Abstract
Reptiles (sauropsids) represent the sister group to mammals, and the basal members of Reptilia may provide a good model for the condition of the common ancestor of both groups. Sex-determining mechanisms (SDM) and organizations of sex chromosomes among genotypically sex-determining (GSD) species vary widely across reptiles. Birds and snakes, for example, are entirely GSD whereas other reptiles, like all crocodilians, exhibit temperature-dependent sex determination (TSD). Here we explore the evolution of sex chromosomes and SDM within reptiles, using family-level analyses of character evolution and applying parsimony, likelihood, Bayesian, and stochastic methods. We find support for the common ancestor of amphisbaenians and whiptail lizards (Laterata) possessing the XY (male heterogametic) GSD mechanism, while the ancestors of Testudines and Crocodylia, as well as the larger group Archosauromorpha (here containing turtles) are inferred to have exhibited TSD. We also find evidence consistent with the hypothesis that the XY system is more labile and evolves faster than does the ZW (female heterogametic) system. Phylogenetic-based speciation tests do not support an association between GSD and speciation, and reject the hypothesis that the presence of the XY system is associated with speciation in reptiles.
Introduction
Heteromorphic sex chromosomes are thought to arise from ancestral autosome, usually by selection acting on sexually antagonistic mutations that initiate divergence between homologous chromosomes (Ohno 1967). Sex-linked mutations are thought to accumulate on nascent sex chromosomes characterized by reduced levels of recombination. The origins of sex chromosomes are not well understood experimentally, or theoretically, and are a fundamental focus in modern biology. All studied amphibians exhibit genotypic sex determination (GSD), and although both heterogametic systems (male heterogametic: XY and female heterogametic: ZW) are present in amphibians, the ZW organization of sex chromosomes appears ancestral for the group (Hillis and Green 1990). Mammals almost universally exhibit XY organization of sex chromosomes. Reptiles (Sauropsida, which refers to the monophyletic group including birds) possess a diverse array of sex-determining mechanisms (SDM), from XY and ZW in closely related GSD species, to temperature-dependent sex determination (TSD). For this reason, reptiles are an excellent model group in which to understand the evolution and diversity of SDM.
Studies of evolutionary biology are frequently challenged by traits that vary in degree, not in kind, but must be classified as discrete to chart their histories. SDM are a classic example of this challenge, especially within Sauropsida, given the diversity of SDM within the group. Once considered discrete states, TSD and GSD are now more often considered ends of a spectrum in which sensitivity of the SDM to incubation temperature decreases from one end of the spectrum (TSD) to the other (GSD) (Sarre et al. 2004; Uller et al. 2007; Radder et al. 2008).
At present, SDM are characterized by a sex-determining response to incubation temperature and, among GSD species, identity of the heterogametic sex. There are three reported TSD types (Ia, Ib, and II) that differ in the direction of bias of sex ratios in response to increasing incubation temperature. In TSD species, increasing incubation temperature either decreases (Type Ia) or increases (Type Ib) proportions of male offspring. Type II species produce a maximal proportion of male offspring at an intermediate temperature whereas either cooler or warmer temperatures yield lower proportions of males. Sauropsids with TSD include all crocodilians, tuatara, and some turtles and lizards (Bull 1983) and this trait has been found to be adaptive in some instances (Warner and Shine 2008). On the other end of the SDM spectrum, there are two reported GSD types (ZZ/ZW and XX/XY) that differ in the identity of the heterogametic sex. Among sauropsids, both types of GSD have been reported in turtles (Ezaz et al. 2006) and lizards whereas all snakes and birds are ZZ/ZW GSD species (Matsubara et al. 2006; Uller et al. 2007).
