Abstract
Transcranial direct current stimulation (tDCS) studies often use one anode to increase cortical excitability in one hemisphere. However, mental processes may involve cortical regions in both hemispheres. This study’s aim was to assess the safety and possible effects on affect and working memory of tDCS using two anodes for bifrontal stimulation. A group of healthy subjects participated in two bifrontal tDCS sessions on two different days, one for real and the other for sham stimulation. They performed a working memory task and reported their affect immediately before and after each tDCS session. Relative to sham, real bifrontal stimulation did not induce significant adverse effects, reduced decrement in vigor-activity during the study session, and did not improve working memory. These preliminary findings suggest that bifrontal anodal stimulation is feasible and safe and may reduce task-related fatigue in healthy participants. Its effects on neuropsychiatric patients deserve further study.
Keywords: tDCS, non-invasive brain stimulation, cognitive function, working memory, affect
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive technique for brain stimulation. It modulates cortical excitability by passing a small direct current (1 ~ 2 mA) to the scalp [1,2]. Recent studies have reported that tDCS may improve mood and reduce craving for substance use in patients with addictive disorders [3-7]. Additionally, tDCS may enhance cognitive functions such as attention and working memory in healthy participants or patients with strokes, depression, or Parkinson’s disease [8-12]. Therefore, tDCS is a promising strategy for enhancing brain functions in patients with neuropsychiatric conditions, including addictive disorders.
Conventional tDCS studies use two large (e.g., 35 cm2) sponge pads as two electrodes (i.e., anode and cathode) to deliver direct current [1,2,13,14], with one “active” electrode over the cortical target and a “return” electrode on another scalp location or other body part (e.g., upper arm). Anode- or cathode-active electrodes are typically presumed to produce opposite effects on cortical excitability. At ~1 mA intensity and for a 5- to 20-minute duration, anodal stimulation increases, whereas cathodal stimulation decreases, cortical excitability as measured by transcranial magnetic stimulation (TMS) motor-evoked potentials [15-17]. Assuming these neurophysiological findings generalize to other brain regions and stimulation intensities, most tDCS studies to date seek to modulate brain function by increasing the excitability of cortical regions adjacent to the anode in one hemisphere.
While this approach holds significant potential, it may not be optimal for all neuropsychiatric conditions because behaviors relevant to these conditions may involve altered functional activities in both hemispheres. For example, nicotine-dependent smokers often experience tobacco withdrawal that includes craving for smoking, negative affect, and impaired cognitive control after abstinence from smoking [18,19]. Neuroimaging studies demonstrate that cognitive control, including craving inhibition, implicates the prefrontal cortex in both hemispheres [20-23]. Furthermore, a recent tDCS study showed that anodal stimulation of either the left or the right dorsolateral prefrontal cortex reduces cue-induced craving for smoking in nicotine-dependent smokers [4]. Thus, the testing of a bifrontal anodal stimulation system is warranted. In other words, it is possible that the efficacy of tDCS for modulating brain functions may be improved by using multiple anodes (relative to using one anode) to increase the excitability of multiple cortical regions in both hemispheres simultaneously. Based on this rationale, we designed a new electrode set for performing tDCS that uses one 35 cm2 sponge pad as a cathode and two high-definition (HD) electrodes [24] as two separate anodes. By placing the two anodes at scalp locations above the left and right dorsal lateral prefrontal cortex (i.e., AF3 and AF4, respectively, of 10/20 EEG system [25,26]) and the cathode at the occipital scalp between Oz and POz, this electrode set should provide extensive anodal stimulation to the prefrontal cortex in both hemispheres during tDCS [27]. We expect that tDCS using this innovative electrode design may increase the excitability and functional activities of the prefrontal cortex in both hemispheres simultaneously.
