Abstract
The purpose of this study was to investigate the age-related NADPH oxidase (arNOX) activity in patients with age-related knee osteoarthritis (OA). Serum and cartilage arNOX activities were determined using an oxidized ferricytochrome C reduction assay. Full-thickness knee joint cartilages obtained through total knee replacement surgery were graded according to the Outerbridge (OB) classification. Radiographic severity of OA was determined on Knee X-rays according to the Kellgren-Lawrence (K/L) grading system. Cartilage β-galactosidase, HIF-1α, and GLUT-1 expression levels were evaluated as markers for tissue senescence, hypoxia, and glycolysis. Higher arNOX activities occurred with higher levels of cartilage β-galactosidase, HIF-1α, and GLUT-1 (P = 0.002). arNOX activity in cartilages with surface defects (OB grade II, III) was higher than in those without the defects (OB grade 0, I) (P = 0.012). Cartilage arNOX activity showed a positive correlation with serum arNOX activity (r = -0.577, P = 0.023). Serum arNOX activity was significantly higher in the OA subgroup with bilateral ROA than in the OA with no or unilateral ROA (2.449 ± 0.81, 2.022 ± 0.251 nM/mL, respectively, P = 0.019). The results of this study demonstrate that OA itself is not a cause to increase arNOX activities, however, arNOX hyperactivity is related to a high degree of cartilage degradation, and a high grade and extent of ROA in age-related OA.
Graphical Abstract

Keywords: ENOX1 Protein, Human; Age-related NOX; Aging; Osteoarthritis; Cartilage
INTRODUCTION
Osteoarthritis (OA) is a degenerative joint disease characterized by articular cartilage degradation, subchondral bony sclerosis, and osteophyte formation leading to chronic joint disability. OA is classified as primary and secondary OA. Regarding primary OA, a combination of various factors including age, obesity, genetic predisposition, female gender, anatomy, and joint instability promotes OA development (1). Among them, age is the most important so that the prevalence of OA increases with aging, and approximately 80% of people older than 75 yr old present OA (2).
Articular cartilage, as an avascular tissue, is ordinarily under hypoxia. Oxygen and glucose for chondrocyte metabolism are provided by diffusion from the adjacent subchondral bone and synovial fluid, and energy production in chondrocytes is controlled by anaerobic glycolysis (3). With aging tissue perfusion declines and metabolic alterations that increase glycolysis occur in a low oxygen environment. The metabolic changes by hypoxia and hypoperfusion would be more prominent in articular cartilage and chondrocytes than other tissues; accordingly, oxidative stress and increased reactive oxygen species (ROS) production are observed in joint tissues and OA cartilage with the advance of age (4).
Surface NADPH oxidase (ENOX) proteins are members of the TM (transmembrane)-9 super-family, which are expressed on the cell surface. Protein disulfide-thiol interchange and NADPH oxidation are common biochemical activities of ENOX proteins (5). ENOX proteins are categorized according to the response to effectors. ENOX1 proteins are associated with hormone responsive activities; ENOX2 proteins with tumor-related activities; ENOX3 proteins with age-related responsive activities. Because of their locational characteristics, ENOX proteins serve as the terminal oxidases of plasma membrane electron transport. ENOX1 and ENOX2 deliver four electrons, reducing molecular oxygen to 2H2O (6). In contrast, ENOX3 generates ROS responsively to aging, as the age-related NOX (arNOX) protein (7). arNOX proteins are weakly bound to plasma membrane, and 30-kDa N-terminal fragments of arNOX proteins are toward the outside of the plasma membrane. Therefore, the N-terminal fragment can be cleaved and detected in body fluids. arNOX proteins are rarely detected below 30 yr of age, however, after that age, increase in correlation with the advance of age, which indicates that as energy source shifts to glycolysis, aging results in a hyperactive plasma membrane oxido-reductase system (6). arNOX hyperactivity of serum and saliva was reported in relation with age-related skin diseases (8).
