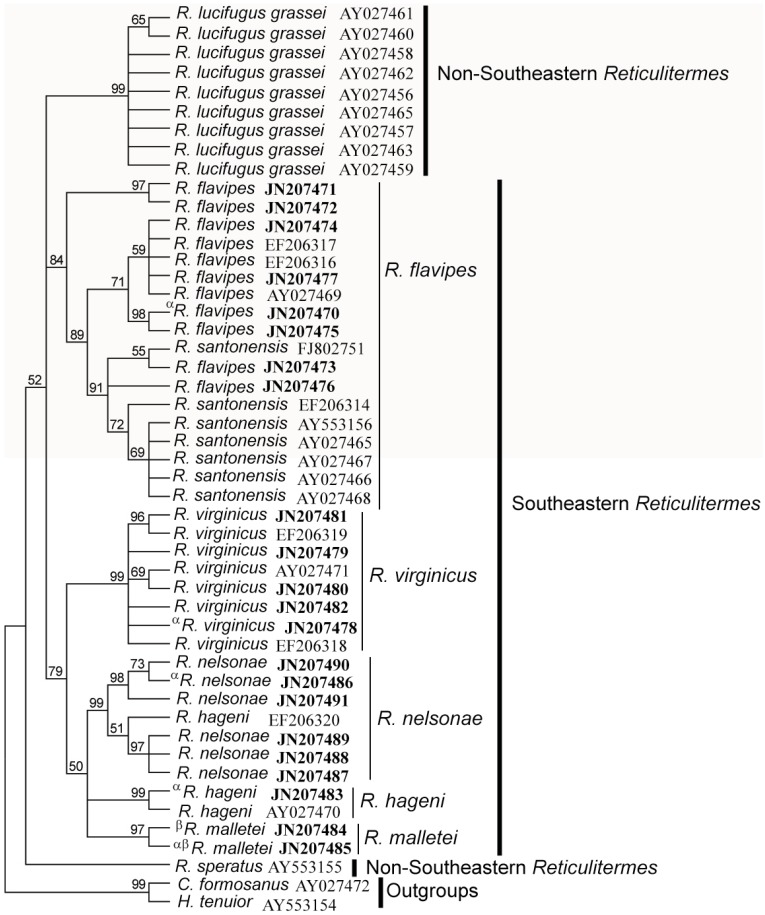
A total of 102 COII and 49 COI sequences were analyzed providing 20 new haplotypes for COII and 21 new haplotypes for COI. The estimated net evolutionary divergence among the five Southeastern Reticulitermes is shown in Table 5a and Table 5b for COII and COI respectively. The values indicate that R. nelsonae evolutionary divergence is comparable to the net evolutionary divergence observed between other described species from that region (Table 5a and Table 5b). Similar clades were recovered in all phylogenies from the COII and COI sequence data (Figure 4, Figure 5, Figure 6 and Figure 7). There are five reference sequences (labeled as α in Figure S1, Figure S2, Figure S3 and Figure S4) obtained from specimens where both alates and soldiers from that same collection point and date matched the described morphological criteria for the respective species (Figure 4, Figure 5, Figure 6 and Figure 7). Two sequences in the R. malletei clade for COII and COI were labeled β (Figure S1, Figure S2, Figure S3 and Figure S4) to signify that these sequences were corroborated with the original 16SrRNA haplotype sequences in Austin et al. (2007).
Table 5a.
Estimate of net evolutionary divergence between species for COII.
| Species | R. flavipes | R. virginicus | R. hageni | R. malletei | R. nelsonae |
|---|---|---|---|---|---|
| R. flavipes | |||||
| R. virginicus | 0.052 | ||||
| R. hageni | 0.047 | 0.033 | |||
| R. malletei | 0.046 | 0.036 | 0.031 | ||
| R. nelsonae | 0.059 | 0.049 | 0.044 | 0.039 |
Table 5b.
Estimate of net evolutionary divergence between species for COI.
| Species | R. flavipes | R. virginicus | R. hageni | R. malletei | R. nelsonae |
|---|---|---|---|---|---|
| R. flavipes | |||||
| R. virginicus | 0.051 | ||||
| R. hageni | 0.048 | 0.040 | |||
| R. malletei | 0.042 | 0.037 | 0.027 | ||
| R. nelsonae | 0.050 | 0.044 | 0.036 | 0.029 |
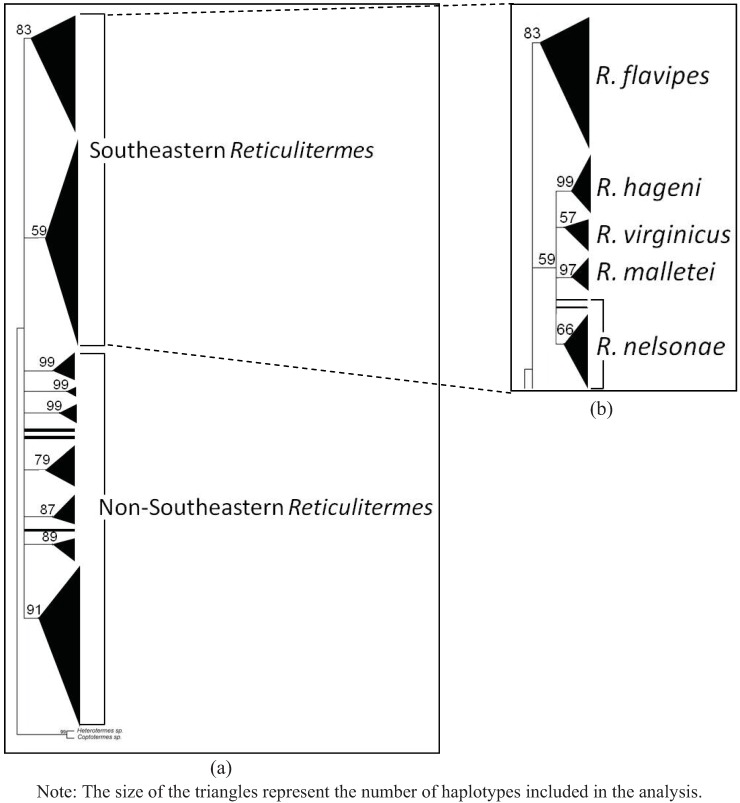
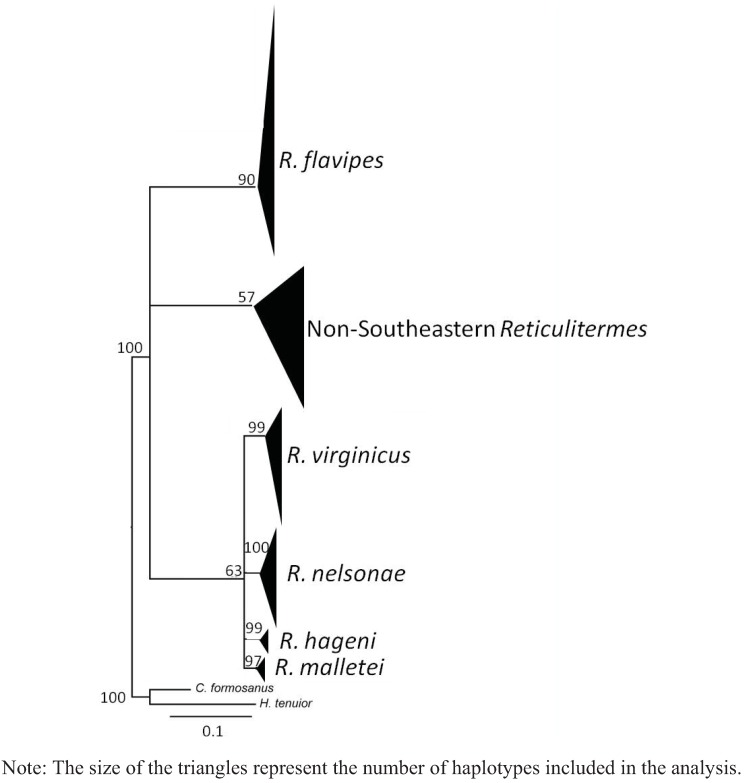
Figure 4.
