Abstract
Fetal lung development normally occurs in a hypoxic environment. Hypoxia-inducible factor (HIF)-1α is robustly induced under hypoxia and transactivates many genes that are essential for fetal development. Most preterm infants are prematurely exposed to hyperoxia, which can halt hypoxia-driven lung maturation. We were to investigate whether the HIF-1α inducer, deferoxamine (DFX) can improve alveolarization in a rat model of bronchopulmonary dysplasia (BPD). A rat model of BPD was produced by intra-amniotic lipopolysaccharide (LPS) administration and postnatal hyperoxia (85% for 7 days), and DFX (150 mg/kg/d) or vehicle was administered to rat pups intraperitoneally for 14 days. On day 14, the rat pups were sacrificed and their lungs were removed and examined. A parallel in vitro study was performed with a human small airway epithelial cell line to test whether DFX induces the expression of HIF-1α and its target genes. Alveolarization and pulmonary vascular development were impaired in rats with BPD. However, DFX significantly ameliorated these effects. Immunohistochemical analysis showed that HIF-1α was significantly upregulated in the lungs of BPD rats treated with DFX. DFX was also found to induce HIF-1α in human small airway epithelial cells and to promote the expression of HIF-1α target genes. Our data suggest that DFX induces and activates HIF-1α, thereby improving alveolarization and vascular distribution in the lungs of rats with BPD.
Graphical Abstract
Keywords: Alveolarization, Bronchopulmonary Dysplasia, Deferoxamine, Hypoxia-Inducible Factor
INTRODUCTION
Fetal lung development occurs in a hypoxic environment (3%-5% O2) (1). However, most very premature infants are exposed to hyperoxia after birth. Even if premature infants breathe room air, their arterial oxygen saturation is far above that of a fetus (1). Hypoxia-inducible factor (HIF)-1α is a transcription factor that orchestrates the expression of a number of genes that are essential for cellular adaptation to hypoxia (2). Hypoxia-inducible factor-1α acts as a master regulator of growth and development in a hypoxic environment during fetal development. Because HIF-1α plays a crucial role in growth and development in a hypoxic environment and rapidly degrades under hyperoxia or normoxia (3), the loss of HIF-1α signaling is likely to be one of the major postnatal physiologic changes that occur after birth in very premature infants born long before lung development is completed. It has been suggested that HIF-1α participates in normal lung development and is down-regulated in the lungs of primate models of bronchopulmonary dysplasia (BPD) (4).
Deferoxamine (DFX) is a bacterial siderophore that has been used as an iron chelator for iron overload-related diseases (5). Deferoxamine has been known to stabilize HIF-1α by inhibiting the activity of prolyl-4-hydroxylases through the depletion of iron (6), and has been shown to improve alveolarization in newborn rats exposed to hyperoxia (7). Recently, DFX has also been reported to upregulate vascular endothelial growth factor (VEGF) expression from adipose-derived stem cells and increase neovascularization and promote wound healing in diabetic rats (8,9). These beneficial effects of DFX were revealed to be mediated by the stabilization of HIF-1α.
The role of HIF-1α in the pathogenesis of BPD has been highlighted by some pioneering researchers (10,11). However, the effect of DFX on BPD has not been explored extensively. In our previous study, we demonstrated that sildenafil improved alveolar and vascular development in a rat model of BPD via the activation of HIF-1α (12). In the present study, we aimed to determine 1) whether DFX, a known stabilizer of HIF-1α, restores alveolarization and pulmonary vascular development using the same BPD model, 2) whether HIF-1α mediates the effect of DFX in an in vitro study.