Age-related OA is primarily a consequence of the aging process, however, how aging affects the joints and what occurs in correlation with aging is not clear. The current study is to evaluate the arNOX activities in age-related knee OA as compared with the severity and extent of bony changes, and the degrees of cartilage senescence, hypoxia and glycolysis.
MATERIALS AND METHODS
Procurement of sera and human cartilages
OA patients fulfilled the American College of Rheumatology (ACR) criteria for knee OA and age-, sex-matched healthy participants who had normal results on a routine medical examination and knee X-rays at the health promotion center were enrolled in this study. Any cases with an erythrocyte sedimentation rate (ESR) higher than 40 mm/hr, positive rheumatoid factor (RF), abnormal results on laboratory tests and chest X-rays, or medical history of diabetes, malignancies, joint trauma or surgery, and other metabolic or inflammatory diseases were excluded. With permission, 10 mL of whole blood was drawn from the participants (n=20 for OA and 20 for control; 22 for no or unilateral ROA and 20 for bilateral ROA), and serum samples were prepared and stored at negative 70 degrees Celsius (℃) for the experiments. Full-thickness human cartilages were obtained from 13 patients with OA who underwent total knee replacement (TKR) surgery.
Assessment of radiological bony changes and cartilage degradation
Regarding OA, bony changes were evaluated on antero-posterior (AP) and 30 degree-flexed lateral knee X-rays using the K/L grading system. The presence and severity of OA bony changes were measured and graded twice by an expert in rheumatology; no abnormal radiological finding was designated as grade 0; no joint space narrowing but suspicious osteophytes, grade 1; minimal joint space narrowing and definite osteophytes, grade 2; joint space less than 3 mm, severe intra-articular irregularity, and prominent osteophytes, grade 3; no visible joint space and severe sclerosis, grade 4. The intra-observer reliability was 0.78 indicating moderate agreement. Full-thickness knee joint cartilages obtained through TKR surgery were graded using the Outerbridge (OB) classification; grade 0: normal cartilage; grade I: cartilage with softening and swelling; grade II: a partial-thickness defect with fissures on the surface that do not reach the subchondral bone or exceed 1.5 cm in diameter; grade III: fissuring to the level of subchondral bone in an area with a diameter greater than 1.5 cm; grade IV, exposed subchondral bone (Fig. 1A) (9).
Fig. 1. Grades and senescence markers of cartilage from knee OA patients. (A) OA cartilages were classified into OB grade 0 to IV according to the degree of cartilage defect. (B) β-galactosidase staining of OA cartilages showed senescent chondrocytes (bright blue cytoplasmic staining) increasing in cartilages of the higher OB grade. (C) HIF-1α and GLUT-1 expressions showed a significant up-regulation in cartilages of the higher OB grade. The changes of cartilage senescence were consistent with cartilage arNOX hyperactivity in OA cartilages. *P < 0.05; †P < 0.01.
Cartilage preparation
Fresh cartilages obtained from TKR surgery were preserved in PBS and stored at negative 20℃ for the histological and biochemical experiments. Full-thickness samples of cartilage, weighing 100 mg, were chopped and ground in 1,000 µL of a lysis buffer containing 1M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, Sigma-Aldrich, Switzerland), 0.5M ethylenediaminetetraacetic acid (EDTA), 0.5M ethylene glycol tetraacetic acid (EGTA), 3M NaCl, 3M NaF, b-glycerophosphate, 0.1M sodium orthovanadate (Na3VO4, pH 9.0), 1M dithiothreitol (DTT), 1M benzamide, 10 mg/mL aprotinin, 10 mg/mL leupeptin, 10 mg/mL pepstatin A, 10% nonidet-P40 (NP-40), 100 mM phenylmethanesulfonylfluoride (PMSF), and glycerol. After mixing for 30 min, the cartilage lysates were separated by centrifugation at 4,000 rpm for 5 min, and then at 13,000 rpm for 15 min at 4℃, consecutively. The supernatants were prepared for the cartilage arNOX analysis.