Evolution of SDM
The adaptive significance and evolutionary history of SDM are not clear. TSD may be beneficial as a means by which parents maintain Fisherian offspring sex ratios in which equal investments are made in sons and daughters because, in some species, fitness of sons and daughters co-vary with the environment (Fisher 1930; Charnov and Bull 1977). If TSD permits sex-ratio compensation for environmental variability, then it may be positively selected (Girondot and Pieau 1999). However, mechanisms by which species of any SDM might manipulate offspring sex ratios have not been clearly demonstrated despite the exciting advances that have been made toward relating maternal effects and sex-ratio models to evolutionary dynamics of SDM (Warner and Shine 2005; Janzen and Phillips 2006; Ferguson-Smith 2007). Likewise, neither GSD nor male/female heterogamety are clearly adaptive. As explained for TSD, GSD may allow parents to invest equally in sons and daughters. Reports of sex ratio bias in GSD species are common, though mechanisms for such a bias are not understood (reviewed by Cockburn et al. 2002). To date, male and female heterogamety also present no clear evolutionary patterns or adaptive significance. Autosomes may be converted to sex chromosomes by the addition or exaptation of a gene that is beneficial to one sex but detrimental to the other (sexual antagonism; see van Doorn and Kirkpatrick 2007) but, excluding the sex-determining region on the mammalian Y chromosome (Charlesworth et al. 2005), the frequency of male-specific and female-specific sexual antagonisms among vertebrates has not been elucidated.
In this report, we reconstruct ancestral states for SDM across sauropsids. We also test for correlations between the SDM character state and speciation rate using a birth-death model. Previous parsimony-based analyses indicate that TSD is ancestral across sauropsids and has been lost at least six times in turtles and arisen at least three times in lizards (Janzen and Krenz 2004). Here, we extend this work by accounting for variability in SDM by organizing sauropsid taxa into families, some of which exhibit multiple SDM. However, we do not explore the mechanisms by which sex chromosomes arise from autosomal ancestors.
In this analysis, we also take advantage of the growing consensus that turtles are the sister group of crocodilians and birds (Shedlock et al. 2007) as well as including information on birds, often still neglected in research on sauropsid evolutionary biology, despite the danger of reaching paraphyletic conclusions. Coupled with information on SDM across Sauropsida (Olmo 2005), we also measured rates of change in SDM using stochastic and Bayesian methods. Taxon sampling is always a concern in phylogenetic and comparative studies. We opted to be as conservative on this matter as possible by using polymorphic character scoring and limiting our taxon sampling to groups for which good data exist. For instance, at least two species that were previously identified as GSD but without heteromorphic sex chromosomes have recently been found to possess cryptic sex chromosomes (Ezaz et al. 2005; Ezaz et al. 2006a). To avoid complication from anticipated changes in recognition of heterogamety in GSD species, we have limited our analyses to GSD species for which heteromorphic sex chromosomes have already been reported. We do not consider GSD species with homomorphic sex chromosomes as this category is likely to decrease, if not disappear, with improved cytogenetic techniques.
Materials and methods
Taxon sampling and character coding
Data on sex chromosomes were obtained primarily from the ChromoRep database (Olmo 2005), which represents ∼ 1300 non-avian sauropsid species. This accounts for nearly 30% of living sauropsid species, but many major groups are sampled, most to the familial level. We obtained additional sex determination data from the literature (Bull 1980, 1983; Donellan 1985; Olmo 1986, 2005; Janzen and Paukstis 1991; Viets et al. 1994; Ewert et al. 2004, 2006a; Janzen and Krenz 2004; Janzen and Phillips 2006; Matsubara et al. 2006). Many sauropsids have not been studied for SDM. However, 38 families are represented in our dataset, providing a broad overview of non-avian reptiles. We included seven families of birds, choosing taxa that represent the major groups (for example, Paleognathae, Galliformes, and Passeriformes), for a total of 45 sauropsid taxa. JMP 6.03 (SAS_Institute 2006) was used to perform statistical analyses and generate graphs. Because the analyses presented in this report were performed at the familial level, we treated the characters as potentially polymorphic (presence or absence could co-occur within families). Our analyses of rates were restricted to Sauropsida by removing outgroups (Xenopus and mammals), although outgroup are used in the character-state reconstructions.