MATERIALS AND METHODS
Animal experiment
A rat model of BPD was produced as previously described (13). Briefly, on gestation day 20, 1.0 µg of lipopolysaccharide (LPS, Escherichia coli 0111:B4; Chemicon International, Temecula, CA, USA) or an equal volume of vehicle (saline) was injected into the amniotic sacs of pregnant Sprague-Dawley rats. The pups were delivered spontaneously 48-72 hr after the injections. The rat pups exposed to LPS in utero were then exposed to 85% O2 (BPD groups), and pups exposed to vehicle were kept in room air (No BPD groups) with their dams for 7 days. Nursing rat dams were rotated between 85% O2 and room air every 24 hr to avoid O2 toxicity. After a 7-day exposure to 85% O2 or room air, all pups were subsequently kept in room air for an additional 7 days until sacrifice on day 14. As the main intervention of the study, DFX (150 mg/kg/d; Sigma-Aldrich, St. Louis, MO, USA) or an equal volume of vehicle (V, 0.5% dimethyl sulfoxide, DMSO) was injected into the peritoneal cavities of rat pups once daily for 14 days beginning on day 1. The dose of DFX was based on the study by Frank (7). Consequently, four experimental groups were established: No BPD+V; No BPD+DFX; BPD+V; BPD+DFX.
Alveolar and pulmonary vascular development
The rat pups were sacrificed on day 14. Lungs were removed and prepared for immunoassays and histological analyses as previously described (13). Morphometric analysis of the alveolarization was performed as previously described (13). To assess pulmonary vascular development, images of platelet endothelial cell adhesion molecule (PECAM)-1-stained slides were captured at 400× magnification. The number of PECAM-1-positive vessels (<100 µm in size) were counted in each high-powered field. The vascular density was expressed as the ratio of the area of blood vessels (PECAM-1-positive area) to the total area of lung parenchyma.
Immunofluorescence and immunohistochemistry
For PECAM-1 immunohistochemistry, the sections were incubated with primary goat anti-PECAM-1 polyclonal antibody (Abcam, Cambridge, MA, USA) followed by incubation with biotin-labeled goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Immune complexes were visualized using a diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA, USA) reaction. A light H&E counterstain was applied. For immunofluorescence analysis of HIF-1α, deparaffinized lung sections were incubated sequentially with anti-HIF-1α antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100), biotin-conjugated anti-rabbit antiserum (1:1,000), and streptavidin-Alexa Fluor 488 (1:1,000). Alexa Fluor 488 fluorescence was detected using the TSA™ kit (Life Technologies, Carlsbad, CA, USA), and nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI).
Cell culture
Human small airway epithelial cells (HSAEpCs) were purchased from PromoCell (Heidelberg, Germany). HSAEpCs were cultured in small airway epithelial cell growth medium (PromoCell) in a humidified atmosphere containing 5% CO2 at 37℃.
Reporter assay
Point five µg of the luciferase reporter plasmid (a kind gift of Dr. Eric Huang at the University of Utah, Salt Lake City, UT, USA) containing the rat VEGF promoter (-1,947 to -1 from the transcription site) was co-transfected with 1 µg of CMV-β-gal plasmid using Lipofectamine LTX-Plus reagent (Invitrogen, Carlsbad, CA, USA). After stabilized for 48 hr, cells were divided and incubated with either DMSO or DFX for 16 hr. Luciferase activity was measured using a Lumat LB 9507 luminometer (Berthold Technologies, Bad Wildbad, Germany), and divided by β-gal activity to normalize the transfection efficiencies. Three independent assays were conducted.
Semi-quantitative RT-PCR
Total RNAs were extracted from cells using TRIzol reagent and were reverse-transcribed at 46℃ for 20 min. Real-time PCR was performed using primers specific for human CA9 (5'-TATGAGGGGTCTCTGACTACA-3', 5'-TTCTCATCTGCACAAGGAAC-3'), LOX (5'-AGGGGTAGGGAGTTGGAGCGG-3', 5'-CCTAAACGTCAGCAGGCGACGG-3') and 18S (5'TTCGTATTGCGCCGCTAGA-3', 5'-CTTTCGCTCTGGTCCGTCTT-3'). To evaluate relative gene expression levels, quantitative real-time PCR was performed using an Applied Biosystems StepOne Real-Time PCR System and a SYBR Green JumpStart Taq ReadyMix qPCR kit (Sigma-Aldrich). The expression level of each target mRNA was normalized to the 18S RNA expression level in the same sample.