Maximum Likehood (ML) estimate of the Reticulitermes phylogeny based on mitochondrial cytochromeoxidase II (COII) gene data (665 bp). The scale bar represents 0.1 substitution/ site. (a) Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes species from the southeastern USA and other regions of the world. (b) Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes from southeastern USA.
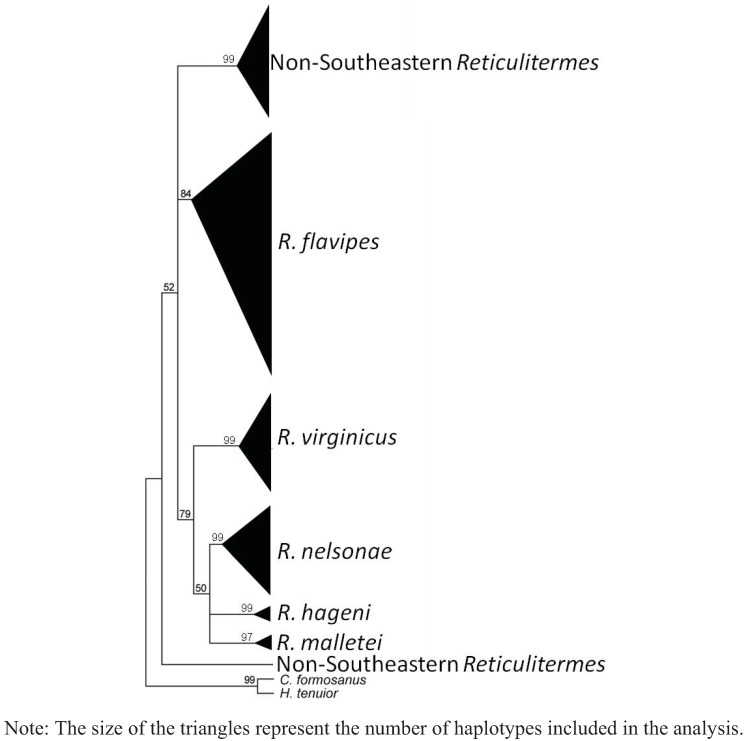
Figure 5.
Maximum parsimony (MP) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COII (665 bp) data. (a) Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes species from the southeastern USA and other regions of the world. (b) Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes from southeastern USA.
Figure 6.
Maximum likelihood (ML) estimate of Reticulitermes phylogeny based on mitochondrial ribosomal gene COI (767 bp) data. Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes.
Figure 7.
Maximum parsimony (MP) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COI (767 bp) data. Collapsed tree topology illustrating the branch support and clades formed by Reticulitermes.
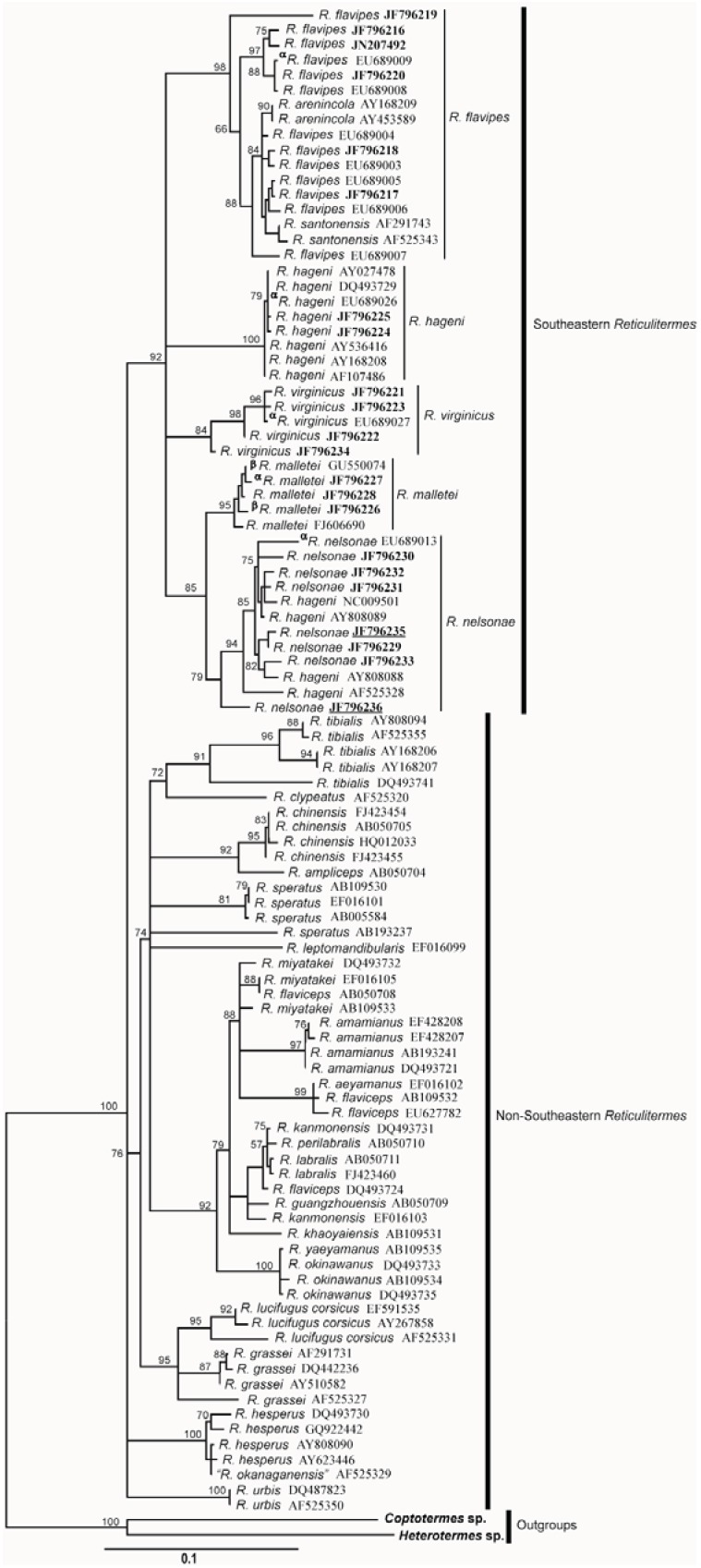
The COII alignment included 665 bp of the 685 bp full length to size-match the length of some sequences retrieved from GenBank. The comparisons yielded 216 variable sites, of which 178 were parsimony-informative. Maximum likelihood (ML) and maximum parsimony (MP) were employed to construct phylogenetic trees that included selected GenBank sequences (as of 8 March 2011) for Reticulitermes. All inferred phylogenetic trees were well resolved for the Southeastern Reticulitermes showing consistent and strongly corroborated topologies with the exception of two haplotypes in the R. nelsonae clade (JF796236 and AF525328) from the MP phylogeny that are recovered as polytomies containing R. nelsonae, R. malletei, R. virginicus and R. hageni (Figure 4, Figure 5, Figure S1 and Figure S2). The inferred ML tree shows high branch support (>84) for all species clades within the Southeastern Reticulitermes group with Ln likelihood = −5075.935 (Figure 4). The MP analysis resulted in 42 most parsimonious trees (length = 854, CI = 0.33, RI = 