Histological assessment of cartilage senescence with β-galactosidase staining
Sliced articular cartilages were embedded in the optimal cutting temperature compound (OCT) vertically to the surface, and cryo-sectioned at 5 µm thickness. The sections were attached to coated slides and fixed in 3.7% paraformaldehyde in PBS for 10 min. After washing with PBS, the slides were treated with senescence-associated beta-galactosidase (SA-β-gal) solution at 37℃ for 16 hr, and then counter-stained with hematoxylin. β-galactosidase can be detected as bright blue cytoplasmic stains. Numbers of total chondrocytes and β-galactosidase positive chondrocytes were counted in three different fields per slide under a low resolution power (×100). The mean percentage of β-galactosidase positive chondrocytes to total chondrocytes was taken as the degree of cartilage senescence.
Western blotting for HIF-1α and GLUT-1
Total lysates of full-thickness human cartilage were prepared in PRO-PREP buffer (iNtRON, Seoul, Korea). Protein concentration of the solutions was determined using a Bradford assay (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as a standard. Twenty micrograms of the cartilage protein and β-actin as a loading control were separated on 10% SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were soaked in 5% non-fat dried milk in TBST (10 mM/L Tris/HCl pH 7.5, 150 mM NaCl and 0.05% Tween-20) for 1 hr and then incubated for 16-18 hr with primary antibodies against HIF-1α, GLUT-1, and β-actin at 4℃. After washing three times with TBST for 10 min, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hr at room temperature. The membranes were then rinsed three times with TBST for 10 min and antigen-antibody complexes were detected using an enhanced chemiluminescence detection system (LAS-3,000; Fujifilm, Tokyo, Japan). The results were analyzed with ImageJ software comparing the density of bands. Mouse anti-HIF-1α monoclonal antibody and Rabbit anti-GLUT-1 polyclonal antibody were purchased from Abcam (Cambridge, MA, USA). Mouse anti-β-actin monoclonal antibody was purchased from Sigma-Aldrich (St. Louis, CA, USA).
arNOX activity analysis
Reduction rate of oxidized ferricytochrome C was measured as the arNOX activity (10,11,12). One hundred microliters sera or 200 µL total lysates of full-thickness cartilage were used to reduce 150 µL of 0.2% oxidized ferricytochrome C (from equine heart, SIGMA, USA) solution in phosphate-buffered saline glucose (PBSG) buffer. The basal absorbance was measured at one minute after mixing the oxidized cytochrome C solution using a UV-160A spectrophotometer (Shimadzu, Kyoto, Japan) in a dual wavelength mode (550/540 nm). And then, adding sera or cartilage lysates into the solution, ferricytochrome C reduction rate was determined by measuring the absorbance at 5, 15, 30, 45, 60, 75, 90, 105, and 120 min. Cartilage arNOX activity was measured using two cartilage samples in each patient; one of the lowest OB grade and another of the highest OB grade. The mean activity of the two samples was considered as the cartilage arNOX activity of a patient.
Statistical analysis
Statistical analysis was performed using SPSS program version 12. Independent sample t-test, Pearson's correlation test for parametric analysis, and Mann-Whitney test, Spearman's correlation test were used for non-parametric analysis with a confidence interval of 95% and P value<0.05.
Ethics statement
The study protocol was approved by the institutional review board of Yeungnam University Hospital (IRB No. YUH-12-0452-083). Informed consent was submitted before the sampling. This study was conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki).
RESULTS
In this study, the healthy control subjects (n=20) included seven males and 13 females, and the OA patients (n=20) included six males and 14 females. No significant difference in age (52.05±12.25 and 55.70±2.49, respectively), body weight, body mass index (BMI), ESR, and C-reactive protein (CRP) was observed between healthy participants and OA patients. As adjusted for age and sex, serum arNOX activity showed no association with OA as compared with non-OA healthy control subjects (2.276± 0.377 nM/mL, 2.208±0.594 nM/mL, respectively) (Table 1).