Immunoblotting
Total proteins were electrophoresed on 8%-12% SDS/polyacrylamide gels, transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA), and blocked with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TTBS) for 60 min. The membranes were then sequentially incubated overnight with anti-HIF-1α antibody (Santa Cruz Biotechnology, 1:1,000) or anti-β-tubulin antibody (1:3,000). The membranes were washed with TTBS and incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000) for 1 hr. The blots were visualized using an Enhanced Chemiluminescence Plus Kit (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
Data are expressed as the means±SEM unless otherwise stated. Comparisons were performed by Kruskall-Wallis analysis of variance; and post-hoc differences were assessed by the Mann-Whitney U test. P values <0.05 were considered statistically significant.
Ethics statement
The animal studies were performed after receiving approval of the institutional animal care and use committee (IACUC) in Seoul National University Bundang Hospital (IACUC approval No. BA1103-079/019-01).
RESULTS
Alveolar development
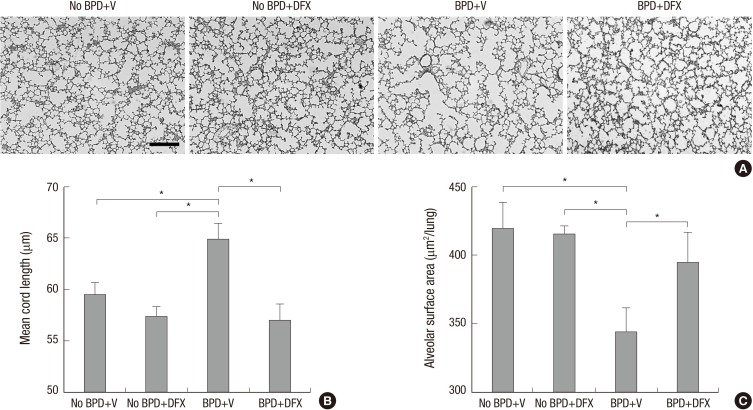
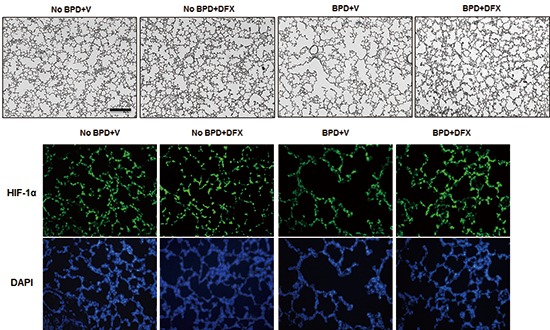
Photomicrographs of lung tissue sections from BPD+V group showed larger and simpler distal air spaces, a hallmark of disrupted alveolarization, compared to No BPD+V group. Deferoxamine appeared to restore alveolarization in animals with BPD, as indicated by the small and complex distal air spaces. However, in the animals without BPD, DFX did not alter alveolar development (Fig. 1A). Mean cord length (Lm), an indicator of average alveolar size, was significantly higher and alveolar surface area (SA) was significantly lower in BPD+V group than No BPD+V group. Deferoxamine significantly decreased Lm and increased SA in the animals with BPD. However, in the animals without BPD, DFX did not alter Lm or SA (Fig. 1B and C).
Fig. 1. Representative photomicrographs of rat lungs on day 14 (A). H&E staining; magnification, 100 ×. Scale bar indicates 200 µm. Morphometric data of rat lungs on day 14 are shown (B, C). n = 5-8 per group. *P <0.05. BPD, bronchopulmonary dysplasia; V, vehicle; DFX, deferoxamine.