0.82) (Figure 5). The composite index for all sites = 0.300, and a composite index for parsimony-informative sites = 0.270 that used 1000 bootstraps to generate the consensus tree (Figure 5). COII sequences were deposited in GenBank (EU689013, JF796229-JF796233, and JF796235-JF796236) and are listed in bold in the supplemental phylogenies (Figure S1 and Figure S2). The ML and MP phylogeny for COII showed separation between Reticulitermes from the southeastern USA and Reticulitermes from other regions (Figure 4 and Figure 5).
The alignment of COI included 767 bp of the 801 bp partial length to size-match with some of the GenBank sequences retrieved for this analysis, Results showed 146 variable sites of which 113 were parsimony-informative. Inferred ML tree shows high branch support for most clades with Ln likelihood = −2905.620 (Figure 6). MP analysis resulted in 33 most parsimonious trees of 366 steps with a consistency index = 0.556, a retention index = 0.892, a composite index for all = 0.565, and a composite index for parsimony-informative sites = 0.496 that used 1000 bootstraps to generate the consensus tree that was similar in tree topology to the ML analysis (Figure 6 and Figure 7). COI sequences also were deposited in GenBank (JN207486-JN207491) and are listed in bold in the supplemental phylogenies (Figure S3 and Figure S4).
The phylogenetic trees from ML and MP analyses using the COI data also recovered genetic separation between Reticulitermes from the southeastern region of the USA and Reticulitermes from other parts of the world (Figure 6 and Figure 7). No COI sequences were found in GenBank for Reticulitermes from the western USA.
4. Systematics
Reticulitermes nelsonae Lim and Forschler, new species
(Figure 1, Figure 3, Figure 8, Figure 9 and Figure 10)
Figure 8.
Reticulitermes nelsonae, diagnostic morphological characters for species identification: (a) Alate, dorsal, scale bar = 1.0 mm, (b) Soldier head capsule, dorsal, scale bar = 0.5 mm, and (c) Soldier mandible pair, dorsal, scale bar = 1.0 mm.
Figure 9.
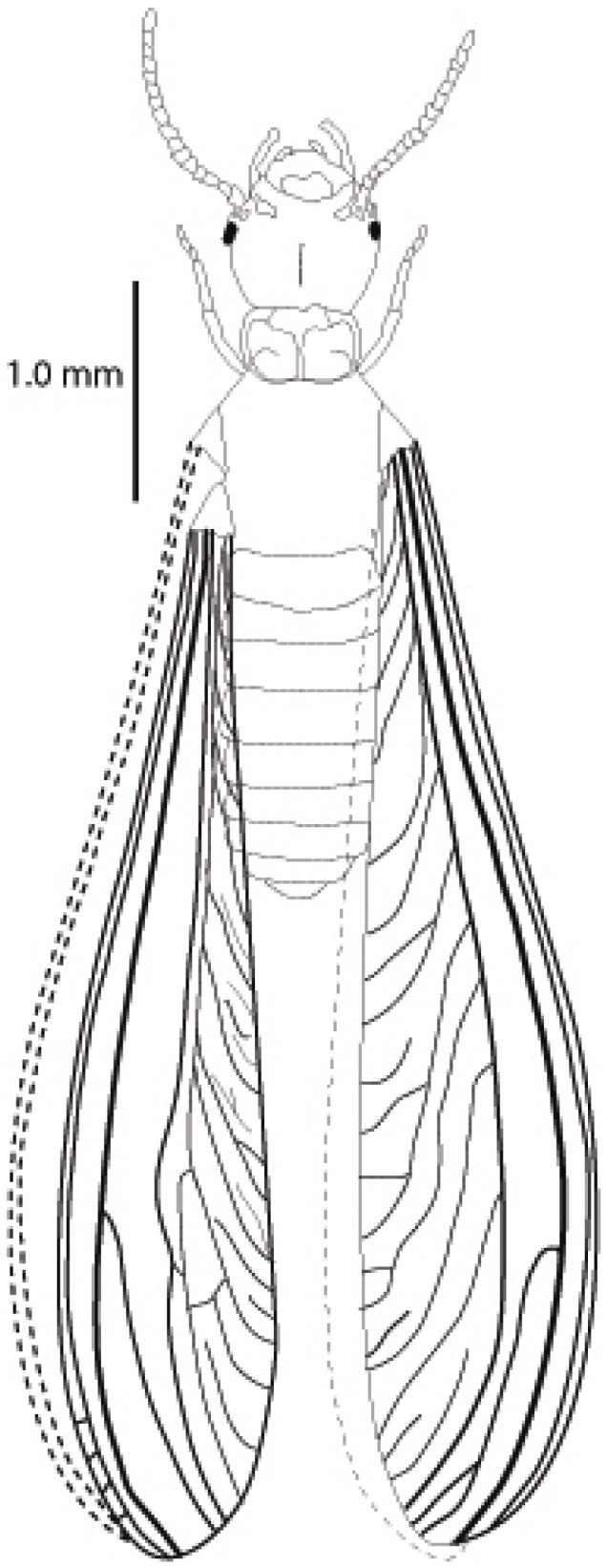
Habitus drawing of R. nelsonae alate, showing fore- and hind wing along with wing venation. Scale bar is 1.0 mm.
