Abstract
Familial Mediterranean fever (FMF) is an auto-inflammatory disease characterised by periodic inflammatory attacks. We investigated changes in monocyte-granulocyte derived S10012A and chitotriosidase in both the attack and silent period of FMF for better estimation of inflammation. Endogenous resolvin was determined for utility to restrict inflammation. This study included 29 FMF patients (15 M/14 F) and 30 healthy controls (15 M/15 F). Serum levels of highly sensitive C-reactive protein, serum amiloid A (SAA), S100A12, chitotriosidase, and resolvin D1 were measured. Age, sex, body mass indexes, and lipids were similar between patients and controls. Biomarkers including hs-CRP, SAA, S100A12, chitotriosidase, and resolvin D1 were higher in the attack period of FMF patients compared to controls (P < 0.001). When FMF patients in the silent period were compared with their attack period, hs-CRP, SAA, and chitotriosidase were found elevated in the attack period (P < 0.001, P < 0.001, and P = 0.02 respectively). Serum levels of SAA, S100A12, chitotriosidase, and resolvin D1 in the silent period of FMF patients were still found elevated compared to healthy controls, indicating subclinical inflammation (P < 0.001, P < 0.001, P = 0.009, and P < 0.001 respectively ). In subgroup analysis, patients with M694V homozygote and heterozygote mutations had higher S10012A and hs-CRP compared to other mutation carriers. Our findings indicate that chitotriosidase and S10012A are useful in diagnosis and detection of subclinical inflammation and/or assessment of disease activity in FMF patients. They could be more informative for inflammation in various disease states compared to hsCRP and SAA. Resolvin D1 is elevated in both the attack and silent periods of FMF. It may be helpful to restrict inflammation.
Graphical Abstract
Keywords: Familial Mediterranean Fever, S100A12, Chitotriosidase, Resolvin D1, Serum Amiloid A
INTRODUCTION
Familial Mediterranean fever (FMF) is the most common autoinflammatory disease prevalent in the Middle East but cases in other ethnic groups in other parts of the world are increasingly presented (1,2,3). It is a multisystemic disease, caused by mutations in the MEFV gene which encodes defective pyrin/marenostrin, predominantly expressed in leukocytes (4). On suitable genetic background, inappropriately activated innate immune system cells are supposed to cause unprovoked short-lived bursts of systemic inflammation that gives to the rise clinical presentation of FMF (5). The most feared long term complication is development of amyloidosis that occurs mostly in untreated patients owing to late diagnosis of disease (3,6). Despite colchicine treatment, some patients still develop complications of disease due to unapparent chronic subclinical inflammation (7). Among the known inflammatory markers, serum amiloid A (SAA) has been used for either diagnostic or disease monitoring purposes (8). Elevated SAA can be found in the silent period of FMF patients, but most of the time is worrisome developing amiloidosis or proteinuria (9). C-reactive protein (CRP) also is a non- specific marker of inflammation and accompanies FMF attacks (10). Chitotriosidase (CTO), a human chitinolytic enzyme, is also a novel biomarker of inflammation, which is increased in parallel to monocyte-macrophage activation in a range of diseases (11,12). Recently, monocyte-granulocyte derived proinflammatory S100A12 (calgranulin C) has received much attention since serum and tissue concentrations are found in high concentration in various auto inflammatory disorders (13,14). In contrary, to restrict or control the inflammation, several natural anti-inflammatory pathways are in action. A sub group of omega-3 fatty acids, primarily eicosapentaenoic (EPA) and docoashexaenoic acids (DHA), are essential fatty acids which have long been known to be important for metabolic dysfunction. They are linked with a range of diseases including inflammatory disorders (15,16). Resolvins are a new area of investigation that may aid in resolution of inflammation in various disorders.
There is high need for new biomarkers aiding in diagnosis and follow-up treatment of FMF patients. In addition, we need new drugs to halt ongoing unapparent inflammation and prevent related complications in those patients. In the current study, we aimed to analyze the role of S100A12 and novel CTO as a potential diagnostic marker of inflammation, detection of flare up and subclinical inflammation. We also intended to define a potential utility of endogenous resolvin D1 (RvD1) in termination of inflammation in FMF patients.
