Abstract
Smoking and obesity represent the largest challenges to public health. There is an established inverse relationship between body mass index (BMI) and smoking, but this relationship becomes more complicated among obese smokers. Smokers with higher BMI consume more cigarettes per day and may be more nicotine-dependent than lean smokers. Rates of obesity are lower among smokers than non-smokers, indicating that chronic exposure to tobacco smoke may prevent excess weight gain in people who would otherwise become obese. Furthermore, obese smokers may be more sensitive to the weight-suppressive and reinforcing effects of nicotine. Consequently, obese smokers may respond differently to reduction in the nicotine content of cigarettes, a tobacco control policy being considered both in the Unites States and abroad. Here, we review the interrelationship between nicotine and obesity in the context of a potential nicotine reduction policy. We discuss the implications of nicotine-induced body weight suppression in obese smokers, as well as the possibility that obesity might increase susceptibility to smoking and nicotine dependence.
Keywords: obesity, nicotine, tobacco, insulin
Introduction
Tobacco use, primarily through cigarette smoking, is the largest cause of preventable death worldwide. Despite the well-publicized health risks associated with smoking, approximately 19 percent of adults in the United States are smokers, and about half of these smokers are predicted to die prematurely due to tobacco-related illnesses [1]. Strategies to reduce the morbidity and mortality caused by tobacco smoke are of immediate need. Nicotine, the primary psychoactive constituent in cigarettes, drives continued use of tobacco products. A potential strategy to improve smoking-related public health outcomes posits that reducing the nicotine content in cigarettes below an addictive threshold would promote quitting in current smokers and prevent initiation of smoking [2,3]. Reduction of nicotine content in cigarettes below an addictive threshold is being considered as a feasible tobacco control policy in the United States and worldwide. The Family Smoking Prevention and Tobacco Control Act in the United States [2,4] and the World Health Organization (WHO) Framework Convention on Tobacco Control call for established guidelines for the regulation of the content of cigarettes [5]. The most recent report of the WHO study group on tobacco product regulation emphasizes nicotine reduction as a strategy to reduce the immense harm caused by tobacco smoke [6]. Recent evidence suggests that very low doses of nicotine do not support self-administration in rats [7,8] and that reduction of nicotine content in experimental very low nicotine content (VLNC) cigarettes reduces smoking in humans [4,9]. Importantly, the use of VLNC cigarettes results in little initial compensatory smoking, suggesting that nicotine reduction may be a safe and effective tobacco control policy [10]. Throughout this brief review, reduction of nicotine dose or content refers to nicotine reduction below an addictive threshold. Such a tobacco control policy could have dramatic implications for the rates of smoking and also may impact other health-related outcomes, such as body weight regulation.
In the United States, the past 35 years have been marked by a slow decline in the number of smokers [1] and a dramatic and rapid increase in the rates of obesity [11]. More than 70 percent of adults and 17 percent of children and adolescents are considered overweight or obese, and deaths due to obesity-related diseases are predicted to surpass mortality caused by tobacco smoke within the decade [11-13]. Together, obesity and smoking represent the largest current obstacles in public health. Epidemiological and empirical studies describe an inverse relationship between tobacco smoking or nicotine use and body weight [14,15], and desired weight loss or maintenance of reduced body weight is commonly cited as a primary reason for smoking [16]. Moreover, ex-smokers typically gain an average of 10 pounds within the first year of abstinence [14]. Oftentimes, even the possibility of weight gain after cessation is a strong enough motive to drive continued use [17,18]. The decline in smoking rates may in part contribute to the increases in obesity [19], and nicotine reduction in cigarettes could potentially increase rates of obesity among smokers. Moreover, the motivation to continue to smoke as a method of weight suppression could be greater in obese smokers [14]. These observations imply that obesity may be a critical determinant of smoking and other related health behaviors as a result of a policy regulating the nicotine content in cigarettes. Though obesity and smoking are interrelated, how one might causally influence the other is essentially unstudied.