Figure 10.
Reticulitermes species alates from the southeastern USA. From left to right: R. virginicus, R. nelsonae, R. hageni, R. malletei, R . flavipes. Scale bar = 1.00 mm.
HOLOTYPE (alate, ♀): “USA: Georgia, McIntosh Co., Sapelo Island, 31°23'43.32"N
81°16'38.23"W, 6.II .2007, D. Sillam-Dussès” (AMNH).
ALLOTYPE (alate, ♂): “USA: Georgia, McIntosh Co., Sapelo Island, 31°23'43.32"N
81°16'38.23"W, 6. II. 2007, D. Sillam-Dussès” (AMNH).
PARATYPES (alate): USA: Georgia: McIntosh Co., Sapelo Island, 31°23'43.32"N
81°16'38.23"W, 6.II.2007, D. Sillam-Dussès (1 ♂, 1♀, UGCA; 1 ♂, 1 ♀, NMNH).
PARATYPES (soldier): USA: Georgia: McIntosh Co., Sapelo Island, 31°23'43.32"N
81°16'38.23"W, 6.II.2007, D. Sillam-Dussès (1 ♂, 1♀, UGCA; 1 ♂, 1 ♀, NMNH).
PARATYPES (worker): USA: Georgia: McIntosh Co., Sapelo Island, 31°23'43.32"N
81°16'38.23"W, 6.II.2007, D. Sillam-Dussès (1 ♂, 1♀, UGCA; 1 ♂, 1 ♀, NMNH).
4.1. Diagnosis
Soldier (Table 4a, Figure 8b and 8c): Head capsule small (mean sl 1.407 mm, mean sw 0.054 mm, mean sl:sw 1.793 mm) (Table 4a).
Alate (Table 4b, Figure 8a, Figure 9 and Figure 10): Body length, with (ablw) and without (abl) wing, small (mean abl 3.93 mm, mean ablw 7.080 mm), body color pale brown and wings non-pigmented (Figure 8a, Figure 9 and Figure 10).
4.2. Description
There is little sexual dimorphism for soldier or alates, males and females can however, be differentiated by the form of the 8th sternal plate (Zimet & Stuart, 1982).
Soldier Head capsule rectangular in shape, typically longer than wide (Figure 8b). Head capsule color yellowish with dark brown to black mandibles (Figure 8b). Body color light yellowish to white. Mean head capsule length (sl) 1.41 mm ± 0.13 (Table 4a), mean width (sw) 0.78 mm ± 0.05 (Table 4a), mean ratio length-to-width (sl : sw) 1.793 ± 0.09 (Table 4a). Mean mandible curvature angles: smac1 = 10.7° ± 2.21, smac2 = 27.27° ± 2.65 (Table 4a).
Alate Body color pale brown with 14 antennal segments (Figure 8a and Figure 10). Wings non-pigmented (Figure 8a and Figure 10). Legs light to dark brownish with 14 antennal segments (Figure 8a and Figure 10). Mean body length without wing (abl) 3.93 mm ± 0.24 (Table 4b). Mean body length with wings (ablw) 7.08 mm ± 0.29 (Table 4b). Mean forewing length (afw) 5.43 mm ± 0.21. Mean hind wing length (ahw) 5.32 mm ± 0.30 (Table 4b).
4.3. Etymology
This patronym was established to honor Lori J. Nelson (USDA Forest Service, Buchanan, CA, USA) who realized in 1996 that specimens collected on Sapelo Island, a barrier island off the Atlantic coast of Georgia, were notably different from all previously described Reticulitermes species based on analysis of cuticular hydrocarbons [17,18,19].
Behavior
Reticulitermes nelsonae is expected to swarm from February to May. Reticulitermes hageni swarms from August to October. Reticulitermes flavipes flights have been recorded from November through April thus overlapping with R. nelsonae [29]. Reticulitermes nelsonae flight times also overlap with R. virginicus and R. malletei, both of which have been recorded in May [10,13,33].
4.4. Distribution
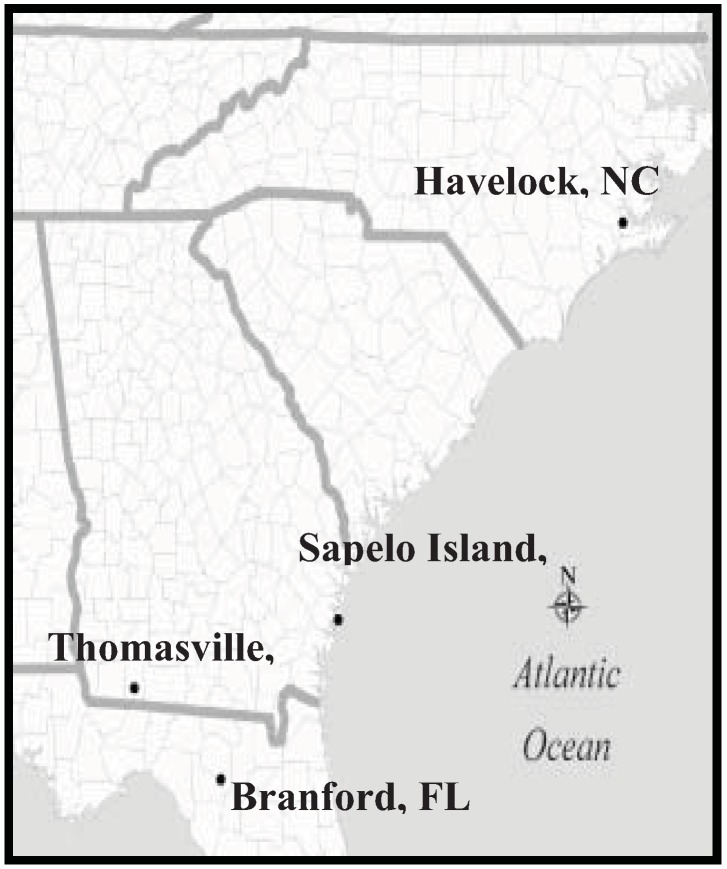
Reticulitermes nelsonae is found in the southeastern region of the United States, in the Atlantic Coastal Flatwoods and South Coastal Plain soil provinces (Table 2, Figure 11). The species has not been detected in the Piedmont soil province despite sampling in that region. In addition to the type locality on Sapelo Island, GA, this species has been collected in Croatan National Forest in Havelock, NC, Greenwood Plantation in Thomasville, GA, and Branford, FL (Table 2, Figure 11).