Table 1. Characteristics and comparison of control and OA patients.
| Parameters | Control | OA |
|---|---|---|
| Age (yr) | 52.05 ± 12.25 (31-78) | 55.70 ± 2.49 (50-77) |
| n (M/F) | 20 (7/13) | 20 (6/14) |
| BW (kg) | 59.19 ± 8.40 | 59.45 ± 7.59 |
| BMI (kg/m2) | 23.04 ± 2.47 | 22.8 ± 2.67 |
| ESR (mm/hr) | 16.1 ± 11.1 | 20.5 ± 12.99 |
| CRP (mg/dL) | 0.228 ± 0.254 | 0.343 ± 0.804 |
| s-arNOX (nM/mL) | 2.276 ± 0.377 | 2.208 ± 0.594 |
Values represent means±SD. s-arNOX, serum age-related NADH oxidase.
With regard to the effects of arNOX activity on the OA bony changes, 42 knee OA patients were enrolled and studied in two subgroups according to the presence and extent of ROA; one with no or unilateral ROA (n=20), and another with bilateral knee ROA (n=22) based on the knee X-rays and the K/L grade (13). ROA of knee joints was defined as K/L grade ≥2 according to the ACR criteria for knee OA. No significant difference in body weight, BMI, ESR, CRP, and age (58.09±5.19 and 61.60± 7.21, respectively) was observed between the OA subgroups (Table 2). Serum arNOX activity, with adjustment for age, sex and BMI showed a significant correlation to the K/L grade of knee OA (β=0.992, P=0.029; β=1.043, P=0.015 for females).
Table 2. Characteristics and comparison of OA subgroups according to the extent of ROA.
| Parameters | Knee ROA | |
|---|---|---|
| No or unilateral | Bilateral | |
| Age (yr) | 58.09±5.19 | 61.60±7.214 |
| n (M/F) | 22 (1/21) | 20 (2/18) |
| BW (kg) | 56.15±6.56 | 59.69±6.50 |
| BMI (kg/m2) | 23.07±2.78 | 23.93±2.90 |
| ESR (mm/hr) | 15.91±8.46 | 20.30±10.29 |
| CRP (mg/dL) | 0.061±0.076 | 0.384±0.812 |
| s-arNOX (nM/mL) | 2.022±0.251 | 2.449±0.810* |
Values represent means±SD. *P<0.05; †P<0.01. ROA, radiological OA; s-arNOX, serum age-related NADH oxidase.
Staining of β-galactosidase revealed histological senescence of cartilages. OB grade 0 and I cartilages showed the intact structure of superficial, middle, and deep zones, except for softening or swelling of the articular surface in grade I. OB grade II and III cartilages accompanied substantial loss of the articular surface and zonal defects. The mean percentage of β-galactosidase positive chondrocytes was 18.9±12.1% in OA cartilages with the surface defects (OB grade II, III), and 1.4%±0.9% in OA cartilages without the defects (OB grade 0, I). The percentage increased with the advance of the OB grade in OA (P=0.012), (Fig. 1A, B).
The expression levels of GLUT-1 and HIF-1α, as markers for tissue hypoxia and glycolytic metabolism, was assessed in human OA cartilages. Western blot analyses showed that the levels of GLUT-1 and HIF-1α were upregulated in the senescent cartilages and in cartilages with the surface defects. Higher levels of HIF-1α and GLUT1 were associated with a higher percentage of β-galactosidase positive chondrocytes and OB grade of OA cartilage (P=0.002, P=0.012, respectively) (Fig. 1C). These results may indicate that cartilage senescence and cartilage defects progress in correlation with cartilage hypoxia and glycolytic metabolism in OA.
