Abstract
Over the past few decades, there has been a rise in the non-medical use of prescription opioids, which has now reached epidemic proportions in the United States. In some cases, this non-medical use progresses to prescription opioid use disorder, heroin use, injection, and inhalation drug use, all of which may have further devastating consequences. The purpose of this review article is to discuss the epidemiology of the non-medical use of prescription opioids; discuss the potential progression to subsequent prescription opioid use disorder; review the state and national efforts in development to address addiction and diversion in the United States; discuss treatment options; and, lastly, to evaluate the impact of the related stigma to the development of opioid use disorder. Many unanswered questions remain, and we will explore future possibilities in how the medical community can play a role in curbing this epidemic.
Keywords: opioid use disorder, prescription opioid use disorder, opioid agonist treatment, substance use disorder
Introduction
Over the past 2½ decades, the use of prescription opioid (PO) pain medication to treat chronic, non-cancer pain has increased dramatically. Since 1990, PO prescribing has nearly tripled globally; however, this is driven by high socio-economic countries such as the United States, which is deemed the highest rank in per capita consumption of POs in the last decade [1]. Coincidentally, opioid misuse, overdose deaths, and the subsequent resurgence of heroin use among those with opioid addiction have reached epidemic proportions in the United States [2]. The term “misuse” was defined by an expert panel consisting of members from the American Society of Addiction Medicine, the American Academy of Pain Medicine, and the American Pain Society as “use of a medication (for a medical purpose) other than as directed or indicated, whether willful or unintentional, and whether harm results or not.” The “non-medical use of POs” is a narrower term and defined as use of medication that was not prescribed to the individual or use only for the experience or feeling it causes [3]. Throughout the remainder of this review, we will use the narrower term: non-medical use of POs.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) refers to any substance use disorder as the recurrent use of alcohol and/or illicit substances that causes clinically and functionally significant impairment in health problems, disability, and failure to meet major responsibilities at work, school, or home [4]. It is based on evidence of impaired control, craving, tolerance (increased amount of substance over time to produce desired effect), physiological dependence (removal of substance produces withdrawal), social impairment, and risky use. It is further characterized as mild (2-3 criteria), moderate (4-5 criteria), or severe (6+ criteria) within a 12-month period. Specifically for PO use disorders, the symptomatology of tolerance and withdrawal are an exception to the criteria for diagnosis, as they will occur in anyone taking medically prescribed opioids for a long enough period of time, since physiologic dependence is inevitable. Physiologic dependence does not constitute addiction, while loss of control does.
An additional rising concern is diversion of POs. As a legal concept, diversion refers to the distribution of a drug into the illicit marketplace, and in terms of illicit substances, it is codified in the Federal Controlled Substances Act that “prescribing of a controlled prescription drug is legal only if it is for a legitimate medical purpose, and performed within the course of usual professional practice” [5]. With the increases in availability of POs due to prescription of POs for chronic non-cancer pain, more POs are accessible to individuals who may use them in a non-medical way.
In this review, we will review the national scope of non-medical use of POs, treatment options, and domestic and local policy to address addiction, diversion, and implications.
National Scope of Non-Medical Use of Prescription Opioids
Over the past few decades, the treatment of pain has received increasing attention. Potential explanations for this increased attention was the “pain as the 5th vital sign” campaign and direct-to-consumer advertising from pharmaceutical companies [6]. There has been substantial variation in the opioid prescribing patterns of primary care providers. Several factors contribute to this variation, including heightened awareness of the under-treatment of pain, reports of non-medical use of certain POs over others, and introduction of pain management guidelines and new analgesics. In addition, physician training of how to appropriately manage chronic pain, issues of documentation, and scrutinizing regulatory effects are all shedding light on prescriber variation [7]. From medical school, residency programs, and continuing medical education courses, the fundamentals of both pain management and addiction screening and treatment are dramatically at a deficit. In 2011, an expert panel convened that developed recommendations focusing on improving substance use training in family medicine and primary care internal medicine residency programs. The overall recommendation centered around integrating substance use competencies, prioritizing substance use teaching the same as for that of other chronic diseases, enhancing faculty development, creating addiction medicine programs in academic medical centers, and integrating substance use screening and management as part of routine primary care [8].