Here, we explore the interaction between nicotine and body weight regulation and consider this in the context of a potential nicotine-reduction policy. We discuss the implications of nicotine-induced body weight suppression in obese smokers, nicotine’s effect on metabolic profile, and how nicotine reinforcement differs between obese and lean smokers. A brief review of epidemiological, public health, clinical, and preclinical science supports the view that obese smokers may be especially susceptible to smoking and the weight-suppressant effects of nicotine.
Nicotine-Induced Body Weight Suppression: Implications for Obesity
In the general population, smokers weigh less than non-smokers and smokers who quit gain approximately 10 pounds within the first year of abstinence [14]; this relationship, however, is not as simple in the obese population. There is a negative correlation between the percentage of smokers and body mass index (BMI) among lean smokers, but this relationship is reversed among overweight, obese, and morbidly obese smokers [20]. Thus, there is a U-shape curve associated with percentages of smokers and smoking status as a function of BMI. Furthermore, several other studies report that moderate smokers weigh less than non-smokers, but heavy smokers (i.e., smoking at higher frequencies) are often obese [21,22].
The relationship between heavy smokers and obesity has not been studied longitudinally or causally and may be explained by several factors. First, obesity may enhance nicotine reinforcement, driving cigarette consumption. This idea is explored in more detail below. Alternatively, the relationship may be explained by clustering of other risk behaviors; for example, higher levels of cigarette consumption are linked with low levels of physical activity, low fruit/vegetable intake, and high alcohol consumption [23]. Thus, high rates of obesity among heavy smokers may be due to other independent health risk factors. Thirdly, obese smokers may smoke at higher rates in an effort to suppress body weight, which would indicate that obesity precedes initiation of smoking. A more complete understanding of the pathways linking smoking and obesity is critical in determining how body weight and smoking may be impacted following the implementation of a nicotine reduction policy.
Despite data suggesting that high BMI is associated with smoking, compared to a rate of more than 35 percent of obesity in the general population, only 5 percent of the general population smokes and is obese [24]. Thus, chronic exposure to nicotine may prevent excess weight gain in individuals who would otherwise be obese. As these data are not available in smokers, animal models might provide important information to fill this gap. A constant subcutaneous infusion of nicotine suppressed body weight gain in rats that become obese when maintained on a densely caloric diet (i.e., diet-induced obesity) [25]. In contrast, oral administration of nicotine via the drinking water had no effect on body weight in obese Zucker fatty rats [26,27], a different animal model of human obesity. The total daily dose of nicotine delivered orally to the Zucker fatty rats [27] was substantially lower than the dose delivered by subcutaneous infusion to diet-induced obese rats [25], which may explain the difference between the two studies. To our knowledge, there are no other reports of the effects of nicotine or smoking on body weight regulation in obese animals.
The impact of nicotine and smoking on obesity must be evaluated beyond an effect solely on body weight or BMI. Chronic smoking can increase fat accumulation, associated with central obesity and insulin resistance [28]. Waist-to-hip ratio (WHR), a measure of central obesity, is often predictive of the development of Type II diabetes and poor health outcomes [29]. Former smokers who relapse lose weight, approximately 2.5 pounds, but display an increase in WHR [29]. Central obesity increases dose-dependently with cigarette consumption, and this increase in abdominal fat accumulation often occurs independently of changes in BMI or body weight [28,29]. Smoking also induces insulin resistance [30], characteristic of Type II diabetes, and it is thought that it is nicotine in tobacco smoke that contributes to the development of insulin resistance [31]. Indeed, smoking and nicotine consumption are associated with the development of Type II diabetes, which may be mediated by central obesity and insulin resistance [32,33]. The contribution of chronic nicotine exposure to the development of insulin resistance is supported in animal models [34]. Thus, it seems the relationship between nicotine, smoking, and obesity is complex; nicotine may reduce BMI in an obese population and prevent the onset of obesity in an otherwise obese smoker, whereas chronic nicotine exposure may increase central obesity and the development of Type II diabetes in lean smokers — and potentially obese smokers as well. The concept that nicotine reduces BMI while increasing central obesity is contradictory and highlights the complexity of the relationship between nicotine and body weight, as well as the need for research directly addressing this question, particularly in the context of nicotine reduction policy.