MATERIALS AND METHODS
Twenty-nine FMF patients in an active disease state and 30 age and sex matched healthy controls were enrolled in this study. All the patients fulfilled diagnostic criteria of Tel-Hashomer for FMF (17). The case definition of active disease or attack included all manifestations of FMF: abdominal pain, chest pain, arthritis or joint pain, skin manifestations, fever, or other symptoms. After clearance of all the signs and symptoms of FMF attack, at least 2 extra weeks were allowed to pass before getting silence period samples. Venous blood samples of FMF patients and healthy controls were preserved at -80℃ until assayed. Except for 4 recently diagnosed cases, all the patients were on colchicines treatment when the samples were drawn and allowed to take non-steroidal anti inflammatory drugs (NSAIDs) on need. None of the patients was proteinuric at time of the study and around 50% had family history of FMF. Subjects who had history of chronic inflammatory disease other than FMF, systemic steroid usage, current pregnancy and infections were excluded from the study.
Laboratory evaluation
At least two separate samples were taken from each subject. Venous blood samples were centrifuged at 2,000 g for 10 min. Reproducibility of laboratory tests was tested by using a different operator, time and sample. Serum total cholesterol, triglyceride, and HDL cholesterol were measured on AU 5800 (Beckman Coulter Inc., Brea, CA, USA) automated chemistry analyzer with commercially available kits from Beckman Coulter Inc.LDL cholesterol was calculated according to Friedewald's formula (LDL=TC-HDL-TG/5.0). Hs-CRP was measured on Immulite XPI 2000 analyzer (Siemens Healthcare Diagnostics, Eshburn, Germany) with the chemiluminescent method. The following ELISA kits were used according to the manufacturer's instructions: IL-1β-EASIA (Cat. No: KAP1211, DIA source, Belgium), S100A12 (Cat. No: SEB08Hu, Cloud-Clone, TX, USA), SAA ELISA (Cat. No: ELHSAA, Ray Biotech, GA, USA), Human Chitotriosidase ELISA (Cat. No: CY-8074, CircuLex, MA, USA), Resolvin D1 EIA (Cat. No: 500380, Ceyman, USA).
Statistical analysis
Statistical analyses were conducted using the statistical package SPSS version 17 (SPSS Inc., Chicago, IL, USA). P value<0.05 was considered as statistically significant. All data were analyzed by Kolmogorov-Smirnov test for normality of distribution. Data were expressed as mean±SD and median (interquartile range). For comparison of biochemical parameters between the FMF attack period and FMF silent period, the paired t test and Wilcoxon signed rank test were used where appropriate. Independent sample t-test and Mann-Whitney U test were used for comparison between FMF patients and healthy controls. Correlation analyses were done by Spearman's rank correlation analysis.
Ethics statement
Patients provided informed and written consent to participate and the study protocol was approved by the ethical committee of Tepecik Teaching and Research Hospital in Izmir (Ethical approval date and number: 30 November 2014; 11).
RESULTS
There were 29 FMF patients (10 males [M], 19 females [F], mean age 26±9.8 yr) and 30 healthy control patients (10 M, 30 F, mean age 25±9.3 yr). Age, sex distribution, body mass index (BMI) and serum lipids (total cholesterol, triglyceride, LDL) were comparable between FMF patients and controls (P>0.05). All patients were carrying MEFV gene mutations. Detailed patients characteristics of FMF patients and controls are shown in Table 1.
Table 1. Patient characteristics of FMF patients and controls.