According to results from the 2013 National Survey on Drug Use and Health (NSDUH), which is an annual self-report survey of the civilian, non-institutionalized population in the United States, nearly 25 million persons aged 12 years or older reported use of any illicit drug within the past month (Figure 1) [9]. Approximately 1.7 percent of those aged 12 years or older have reported non-medical use of a PO in the past month. Survey respondents reported the majority of POs obtained for non-medical use are free from a friend or relative (53 percent), taken or bought from a friend or relative (15 percent), prescribed by one physician (21 percent), prescribed by more than one physician (3 percent), bought from a drug dealer or stranger (4.6 percent), purchased on the Internet (<0.5 percent), and via forgery of stolen prescription pads (4 percent) (Figure 2) [9]. The NSDUH employs sampling methodology to develop a nationally representative sample and uses computerized self-administration methods (ACASI) to minimize under-reporting.
Figure 1.
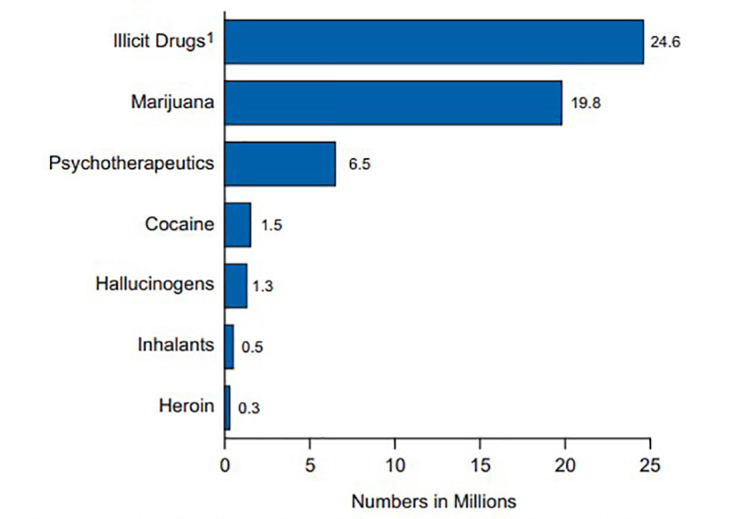
Past Month Illicit Drug Use among Persons Aged 12 or Older: 2013. Illicit Drugs include marijuana/hashish, cocaine (including crack), heroin, hallucinogens, inhalants, or prescription-type psychotherapeutics used nonmedically. Image courtesy of SAMHSA.
Figure 2.
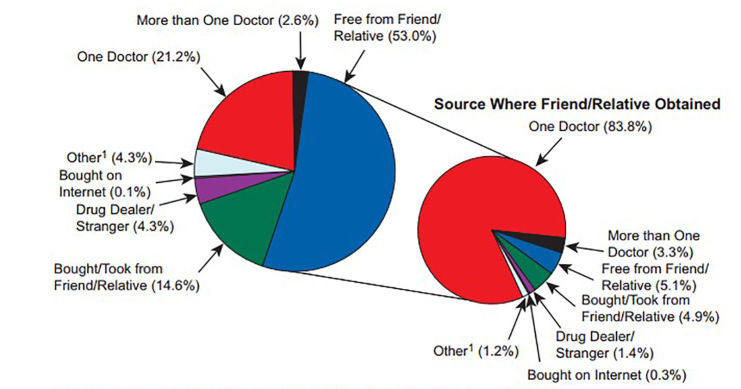
Source Where Pain Relievers Were Obtained for Most Recent Nonmedical Use among Past Year Users Aged 12 or Older: 2012-2013. 1Other includes the sources "Wrote Fake Prescription," "Stole from Doctor's Office/Clinic/Hospital/ Pharmacy," and "Some Other Way." Note: The percentages do not add to 100 percent due to rounding. Image courtesy of SAMHSA.