Phylogenetic framework and divergence times
Mesquite v2.01 (Maddison and Maddison 2007) was used to create the phylogenetic trees. Divergence times and topologies were drawn from various sources for the following groups: Amphibia (Evans et al. 2004; Marjanović and Laurin 2007), Mammalia (Springer et al. 2004), Sauropsida (Rest et al. 2003), Squamata (Rest et al. 2003; Vidal and Hedges 2005; Wiens et al. 2006; Kumazawa 2007), Serpentes (Lawson et al. 2005; Lee et al. 2007; Vidal et al. 2007), Testudines (Fujita et al. 2004; Near et al. 2005; Fritz and Bininda-Emonds 2007), and Aves (Ericson et al. 2006), although portions of the avian tree are still unresolved (Brown et al. 2007), our tree is likely robust given our taxon sampling. The StratAdd (Faure et al. 2006) package for Mesquite was used to anchor the phylogenies within the geologic timescale (Gradstein et al. 2004). Uncertain divergence times were estimated as half the span from the last ancestor to the first descendant node. Because SIMMAP (stochastic mutational mapping on phylogenies) and the BiSSE (binary-state speciation and extinction) module in Mesquite cannot handle polymorphic data we analyzed two datasets, one biased toward GSD (at least one member within the family exhibited GSD) and one biased toward TSD (at least one member within the family contained TSD) and looked for concordance.
Analysis of character evolution
We used several methods to explore the evolution of SDM, including parsimony, likelihood, Bayesian, and stochastic approaches. For the parsimony analysis, characters were traced in Mesquite v2.01 (Maddison and Maddison 2007) with the states unordered. Stochastic character-mapping was implemented using the SIMMAP software package (Build 29072007-1.0-B2.3.2; Bollback 2006), which uses a mutational approach to reconstruct character history (Nielsen 2002; Huelsenbeck et al. 2003). The SIMMAP prior on the bias parameter was uninformative (α = 1, K = 19), while the rate parameter was set to α = 3, β = 2, K = 60. The tree was rescaled before applying the priors. The number of realizations was set to 1000.
The BayesMultiState (Pagel and Meade 2004) component of the BayesTraits program (http://www.evolution.rdg.ac.uk/BayesTraits.html) was used to estimate the rate of character change as well as to reconstruct ancestral character states using maximum likelihood and Bayesian approaches. The maximum likelihood implementation used 50 likelihood attempts per tree. The Bayesian implementation settings were 5 000 000 iterations, 100 000 burnin, and sampling every 100 iterations. Rate deviation priors were selected to achieve a ∼0.20–0.40 acceptance rate. The multistate analysis (0 = TSD, 1 = XY, 2 = ZW) used a rate deviation of 0.01 and an exponential (0.00, 0.50) reverse jump hyperprior for model selection. The binary analysis (0 = TSD, 1 = GSD) used a rate deviation of 0.1 and an exponential (0.00, 0.10) reverse jump hyperprior. Bayesian analyses were run at least twice and repeated with a gamma reverse-jump hyperprior (mean: 0.00, 0.50; variance: 0.00, 0.50). There was no appreciable difference in the posterior distributions between analyses using the exponential distribution prior and the gamma distribution prior.
For the Bayesian ancestral-state reconstructions, nodes can be constrained to a given state (0, 1, or 2) and a harmonic mean produced. By comparing the harmonic means of these three models, we can evaluate the following hypotheses. Hypothesis (H) 1: there is support for either TSD (state 0) or XY (state 1) H2: there is support for either TSD (state 0) or ZW (state 2). H3: there is support for either XY (state 1) or ZW (state 2).
Character association with speciation
The BiSSE module for mesquite (Maddison and Maddison 2007) was used to determine whether there is a significant association between the presence or absence of sex chromosomes and speciation in extant reptiles. The model contains six parameters: two speciation rates (for states 0 and 1), two extinction rates (for states 0 and 1), and two rates of character change (q01 and q10) (Maddison et al. 2007). The module estimates the parameters and calculates a maximum likelihood value given a tree. Significance is determined by constraining a parameter and recalculating the likelihood [likelihood ratio test (LRT)]. We scaled our tree with branch lengths in absolute time uniformly by 1/100 to perform this analysis because the original branch lengths in time were computationally difficult for the BiSSE package. We also scaled our tree by 1/10 in a reanalysis to assess the impact of scaling on our results. For a stringent analysis, the number of divisions in each branch was set to 1000 and the number of random starting points for likelihood optimization was set to 20. The underflow checking frequency was performed each iteration and the analysis was repeated at least three times to confirm the likelihood results.