Figure 11.
Distribution map of locales where the new species, Reticulitermes nelsonae have been collected.
4.5. Comments/Remarks
Soldier Reticulitermes nelsonae head capsule length (sl) is at least 0.2 mm shorter than R. flavipes and R. virginicus (Table 4a). Reticulitermes nelsonae head capsule width (sw ) at least 0.1 mm smaller than R. flavipes and R. virginicus (Table 4a). The sl and sw are also more than 0.1 mm smaller than R. malletei (Table 4a). The R. nelsonae smac2 is more than 24° (typically 24°–30°), while R. hageni smac2 is less than 25° (typically 22°–25°) (Table 4a).
Alate Reticulitermes nelsonae abl and ablw are typically 3.7 mm–4.2 mm and 6.8 mm–7.4 mm, whereas in R. flavipes those same characters are more than 4.4 mm and 8.6 mm, respectively (Table 4b). Reticulitermes nelsonae afw and ahw are 1.0 mm shorter than R. flavipes (Table 4b). Body color differs from that of R. hageni’s yellowish to yellowish-brown body color (Figure 10). Reticulitermes nelsonae wings are non-pigmented, while R. malletei has pigmented wings (Austin et al., 2007) and forewing (afw) measurements are 0.4 mm shorter than R. malletei. Reticulitermes nelsonae and R. virginicus share similar morphometric ranges, but differ in body color, with R. nelsonae having light brown color and R. virginicus dark brown. The ratio of mean body length including wings to mean forewing length (ablw: afw) is typically 1.27–1.31 for R. nelsonae, while for R. virginicus it is 1.32–1.37 (Table 4b).
4.6. Genetics
Sequences from cytochrome oxidase I (COI) and cytochrome oxidase II (COII) genes generated data that showed R. nelsonae was genetically unique as its haplotypes consistently formed a separate clade from the other described species (Figure 4, Figure 5, Figure 6 and Figure 7).
5. Discussion
The genus Reticulitermes is in need of a thorough taxonomic revision, so describing new species is a task beset with numerous difficulties. Broad intraspecific morphological variation exacerbates the issue of species discrimination, yet multiple independent lines of evidence (listed below) support recognition of R. nelsonae as a new species. The description of a single new species within taxa of economic and ecological significance, like Reticulitermes, is justifiable outside of the context of an exhaustive revision. It is our hope that this work will serve as a foundation toward a revision of Reticulitermes.
5.1. Cuticular Hydrocarbon
An examination of Haverty et al. (1999) [18] and Jenkins et al. (2000) [19] indicates that the hydrocarbon phenotypes GA-L and GA-I most likely correspond to R. nelsonae. Jenkins et al. (2000) [19] observed that two of their collections were “different morphologically, chemically and genetically”. We now believe these samples, identified as haplotypes BH25 (JF796235) and HH11 (JF796236), are R. nelsonae because we recovered those haplotypes in the R. nelsonae clade for the COII gene (haplotypes are underlined in Figure S1 and Figure S2). We, therefore, reason that the cuticular hydrocarbon phenotypes GA-L and GA-I reported by [17,18] belong to R. nelsonae.
5.2. Morphology
Morphological separation of Reticulitermes species is notoriously difficult. Our morphometric measurements provided a range of overlap consistent with past reports for the genus [13,14,16,17,21,34]. Dichotomous keys for soldiers and alates of Reticulitermes species collected in Georgia (Appendix 1 and Appendix 2) were prepared to distinguish the five species endemic to the southeastern USA. The measurements used to build the soldier key can separate R. nelsonae soldiers from all previously described species with the exception of R. hageni, which overlap on all measures at the upper range for R. nelsonae (Table 4a and Appendix 1). Alates of all species can be separated based on the combination of body color, morphometric measurements, and flight times (Table 4b and Appendix 2).
The first author has prepared a more extensive study of the literature on morphological variation in Reticulitermes species from the southeastern USA [29]. We recommend that 6 alates and/or 29 soldiers specimens be used to achieve a 95% confidence in morphometric-based species diagnosis [29].
5.3. Genetics
Congruent and similar phylogenies were obtained from ML and MP analyses for both COII and COI sequences (Figure 4, Figure 5, Figure 6 and Figure 7). Genetics of Reticulitermes from the southeastern USA were consistently differentiated from that of Reticulitermes from other regions of the world and haplotypes of Reticulitermes nelsonae were further differentiated within the ‘Southeastern Reticulitermes’ grouping and thus are genetically unique from previously described Reticulitermes (Figure 4, Figure 5, Figure 6 and Figure 7). The net evolutionary distance dataset demonstrates that the ‘genetic uniqueness’ of R. nelsonae is within the range of the already described species (Table 5a and Table 5b).
Three Reticulitermes species (R. virginicus, R. hageni and R. malletei) from the southeastern USA have clear separation between COII haplotype designations within their respective species clades, while mixed haplotype designations were observed in the R. flavipes and R. nelsonae clade (see supplementary Figure S1 and Figure S2). The R. flavipes clade includes R. santonensis (which has been synonymized as R. flavipes) and R. arenincola haplotypes (Figure S1 and Figure S2). Previous reports on R. arenincola support our findings showing R. arenincola is genetically similar to R. flavipes [35,36,37].The R. nelsonae clade includes four GenBank sequences that were designated as R. hageni (NC009501, AY808088, AY808089, and AF525328) (Figure S1 and Figure S2). Voucher specimens for these GenBank accessions have been requested and to date two examined (AY808088, AY808089). The mistaken GenBank accessions are most likely the result of the fact that only 3 of the 5 species endemic to the southeastern USA are listed in published keys and the available taxonomic keys would have identified R. nelsonae specimens as R. hageni [13,14,15].
The 767 bp COI gene phylogenies also indicate that haplotypes of Reticulitermes species from the southeastern USA are different from Reticulitermes found in other areas of the USA (Figure 4, Figure 5, Figure 6 and Figure 7). Reticulitermes santonensis was again recovered within the R. flavipes clade further supporting junior synonym status (Figure S3 and Figure S4). One GenBank R. hageni sequence (EF 206320) was recovered within the R. nelsonae clade (Figure S3 and Figure S4) which is not surprising, and as mentioned, the specimen would have been identified as R. hageni based on published keys [13,14,15].