The specific aim of this study was to assess whether tDCS, using this novel electrode design, is safe and may enhance prefrontal cortical function in healthy participants. Stimulation was applied in two sessions: one for real and the other for sham stimulation, with assessments for adverse effects, mood, and working memory obtained from participants immediately before and after each stimulation session. We assessed the tolerability and safety of the new electrode design by comparing the reported adverse effects of real and sham stimulation. We predicted that this bifrontal tDCS would be well tolerated and real stimulation would improve mood and working memory relative to sham stimulation.
Materials and Methods
Participants
Potential participants were recruited from communities around Yale University through flyers and ads placed on Craigslist. All participants provided written informed consent approved by the Human Investigation Committee at the Yale School of Medicine. The inclusion criteria included good general physical health and ages between 18 and 60 years, inclusive. Thirty-eight participants were recruited. Among them, 31 (15 females) completed two tDCS sessions as scheduled. Their mean age was 27.2 years (standard deviation (SD) = 8.4; range: 18 to 49).
Procedure
Subsequent to baseline assessments, subjects participated in two stimulation sessions, one for real tDCS and the other for sham with a minimal interval between the two of 48 hours. The sequence of the two tDCS sessions was counterbalanced among participants. After arriving at the lab, participants completed the Profile of Mood States (POMS) [28], performed a computerized n-back task, received tDCS (either real or sham) during a resting condition, completed the tDCS Adverse Effects Questionnaire, performed the n-back task, and completed the POMS again.
tDCS
We used a 1 x 1 Low-Intensity DC Stimulator, Model 1224-B (Soterix Medical, New York, USA) to deliver stimulation. The new electrode design employed one 35 cm2 sponge pad as a cathode and two high-definition (HD) electrodes [24] as anodes. The size of each HD electrode was 2 x 2 cm2. For bilateral anodal stimulation, a 2 x 1 passive adaptor converted the anode line to two HD outputs. During set-up, the stimulator ensures equal current carried by the two anodes by checking the quality of scalp contact of each anode. Using multiple small HD electrodes facilitated the targeting of specific brain structures [14,27] and produced equal or less skin irritation relative to conventional sponge pads [29].
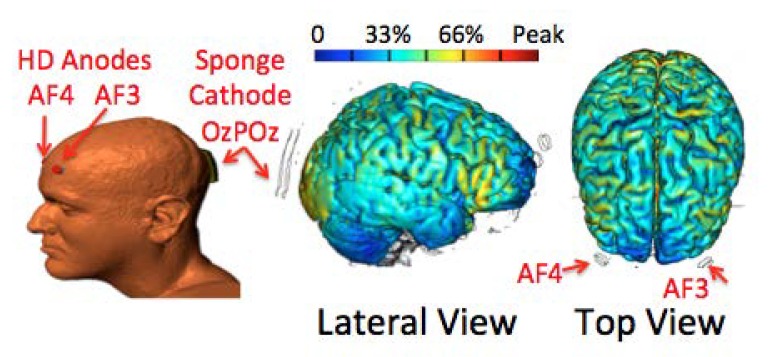
As described in a recent publication [27], high-resolution computer-based MRI-derived Finite Element modeling [27,30,31] was used to model the electric field generated by different electrode arrangements. With the arrangement of two HD electrodes placed at AF3 and AF4 of a 10/20 EEG system [25,26] and the cathode placed between Oz and POz, the modeled electric field covered the ventral and dorsal frontal and parietal cortices and the occipital cortex beneath the sponge cathode in both hemispheres [27] (Figure 1). Therefore, this electrode arrangement provided extensive anodal stimulation to the frontal and parietal cortices in both hemispheres during tDCS [27]. In the present study, this electrode placement was employed for real and sham tDCS. Real stimulation used a current of 2.0 mA for 20 min, with a current ramping up for the first 30 s to reduce skin sensations. Sham stimulation also lasted for 20 min with a current ramping up to 2.0 mA and back to 0 during the first 30 s, and then the stimulator would automatically turn off. A similar procedure has been used regularly to keep participants blind to the real and sham stimulation [4,32,33]. Therefore, this was a single-blind study.
Figure 1.