The Effects of Obesity on Nicotine Reinforcement
Evidence also points to the potential impact of obesity on the degree to which nicotine may reinforce behavior. The mechanistic link between the drive for food and psychoactive drugs is clear in humans and rodents, which may underlie the co-occurrence of obesity and substance abuse disorders [35-37]. As mentioned above, higher BMI is associated with smoking more cigarettes per day, which may be linked with higher levels of nicotine dependence. In female smokers, childhood-onset obesity is associated with earlier smoking initiation and more severe withdrawal symptoms, but not increases in nicotine dependence or cigarettes consumed per day [38]. Further, craving for cigarettes significantly increased following 2-day abstinence in high-BMI female smokers compared to lean counterparts [39]. A longitudinal survey study found that female adolescent obesity is linked to higher levels of nicotine dependence later in life [40]. However, a separate study found a positive correlation between nicotine dependence and BMI in adult men but not women [41]. Results from these studies are not totally consistent, perhaps due to differing data collection methods or measures of dependence, but generally support the notion that obesity might contribute to increased uptake of cigarette use or nicotine dependence.
To our knowledge, there is only one controlled laboratory study investigating the effects of body weight on nicotine reinforcement in human smokers [42]. Non-obese and obese non-deprived smokers were asked to take 16 total puffs from two cigarettes differing in nicotine content: a normal nicotine content (NNC) and a VLNC cigarette. Measures of nicotine dependence and cigarettes per day were slightly elevated in the obese smokers. However, nicotine reward, measured by the percentage of total puffs taken from the NNC cigarette, was lower in the obese subjects. Ratings of liking for the VLNC cigarette, while lower than the ratings of liking for the NNC cigarette, were elevated in the obese subjects. The data describing a relationship between obesity and smoking behavior are limited and not entirely consistent across studies, but a picture emerges that might support the view that obese smokers may be more nicotine dependent and susceptible to smoking but derive less reward or liking from NNC cigarettes. More importantly, perhaps, is that these data highlight the possibility that obese smokers may derive more reward from VLNC cigarettes than non-obese smokers, indicating the potential for increased acceptance and use of reduced nicotine content cigarettes in obese smokers.
Recent efforts using animal models have focused on the concept that consumption of a densely caloric diet may increase motivated behaviors, such as drug-seeking. Evidence supports the idea that high-fat diet exposure may increase motivation for nicotine reward. In an outbred population of Sprague-Dawley rats that become obese when maintained on a densely caloric diet, only a subset develops insulin resistance [43]. Obese insulin-resistant rats, modeling Type II diabetes, displayed a strong place preference for an environment previously paired with nicotine [43]. Interestingly, the obese insulin-sensitive rats did not show a nicotine conditioned place preference. Similarly, in a separate report that that did not consider insulin sensitivity, lean mice fed a standard chow diet showed conditioned place preference for nicotine, but this was not observed in mice fed a high-fat diet [42]. Further, rats that become hypoinsulinemic by injection of Streptozotocin, modeling Type I or advanced stage Type II diabetes, show enhanced nicotine self-administration across doses and schedules of reinforcement [44]. These data indicate that perhaps obesity-induced insulin resistance, and not obesity itself, enhances nicotine reinforcement. Limited data from humans are consistent with these claims. More than 40 percent of adolescents with diabetes report to be smokers [45], and quit rates among diabetic smokers are very low [46]. Further studies investigating specifically whether diet-induced diabetes enhances acquisition and maintenance of nicotine self-administration and smoking behavior is warranted.