Molecular phylogenies are an estimation of plausible species relationships and therefore detailed research and comparison is warranted to accurately identify species designations for gene sequence data. Cytochrome oxidase II (COII), with a length of 685 bp, has been a valuable marker for identification of Reticulitermes species [19,22,38,39] as evidenced by Sillam-Dusses and Forschler (2010) who reported an undescribed species based on genetics alone [22]. We recovered R. arenincola Goellner 1931 in the R. flavipes clade lending support for synonymy with R. flavipes (Figure 4 and Figure 5) [12,35,36,37,40].
We suggest that future genetic analysis provide reference sequences for COII and COI genes that are from specimens corroborated with morphological descriptions for Reticulitermes species (as denoted by α in Figure S1, Figure S2, Figure S3 and Figure S4).
6. Conclusions
We echo past recommendations that species discrimination based on morphology should include data from both alates and soldiers, from the same collection, for accurate identification [13,14]. A prominent conclusion from this study is that Reticulitermes species discrimination should be attempted using morphometric characters from both castes accompanied by genetic and or other chemical evidence [21,41,42,43]. Based on the data obtained in this study, R. nelsonae is a true entity that satisfies the following species concepts: the morphological [44], phylogenetic [44,45], genetic [46], ecological and reproductive isolation species concepts [44,47]. Further examination will determine if the distribution of R. nelsonae is restricted, as currently described to the Atlantic Coastal Flatwoods and South Coastal Plain soil provinces across the southeastern United States.
Additional studies of the morphological and haplotype diversity of Reticulitermes are needed throughout the distributional range. Data sets combining cuticular hydrocarbon phenotypes, genetics, and morphology, should be explored further to facilitate identification of species within this taxonomically challenging genus.
Acknowledgments
We would like to thank all individuals who helped in this project, especially Joseph V. McHugh, Tracie M. Jenkins, Michael I. Haverty and Lori J. Nelson and several anonymous reviewers for their critical review, comments and suggestions. The authors also would like to thank Joe Eger, Marty Jurgensen, Debbie Dumroese, Joanne Tirocke, Robert Eaton, Felipe Sanchez, Tabitha Hayes, and Charles Dixon and the Dixon Exterminating team for their help in obtaining samples from Branford, FL, Havelock, NC and Thomasville, GA. We also would like to thank David Grimaldi, Kumar Krishna and Tam Nguyen at the American Museum of National History and the late Margaret Collins at the National Museum of National History for their kind assistance in allowing access to type specimens of Reticulitermes species. We also thank Rudolf Scheffrahn, Seemanti Chakrabarti and Nan-Yao Su for loaning voucher specimens from their study. The authors would also like to thank the University of Georgia Marine Institute (UGA MI) and Sapelo Island National Estuary Research Reserve (SINERR) for their assistance and access to land use. Finally, we thank the Georgia Department of Agriculture for partial funding pursuant to Rule 620-3-.01(2) of the Georgia Pest Control Act.
Appendix 1: Key 1
1 Head capsule length without mandible (sl) typically ≥ 1.56 mm (Figure 1) ………… 2
- Head capsule length without mandible (sl) typically ≤ 1.55 mm (Figure 1) ………… 3
2 Head capsule width (sw) mean 0.92 mm typically 0.88–0.96 mm (Figure 1). Right mandible curvature angle (smac2) mean 32.6° typically ≥ 31° (31°–35°) (Figure 2) ……………….R. virginicus
- Head capsule width (sw) mean 1.0 mm typically 0.97–1.12mm (Figure 1). Right mandible curvature angle (smac2) mean 27.3° typically ≤ 25° (25°–30°) (Figure 2)..………………….………R. flavipes
3 Head capsule width (sw) mean 0.86 mm typically 0.82–0.90 mm (Figure 1).
Right mandible angle of curvature (smac2) mean 23.39° typically ≤ 25° (22°–25°) (Figure 2).
Head capsule length without mandible (sl) mean 1.43 mm typically 1.34–1.53 mm (Figure 1).
………………………………………………………………………………... R. hageni
- Head capsule width (sw) mean 0.88 mm typically 0.85–0.91 mm (Figure 1).
Right mandible angle of curvature (smac2) mean 25.85° typically ≥ 25° (25°–30°) (Figure 2).
Head capsule length without mandible (sl) mean 1.49 mm typically ≥1.43 mm (1.43–1.55 mm) (Figure 1).……………………………………………………………………….. R. malletei
- Head capsule width (sw) mean 0.78 mm typically 0.73 mm–0.84 mm (Figure 1).
Right mandible angle of curvature (smac2) mean 27.27° typically 24–28° (Figure 2). Head capsule length without mandible (sl) mean 1.41mm typically ≤1.42 mm (1.28–1.42 mm) (Figure 1).………………………………………………………………………………..R. nelsonae
Appendix 2: Key 2
1- Body color light yellowish brown, pigmented wings (Figure 11). Swarm dates August–October
…………………………………………………………………………………………... R. hageni
- Body color pale brown to dark brown, blackish, pigmented or non-pigmented wings (Figure 11).
Swarm dates November - May ….……………………………………………………. 2
2- Body length including wings (ablw) mean 8.97 mm typically ≥ 8.57 mm (8.57–9.38 mm) (Figure 6 and Figure 7). Body length without wings (abl) mean 4.78 mm typically ≥ 4.40 mm (4.40–5.17 mm) (Figure 6 and Figure 7)……………………………………………… R. flavipes
- Body length including wings (ablw) typically ≤ 8.57 mm (6.79–8.57 mm) (Figure 6 and Figure 7).
Body length without wings (abl) typically ≤ 4.41mm (3.69–4.41 mm) (Figure 6 and Figure 7)….3
3- Average forewing length (afw) mean 6.37 mm ≥ 6.04 mm (6.04–6.70 mm) (Figure 6 and Figure 7).
Average hind wing length (ahw) mean 6.10 mm typically ≥ 5.76 mm (5.76–6.44 mm) (Figure 6 and Figure 7)……………………………………………………………………………………R. malletei
- Average forewing length (afw) ≤ 5.70 mm (5.22–5.73 mm) (Figure 6 and Figure 7).
Average hind wing length (ahw) typically ≤ 5.61 mm (5.02–5.61 mm) (Figure 6 and Figure 7)…… 4
4-Body color dark brown to black, non-pigmented wings (Figure 10). Ratio of body length with wing (ablw) to average forewing (afw) typically 1.32–1.37. Swarm dates April–May ……. R. virginicus
- Body color pale brown, non-pigmented wings (Figure 10). Ratio of body length with wing (ablw) to average forewing (afw) typically 1.27–1.31. Swarm season February–May….……… R. nelsonae
Supplementary 1.
Maximum likelihood (ML) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COII (665 bp) data. Bold Accession numbers indicate sequence from specimens matched with collections used in the morphometric portion of this study. Underlined Accession numbers are BH25 = JF796235, HH11 = JF796236.