Pulmonary vascular development
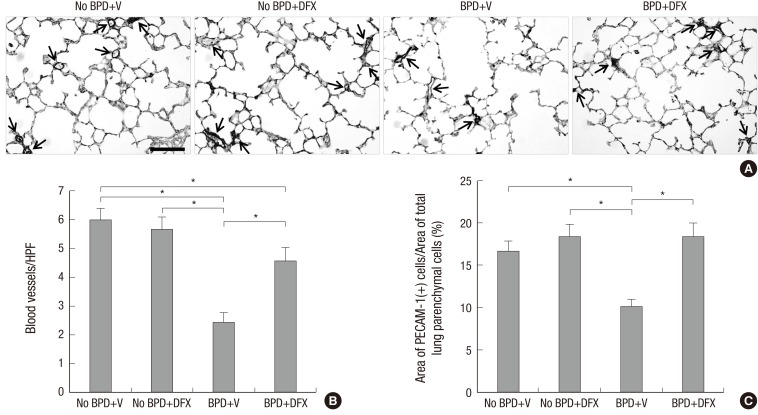
Photomicrographs of the lung tissue sections immunostained with PECAM-1 revealed a significantly lower number and density of vessels in BPD+V group than in No BPD+V group. Deferoxamine significantly increased the number and density of vessels in the animals with BPD. However, in the animals without BPD, DFX did not cause significant changes in the number or density of vessels (Fig. 2A-C).
Fig. 2. Representative photomicrographs illustrating the results of immunohistochemical analysis of PECAM-1 in rat lungs on day 14 (A). Arrows indicate pulmonary vessels stained with PECAM-1. Magnification, 400 ×. Scale bar indicates 50 µm. The number of pulmonary vessels per high-power field (HPF) (B) and pulmonary vascular density (C) are displayed in a bar graph. n = 5-8 per group. *P < 0.05. PECAM, platelet endothelial cell adhesion molecule; BPD, bronchopulmonary dysplasia; V, vehicle; DFX, deferoxamine.
Effect of deferoxamine on hypoxia-inducible factor-1α expression
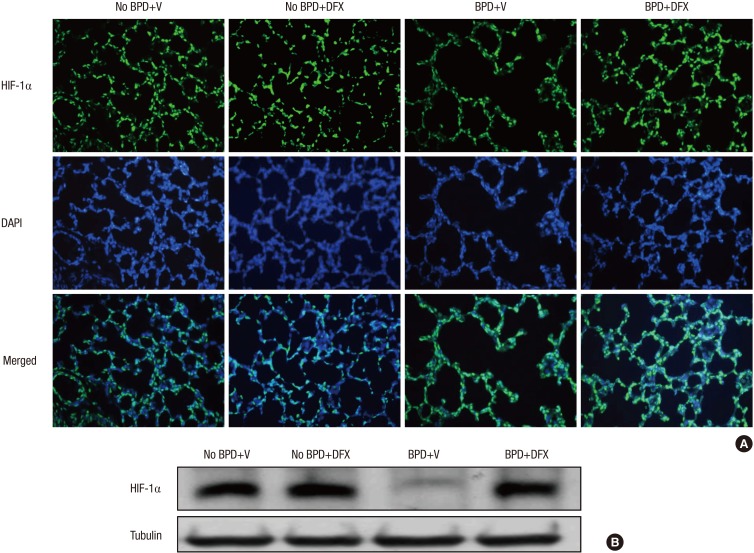
Immunofluorescence analysis showed that HIF-1α expression was substantially decreased in the lungs of the animals with BPD; however, HIF-1α expression was recovered by DFX (Fig. 3A). To quantify HIF-1α levels, we detected HIF-1α by Western blotting and found that the suppression of HIF-1α in the animals with BPD was effectively rescued by DFX (Fig. 3B).