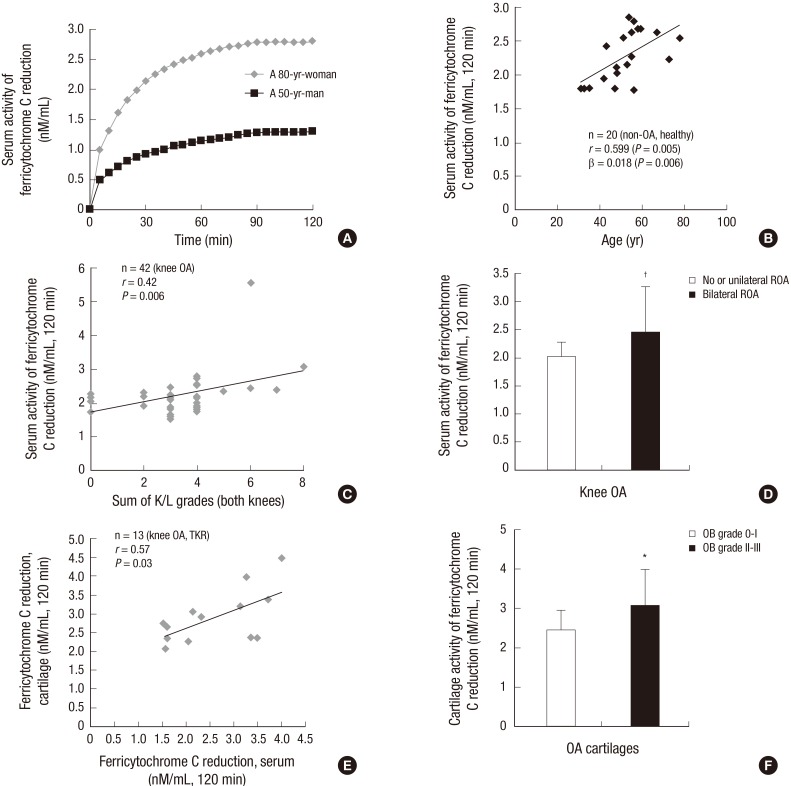
Oxidized ferricytochrome C reduction assay revealed that serum arNOX activity was higher in older participants as compared with younger participants (Fig. 2A), showing a positive correlation between arNOX activity and age in healthy control subjects (r=0.599, β=0.018, P=0.006) (Fig. 2B). The K/L grades of the right and left knees were added as 0 to 8 to assess the overall bony status of the knees in a patient. Serum arNOX activity showed a significant positive correlation with the radiographic scores in OA patients (r=0.42, P=0.006) (Fig. 2C). Regarding the extent of OA bony changes, a significantly higher serum arNOX activity was observed in the OA subgroup with bilateral ROA as compared to the subgroup with no or unilateral ROA (2.449± 0.81, 2.022±0.251 nM/mL, respectively, P=0.024) (Table 2 and Fig. 2D).
Fig. 2. arNOX activities of serum in healthy control subjects and those of serum and cartilage in OA patients. (A) Ferricytochrome C reduction assay showed a significantly different activity in serum between a healthy younger man and an older woman. (B) Serum arNOX activity showed a steady increase in correlation with age in healthy control subjects. (C) Serum arNOX activity in OA patients increased in correlation with the K/L grade of both knees. (D) Serum arNOX activity was significantly higher in patients with bilateral ROA as compared to those with no or unilateral ROA. (E) Cartilage arNOX activity showed a strong positive correlation with serum arNOX activity in OA patients. (F) Cartilage arNOX activity was more active in cartilages with surface defects (OB grade II, III) than in those without defects (OB grade 0, I). *P < 0.05; †P < 0.01.
Cartilage arNOX activity was consistently associated with higher levels of β-galactosidase, GLUT-1, and HIF-1α in OA cartilages (P=0.005). In a multivariate regression analysis with adjustment for age and BMI, cartilage arNOX activity was positively correlated to the OB grade (P=0.005) and serum arNOX activity in OA (r=0.577, P=0.03) (Fig. 2E). Higher serum arNOX activity was observed in patients with the cartilage defects (3.058 ±0.939 nM/mL for OB grade II, III) than in those without the defects (2.466±0.485 nM/mL for OB grade 0, I) (P=0.026) (Fig. 2F). The results may indicate that arNOX hyperactivity correlates to the severity and extent of cartilage degradation and ROA in age-related OA.
DISCUSSION
In the current study, serum and cartilage arNOX activities were analyzed as compared with the degrees of cartilage senescence, hypoxia, and glycolysis in age-related OA. The results showed a significant correlation of arNOX hyperactivity with aging. Even though OA was not a causal condition for arNOX hyperactivity, a higher arNOX activity was observed in OA patients with severe cartilage and bony changes.