Supplementary 2.
Maximum parsimony (MP) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COII (665 bp) data. Bold Accession numbers indicate sequence from specimens matched with collections used in the morphometric portion of this study. Underlined Accession numbers are BH25 = JF796235, HH11 = JF796236.
Supplementary 3.
Maximum likelihood (ML) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COI (767 bp) data.Bold Accession numbers indicate sequence from specimens matched with collections used in the morphometric portion of this study.
Supplementary 4.
Maximum parsimony (MP) estimate of the Reticulitermes phylogeny based on mitochondrial ribosomal gene COI (767 bp) data. Bold Accession numbers indicate sequence from specimens matched with collections used in the morphometric portion of this study.
References
- 1.Miller E.M. Biology of Termites. University of Miami Press; Coral Gables, FL, USA: 1964. p. 30. [Google Scholar]
- 2.Thorne B.L., Russek-Cohen E., Forschler B.T., Breisch N.L., Traniello J.F.A. Evaluation for mark-release-recapture methods for estimating forager population size of subterranean termite (Isoptera: Rhinotermitidae) colonies. Environ. Entomol. 1996;25:938–951. [Google Scholar]
- 3.Su N.-Y., Scheffrahn R.H. Economically important termites in the United States and their control. Sociobiology. 1990;17:77–94. [Google Scholar]
- 4.Mallis A. Handbook of Pest Control. 10 ed. The Mallis Handbook Company; Cleveland, Ohio, USA: 2011. p. 1599. [Google Scholar]
- 5.Thorne B.L., Forschler B.T. NPMA Research Report on Subterranean Termite: Biology of Subterranean Termites of the Genus Reticulitermes & Subterranean Termite Biology in Relation to Prevention and Removal of Structural Infestation. NPMA; Dunn Loring, VA, USA: 1999. [Google Scholar]
- 6.Abe T., Bignell D.E., Higashi M. Termites: Evolution, Sociality, Symbioses, Ecology. Kluwer Academic Publishers; Dordrecht, The Netherlands; Boston, MA, USA: 2000. p. xxii.p. 466. [Google Scholar]
- 7.Su N.Y., Scheffrahn R.H., Cabrera B. Native Subterranean Termites: Reticulitermes Flavipes (Kollar),Reticultiermes Virginicus (Banks),Reticulitermes Hageni Banks (Insecta: Isoptera: Rhinotermitidae) University of Florida Cooperative Extension Service; Lackawanna, PA, USA: 2001. Publication #EENY-212. [Google Scholar]
- 8.Logan J.W.M., Rajagopal D., Wightman J.A., Pearce M.J. Control of termites and other soil pests of groundnuts with special reference to controlled release formulations of non-persistent insecticides in India and Sudan. Bull. Environ. Res. 1992;82:57–66. doi: 10.1017/S000748530005149X. [DOI] [Google Scholar]
- 9.Pearce M.J. Termites: Biology and Pest Management. CAB International; Cambridge, MA, USA: 1997. [Google Scholar]
- 10.Banks N., Snyder T.E. A Revision of the Nearctic Termites. Vol. 108. Smithsonian Institution, United States National Museum; Washington DC, USA: 1920. p. 228. [Google Scholar]
- 11.Clément J.L., Howard R., Blum M., Lloyd H. L'isolement spécifique des termites du genre Reticulitermes (Isoptera) du sud-est des États-Unis. Mise en evidence grace a la chimie et au portement d' une espéce jumelle de R. virginicus = R. malletei sp.nov. et d' une semi-species de R. flavipes. Comptesrendus de l'Académie des sciences.Série 3, Sciences de la vie. 1986;302:67–70. [Google Scholar]
- 12.Austin J.W., Bagnères A.-G., Szalanski A.L., Scheffrahn R.H., Heintschel B.P., Messenger M.T., Clement J.-L., Gold R.E. Reticulitermesmalletei (Isoptera: Rhinotermitidae): A valid Nearctic subterranean termite from Eastern North America. Zootaxa. 2007;1554:1–26. [Google Scholar]
- 13.Weesner F.M. The Termites of the United States: A Handbook. The National Pest Control Association; Elizabeth, NJ, USA: 1965. p. 70. [Google Scholar]
- 14.Nutting W.L. Insecta: Isoptera. In: Dindal D.L., editor. Soil Biology Guide. Vol. 33. John Wiley & Sons; New York, NY, USA: 1990. pp. 997–1032. [Google Scholar]
- 15.Scheffrahn R.H., Su N.-Y. Keys to soldier and winged adult termites (Isoptera) of Florida. Fla. Entomol. 1994;77:460–474. [Google Scholar]
- 16.Hostettler N.C., Hall D.W., Scheffrahn R.H. Intracolonymorphometric variation and labral shape in Florida Reticulitermes (Isoptera: Rhinotermitidae) soldiers: Significance for identification. Fla. Entomol. 1995;78:119–129. [Google Scholar]
- 17.Haverty M.I., Forschler B.T., Nelson L.J. An assessment of the taxonomy of Reticulitermes (Isoptera: Rhinotermitidae) from the Southeastern United States based on cuticular hydrocarbons. Sociobiology. 1996;28:287–318. [Google Scholar]
- 18.Haverty M.I., Nelson L.J., Forschler B.T. New cuticular hydrocarbon phenotypes of Reticulitermes (Isoptera: Rhinotermitidae) from the United States. Sociobiology. 1999;34:1–21. [Google Scholar]
- 19.Jenkins T.M., Haverty M.I., Basten C.J., Nelson L.J., Page M., Forschler B.T. Correlation of mitochondrial haplotypes with cuticular hydrocarbon phenotypes of sympatric Reticulitermes species from the southeastern United States. J. Chem. Ecol. 2000;26:1525–1542. doi: 10.1023/A:1005548111591. [DOI] [Google Scholar]
- 20.Nelson L.J., Cool L.G., Forschler B.T., Haverty M.I. Correspondence of soldier defense secretion mixtures with cuticular hydrocarbon phenotypes for chemotaxonomy of the termite genus Reticulitermes in North America. J. Chem. Ecol. 2001;27:1449–1479. doi: 10.1023/A:1010325511844. [DOI] [PubMed] [Google Scholar]
- 21.Nelson L.J., Cool L.G., Solek C.W., Haverty M.I. Cuticular hydrocarbons and soldier defense secretions of Reticulitermes in Southern California: A critical analysis of taxonomy of the Genus in North America. J. Chem. Ecol. 2008;34:1452–1475. doi: 10.1007/s10886-008-9548-6. [DOI] [PubMed] [Google Scholar]