Conclusions and Outlook
The relationship between obesity and nicotine is complex. Smoking among the obese population is high [14,20]. This may be due to many factors. The explanation might be as simple as a clustering of unhealthy behaviors, such that, for example, people eating densely caloric foods also smoke [23]. Alternatively, many obese individuals may use smoking as a method of weight reduction [14]. Finally, obesity has been linked to increased reward-seeking behaviors. This could lead to increased nicotine-seeking or smoking behavior and augment seeking for food reward, causing excess weight gain [47]. Nicotine reduces BMI while at the same time increases fat accumulation, central obesity, and insulin resistance [29], which could contribute to the development of obesity. Moreover, insulin resistance is thought to enhance nicotine reinforcement [43,44], potentially driving smoking behavior. Together, we propose these factors create a cycle promoting nicotine-seeking in the obese population (Figure 1).
Figure 1.
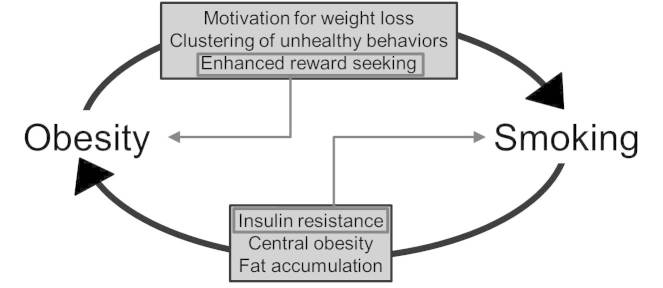
A simplistic model of the relationship between obesity and smoking. Obesity may lead to increased rates of smoking, mediated by several factors: a clustering of unhealthy behaviors, such as consumption of densely caloric foods; motivation for weight loss; or enhanced reward seeking. Smoking causes insulin resistance, central obesity, and fat accumulation, which could contribute to the development of obesity among smokers. These factors may create a cycle promoting smoking in the obese population. The proposed cycle is enhanced by two specific interrelated factors, enclosed in grey boxes. Increased reward seeking may lead to further excess weight gain by enhancing food-seeking behaviors, exacerbating obesity. Insulin resistance, which can be caused by chronic smoke exposure, is thought to augment nicotine reinforcement, which may lead to increased smoking behavior. For clarity, the well-characterized relationships among obesity, clustering of unhealthy behaviors, and insulin resistance have been omitted from the figure.
Obese smokers represent a unique population and may be especially susceptible to smoking and the weight-suppressive effects of nicotine. Thus, obese smokers should be considered a vulnerable population in a tobacco-reduction policy. It is possible that chronic nicotine exposure may reduce the onset of obesity in a subset of people who are otherwise predisposed to overweightness or obesity. Preliminary work from human and rodent models supports the possibility that reduction of nicotine dose or content will result in substantial weight gain in lean populations [48]. It is possible that nicotine reduction may result in the development of obesity and its associated co-morbidities in a subset of smokers. Several lines of research suggest that diet-induced insulin resistance increases susceptibility to smoking and nicotine reinforcement [43,44], raising the possibility that obese individuals might be more likely to initiate smoking of reduced nicotine content cigarettes, potentially increasing acceptance and use of VLNC cigarettes. On the other hand, insulin-resistant obese smokers may maximally benefit from nicotine reduction, as insulin resistance is a greater determinant of their behavior. Our argument is limited by the number of controlled, experimental studies focused on obese smokers or animal models of obese smokers. This gap in the literature restricts our ability to discern causal from correlative effects and demands future attention to this population, both in human and animal models. A better understanding of how weight, insulin resistance, and smoking behaviors will be impacted by a potential transition to VLNC cigarettes is critical. Analyses from surveys, such as the National Health and Nutrition Examination Survey, examining the differences in weight at the initiation of and following the cessation of smoking between lean and obese individuals could provide a useful foundation for future work. The initiation of smoking behaviors cannot be experimentally evaluated in humans; thus, animal models are needed to test the self-administration of low doses of nicotine thought to be below the threshold of addiction [8] in a model of diet-induced obesity and hypoinsulinemia. The ability to ask these questions in an animal model may lead to a more mechanistic understanding of the link between obesity and smoking. Until we gain a more comprehensive picture of the relationship between nicotine and obesity, careful consideration of obese smokers in nicotine-reduction policy is necessary and future work focusing on this population in human and animal models is of immediate importance.