In many cases, non-medical use of POs manifests with concurrent use of other illicit substances. One study found that for both genders with non-medical use of POs, there was an association with concurrent alcohol, marijuana, hallucinogen, cocaine, non-medical stimulant, and sedative use [10]. Women with non-medical use of POs had a higher prevalence of serious mental illness, illicit use beginning at age 24 or older, and cigarette smoking. It is important to note that these studies are all cross-sectional, so trajectories of substance use cannot be inferred.
Mental illness often co-occurs in individuals with non-medical use of POs. One study found that those with past year non-medical use of POs were more likely to have panic, depressive, and agoraphobic symptoms [11]. In addition, they were more likely to report fair to poor health, misuse of another class of prescription medication, illicit use of heroin, and substance use initiation before the age of 13.
In a recent study by Manubay et al., 162 patients (120 men and 42 women) with PO use disorder and chronic pain were evaluated based on sex differences [12]. It was found that men reported more aberrant behaviors such as abuse of alcohol or other illicit drugs concurrently, in addition to history of more arrests. Women manifested more symptoms of depressed mood, decreased ambulation, and impaired capacity for interpersonal relations.
Over the past 11 years, there has been a 300 percent increase in the sales rate of POs in the United States, which results in more POs available for potential diversion. Diversion is concerning for many reasons, including its contribution to overdose [13]. Studies assessing trends in PO use have confirmed the relationship between increasing medical use of opioids with increasing fatalities (Figure 3). These overdoses are due to POs, heroin, or often opioids plus other substances, such as benzodiazepines. Deaths from POs increased fivefold between 1999 and 2010 for women and 3.6 times for men [14]. In 2013, of the 43,982 drug overdose deaths in the United States, 22,767 (51.8 percent) were related to prescription medications: 16,235 (71.3 percent) involved POs, and 6,973 (30.6 percent) involved benzodiazepines [14].
Figure 3.
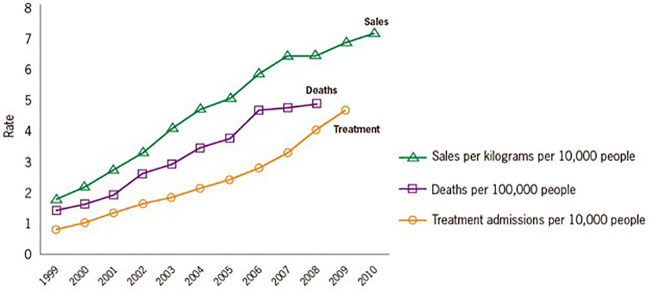
Prescription Opioid Sales, Deaths and Substance Abuse Treatment Admissions. Image courtesy of SAMHSA.
Treatment of Prescription Opioid Use Disorder
Addressing non-medical PO use and concerns over diversion requires a thorough and comprehensive plan that is concrete and well-communicated with the patient. Approaches to treatment for PO use disorder include both pharmacologic and non-pharmacologic treatment models. Treatment with opioid agonist therapy (OAT) for PO use disorder is more effective than non-pharmacologic approaches or detoxification strategies [15].
OAT with either methadone or buprenorphine is the most effective treatment and is associated with individual and societal benefits [16]. Both methadone and buprenorphine help stabilize neuronal systems, provide narcotic blockade, and produce improved outcomes when given as maintenance therapy over a prolonged period of time [17]. Methadone is a long-acting, mu opioid full agonist and NMDA receptor antagonist, which inhibits ascending pain pathways, preventing withdrawal symptoms for more than 24 hours or longer; reduces craving for opioids; and, by maintaining high levels of opioid tolerance, reduces the euphoric effects of subsequent illicit opioid use [17]. Methadone for opioid use disorder is administered once daily only from federally licensed outpatient dispensaries and is incorporated with clinician counseling and group therapies. Overall, methadone is an effective option for the treatment of opioid use disorder in terms of treatment retention and reduction of illicit opioid use [18].