| Parameters | FMF patients (n = 29) | Controls (n = 30) | P value |
|---|---|---|---|
| Age (yr) | 26 ± 10 | 25 ± 9 | 0.630 |
| Sex (M/F) | 19/10 | 20/10 | 0.926 |
| BMI (kg/m2) | 23 ± 4 | 23 ± 4 | 0.724 |
| Total Cholesterol (mg/dL) | 167 ± 34 | 179 ± 44 | 0.223 |
| Triglyceride (mg/dL) | 116 (95-153) | 101 (72-134) | 0.200 |
| LDL Cholesterol (mg/dL) | 108 ± 27 | 114 ± 31 | 0.436 |
| HDL Cholesterol (mg/dL) | 31 (25-38) | 39 (34-46) | < 0.001 |
| Mutations | 8 × M694V/M694V | ||
| 3 × M694V/R761H | |||
| 3 × M694V/M680I | |||
| 2 × M694V/R202Q | |||
| 2 × M694V/E148Q | |||
| 1 × M694I/M694I | |||
| 1 × M694V | |||
| 2 × R202Q/E148Q | |||
| 2 × R202Q | |||
| 1 × M680I/V726A | |||
| 1 × M680I/M680I | |||
| 1 × E167D/F479L | |||
| 1 × R761H | |||
| 1 × V726A |
Comparison of inflammatory biomarkers of the attack and silent periods of FMF patients and healthy controls
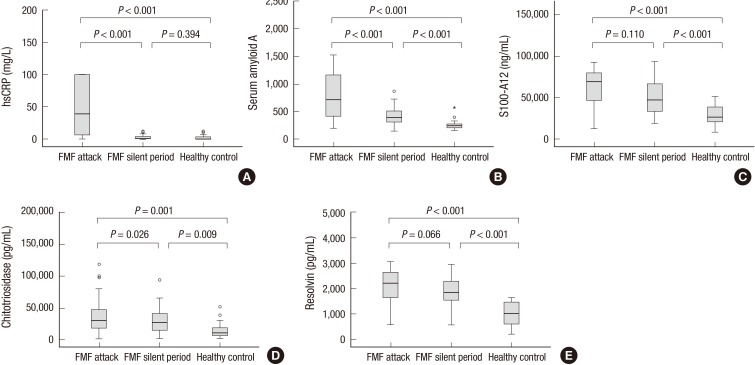
Analysis of FMF patients according to disease activity showed that among the studied inflammatory biomarkers hs-CRP, SAA and CTO were significantly increased in the attack compared to the attack free period of the same patients (44 [9.4-100] vs. 4.2 [0.6-15.8] mg/L, P<0.001 and 716 [411-1,198] vs. 408 [316-554] mg/L, P<0.001 and 30,336 [17,731-47,507] mg/L vs. 25,816 [14,237-41,403] pg/mL, P=0.026; respectively). But, the difference in circulating S100A12 and RvD1 did not reach statistically significant values regarding disease activity (69,528 [46,616-80,042] vs. 54,602 [28,208-73,442] ng/mL, P=0.110 and 2,112± 640 vs. 1,909±523 pg/mL, P=0.066; respectively). Similarly, concentration of hs-CRP, SAA, S100A12, CTO, and RvD1 were significantly higher in the attack period of FMF patients compared with the healthy control group (44 [9.4-100] vs. 1.2 [0.2-3.9] mg/L, P<0.001; and 716 [411-1,198] vs. 246 [216-281] ng/mL, P<0.001; and 69,528 [46,616-80,042] vs. 26,672 [20,332-40,224] ng/mL, P<0.001; and 30,336 [17,731-47,507] vs. 10,625 [6,128-19,911] pg/mL, P=0.001; and 2,112±640 vs. 1,005±464 pg/mL, P<0.001; respectively). Meanwhile, circulating levels of SAA, S100A12, CTO, and RvD1 in the silent period of FMF patients were higher compared with the healthy control group (408 [316-554] vs. 246 [216-281] ng/mL, P<0.001; and 54,602 [28,208-73,442] vs. 26,672 [20,332-40,224] ng/mL, P<0.001; and 25,816 [14,237-41,403] vs. 10,625 [6,128-19,911] pg/mL, P=0.009; and 1,909±523 vs. 1,005±464 pg/mL, P<0.001; respectively). When hs-CRP of the silent period of FMF patients was compared with healthy controls, no difference was found (4.2 [0.6-15.8] vs. 1.2 [0.2-3.9] mg/L, P=0.394; respectively) (Fig. 1A-E).
Fig. 1. Various inflammatory markers in Familial Mediterranean Fever (FMF). Serum level of highly sensitive-C reactive protein (hs-CRP) (A), serum amiloid A protein (SAA) (B), S100A12 (calgranulin C) (C), chitotriosidase (D), and resolvin D1 (E) were determined in the attack and silent period of FMF patients and compared with healthy controls.