The LRT and Bayes factor (BF) tests were used to evaluate significance. For BFs, values >2 are taken as positive evidence, >5 as strong evidence, and >10 as compelling evidence for the better fitting model. For LRTs, twice the difference between the log likelihoods was used to calculate P-values, assuming a chi-squared distribution and degrees of freedom equal to the difference between the numbers of free parameters in the nested models. For the rate of character change in the Bayesian analysis, the frequency in the posterior distribution represents the support, or belief in, the estimated parameter value.
Results and discussion
When sex determination is coded as a binary variable, parsimony reconstruction takes 14 steps. The SDM of the family-level ancestor of Lepidosauromorpha is equivocal, but the nodes along the entire backbone of this group are inferred to have GSD (Fig. 1A). Parsimony reconstruction also fails to unequivocally reconstruct the SDM of the archosauromorph and sauropsid family level ancestor. When SDM are coded as a multistate variable (Fig. 1B), parsimony reconstruction takes 21 steps and unequivocally reconstructs TSD as the ancestral family-level condition of sex determination in Archosauromorpha, but with GSD in Lacertilia and other deep nodes being equivocal.
Fig. 1.
Parsimony reconstruction with sex determination coded as a polymorphic variable across many families of reptiles. Mammals and amphibians are represented at the species level. (A) Parsimony reconstruction of sex determination coded as a binary variable takes 14 steps and is equivocal concerning the ancestral family-level condition of sex determination in the deepest nodes within Sauropsida, but reconstructs Squamata as genotypic sex determination. (B) Parsimony reconstruction of sex determination coded as a multistate variable takes 21 steps and is equivocal concerning the ancestral family-level condition of sex determination in the deepest nodes within Sauropsida, but reconstructs Lacertilia as having an XY system and Archosauromorpha as having temperature-dependent sex determination.
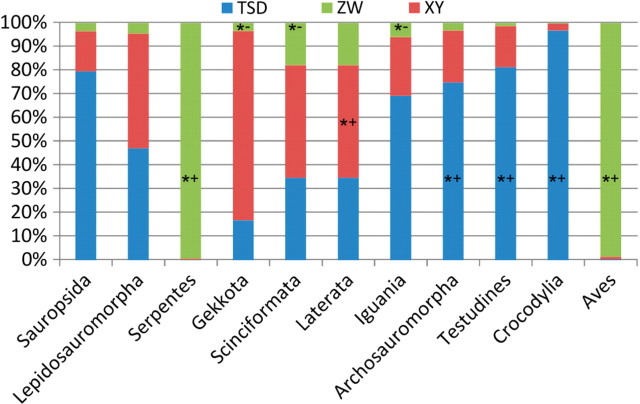
For the Bayesian analysis (Fig. 2), reconstruction of Sauropsida is equivocal (TSD-XY BF = 0.1, TSD-ZW BF = 0.7, XY-ZW BF = 0.6). The two deepest nodes within reptiles are Archosauromorpha (birds, crocodilians, and here turtles) and Lepidosauromorpha (tuatara, lizards, and snakes). For Lepidosauromorpha the Bayesian analysis is equivocal as well (TSD-XY BF = 2.0, TSD-ZW BF = 1.0, XY-ZW BF = −1.1). Within Lepidosauromorpha, inferred ancestral family-level states are a little clearer with Gekkota, Iguania, and Scinciformata equivocal between TSD and XY, but rejecting ZW (Gekkota: TSD-ZW BF = 5.0, XY-ZW BF = 5.7, Iguania: TSD-ZW BF = 4.3, XY-ZW BF = 4.2, Scinciformata: TSD-ZW BF = 4.6, XY-ZW BF = 5.38). Better resolved reconstructions are produced for the family-level ancestor of Laterata as XY (TSD-XY BF = −3.2, XY-ZW BF = 14.7) and Serpentes as ZW (TSD-ZW BF = −6.8, XY-ZW BF = −6.0).
Fig. 2.
Bayesian posterior-probability fraction of sex determination coded as a multistate character for the family-level ancestor in several important nodes of the sauropsid tree. “*+” indicates significant support for the designated type of sex determination while “*−” indicates significant rejection (two of the three pair-wise comparisons rejected).