Another effective form of OAT is buprenorphine, which is a partial mu opioid agonist. Buprenorphine has a high affinity for the mu opioid receptor, which prevents other opioids from binding and, ultimately, reduces cravings [19]. It is usually co-administered with the opioid antagonist naloxone, which when taken via sublingual route is not bioavailable but if crushed and injected would cause abrupt withdrawal. It is administered in the form of sublingual tablets or films, or buccal films, and can be prescribed by a certified physician who has registered with the Center for Substance Abuse Treatment (CSAT) of the Substance Abuse and Mental Health Services Administration (SAMHSA) and with the Drug Enforcement Administration (DEA) in the United States. Buprenorphine has been proven to have substantial efficacy. In several well-designed trials, patients maintained on buprenorphine were more likely to be retained in treatment, had decreased cravings for illicit opioids, and had fewer urine toxicology screens positive for illicit opioids than placebo [20]. Of note, buprenorphine-naloxone maintenance treatment has improved outcomes than that of a 12-week buprenorphine-naloxone taper, which has a 90 percent relapse rate [21]. Buprenorphine-naloxone maintenance therapy is more effective in those with exclusively PO use disorders than in those with exclusively heroin use [22].
The third approved medication for opioid use disorder is naltrexone, which is a high-affinity opioid receptor antagonist that produces potent blockade of the effects of opioids [23]. It is prescribed in an oral formulation taken daily or an extended-release intramuscular formulation given monthly. It is meant for relapse prevention and is generally given to those who are highly motivated or legally bound to adhere to treatment. There has been a lack of efficacy with oral naltrexone, due to low rates of retention and adherence, which has led to extended release formulations including implants and depot injections [17].
In addition to the three medications approved for PO and opioid use disorders, adjunctive counseling and group therapy are vital to substance use treatment as a whole. Examples of these standardized approaches include: 1) motivational interviewing, which is a directive, patient-centered approach that aims to change unhealthy behaviors via enhancing intrinsic motivation to change by exploring and resolving ambivalence [24]; 2) cognitive behavioral therapy (CBT) which is a structured, goal directed, problem-focused, and time-limited (often 10 to 20 sessions) therapy in which patients learn how their thoughts contribute to symptoms of their disorder and how to modify these thoughts; and 3) intensive outpatient programs that constitute a higher level of care with participation in group therapy and individual counseling on a more frequent, weekly basis. These standardized counseling strategies are often bundled into comprehensive OAT programs. While counseling and therapy remain a key component to substance use treatment, the frequency with which they occur may be adjusted. One study found that extended thrice weekly counseling in an outpatient buprenorphine clinic was no more effective than brief weekly counseling in enhancing adherence [25].
Educating providers and the public about overdose prevention is crucial. Treatment of PO use disorder is an essential component to overdose prevention. However, community-based and public health organizations have developed Overdose Education and Naloxone Distribution (OEND) programs to prevent opioid overdose fatalities. Naloxone, which is given intramuscularly and intravenously or off-label via the intranasal or inhalation route, antagonizes the effects of opioids by displacing opioid agonists from their receptors in the central nervous system, thus reversing potential respiratory depression [26]. Current and existing research suggest training those at risk for overdose in OEND is feasible and effective in preventing mortality from overdose [26].
Domestic and Local Policy
Introduction of state and federal legislation and local policy to address the PO epidemic has increased over the past decade and a half. Some measures that have been introduced include prescription drug monitoring programs (PMP) and implementation of controlled substance use agreements in clinical settings.