Abbreviations
- BMI
body mass index
- NNC
normal nicotine content
- WHO
World Health Organization
- WHR
waist-to-hip ratio
- VLNC
very low nicotine conten
Author contributions
LER, ECD, and AFS conceptualized arguments presented in the manuscript. The manuscript was written by LER, with comments and editing provided by ECD and AFS. Funding: 5T32DA031111-04 (LER); U54DA031659 (ECD and AFS) The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
References
- Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged >/=18 years--United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207–1212. [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D. et al. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14(11):1319–1338. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny EC, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013;15(6):1003–1013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Study Group on Tobacco Product Regulation. Report on the scientific basic of tobacco product regulation: fourth report of a WHO study group. World Health Organ Tech Rep Ser. 2012;(967):1–83. [PubMed] [Google Scholar]
- World Health Organization Study Group on Tobacco Product Regulation. WHO Study Group on Tobacco Product Regulation. Report on the scientific basis of tobacco product regulation: fifth report of a WHO study group. World Health Organ Tech Rep Ser. 2015 [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15(11):1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Low-dose nicotine self-administration is reduced in adult male rats naive to high doses of nicotine: implications for nicotine product standards. Exp Clin Psychopharmacol. 2014;22(5):453–459. doi: 10.1037/a0037396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22 Suppl 1:i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24(2):472–476. doi: 10.1158/1055-9965.EPI-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (NY) 2010;6(12):780–792. [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: actual causes of death in the United States, 2000. JAMA. 2005;293(3):293–294. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90(1):164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DR Jr., Gottenborg S. Smoking and weight: the Minnesota Lipid Research Clinic. Am J Public Health. 1981;71(4):391–396. doi: 10.2105/ajph.71.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J Adolesc Health. 2003;32(4):306–313. doi: 10.1016/s1054-139x(02)00566-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol Behav. 2011;104(1):143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5(2):95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Chou SY, Grossman M, Saffer H. An economic analysis of adult obesity: results from the Behavioral Risk Factor Surveillance System. J Health Econ. 2004;23(3):565–587. doi: 10.1016/j.jhealeco.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Chatkin R, Mottin CC, Chatkin JM. Smoking among morbidly obese patients. BMC Pulm Med. 2010;10:61. doi: 10.1186/1471-2466-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolero A, Jacot-Sadowski I, Faeh D, Paccaud F, Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring) 2007;15(5):1311–1318. doi: 10.1038/oby.2007.153. [DOI] [PubMed] [Google Scholar]
- Nielsen TL, Wraae K, Brixen K, Hermann AP, Andersen M, Hagen C. Prevalence of overweight, obesity and physical inactivity in 20- to 29-year-old, Danish men. Relation to sociodemography, physical dysfunction and low socioeconomic status: the Odense Androgen Study. Int J Obes (Lond) 2006;30(5):805–815. doi: 10.1038/sj.ijo.0803197. [DOI] [PubMed] [Google Scholar]
- Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: a population-based survey. Prev Med. 2006;42(5):348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. BMJ. 2006;333(7557):25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane-Collazo P, Martinez de Morentin PB, Ferno J, Dieguez C, Nogueiras R, Lopez M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155(5):1679–1689. doi: 10.1210/en.2013-1839. [DOI] [PubMed] [Google Scholar]
- Liu RH, Mizuta M, Matsukura S. Oral nicotine administration decreases tumor necrosis factor-alpha expression in fat tissues in obese rats. Metabolism. 2001;50(1):79–85. doi: 10.1053/meta.2001.19436. [DOI] [PubMed] [Google Scholar]