Electrode placements and modeled electric fields. The diagram of the head shows electrode locations (anodes: AF3 and AF4; cathode: between Oz and POz). Colors on the brain show electric fields generated by this electrode set. The color bar indicates the percentage of maximum strength of the electric current in the brain induced by the specified tDCS in an arbitrary unit. Abbreviations: HD: high-definition.
Measures
The POMS consists of 65 five-point mood-related items [28]. They were used to calculate scores for six subscales relating to tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment. For all subscales, except for vigor-activity, a higher score indicated a more negative mood state. This study calculated a score for total mood disturbance by adding the scores of all subscales except the vigor-activity subscale items. A tDCS Adverse Effect Questionnaire was used to assess tDCS adverse effects, including headache, neck pain, scalp pain, scalp irritation, tingling, skin redness, sleepiness, trouble concentrating, and acute mood change [34,35].
The n-back task consisted of 1- and 2-back conditions and used individual digits from 0 to 9 presented on the screen as stimuli. It used a block design with a 5 s instruction screen between blocks. During each task block, each digit was presented for 500 ms and the interstimulus interval (ISI) was 1000 ms. During the 1- and 2-back conditions, targets were repetitions of the digits presented one and two trials before, respectively. A total of 40 digits were presented in each block, and 20 percent of these digits were targets. Participants were instructed to press a button on a Mac laptop keyboard as soon as possible when they detected a target. The 1-back condition imposed little working-memory load, while the 2-back condition required both effortful attention and considerable working-memory. Parameters measuring task performance included reaction time (RT) and frequencies of omission errors and commission errors. Omission errors involved missed responses to targets, with the frequency equaling the number of missed responses divided by the total number of targets in the task. Commission errors involved incorrect responses to non-target trials, with the frequency equaling the number of incorrect responses divided by the number of total non-target trials in the task.
Data Analysis
Frequencies of tDCS-related adverse effects between the real and sham sessions were compared using SPSS Chi-square tests. The scores on POMS and RTs and error rates on the n-back task were analyzed separately using the SPSS general linear model (GLM) for repeated measures. These scores and performance parameters were dependent variables, and test sessions (real versus sham) and blocks (pre- versus post-tDCS) were within-subject variables. Statistical analyses were performed to assess whether dependent variables showed significant differences in change after real versus sham tDCS and/or interaction effects with respect to blocks and sessions. The statistical significance threshold was set at p < 0.05.
Results
Participants did not report any clinically significant adverse effects after either real or sham stimulation, and no participant quit the study due to adverse effects. Relative to sham stimulation, real stimulation was more likely to be associated with tingling (Pearson Chi-Square Value = 4.4; df = 1; p = 0.036, Cramer’s V = 0.28) (Table 1). No other between-condition measures of adverse effects differed at p < 0.05.
Table 1. tDCS adverse effects (%*) in real and sham stimulation sessions.
| Headache | Neck Pain | Scalp Pain | Scalp Irritation | Tingling | Skin Redness | Sleepiness | Trouble Concentrating | Mood Changes | |
| Real | 23.3 | 3.3 | 16.7 | 26.7 | 60.0 | 10.0 | 16.7 | 6.7 | 33.3 |
| Sham | 27.6 | 6.9 | 17.2 | 13.8 | 34.5 | 10.3 | 31.0 | 3.4 | 17.2 |
*percent of total participants in real or sham study session reporting any adverse effect
RTs on the n-back task showed a main effect of block. RTs reduced significantly post- relative to pre-tDCS at both 1-back (F(1, 30) = 9.4; p = 0.005, Partial Eta Squared = 0.24) and 2-back conditions (F(1, 30) = 8.7; p = 0.006, Partial Eta Squared = 0.23). RTs did not differ according to session (For 1-back: F(1, 30) = 1.3; p = 0.27, Partial Eta Squared = 0.04; For 2-back: F(1, 30) = 0.3; p = 0.56, Partial Eta Squared = 0.01), and no significant interactions between block and session were observed (For 1-back: F(1, 30) = 0.4; p = 0.51, Partial Eta Squared = 0.02; For 2-back: F(1, 30) = 1.1; p = 0.30, Partial Eta Squared = 0.04). Both omission and commission error frequencies did not show significant effects at either load condition.