PMPs are statewide electronic databases that collect and distribute data on all controlled substances and are regulated at the state level. Interstate data exchange and their analysis systems are, however, federally funded by the Bureau of Justice [27]. New initiatives are being pursued by the National Association of Boards of Pharmacy and Alliances of States with Prescription Monitoring Programs, in order to minimize the heterogeneity among data reporting by each state.
To date, 49 states have enacted PMPs, which serve to mitigate and detect aberrant prescription medication-filling behavior. The U.S. Office of National Drug Control Policy (ONDCP) has recommended evaluating “current databases that measure the extent of prescription drug use, misuse, and toxicity” [28].
In 2011, the ONDCP issued a response to the growing problem of non-medical use of POs. It outlined four different, mandatory domains: increased patient and clinician education, increased prescription drug tracking and monitoring, proper medication disposal, and reductions in illegitimate prescription abuse [28]. Most specifically, this response highlighted the mandatory usage of PMPs. However, the evidence for PMPs decreasing opioid overdose mortality is equivocal. A 2011 analysis of PO-induced deaths ranging from all DEA schedules found no difference in opioid overdose mortality between states with and without PMPs [29]. Thus, PMPs require intense scrutiny and homogenization in order to function and have the desired outcome.
Controlled substance treatment agreements are another measure often implemented locally within clinical systems to limit unsanctioned dose escalations and prevent multiple prescribers to a single individual. A systematic review on the efficacy of treatment agreements and urine drug screening to reduce non-medical PO use in chronic pain patients found that in four studies with comparison groups, non-medical use was modestly reduced (7 percent to 23 percent) after treatment agreements with or without urine drug testing [30]. In seven other studies, the proportion of patients with opioid misuse after treatment agreements, urine drug testing, or both varied widely (3 pecent to 43 percent), further demonstrating the lack of evidence to support the effectiveness of opioid treatment agreements and urine drug testing in reducing non-medical PO use by patients with chronic pain [13].
Potential future measures being considered are: 1) limits on physicians prescribing POs, 2) requirements to complete Continuing Medical Education courses in opioid prescribing, and 3) electronic prescribing of all controlled medications and POs, thus eliminating the need for actual prescription pads and reducing the possibility of forgery.
Implications and Future Directions
In 2012, more than 23 million Americans needed specialized addiction treatment; however, only 2.5 million (about 11 percent) actually received treatment [9]. The reasons for this gap are innumerable and primarily centered on addiction-related stigma, lack of education related to addiction in the health care workforce, and limited access to specialized care vis-à-vis lack of state Medicaid-funded programs nationally. Increased clinician awareness is essential in helping reduce inappropriate PO prescribing while concurrently improving referral and access to evidence-based treatment for patients who may develop PO use disorder.
As a medical community, it is vital that we recognize at-risk PO use, perform risk assessment prior to prescribing POs and frequently re-evaluate treatment plans, utilize risk reduction strategies, including use of PMPs, and engage in inter-professional collaboration, especially when caring for patients at heightened risk.
Providers should screen all patients who are being prescribed opioids for psychiatric comorbidities and substance use and identify those at high risk for non-medical use. In 2009, an expert panel from the American Academy of Pain Medicine and American Pain Society concluded that before initiating chronic opioid therapy, clinicians should conduct a thorough clinical history, physical examination, and appropriate testing, including an assessment of risk of substance abuse, misuse, or addiction [8,31]. In addition, a comprehensive benefit-to-harm evaluation, weighing the potential positive effects of opioids on pain and function against potential risks, should be performed and documented continuously during the treatment period. Prior to initiation of chronic opioid therapy, it is invaluable and necessary to risk-stratify all patients based on their risk factors for developing addictions, such as genetic heritability, previous substance use disorders, family history, and concurrent psychiatric comorbidities, among others. While there are several different screening tools such as the Opioid Risk Tool (ORT) and Current Opioid Misuse Measure (COMM), a lack of consensus remains on one validated tool to implement for screening for risk prior to initiation of opioid treatment [31,32].