- Liu RH, Mizuta M, Matsukura S. Long-term oral nicotine administration reduces insulin resistance in obese rats. Eur J Pharmacol. 2003;458(1-2):227–234. doi: 10.1016/s0014-2999(02)02726-7. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT. Cigarette smoking and increased central adiposity. Ann Intern Med. 1989;111(10):783–787. doi: 10.7326/0003-4819-111-10-783. [DOI] [PubMed] [Google Scholar]
- Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA. 1989;261(8):1169–1173. [PubMed] [Google Scholar]
- Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance--a potential link with the insulin resistance syndrome. J Intern Med. 1993;233(4):327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Taskinen MR, Smith U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation. 1996;94(5):878–881. doi: 10.1161/01.cir.94.5.878. [DOI] [PubMed] [Google Scholar]
- Liu T, Chen WQ, David SP, Tyndale RF, Wang H, Chen YM. et al. Interaction between heavy smoking and CYP2A6 genotypes on type 2 diabetes and its possible pathways. Eur J Endocrinol. 2011;165(6):961–967. doi: 10.1530/EJE-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XT, Liu Q, Wu J, Wakui M. Impact of cigarette smoking in type 2 diabetes development. Acta Pharmacol Sin. 2009;30(6):784–787. doi: 10.1038/aps.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z. et al. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med. 2015;21(4):373–382. doi: 10.1038/nm.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W. et al. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33(2):136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M. et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21(4):1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Saules KK, Levine MD, Marcus MD, Pomerleau CS. Differences in smoking patterns among women smokers with childhood versus later onset of weight problems. Eat Behav. 2007;8(3):418–422. doi: 10.1016/j.eatbeh.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saules KK, Pomerleau CS, Snedecor SM, Pomerleau CS, Brouwer RN, Rosenberg EE. Effects of disordered eating and obesity on weight, craving, and food intake during ad libitum smoking and abstinence. Eat Behav. 2004;5(4):353–363. doi: 10.1016/j.eatbeh.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hussaini AE, Nicholson LM, Shera D, Stettler N, Kinsman S. Adolescent obesity as a risk factor for high-level nicotine addiction in young women. J Adolesc Health. 2011;49(5):511–517. doi: 10.1016/j.jadohealth.2011.04.001. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Relationships of psychiatric disorders with sleep duration in an adult general population sample. J Psychiatr Res. 2005;39(6):577–583. doi: 10.1016/j.jpsychires.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R. et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology. 2005;180(2):306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Pipkin JA, O’Dell LE, Nazarian A. Insulin resistant rats display enhanced rewarding effects of nicotine. Drug Alcohol Depend. 2014;140:205–207. doi: 10.1016/j.drugalcdep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Natividad LA, Pipkin JA, Roman F, Torres I, Jurado J. et al. Enhanced nicotine self-administration and suppressed dopaminergic systems in a rat model of diabetes. Addict Biol. 2014;19(6):1006–1019. doi: 10.1111/adb.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Liese AD, Anderson AM, Dabelea D, Standiford D, Daniels SR. et al. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. J Pediatr. 2011;158(4):594–601.el. doi: 10.1016/j.jpeds.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill GV, Morgan C, MacFarlane IA. Awareness and use of smoking cessation treatments among diabetic patients. Diabet Med. 2005;22(5):658–660. doi: 10.1111/j.1464-5491.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Donny EC, Sved AF. Body weight gain is suppressed by low doses of nicotine independent of food intake; Poster session presented at: 22nd Annual Meeting of the Society for the Study of Ingestive Behavior; 2014 Jul 29-Aug 2; Seattle, WA. [Google Scholar]