Cartilage senescence was assessed by the expression and accumulation of β-galactosidase. The senescent chondrocytes were observed in age-related OA cartilages, prominently in those with defects of the articular surface. β-galactosidase is known to be specifically expressed in senescent cells. Dimri et al. (14), who first identified senescent cells using a SA-β-galactosidase staining, reported that the number of senescent cell increases in aging skin. Price et al. (15) confirmed SA-β-galactosidase positive chondrocytes in human articular cartilages and that the number increased with the severity of cartilage damage in OA. Consistently, in this study, senescent chondrocytes were identified in OA cartilages by SA-β-galactosidase staining. The number was also found to increase with the advance of surface defect. The results suggest that chondrocyte senescence is related with cartilage degradation in OA.
The enhanced expressions of cartilage HIF-1α and GLUT-1 in correlation with the OB grade may suggest that more oxygen and glucose are needed in higher OB grade and more senescent cartilage. As a result of the ordinary hypoxic and glycolytic conditions of articular cartilage, chondrocytes are prone to exposure to a profound hypoxia/ischemia with aging as compared to other tissues. Additionally, a further decrease of the oxygen tension was accompanied by OA (16). HIF-1α and GLUT-1 are the critical factors for mammalian cells to survive ischemia and hypoxia (17). HIF-1α expression is required for the cellular adaptation to tissue hypoxia, and GLUT-1 and -3 are hypoxia responsive isoforms of transmembranous glucose transporter as the first rate-limiting molecule of glucose metabolism. Upregulation of HIF-1α and GLUT-1 contents in a hypoxic environment has been demonstrated in chondrocytes (18,19). The current study may inform that cartilage senescence accompanies a worsening of hypoxia and glycolytic metabolism in correlation with the severity of cartilage defect.
Previous studies reported arNOX hyperactivities in senescence of human skin with a causal relationship (12) in which arNOX propagated the aging cascade to adjacent cells in response to aging. As a possible link between arNOX and age-related diseases, the current study evaluated arNOX activity in OA. In this study serum arNOX activity was not directly responsive to the presence of knee OA. However, arNOX activity was consistently higher in senescent, defective, profoundly hypoxic and glycolytic cartilages, and in OA with severe or extensive radiographic bony change. Cells with functionally deficient mitochondria become characterized by an anaerobic metabolism (20). In these cells, NADH accumulates from the glycolytic production of ATP, and hyperactivity of the plasma membrane electron transport system results in an NADH oxidase activity capable of cell surface generation of ROS, as the consequence of ENOX activity (21). The elevated plasma membrane electron transport activity in hypoxia and mitochondrial damages is critical to maintaining the NAD+/NADH homeostasis essential for survival (22). As the terminal oxidase, arNOX hyperactivity can be a potential source for age-related oxidative damage, generating superoxide and other ROS in human tissues and body fluids (23). This study has several limitations as a cross-sectional, case-control study in a small numbered population, and a prospective study with large populations would be required to confirm the effect of arNOX on cartilage degeneration in OA.
However, the current study may suggest that arNOX hyperactivity is correlated with cartilage senescence in association with hypoxia and glycolytic metabolism. The increasing arNOX activities may be related to the severity of cartilage defects and radiographic bony changes in age-related OA.
Footnotes
Funding: This research was supported by a grant of Yeungnam University Medical Center (2013).
DISCLOSURE: All authors declare that there are no conflicts of interest concerning to this work.
AUTHOR CONTRIBUTION: Study concepts & design: Kim MJ, Kim HJ, Hong YH, Lee CK, Shon OJ. Data collection: Kim MJ, Kim HJ, Hong YH, Lee CK, Kim YW, Shon OJ, Song IH. Analysis and interpretation of results, writing: Kim MJ, Kim HJ, Hong YH, Lee CK, Kim YW, Song IH. Review and revising the manuscript: Kim MJ, Kim HJ, Hong YH, Lee CK, Kim YW, Song IH. Final approval: all authors.