Comparison of inflammatory biomarkers with respect to different mutations
When the level of inflammatory markers in the silent period were compared with each other according to different mutations, both homozygotes M694V or heterozygotes with M694 mutations seemed to have more pronounced hs-CRP and S100 A12 levels. Due to the limited number of FMF patients with M694V mutations, the results were shown as a median (interquartile range), statistical analysis was not done. Data for circulating biomarkers in respect to mutations are shown in Table 2.
Table 2. Circulating level of various biomarkers in the silent period of FMF with respect to different mutations.
| Genotypes | hsCRP (mg/L) | SAA (mg/L) | S100A12 (ng/mL) | Chitotriosidase (pg/mL) | Resolvin (pg/mL) |
|---|---|---|---|---|---|
| M694V-M694V (n = 8) | 6.0 (1.3-15.8) | 382 (233-605) | 62,600 (40,189-81,101) | 26,236 (15,169-41,333) | 2,014 (1,750-2,792) |
| M694V-Other (n = 11) | 12.9 (1.0-35.5) | 481 (319-599) | 65,139 (42,102-75,845) | 32,247 (18,827-54,894) | 1,774 (1,427-1,978) |
| Other-Other (n = 5) | 2.7 (0.7-5.3) | 448 (347-665) | 51,729 (28,832-75,635) | 14,447 (4,755-35,928) | 1,840 (1,481-2,643) |
| Other-Non (n = 5) | 0.5 (0.3-5.8) | 335 (276-408) | 33,350 (20,593-45,818) | 26,469 (11,371-53,309) | 2,205 (1,660-2,414) |
Correlation analysis
Upon correlation analysis, S100A12 was found to be correlated with hs-CRP, SAA, and CTO (P<0.05; r=0.6, 0.6, and 0.3, respectively). CTO was correlated with hs-CRP, SAA, S100A12, and RvD1 (P<0.05; r=0.3, 0.3, 0.3, and 0.5, respectively). RvD1 also showed correlation with hs-CRP and SAA (P<0.05; r=0.4 and 0.5, respectively).
DISCUSSION
In our study, we demonstrated that the human chitinolytic enzyme (CTO) level was elevated in FMF patients and also showed good correlation with inflammation markers like hs-CRP, SAA, and S100A12. In this regard, it may be useful in diagnosis of FMF in addition to known biomarkers of inflammation. The serum levels of CTO were also remarkably increased in the FMF attack compared to the silent period of the disease, which may afford to differentiate a relapse (Fig. 1D). Another interesting finding was continuous CTO function in the silent period of FMF that is also confirmatory of chronic macrophage activation and persistent subclinical inflammation in these patients with resulting long term complications. CTO may also aid to control effectiveness of treatment by detection of inflammation in the silent period of FMF. In several studies, CTO was found augmented in diseases like gaucher and sarcoidosis, in which chronically activated macrophages and neutrophils play a major role in pathogenesis (11,12). To our knowledge, this is the first study which evaluates CTO function in FMF patients.
S10012A could be a relatively more specific marker of inflammation in FMF. The systemic form of juvenile idiopathic arthritis (JIA) is a well-known disease with massively elevated S10012A in blood (14). In a study (18), S10012A emerged as an important biomarker to differentiate fever of unknown origin from other causes, including infections, as well as define risk of relapse in JIA patients. Another study by Kallinich et al. (13) demonstrated that S10012A in untreated pediatric FMF patients were significantly increased compared to age matched healthy controls. The relatively high S10012A level in homozygote for M694V was another important finding. In the present study, we confirmed that S10012A is a sensitive biomarker of inflammation and well expressed in the attack period of FMF. It has good correlation with hs-CRP and SAA, which demonstrates diagnostic utility in the disease. In addition, it can detect subclinical inflammation in the silent period of the disease and comes out as a helpful biomarker for treatment monitoring (Fig. 1C). On the contrary, we did not find significant differences in S10012A levels between the silent and attack periods of FMF, which excludes an indicator for detection of the relapse. Patients with homozygote and heterozygote mutations of M694V had elevated S10012A levels. It may show relatively higher pro-inflammatory potential in these patients (Table 2). Because of the small number of FMF patients in the subgroups, this finding should be confirmed with statistical analysis in further studies.