On the other side of the sauropsid tree, the Bayesian analysis supports TSD over XY (BF = 5.8) and ZW (BF = 8.7) for the SDM in Archosauromorpha, as well as turtles (TSD-XY BF = 2.2, TSD-ZW BF = 7.9) and crocodilians (TSD-XY BF = 7.4, TSD-ZW BF = 12.8) within it. The avian ancestor, not surprisingly, is predicted to have had the ZW system of sex determination (TSD-ZW BF = −7.4, XY-ZW BF = −10.5), although we have no idea how far back this trait extends into Dinosauria or Ornithodira.
When sex determination is coded as a binary trait, TSD changes into GSD faster than the reverse (mean of stochastic posterior q01 = 18.6, q10 = 1.5; mean of Bayesian posterior probability q01 = 0.003, q10 = 0.0002), though under maximum likelihood this difference is marginally significant (maximum likelihood q01 = 0.002, q10 = 0, P = 0.06). When sex determination is coded as a multistate character, the same basic pattern is detected, suggesting that the analyses presented here are robust to character coding. A more detailed picture emerges, however, from the multistate character coding scheme. For example, although the XY system is relatively uncommon in reptiles, it is convergently distributed, from skinks to some iguanids and turtles (Modi and Crews 2005; Ezaz et al. 2006). The XY system of sex determination evolves from TSD and back at equivalent rates (for example, mean of PP of q01 = 0.003, mean of PP of q10 = 0.003), suggesting that this system has a low degree of phylogenetic inertia. However, the ZW system appears to evolve from TSD (mean of PP of q02 = 0.001) an order of magnitude faster than in the reverse direction (mean of PP of q20 = 0.0002). These results suggest that XY systems evolve more readily than do ZW systems within reptiles and that a ZW ancestor is less likely to give rise to a TSD descendant. The rate of inferred evolution among the two GSD types suggests that the ZW system potentially evolves from an XY system an order of magnitude faster than in the reverse direction (mean of PP of q12 = 0.002; mean of PP of q21 = 0.0004). Interestingly, these results are consistent with the scenario of ZW systems evolving from XY systems faster than ZW systems evolve from TSD, implying a complex evolutionary mechanism for the evolution of sex chromosomes over simpler ideas of GSD evolving directly from TSD systems. A difference in the evolutionary rates between XY and ZW sex-chromosomal systems suggests a greater instability in XY systems than is detected in ZW systems. This difference is intriguing because both are believed to have evolved from the same principal cause of sexual antagonism. Sexual antagonisms that block recombination in XY systems could be less evolutionarily stable.
As for Y-linked genes in XY species, W-linked genes have been found to have higher dn/ds ratios than paralogous Z-linked genes (Berlin and Ellegren 2006), demonstrating that non-recombining regions undergo high rates of non-synonymous substitutions regardless of male or female heterogamety. Furthermore, exchanging X chromosomes during interbreeding between two diverging species is more likely to produce infertile hybrids than when autosomes or Y chromosomes are exchanged (Masly and Presgraves 2007). Dubbed the “large X-effect”, this phenomenon is thought to be an important force during speciation in XY species. A “large Z-effect” almost surely also exists (Kirkpatrick and Hall 2004), suggesting that the presence of GSD systems plays an important part in reproductive isolation and hence in speciation. Given the central role of SDM in reproductive isolation, we hypothesized that there would be significant association between GSD and speciation rates across sauropsids. We found no support for this hypothesis, however, when we coded SDM biased toward GSD (BiSSE; at least one member within the family contained GSD; P = 0.5) or when we coded biased toward TSD (BiSSE; at least one member within the family contained TSD; P = 0.8). Because we scaled the tree for the speciation test, we do not estimate rates of character change, but limit our analysis to evaluating hypotheses about the relative magnitude of speciation rates. We performed additional BiSSE tests to evaluate the hypothesis that the majority of the relationship between speciation and GSD was due to the presence of the ZW system of sex determination in more specious groups. The results reject the hypothesis that the presence of the XY system is associated with speciation in sauropsids (P = 0.002). The ZW system is associated with speciation (λ0 : 0.1, λ1 : 1.0), but the result is not significant (P = 0.2). In a reanalysis to assess the impact of scaling on our results, we scaled our tree again by 1/10 and obtained concordant results (not shown).