- 22.Sillam-Dussès D., Forschler B.T. A dominant and undescribed species of Reticulitermes in Sapelo Island (Georgia, USA) Sociobiology. 2010;46:137–147. [Google Scholar]
- 23.Kollar V. Naturgeschichte der Schadlichen Insekten. Verh. Landwirthschaft Gesellshaft in Wien. 1837;5:411. [Google Scholar]
- 24.Banks N. A New Species of Termes; Entomological News and Proceedings of the Entomological Section Academy of Natural Sciences of Philadephia; Philadelphia, PA, USA: Academy of Natural Sciences of Philadelphia; 1907. pp. 392–393. [Google Scholar]
- 25.Scheffrahn R.H., Su N.-Y., Chase J.A., Forschler B.T. New termite (Isoptera: Kalotermitidae, rhinotermitidae) records from Georgia. J. Entomol. Sci. 2001;36:109–113. [Google Scholar]
- 26.Feytaud J. Le termite de saintonge. C.R. Acad. Sc. Paris. 1924;178:241–244. [Google Scholar]
- 27.Austin J.W., Szalanski A.L., Scheffrahn R.H., Messenger M.T., Dronnet S., Bagneres A.-G. Genetic evidence for the synonymy of two reticulitermes species: Reticulitermes flavipes and Reticulitermes santonensis. Ann. Entomol. Soc. Am. 2005;98:395–401. doi: 10.1603/0013-8746(2005)098[0395:GEFTSO]2.0.CO;2. [DOI] [Google Scholar]
- 28.Jenkins T.M., Dean R.E., Verkerk R., Forschler B.T. Phylogenetic analyses of two mitochondrial genes and one nuclear intron region illuminate European subterranean termite (Isoptera: Rhinotermitidae) gene flow, taxonomy, and introduction dynamics. Mol. Phylogenet. Evol. 2001;20:286–293. doi: 10.1006/mpev.2001.0966. [DOI] [PubMed] [Google Scholar]
- 29.Lim S.Y. Taxonomic Review and Biogeography of Reticulitermes (Rhinotermitidae) Termites of Georgia. University of Georgia; Athens, GA, USA: 2011. [Google Scholar]
- 30.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011 doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayar J.-F., Guindon S., Lefort V., Lescot M., et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;1:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molecular evolution, phylogenetics and epidemiology. FigTree. [(accessed on 2 December 2011)]. Available online: http://tree.bio.ed.ac.uk/software/figtree/
- 33.Krishna K., Weesner F.M. Biology of Termites. Academic Press Inc.; New York, NY, USA: 1970. [Google Scholar]
- 34.Wang C., Zhou X., Li S., Schwinghammer M., Scharf M., Buczkowski G., Bennett G. Survey and Identification of Termites (Isoptera: Rhinotermitidae) in Indiana. Ann. Entomol. Soc. Am. 2009;102:1029–1036. doi: 10.1603/008.102.0611. [DOI] [Google Scholar]
- 35.Austin J.W., Szalanski A.L., Cabrera B.J. Phylogenetic analysis of the subterranean termite family Rhinotermitidae (Isoptera) by using the mitochondrial cytochromeoxidase II gene. Ann. Entomol. Soc. Am. 2004;97:548–555. doi: 10.1603/0013-8746(2004)097[0548:PAOTST]2.0.CO;2. [DOI] [Google Scholar]
- 36.Austin J.W., Szalanski A.L., Scheffrahn R.H., Messenger M.T. Genetic variation of Reticulitermes flavipes (Isoptera: Rhinotermitidae) in north america applying the mitochondrial rRNA 16S gene. Ann. Entomol. Soc. Am. 2005;98:980–988. doi: 10.1603/0013-8746(2005)098[0980:GVORFI]2.0.CO;2. [DOI] [Google Scholar]
- 37.Vargo E.L., Husseneder C. Biology of subterranean termites: Insights from molecular studies of Reticulitermes and Coptotermes. Annu.Rev. Entomol. 2009;54:379–403. doi: 10.1146/annurev.ento.54.110807.090443. [DOI] [PubMed] [Google Scholar]
- 38.Copren K.A., Nelson L.J., Vargo E.L., Haverty M.I. Phylogenetic analyses of mtDNA sequences corroborate taxonomic designations based on cuticular hydrocarbons in subterranean termites. Mol. Phylogenet. Evol. 2005;35:689–700. doi: 10.1016/j.ympev.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Cameron S.L., Whiting M.F. Mitochondrial genomic comparisons of the subterranean termites from the Genus Reticulitermes (Insects: Isoptera: Rhinotermitidae) Genome. 2007;50:188–202. doi: 10.1139/g06-148. [DOI] [PubMed] [Google Scholar]
- 40.Ye W., Lee C.-Y., Scheffrahn R.H., Aleong J.M., Su N.-Y., Bennett G.W., Scharf M.E. Phylogenetic relationships of neartic Reticulitermes species (Isoptera: Rhinotermitidae) with particular reference to Reticulitermes arenincola Goellner. Mol. Biol. Evol. 2004;30:815–822. doi: 10.1016/S1055-7903(03)00230-6. [DOI] [PubMed] [Google Scholar]
- 41.Forschler B.T., Jenkins T.M. Evaluation of subterranean termite biology using genetic, chemotaxonomic, and morphometric markers and ecological data: A testimonial for multi-disciplinary efforts. Trends Entomol. 1999;2:71–80. [Google Scholar]
- 42.Lee M.S.Y. The molecularisation of taxonomy. Invertebr.Syst. 2004;18:1–6. doi: 10.1071/IS03021. [DOI] [Google Scholar]
- 43.Cognato A.I. Standard percent DNA sequence difference for insects does not predict species boundaries. Entomol.Soc. Am. 2006:1037–1045. doi: 10.1603/0022-0493-99.4.1037. [DOI] [PubMed] [Google Scholar]
- 44.Winston J.E. Describing Species: Practical Taxonomic Procedure for Biologists. Columbia University Press; New York, NY, USA: 1999. p. 513. [Google Scholar]
- 45.Cracraft J. Speciation and Its Ontology: The Empirical Consequences of Alternative Species Concepts for Understanding Patterns and Processes of Differentiation. In: Otte D., Endleer J.A., editors. Speciation and Its Consequences. Sinauer Associates; Sunderland, MA, USA: 1989. pp. 28–59. [Google Scholar]
- 46.Baker R.J., Bradley R.D. Speciation in mammals and the genetic species concept. J. Mammal. 2006;87:643–662. doi: 10.1644/06-MAMM-F-038R2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkins S.J. Defining Species: A Sourcebook from Antiquity to Today. Peter Lang Publishing; New York, NY, USA: 2009. p. 224. [Google Scholar]