Among the inflammatory biomarkers, SAA and CRP have been used for detection of relapse so far in FMF patients. Duzova et al. (8) observed elevated mean SAA levels in 183 silent FMF patients compared to healthy controls. It is presumed to be owed to subclinical inflammation (8,9). In our study; hs-CRP was sensitive only for showing a relapse in FMF patients. Meanwhile, SAA enabled to demonstrate both attack and subclinical inflammation similar to S10012A and CTO (Fig. 1A-D). What we do not know right now is which markers are the best or most sensitive and specific. Research on correlations of biomarkers with co- morbid conditions like amiloidosis and response to various treatments in FMF patients may answer these questions in future.
Bioactive derivatives of omega-3 fatty acids, mainly EPA and DHA, are essential for the innate immune system and have natural anti-inflammatory, pro-resolving properties (19). Resolvins mainly switch inflammation to the resolution phase by inhibition of endothelial migration and infiltration of leukocytes, as well as promotion of clearance of apoptotic polymorphonuclear leukocytes (20). The efficacy of endogenous RvD1 has been tested for anti-inflammatory actions in several in vivo experimental models. In the study by Krishnamoorthy et al. (21), RvD1 specifically interacts with specific receptors on phagocytes and plays an important role in resolving acute inflammation. FMF is primarily a disease of the innate immune system affecting leucocytes (5). The proposed mechanisms of action for resolvins make them a suitable agent to control inflammation in FMF patients. In a recent study, Recchiuti et al. (22) showed that after oral supplement of RvD1, experimentally induced peritonitis in mouse models have resolved faster with reduced leukocyte infiltration and shifting macrophages into the resolution phase. In our study, the serum levels of RvD1 were increased in both the attack and silent periods of FMF and correlated with classical biomarkers of inflammation such as hs-CRP and SAA. This is a remarkable observation, since RvD1 activation is accompanied with a degree of inflammation in FMF patients. Further studies should focus on not only the blood level but function of RvD1in resolution of inflammation in FMF patients. Whether RvD1treatment shorten FMF attack or improves subclinical inflammation, or both, is a subject of new research.
In summary, both S10012A and CTO are proposed as new laboratory tests and could be helpful in differential diagnosis and detection of subclinical inflammation and/or define flare up of FMF patients. RvD1 has a good correlation with inflammatory markers and may be useful to restrict inflammation and aid in treatment of FMF patients.
Footnotes
DISCLOSURE: The authors declare that there are no conflicts of interest regarding the publication of this paper.
AUTHOR CONTRIBUTION: Conception and design of this study: all authors. Interpretation of results and drafting the manuscript: Taylan A, Toprak B. Analysis and interpretation of data: Toprak B, Gurler O, Sari I. Acquisition of data: Sisman AR, Colak A, Yalcin H. Critical revision of manuscript: Taylan A, Toprak B. Manuscript approval: all authors.
References
- 1.Lee CG, Lim YJ, Kang HW, Kim JH, Lee JK, Koh MS, Lee JH, Huh HJ, Lee SH. A case of recurrent abdominal pain with fever and urticarial eruption. Korean J Gastroenterol. 2014;64:40–44. doi: 10.4166/kjg.2014.64.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Lim AL, Jang HJ, Han JW, Song YK, Song WJ, Woo HJ, Jung YO, Kae SH, Lee J. Familial Mediterranean fever: the first adult case in Korea. J Korean Med Sci. 2012;27:1424–1427. doi: 10.3346/jkms.2012.27.11.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo KY, Park SJ, Wang JY, Shin JI, Jeong HJ, Lim BJ, Lee JS. The first case of familial Mediterranean fever associated with renal amyloidosis in Korea. Yonsei Med J. 2012;53:454–458. doi: 10.3349/ymj.2012.53.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Zvi I, Livneh A. Chronic inflammation in FMF: markers, risk factors, outcomes and therapy. Nat Rev Rheumatol. 2011;7:105–112. doi: 10.1038/nrrheum.2010.181. [DOI] [PubMed] [Google Scholar]