The BiSSE method, like other birth-death models, has low statistical power at small tree sizes (our tree contains 45 taxa and is likely robust to this assumption). Moreover, this test, as well as the comparative-rate and ancestral-state tests above, assumes that the tree under study is entirely known and that it contains all extant taxa within the group. The assumption of including all living descendant species becomes increasingly difficult the broader the comparative study becomes. We have not included all sauropsid families within these analyses and the SDM in many sauropsid species is unknown, making matters more difficult. But the limits of available data should not preclude an exploration of the evidence. We attempted to account for these problems by focusing at the familial level and performing a variety of character-coding schemes. Our conclusions would likely remain unchanged if more complete sampling were possible given the fixation of the ZW system in the diverse clades Aves and Serpentes. However, the results presented here will surely shift as new discoveries are made, but they also point in important directions and make quantitative hypotheses about the evolution of sex determination, and may be useful for formulating future research as the biology of sauropsid genomes becomes illuminated through cytological and genomic advances.
Conclusions
Our conclusions expand upon previously reported parsimony-based analyses (Janzen and Krenz 2004). TSD is reconstructed as the ancestral family-level condition for Archosauromorpha. Within this major clade, the ancestral state for Testudines and Crocodylia are also reconstructed as TSD while Aves is reconstructed as genotypically sex-determined (GSD) with ZW sex chromosomes. In contrast, within Lepidosauria, TSD is inferred to have evolved from an ancestral family that is inferred to have been universally GSD according to our parsimony analy. Within Lepidosauromorpha, Bayesian analysis reconstructs Serpentes as ZW and Laterata as XY, though the ZW system is rejected for Gekkota, Scinciformata, and Iguania. Whereas the XY system evolves to and from TSD at equal rates, ZW evolves an order of magnitude faster from TSD than vice-versa. As a result, XY systems in Sauropsida evolve more readily than do ZW systems and a ZW ancestor is less likely than an XY ancestor to give rise to a TSD descendant.
Acknowledgments
This research was first presented at the Symposium on Reptile Genomics during the 2008 conference for the Society of Integrative and Comparative Biology (SICB) in San Antonio, Texas. Peter Midford, and Nicole Hobbs deserve our gratitude for providing useful comments and support. Two divisions within SICB provided funding for the symposium, which helped facilitate this work: Developmental and Cell Biology (DDCB) and Evolutionary Developmental Biology (DEDB). Additional symposium funding was provided by the National Science Foundation's Division of Integrative Organismal Systems (NSF Grant # 0809547 to Nicole Valenzuela, Dan Janes, and Chris Organ). Primary funding was provided by NIH NRSA Postdoctoral Fellowships granted to Christopher L. Organ (5F32GM075490) and Daniel E. Janes (5F32GM072494).
References
- Berlin S, Ellegren H. Accumulation of deleterious mutations on the female-specific W chromosome in birds. J Mol Evol. 2006;62:66–72. doi: 10.1007/s00239-005-0067-6. [DOI] [PubMed] [Google Scholar]
- Bollback JP. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics. 2006;7:88. doi: 10.1186/1471-2105-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Payne RB, Mindell DP. Nuclear DNA does not reconcile ‘rocks’ and ‘clocks’ in Neoaves: a comment on Ericson et al. Biol Lett. 2007;3:257–9. doi: 10.1098/rsbl.2006.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull JJ. Sex determination in reptiles. Q Rev Biol. 1980;55:3–21. [Google Scholar]
- Bull JJ. Menlo Park, CA: Benjamin/Cummings; 1983. Evolution of sex determining mechanisms. [Google Scholar]
- Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. 2005;95:118–28. doi: 10.1038/sj.hdy.6800697. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Bull J. When is sex environmentally determined. Nature. 1977;266:829–30. doi: 10.1038/266828a0. [DOI] [PubMed] [Google Scholar]