Risk management and harm reduction strategies are used for monitoring and controlling non-medical PO use. Constant reassessment of treatment plans and employment of many different measures to monitor adherence to treatment such as urine drug screenings, pill counts, signed treatment agreements, and prescription of small frequent quantities should be employed on an ongoing basis [33]. Public health interventions to achieve a balance between safeguarding legitimate access to safe treatment of chronic pain while also reducing non-medical use of POs and other controlled substances are vital. Routine use of PMPs, interdisciplinary communication among all health care providers, and discussion about overdose prevention and harm reduction are essential to safe opioid prescribing. Overdose deaths and emergency department visits related to POs either alone or in combination with other controlled medications or illicit substances continue to be unacceptably high, and targeted efforts are needed to reduce the number of fatalities [34]. Specifically, primary care, pediatric, and emergency medicine providers are on the frontlines of the workforce who interact with patients with non-medical use of POs and may play a vital role addressing this epidemic. Providers need to risk assess all of their patients on each visit for potential aberrancy, non-medical use of controlled substances, other illicit substance use, and adherence to all treatment regimens, both medical and psychiatric. Checking state PMPs before long-term prescribing of controlled substances should be a standard of care.
The increasing prevalence of PO and opioid use disorders, in addition to diversion, make it challenging to provide appropriate treatment for pain, both chronic and acute. Utilizing the tools in our arsenal, such as PMPs, risk stratification, and recognition and treatment of psychiatric illness, as well as developing the trust and rapport with patients is essential in reducing non-medical use of POs while also providing patients with appropriate treatment. The need to strike a balance between adequate treatments of pain in the background of safe and effective prescribing of opioids is fundamental. Under-treatment of pain is a potential consequence of regulation, and physician education regarding pain management need not focus on regulatory scrutiny nor fears of diversion, rather achieving a proper balance between safe usages and attaining functional goals [35].
Conclusions
Non-medical use of PO has reached epidemic proportions in this country. This has been fueled by the medical community’s over-reliance on the use of POs to treat both acute and chronic pain. In addition, lack of formal curricula in pain management and addiction during all levels of medical training also plays a role. Of concern, non-medical use of POs can lead to the development of a PO use disorder, progression to the use of heroin and other drugs, and even death. Local and national policies have been put in place in an attempt to mitigate the sheer number of POs that may be available for illicit use and diversion. As a medical community, we need to place more emphasis on evidence-based treatment of pain in general medical settings, screening for opioid misuse, referring patients who do meet criteria for opioid use disorder for opioid agonist treatment or other specialized treatment, performing risk assessment on all patients being prescribed POs, utilizing evidence-based risk reduction strategies, and collaborating with other health care professionals to care for high-risk patients. Lastly, improved education regarding screening for and treatment of nonmedical PO use and PO use disorder is an initial step to addressing the treatment gap that has been noted regarding non-medical use of PO.