Fig. 3. Representative photomicrographs illustrating the results of immunofluorescence analysis of HIF-1α in rat lung on day 14 (A). Treatment with DFX recovered the expression of HIF-1α in the lungs of BPD rats. Tissue sections were serially cut at 4 µm and subjected to immunofluorescence staining of HIF-1α. Lung tissues in the four experimental groups were homogenized, and 20 µg of protein was subjected to Western blotting using an anti-HIF-1α antibody (B). Tubulin was used as a loading control. DFX, deferoxamine; HIF, hypoxia-inducible factor; BPD, bronchopulmonary dysplasia; V, vehicle; DAPI, 4',6-diamidino-2-phenylindole.
Effect of deferoxamine on hypoxia-inducible factor-1α expression in lung epithelial cells
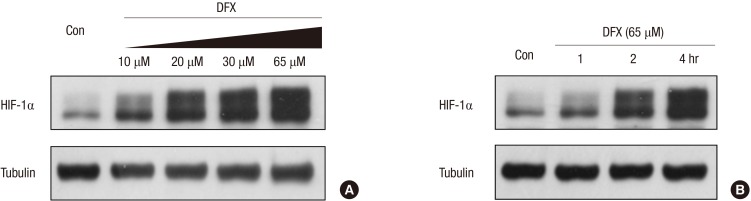
The induction of HIF-1α by DFX was confirmed in human small airway epithelial cells (HSAEpC). When the cells were treated with DFX, HIF-1α was induced in a dose-dependent manner, even under normoxia (Fig. 4A). Temporally, induction of HIF-1α began as early as 2 hr after DFX treatment (Fig. 4B).
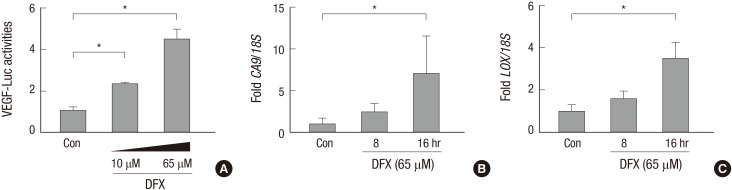
Fig. 4. Deferoxamine induced HIF-1α in a dose-dependent manner in human small airway epithelial cells (HSAEpCs) (A). HSAEpCs were incubated with 10, 20, 30 or 65 µM DFX for 8 hr, and HIF-1α expression was evaluated by Western blotting. HSAEpCs were incubated with 65 µM DFX for the indicated times (B). The expression of HIF-1α was analyzed by Western blotting. DFX, deferoxamine; HIF, hypoxia-inducible factor.
Effect of deferoxamine on hypoxia-inducible factor-1α-driven gene expression in lung epithelial cells
To evaluate whether the DFX-induced HIF-1α was active, we analyzed its transcriptional activity using a VEGF promoter-luciferase reporter system; and we found that VEGF promoter activity was enhanced by DFX (Fig. 5A). Subsequently, we analyzed the mRNA levels of the HIF-1α target genes CA9 and LOX by semi-quantitative RT-PCR, and we found that CA9 and LOX mRNA expression was induced by DFX in a time-dependent manner (Fig. 5B and C).
Fig. 5. Deferoxamine-induced HIF-1α was active. Deferoxamine activated VEGF promoter (A). Human small airway epithelial cells (HSAEpCs) were co-transfected with 1 µg of VEGF promoter-luciferase plasmid and β-galactosidase plasmid. After 48 hr, the cells were treated with 10 or 65 µM DFX for 16 hr and lysed for the luciferase assay. Luciferase activity was normalized to β-galactosidase activity. Bars represent the mean±SD (n = 4) of luciferase activity. Deferoxamine induces the mRNA expression of HIF-1α target genes (B, C). Cells were treated with 65 µM DFX for the indicated times and subsequently harvested. Total RNAs were extracted from the cells, and the mRNA levels of CA9 (B), LOX (C), and 18S were analyzed by RT-qPCR. Each bar represents the mean±SD of four separate experiments. *P < 0.05. DFX, deferoxamine; HIF, hypoxia-inducible factor.