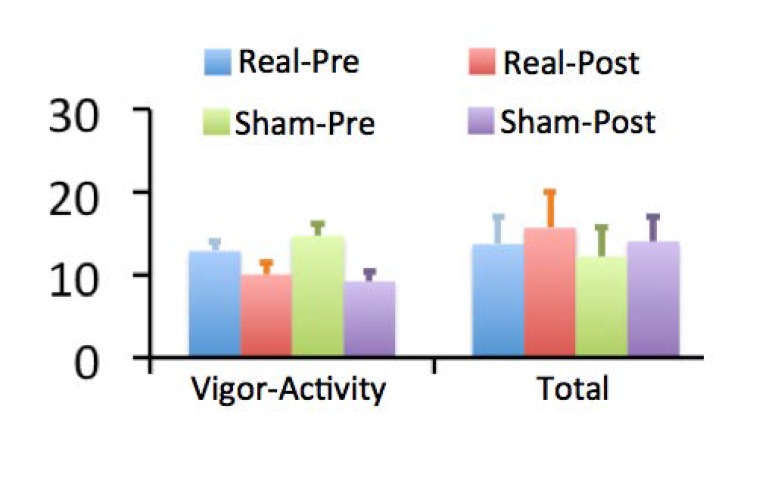
Figure 2 shows POMS scores. Participants showed a significant block effect on scores of vigor-activity subscale; i.e., reduced at the end relative to at the beginning of both tDCS sessions (F(1, 30) = 38.8; p < 0.001, Partial Eta Squared = 0.56). The scores on this subscale showed a significant session x block two-way interaction effect. Specifically, there was a greater reduction in vigor-activity during the sham relative to real session (F(1, 30) = 4.7; p = .038, Partial Eta Squared = 0.14). This subscale includes items relating to how lively, active, energetic, cheerful, alert, full of pep, carefree, and vigorous individuals rate themselves. A greater score on this subscale generally indicates that one feels more energetic, less fatigued, and in a better mood. Total mood-disturbance scores showed a significant block effect, with scores increased at the end relative to at the beginning of both tDCS sessions (F(1, 30) = 21.0; p < 0.001, Partial Eta Squared = 0.41). However, the scores did not show significant main effects of session or interaction effects between block and session (F(1, 30) = 0.8; p = 0.38, Partial Eta Squared = 0.03).
Figure 2.
Scores on the Profile of Mood State (POMS). Bar graphs show scores on the vigor-activity subscale and total scale of negative affect of POMS before and after tDCS during real and sham stimulation sessions. Error bars indicate standard errors of the mean. The vigor-activity subscale included items relating to how lively, active, energetic, cheerful, alert, full of pep, carefree, and vigorous individuals feel. The total score included all items except the vigor-activity subscale items.
Discussion
To our best knowledge, this is the first study to assess the feasibility of bilateral anodal stimulation of the prefrontal cortex using HD electrodes. The main findings were that: 1) real stimulation was tolerable and did not generate significant adverse effects; and 2) real stimulation relative to sham stimulation attenuated decreases in score on a vigor-activity subscale in participants during the study session. However, it did not improve performance on the n-back working-memory task.
Bifrontal Stimulation
For enhancing cognitive function including working memory, tDCS studies usually place the anode above the prefrontal cortex in one hemisphere and the cathode at the contralateral supra-orbital area or extracranial body part to avoid the potential negative effect of cathodal stimulation on brain regions [36-39]. More recently, several studies placed the cathode above the prefrontal cortex opposite to the anodal stimulation and named this electrode arrangement as bifrontal stimulation [40-42]. Because cathodal stimulation decreases while anodal stimulation increases cortical excitability [15-17], the bifrontal stimulation used in the current study is different from the bifrontal stimulation employed in these prior studies.