- 5.Ozen S, Uckan D, Baskin E, Besbas N, Okur H, Saatci U, Bakkaloglu A. Increased neutrophil apoptosis during attacks of familial Mediterranean fever. Clin Exp Rheumatol. 2001;19:S68–S71. [PubMed] [Google Scholar]
- 6.Sevoyan MK, Sarkisian TF, Beglaryan AA, Shahsuvaryan GR, Armenian HK. Prevention of amyloidosis in familial Mediterranean fever with colchicine: a case-control study in Armenia. Med Princ Pract. 2009;18:441–446. doi: 10.1159/000235892. [DOI] [PubMed] [Google Scholar]
- 7.Yüksel S, Ayvazyan L, Gasparyan AY. Familial mediterranean Fever as an emerging clinical model of atherogenesis associated with low-grade inflammation. Open Cardiovasc Med J. 2010;4:51–56. doi: 10.2174/1874192401004020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duzova A, Bakkaloglu A, Besbas N, Topaloglu R, Ozen S, Ozaltin F, Bassoy Y, Yilmaz E. Role of A-SAA in monitoring subclinical inflammation and in colchicine dosage in familial Mediterranean fever. Clin Exp Rheumatol. 2003;21:509–514. [PubMed] [Google Scholar]
- 9.Yalçinkaya F, Cakar N, Acar B, Tutar E, Güriz H, Elhan AH, Oztürk S, Kansu A, Ince E, Atalay S, et al. The value of the levels of acute phase reactants for the prediction of familial Mediterranean fever associated amyloidosis: a case control study. Rheumatol Int. 2007;27:517–522. doi: 10.1007/s00296-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 10.Guzel S, Andican G, Seven A, Aslan M, Bolayirli M, Guzel EC, Hamuryudan V. Acute phase response and oxidative stress status in familial Mediterranean fever (FMF) Mod Rheumatol. 2012;22:431–437. doi: 10.1007/s10165-011-0517-5. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, He W, Boer AM, Wevers RA, de Bruijn AM, Groener JE, Hollak CE, Aerts JM, Galjaard H, van Diggelen OP. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J Inherit Metab Dis. 1995;18:717–722. doi: 10.1007/BF02436762. [DOI] [PubMed] [Google Scholar]
- 12.Harlander M, Salobir B, Zupančič M, Dolenšek M, Bavčar Vodovnik T, Terčelj M. Serial chitotriosidase measurements in sarcoidosis--two to five year follow-up study. Respir Med. 2014;108:775–782. doi: 10.1016/j.rmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Kallinich T, Wittkowski H, Keitzer R, Roth J, Foell D. Neutrophil-derived S100A12 as novel biomarker of inflammation in familial Mediterranean fever. Ann Rheum Dis. 2010;69:677–682. doi: 10.1136/ard.2009.114363. [DOI] [PubMed] [Google Scholar]
- 14.Wittkowski H, Frosch M, Wulffraat N, Goldbach-Mansky R, Kallinich T, Kuemmerle-Deschner J, Frühwald MC, Dassmann S, Pham TH, Roth J, et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008;58:3924–3931. doi: 10.1002/art.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk JM, Turk HF, Fan YY, Callaway E, Weeks B, Yang P, McMurray DN, Chapkin RS. Antagonizing arachidonic acid-derived eicosanoids reduces inflammatory Th17 and Th1 cell-mediated inflammation and colitis severity. Mediators Inflamm. 2014;2014:917149. doi: 10.1155/2014/917149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin CR, Blanco PG, Keach JC, Petz JL, Zaman MM, Bhaskar KR, Cluette-Brown JE, Gautam S, Sheth S, Afdhal NH, et al. The safety and efficacy of oral docosahexaenoic acid supplementation for the treatment of primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2012;35:255–265. doi: 10.1111/j.1365-2036.2011.04926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, Migdal A, Padeh S, Pras M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–1885. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 18.Gerss J, Roth J, Holzinger D, Ruperto N, Wittkowski H, Frosch M, Wulffraat N, Wedderburn L, Stanevicha V, Mihaylova D, et al. Paediatric Rheumatology International Trials Organization (PRINTO) Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann Rheum Dis. 2012;71:1991–1997. doi: 10.1136/annrheumdis-2012-201329. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. Novel omega -- 3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recchiuti A, Codagnone M, Pierdomenico AM, Rossi C, Mari VC, Cianci E, Simiele F, Gatta V, Romano M. Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB J. 2014;28:3090–3102. doi: 10.1096/fj.13-248393. [DOI] [PubMed] [Google Scholar]