DISCUSSION
Fetal lung development occurs in a hypoxic environment; airway branching and pulmonary vascular development are promoted in such hypoxic environments (14). BPD occurs exclusively in very premature infants whose lungs should have been actively developing in the hypoxic environment in utero (15). After premature birth, this active lung development is disrupted by various detrimental stimuli such as hyperoxia, mechanical injury and inflammation (16,17). Even room air is relatively hyperoxic to these premature infants compared to the normal hypoxic environment in utero (18). Moreover, the limited antioxidant defense of premature infants makes them vulnerable to oxidative injury, even at lower oxygen tensions (17).
The pathologic hallmark of BPD is arrested alveolar and pulmonary vascular development (19). Alveolar development and pulmonary vascular development are interlocked processes, as evidenced by the finding that experimental inhibition of angiogenesis during fetal lung development results in the arrest of distal airspace development (20). The primary transcriptional regulator of vascular development in the hypoxic fetal environment is HIF-1α (3). After birth, HIF-1α degrades rapidly; thus, angiogenic activity decreases due to the relatively oxygen-rich environment (18). Asikainen et al. demonstrated the downregulation of HIF-1α after preterm birth in a preterm baboon model of BPD (4). Taken together, these data indicate that stabilization of HIF-1α could be an approach for the prevention of BPD. By stabilizing HIF-1α, the fetal hypoxic environment could be mimicked; thus, pulmonary vascular development and distal airspace development could be enhanced in the extra-uterine hyperoxic environment.
The pharmacologic stabilization of HIF-1α can be achieved by selective and non-selective inhibitors of prolyl 4-hydroxylase (PHD). FG-4095 is a specific inhibitor of PHD, while dimethyloxaloylglycine and DFX are non-specific inhibitors (18,20). In our study, we activated HIF-1α with DFX. In an in vitro study, we confirmed that DFX increases HIF-1α production and its transcriptional activity in a dose- and time-dependent manner. Also, DFX increased HIF-1α expression in the lungs and improved pulmonary vascular development and alveolarization. These findings suggest that DFX could be an approach for stabilizing HIF-1α and relieving the pathologic consequences of disrupted lung development.
Deferoxamine is a well-known stabilizer of HIF-1α and, consequently, an inducer of HIF-1α-dependent genes, including genes involved in angiogenesis; however, the mechanism of HIF-1α stabilization is not clearly understood (21). Iron takes part in redox reactions that produce reactive oxygen species (22). As an iron chelator, inhibition of PHD by depleting iron from the catalytic center could be a plausible mechanism of HIF-1α stabilization by DFX (20). Because we exposed the animals to hyperoxia to induce BPD in this study, the positive effects of DFX on alveolar and pulmonary vascular development might be a result of the reduction of oxidative stress by the iron-chelating effect of DFX. However, in a study with adipose-derived stem cells, it was shown that DFX stabilizes HIF-1α in a redox-independent manner, in which other antioxidants failed to reproduce or alter the effect of DFX on the transcriptional activity of HIF-1α (8). When we evaluated the oxidative stress in the lungs, the level of protein carbonyl was not different between DFX-treated animals and untreated animals (data not shown). Therefore, it is not probable that reduced oxidative stress is the main action mechanism of DFX in our study.