As mentioned in the introduction, there are at least three lines of evidence that indicate that the bilateral anodal stimulation of the prefrontal cortex might be more effective than unilateral stimulation in enhancing cognitive function and/or ameliorating psychiatric symptoms. The first is that many cognitive functions such as working memory and top-down control often involve the prefrontal cortex bilaterally, albeit in some cases to varying degrees. Functional magnetic resonance imaging (fMRI) studies regularly show task-related activity in the prefrontal cortex in both hemispheres while healthy participants perform cognitive tasks [43-45]. The second is that many psychiatric conditions show complex features that may involve brain regions in both hemispheres. For example, fMRI studies show that tobacco craving after overnight smoking abstinence is associated with increased activity in the left lateral prefrontal cortex [22,46], while impaired cognitive control is associated with increased activity in the right lateral prefrontal cortex [23,47]. The third is that anodal stimulation of either the left or the right prefrontal cortex enhances similar cognitive functions such as working memory and top-down attention control [4,36,48], suggesting that increasing excitability of the prefrontal cortex in both hemispheres simultaneously may enhance certain brain functions.
Tolerability
Participants in the current study did not report significant adverse effects induced by tDCS. A common adverse effect, and the only one to show a between-condition difference, was skin tingling. Two recent tDCS studies also used HD electrodes and found no significant adverse effects [13,49]. Therefore, the new electrode arrangement used in the current study appears safe and tolerable to participants.
Affect
The effects of “traditional” forms of tDCS on affect/emotion in healthy participants and patients have been assessed previously. Among healthy participants, tDCS reduced stress and negative affect induced by unpleasant pictures [42,50-53] and attenuated decrements in vigilance during a sustained attention task [54]. Among patients, tDCS improved symptoms in patients with depression [10,40,41], reduced intensity of distress related to tinnitus [55], and alleviated chronic neuropathic pain [56], although not all studies have reported significant effects of tDCS on affect and emotion [50,57,58]. The current study showed a promising effect of attenuating decrements in vigor-activity after real relative to sham stimulation. This finding is in line with the finding of attenuated decrements in performance on a vigilance task in healthy participants after unilateral anodal stimulation [54] and supports our prediction that bilateral anodal stimulation might reduce task-related fatigue as indexed by scores on the vigor-activity subscale. This finding warrants additional investigation in patients that might be particularly sensitive to stress-related fatigue, including individuals with addictions.
Working Memory
Multiple studies have assessed the effects of anodal stimulation of the prefrontal cortex on working memory. Findings from these studies are not fully consistent. Several studies report improved performance on working-memory tasks after real relative to sham stimulation [9,38,39,59], whereas several studies do not find significant effects of real stimulation [37,57,60]. Furthermore, among those reporting improved performance, some studies report increased accuracy [8,32,61] while others report reduced RTs [59,62]. The reasons for the different findings from different studies are not clear and may involve differences in working-memory tasks, working-memory capacities of participants, electrode arrangements, and stimulation parameters used in different studies [36,37,63,64]. The present study did not find significant improvement on task performance after real relative to sham stimulation.
Limitation
One limitation is that this study did not compare the effect of unilateral and bilateral anodal stimulation. Therefore, it did not provide data indicating whether the bifrontal stimulation is more or less effective in influencing brain function than unilateral stimulation. The negative findings of real stimulation on n-back task performance did not support our prediction that bilateral anodal stimulation of the prefrontal cortex would enhance working-memory performance. An additional limitation is the relatively small sample size, which may have limited power for detecting between-condition effects.
In summary, participants did not report significant adverse effects after bilateral tDCS using two anodes. They reported attenuated decrements in self-reported vigor-activity during the study session after real relative to sham stimulation. These findings support the feasibility of bilateral anodal stimulation and indicate that this novel electrode arrangement may reduce fatigue as indexed by a vigor-activity subscale in healthy participants. Its potential for helping individuals with substance-use or other neuropsychiatric disorders deserves further study.