Abbreviations
- PO
prescription opioid
- PMP
Prescription Drug Monitoring Program
- OAT
Opioid Agonist Treatment
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- NSDUH
National Survey on Drug Use and Health
- CSAT
Center for Substance Abuse Treatment
- SAMHSA
Substance Abuse and Mental Health Services Administration
- DEA
Drug Enforcement Administration
- CBT
cognitive behavioral therapy
- OEND
Overdose Education and Naloxone Distribution
- ONDCP
Office of National Drug Control Policy
- ORT
Opioid Risk Tool
- COMM
Current Opioid Misuse Measure
References
- Imtiaz S, Shield KD, Fischer B, Rehm J. Harms of prescription opioid use in the United States. Subst Abuse Treat Prev Policy. 2014;9:43. doi: 10.1186/1747-597X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouse AB, Compton P. The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother. 2015;29(2):102–114. doi: 10.3109/15360288.2015.1037521. [DOI] [PubMed] [Google Scholar]
- Katz NP, Adams EH, Chilcoat H, Colucci RD, Comer SD, Goliber P. et al. Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007;23(8):648–660. doi: 10.1097/AJP.0b013e318125c5e8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Gilson AM, Joranson DE. U.S. policies relevant to the prescribing of opioid analgesics for the treatment of pain in patients with addictive disease. Clin J Pain. 2002;18(4 Suppl):S91–S98. doi: 10.1097/00002508-200207001-00011. [DOI] [PubMed] [Google Scholar]
- Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by u.s. primary care physicians from 1992 to 2001. J Pain. 2006;7(4):225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- O’Connor PG, Nyquist JG, McLellan AT. Integrating addiction medicine into graduate medical education in primary care: the time has come. Ann Intern Med. 2011;154(1):56–59. doi: 10.7326/0003-4819-154-1-201101040-00008. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: SAMHSA; 2014. [Google Scholar]
- Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE. Gender and non-medical use of prescription opioids: results from a national US survey. Addiction. 2008;103(2):258–268. doi: 10.1111/j.1360-0443.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94(1-3):38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Manubay J, Davidson J, Vosburg S, Jones J, Comer S, Sullivan M. Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J Addict Med. 2015;9(1):46–52. doi: 10.1097/ADM.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17(2):E119–E128. [PubMed] [Google Scholar]
- CDC. Vital signs: overdoses of prescription opioid pain relievers and other drugs among women--United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62(26):537–542. [PMC free article] [PubMed] [Google Scholar]
- Clausen T. Coherent long-term treatment approaches–superior in the treatment of opioid dependence. Addiction. 2015;110(6):1006–1007. doi: 10.1111/add.12922. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Sys Rev. 2011;(8):CD004145. doi: 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Fiellin DA. Current and potential pharmacological treatment options for maintenance therapy in opioid-dependent individuals. Drugs. 2012;72(2):217–228. doi: 10.2165/11597520-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Sys Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski M, Schottenfeld RS. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse. 2002;28(2):231–241. doi: 10.1081/ada-120002972. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K. et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O’Connor PJ. Primary care–based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947–1954. doi: 10.1001/jamainternmed.2014.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PJ. et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, Fiellin DA. Current and potential pharmacological treatment options for maintenance therapy in opioid-dependent individuals. Drugs. 2012;72(2):217–228. doi: 10.2165/11597520-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll K. Motivational interviewing for substance use disorder. UpToDate [Internet] 2015. Available from: http://www.uptodate.com/contents/motivational-interviewing-for-substance-use-disorder.
- Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG. et al. Counseling plus buprenorphine–naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Subst Abus. 2015;36(2):240–253. doi: 10.1080/08897077.2015.1010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugelmann HM, Perrone J. Can prescription drug monitoring programs help limit opioid abuse? JAMA. 2011;306(2):258–259. doi: 10.1001/jama.2011.1712. [DOI] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. Washington, DC: Office of National Drug Control Policy; 2011. [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Desai HA. Prescription Drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- Starrels JL, Becker WC, Alford DP, Kapoor A, William AR, Turner BJ. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712–720. doi: 10.7326/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- Portenoy RK. In: Lowinson and Ruiz’s Substance abuse: a comprehensive textbook. 5th ed. Ruiz P, Strain E, editors. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. Acute and chronic pain; pp. 695–720. [Google Scholar]
- Dwyer K, Walley AY, Langlois BK, Mitchell PM, Nelson KP, Cromwell J. et al. Opioid education and nasal naloxone rescue kits in the emergency department. West J Emerg Med. 2015;16(3):381–384. doi: 10.5811/westjem.2015.2.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann DE, Tarzian AJ. Achieving the right balance in oversight of physician opioid prescribing for pain: the role of state medical boards. J Law Med Ethics. 2003;31(1):21–40. doi: 10.1111/j.1748-720x.2003.tb00057.x. [DOI] [PubMed] [Google Scholar]