References
- 1.Di Cesare PE, Haudenschild DR, Samuels J, Abramson SB, Firestein GS. Pathogenesis of osteoarthritis. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, Kelley WN, editors. Kelley's textbook of rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013. pp. 1617–1633. [Google Scholar]
- 2.van der Kraan PM. Osteoarthritis year 2012 in review: biology. Osteoarthritis Cartilage. 2012;20:1447–1450. doi: 10.1016/j.joca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Peansukmanee S, Vaughan-Thomas A, Carter SD, Clegg PD, Taylor S, Redmond C, Mobasheri A. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J Orthop Res. 2009;27:529–535. doi: 10.1002/jor.20772. [DOI] [PubMed] [Google Scholar]
- 4.Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23:492–496. doi: 10.1097/BOR.0b013e3283494005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morré DJ, Chueh PJ, Lawler J, Morré DM. The sulfonylurea-inhibited NADH oxidase activity of HeLa cell plasma membranes has properties of a protein disulfide-thiol oxidoreductase with protein disulfide-thiol interchange activity. J Bioenerg Biomembr. 1998;30:477–487. doi: 10.1023/a:1020594214379. [DOI] [PubMed] [Google Scholar]
- 6.Morré DM, Lenaz G, Morré DJ. Surface oxidase and oxidative stress propagation in aging. J Exp Biol. 2000;203:1513–1521. doi: 10.1242/jeb.203.10.1513. [DOI] [PubMed] [Google Scholar]
- 7.Morré DJ, Morré DM. Cell surface NADH oxidases (ECTO-NOX proteins) with roles in cancer, cellular time-keeping, growth, aging and neurodegenerative diseases. Free Radic Res. 2003;37:795–808. doi: 10.1080/1071576031000083107. [DOI] [PubMed] [Google Scholar]
- 8.Morré DJ, Morré DM. ECTO-NOX proteins growth, cancer, and aging. New York, NY: Springer; 2013. [Google Scholar]
- 9.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 10.Butler J, Koppenol WH, Margoliash E. Kinetics and mechanism of the reduction of ferricytochrome c by the superoxide anion. J Biol Chem. 1982;257:10747–10750. [PubMed] [Google Scholar]
- 11.Morré DM, Morré DJ. Specificity of coenzyme Q inhibition of an aging-related cell surface NADH oxidase (ECTO-NOX) that generates superoxide. Biofactors. 2003;18:33–43. doi: 10.1002/biof.5520180205. [DOI] [PubMed] [Google Scholar]
- 12.Morré DM, Meadows C, Hostetler B, Weston N, Kern D, Draelos Z, Morré DJ. Age-related ENOX protein (arNOX) activity correlated with oxidative skin damage in the elderly. Biofactors. 2008;34:237–244. doi: 10.3233/BIO-2009-1077. [DOI] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 16.Pfander D, Cramer T, Swoboda B. Hypoxia and HIF-1alpha in osteoarthritis. Int Orthop. 2005;29:6–9. doi: 10.1007/s00264-004-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong YH, Park CW, Kim HS, Won KC, Kim YW, Lee CK. Effects of hypoxia/ischemia on catabolic mediators of cartilage in a human chondrocyte, SW1353. Biochem Biophys Res Commun. 2013;431:478–483. doi: 10.1016/j.bbrc.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Ren BF, Deng LF, Wang J, Zhu YP, Wei L, Zhou Q. Hypoxia regulation of facilitated glucose transporter-1 and glucose transporter-3 in mouse chondrocytes mediated by HIF-1alpha. Joint Bone Spine. 2008;75:176–181. doi: 10.1016/j.jbspin.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 21.Morré DM, Meadows C, Morré DJ. arNOX: generator of reactive oxygen species in the skin and sera of aging individuals subject to external modulation. Rejuvenation Res. 2010;13:162–164. doi: 10.1089/rej.2009.0919. [DOI] [PubMed] [Google Scholar]
- 22.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 23.Morré DJ, Morré DM. Aging-related cell surface ECTO-NOX protein, arNOX, a preventive target to reduce atherogenic risk in the elderly. Rejuvenation Res. 2006;9:231–236. doi: 10.1089/rej.2006.9.231. [DOI] [PubMed] [Google Scholar]