Acknowledgments
This study is supported by NIH grant K01DA027750, P20 DA027844, the Wallace H Coulter Foundation and the National Center for Responsible Gaming. We thank Dr. Stephanie O’Malley for her advice about study design.
Abbreviations
- EEG
electroencephalography
- fMRI
functional magnetic resonance imaging
- GLM
general linear model
- HD
high-definition
- POMS
Profile of Mood States
- RT
reaction time
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
- SD
standard deviation
Author contributions
Xu contributed to all aspects of the study, including study design, data collection and analysis, and manuscript preparation. Healy contributed to data collection. Truong, Datta, Bikson, and Potenza contributed to manuscript preparation.
Conflict of interest
Drs. Xu and Truong and Mr. Healy report no conflicts of interest. Drs. Bikson and Datta have equity in Soterix Medical Inc., a company developing tDCS-related equipment. Dr. Potenza has consulted for and advised Somaxon, Boehringer Ingelheim, Lundbeck, Ironwood, Shire, INSYS and RiverMend Health and has received research support from Mohegan Sun Casino, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, Pfizer, and Psyadon pharmaceuticals.
References
- Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6(2):188–191. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A. et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Fraser PE, Rosen AC. Transcranial direct current stimulation and behavioral models of smoking addiction. Front Psychiatry. 2012;3:79. doi: 10.3389/fpsyt.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A. et al. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. 2008;69(1):32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2472–2480. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G. Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci. 2014;8:661. doi: 10.3389/fnhum.2014.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Fregni F, Brody AL, Rahman AS. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry. 2013;4:112. doi: 10.3389/fpsyt.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E. et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166(1):23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4(2):84–89. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200(1):52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Goncalves OF. Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS One. 2011;6(9):e24140. doi: 10.1371/journal.pone.0024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012;5(3):231–241. doi: 10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V. et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13(2):112–120. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(4):201-7, 207 e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011;29(6):463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL. et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58(2):143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, London ED. et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–1661. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL. et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB FB, Chuzi S, Pachas G. et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS. et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16(4):654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, McCabe E, Jones J, Clancy L, Garavan H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage. 2011;56(4):2258–2275. doi: 10.1016/j.neuroimage.2011.03.054. [DOI] [PubMed] [Google Scholar]
- Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A. et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. 2010;190(2):188–197. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Truong DQ, Datta A, Xu J, Bikson M. Prefrontal cortex transcranial direct current stimulation via a combined high definition and conventional electrode montage: a FEM modeling studying. Engineering in Medicine and Biology Society (EMBC), 34th Annual International Conference of the IEEE; 2012 Aug 28-Sep 1; San Diego, CA. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EDITS manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Petree L, Bullard L, Jung RE, Paulson K, van der Merwe A, Vakhtin A. Alternative electrode methodology for the administration of transcranial direct current stimulation (TDCS). 41st Annual Society For Neuroscience Annual Meeting; 2011 Nov 12-16; Washington, DC. [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35(3):1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Sadleir RJ, Vannorsdall TD, Schretlen DJ, Gordon B. Transcranial direct current stimulation (tDCS) in a realistic head model. Neuroimage. 2010;51(4):1310–1318. doi: 10.1016/j.neuroimage.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A. et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249(1):31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A. et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92(1-3):55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- Jeon SY, Han SJ. Improvement of the working memory and naming by transcranial direct current stimulation. Ann Rehabil Med. 2012;36(5):585–595. doi: 10.5535/arm.2012.36.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MF, Nitsche MA. Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci. 2012;43(3):192–199. doi: 10.1177/1550059412444975. [DOI] [PubMed] [Google Scholar]
- Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol Psychiatry. 2013;73(7):646–651. doi: 10.1016/j.biopsych.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jancke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 2011;12:2. doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A. et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70(4):383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M. et al. Transcranial direct current stimulation in severe, drug-resistant major depression. J Affect Disord. 2009;118(1-3):215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Zanao TA, Ferrucci R, Priori A, Valiengo L, de Oliveira JF. et al. Bifrontal tDCS prevents implicit learning acquisition in antidepressant-free patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:146–150. doi: 10.1016/j.pnpbp.2012.12.019. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 2003;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp. 2006;27(11):837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y. et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozink RV, Lutz AM, Rose JE, Froeliger B, McClernon FJ. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addict Biol. 2010;15(4):480–490. doi: 10.1111/j.1369-1600.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiron O, Lavidor M. Unilateral prefrontal direct current stimulation effects are modulated by working memory load and gender. Brain Stimul. 2013;6(3):440–490. doi: 10.1016/j.brs.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A. et al. Focal modulation of the primary motor cortex in fibromyalgia using 4x1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain. 2013;14(4):371–383. doi: 10.1016/j.jpain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Koschack J, Pohlers H, Hullemann S, Paulus W, Happe S. Effects of frontal transcranial direct current stimulation on emotional state and processing in healthy humans. Front Psychiatry. 2012;3:58. doi: 10.3389/fpsyt.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci Lett. 2012;512(1):12–16. doi: 10.1016/j.neulet.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Pena-Gomez C, Vidal-Pineiro D, Clemente IC, Pascual-Leone A, Bartres-Faz D. Down-regulation of negative emotional processing by transcranial direct current stimulation: effects of personality characteristics. PLoS One. 2011;6(7):e22812. doi: 10.1371/journal.pone.0022812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) Neuropsychologia. 2009;47(1):212–217. doi: 10.1016/j.neuropsychologia.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Nelson JT, McKinley RA, Golob EJ, Warm JS, Parasuraman R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS) Neuroimage. 2014;85 Pt 3:909–917. doi: 10.1016/j.neuroimage.2012.11.061. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S. EEG driven tDCS versus bifrontal tDCS for tinnitus. Front Psychiatry. 2012;3:84. doi: 10.3389/fpsyt.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arul-Anandam AP, Loo C, Martin D, Mitchell PB. Chronic neuropathic pain alleviation after transcranial direct current stimulation to the dorsolateral prefrontal cortex. Brain Stimul. 2009;2(3):149–151. doi: 10.1016/j.brs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Tadini L, El-Nazer R, Brunoni AR, Williams J, Carvas M, Boggio P. et al. Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. J ECT. 2011;27(2):134–140. doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- Plazier M, Joos K, Vanneste S, Ost J, De Ridder D. Bifrontal and bioccipital transcranial direct current stimulation (tDCS) does not induce mood changes in healthy volunteers: a placebo controlled study. Brain Stimul. 2012;5(4):454–461. doi: 10.1016/j.brs.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Mulquiney PG, Hoy KE, Daskalakis ZJ, Fitzgerald PB. Improving working memory: exploring the effect of transcranial random noise stimulation and transcranial direct current stimulation on the dorsolateral prefrontal cortex. Clin Neurophysiol. 2011;122(12):2384–2389. doi: 10.1016/j.clinph.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Mylius V, Jung M, Menzler K, Haag A, Khader PH, Oertel WH. et al. Effects of transcranial direct current stimulation on pain perception and working memory. Eur J Pain. 2012;16(7):974–982. doi: 10.1002/j.1532-2149.2011.00105.x. [DOI] [PubMed] [Google Scholar]
- Oliveira JF, Zanao TA, Valiengo L, Lotufo PA, Bensenor IM, Fregni F. et al. Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neurosci Lett. 2013;537:60–64. doi: 10.1016/j.neulet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Teo F, Hoy KE, Daskalakis ZJ, Fitzgerald PB. Investigating the role of current strength in tDCS modulation of working memory performance in healthy controls. Front Psychiatry. 2011;2:45. doi: 10.3389/fpsyt.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Berryhill ME. Parietal contributions to visual working memory depend on task difficulty. Front Psychiatry. 2012;3:81. doi: 10.3389/fpsyt.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521(2):148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]