We confirmed that DFX increased the production of HIF-1α in both in vivo and in vitro experiments. The dose-dependent induction of HIF-1α and its transcriptional activity by DFX was demonstrated in in vitro study. Small airway epithelial cells, which were used in in vitro study, are directly exposed to room air or hyperoxia after preterm birth and play an important role in alveolar and pulmonary vascular development via epithelial-mesenchymal interactions (23). In this regard, the results from in vitro study have the biological relevance and complement our in vivo study. The increased pulmonary vascular development observed in DFX-treated animals is likely to be the effect of HIF-1α stabilization by DFX, because HIF-1α is known to mediate angiogenesis and activate VEGF expression. Whereas VEGF is a specific regulator of angiogenesis, HIF-1α is a master regulator involved not only in angiogenesis but also in various other processes including cellular metabolism, proliferation, and survival (2,24). In fact, administration of VEGF alone has been shown to produce abnormal vessels with excessively high permeability and tortuosity (25). However, the overexpression of HIF-1α has been shown to result in increased vascularity with normal permeability (26). Because HIF-1α acts in a pleiotropic manner, it is expected that pharmacologic stabilization of HIF-1α will lead to more balanced angiogenesis than the administration of one or several angiogenic factors.
It is noteworthy that DFX caused an increase in the expression of HIF-1α in the lung tissues of animals with BPD as well as those without BPD. However, DFX did not cause further improvement in alveolar or pulmonary vascular development in the animals without BPD. This finding suggests that the pharmacological stabilization of HIF-1α may have little effect on alveolar and pulmonary vascular development under normal physiologic condition. Further studies are required to determine the optimal usage of DFX for the prevention and/or treatment of BPD.
Footnotes
Funding: This work was supported by the SNUBH Research Fund (grant No. 03-2007-007) and by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2010-0021644).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study conception and design: Choi CW, Chun YS, Kim BI. Animal experiments: Choi CW, Lee HJ, Park HS. Acquisition of data: Choi CW, Lee HJ, Park HS, Chun YS. Data analyses: Choi CW, Chun YS, Kim BI. Drafting and writing the manuscript: Choi CW, Lee J, Park HS, Chun YS, Kim BI. Final manuscript approval: all authors.
References
- 1.Stenmark KR, Gebb SA. Lung vascular development: breathing new life into an old problem. Am J Respir Cell Mol Biol. 2003;28:133–137. doi: 10.1165/rcmb.F259. [DOI] [PubMed] [Google Scholar]
- 2.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med. 1998;131:207–214. doi: 10.1016/s0022-2143(98)90091-9. [DOI] [PubMed] [Google Scholar]
- 4.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol. 2005;40:538–546. doi: 10.1002/ppul.20321. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 6.Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172:738–747. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank L. Hyperoxic inhibition of newborn rat lung development: protection by deferoxamine. Free Radic Biol Med. 1991;11:341–348. doi: 10.1016/0891-5849(91)90149-w. [DOI] [PubMed] [Google Scholar]
- 8.Liu GS, Peshavariya HM, Higuchi M, Chan EC, Dusting GJ, Jiang F. Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1796. [DOI] [PubMed] [Google Scholar]
- 9.Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res Clin Pract. 2013;101:62–71. doi: 10.1016/j.diabres.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;291:L588–L595. doi: 10.1152/ajplung.00098.2006. [DOI] [PubMed] [Google Scholar]
- 11.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J. 2006;20:1698–1700. doi: 10.1096/fj.06-5887fje. [DOI] [PubMed] [Google Scholar]
- 12.Park HS, Park JW, Kim HJ, Choi CW, Lee HJ, Kim BI, Chun YS. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am J Respir Cell Mol Biol. 2013;48:105–113. doi: 10.1165/rcmb.2012-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res. 2009;65:323–327. doi: 10.1203/PDR.0b013e318193f165. [DOI] [PubMed] [Google Scholar]
- 14.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288:L167–L178. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D, Hack M. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 16.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 17.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 18.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Günzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci U S A. 2005;102:10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 20.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Günzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Hirsilä M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 2005;19:1308–1310. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- 22.Galaris D, Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 23.Wessells NK. Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–466. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- 24.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 25.Larcher F, Murillas R, Bolontrade M, Conti CJ, Jorcano JL. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene. 1998;17:303–311. doi: 10.1038/sj.onc.1201928. [DOI] [PubMed] [Google Scholar]
- 26.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]