- Cockburn A, Legge S, Double MC. Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy ICW, editor. Sex Ratios: Concepts and Research Methods. Cambridge, UK: Cambridge University Press; 2002. pp. 266–86. [Google Scholar]
- Donellan SC. The evolution of sex chromosomes in scincid lizards. Australia (NSW): Macquarie University; 1985. [Google Scholar]
- Ericson PGP, Anderson CL, Britton T, Elzanowski A, Johansson US, Kallersjo M, Ohlson JI, Parsons TJ, Zuccon D, Mayr G. Diversification of Neoaves: integration of molecular sequence data and fossils. Biol Lett. 2006;2:543–7. doi: 10.1098/rsbl.2006.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatellae DC. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol. 2004;33:197–213. doi: 10.1016/j.ympev.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Ewert BJ, Etchberger CR, Nelson CE. Turtle sex-determining modes and TSD patterns, and some TSD pattern correlates. In: Valenzuela N, Lance VA, editors. Temperature-dependent sex determination in vertebrates. Washington, DC: Smithsonian Books; 2004. pp. 21–32. [Google Scholar]
- Ezaz T, Quinn AE, Miura I, Sarre SD, Georges A, Graves JAM. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res. 2005;13:763–76. doi: 10.1007/s10577-005-1010-9. [DOI] [PubMed] [Google Scholar]
- Ezaz T, Valenzuela N, Grutzner F, Miura I, Georges A, Burke RL, Graves JAM. An XX/XY sex microchromosome system in a freshwater turtle, Chelodina longicollis (Testudines: Chelidae) with genetic sex determination. Chromosome Res. 2006;14:139–50. doi: 10.1007/s10577-006-1029-6. [DOI] [PubMed] [Google Scholar]
- Faure E, Lony E, Lovigny R, Menegoz A, Ting Y, Laurin M. StratAdd module for Mesquite. 2006. ( http://tolweb.org/notes/?note_id=3669)
- Ferguson-Smith M. The evolution of sex chromosomes and sex determination in vertebrates and the key role of DMRT1. Sex Dev. 2007;1:2–11. doi: 10.1159/000096234. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Oxford University Press; 1930. The genetical theory of natural selection. [Google Scholar]
- Fritz U, Bininda-Emonds ORP. When genes meet nomenclature: tortoise phylogeny and the shifting generic concepts of Testudo and Geochelone. Zoology. 2007;110:298–307. doi: 10.1016/j.zool.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Fujita MK, Engstrom TN, Starkey DE, Shaffer HB. Turtle phylogeny: insights from a novel nuclear intron. Mol Phylogenet Evol. 2004;31:1031–40. doi: 10.1016/j.ympev.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Girondot M, Pieau C. A fifth hypothesis for the evolution of TSD in reptiles. Trends Ecol Evol. 1999;14:359–60. doi: 10.1016/s0169-5347(99)01681-x. [DOI] [PubMed] [Google Scholar]
- Gradstein FM, et al. A geologic time scale 2004. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Hillis DM, Green DM. Evolutionary changes of heterogametic sex in the phylogenetic history of amphibians. J Evolution Biol. 1990;3:49–64. [Google Scholar]
- Huelsenbeck JP, Nielsen R, Bollback JP. Stochastic mapping of morphological characters. Syst Biol. 2003;52:131–58. doi: 10.1080/10635150390192780. [DOI] [PubMed] [Google Scholar]
- Janzen FJ, Krenz JG. Phylogenetics: which was first, TSD or GSD? In: Valenzuela N, Lance VA, editors. Temperature-dependent sex determination in vertebrates. Washington, DC: Smithsonian Books; 2004. pp. 121–30. [Google Scholar]
- Janzen FJ, Paukstis GL. Environmental sex determination in reptiles - ecology, evolution, and experimental-design. Q Rev Biol. 1991;66:149–79. doi: 10.1086/417143. [DOI] [PubMed] [Google Scholar]
- Janzen FJ, Phillips PC. Exploring the evolution of environmental sex determination, especially in reptiles. J Evol Biol. 2006;19:1775–84. doi: 10.1111/j.1420-9101.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Hall DW. Sexual selection and sex linkage. Evolution. 2004;58:683–91. doi: 10.1111/j.0014-3820.2004.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y. Mitochondrial genomes from major lizard families suggest their phylogenetic relationships and ancient radiations. Gene. 2007;388:19–26. doi: 10.1016/j.gene.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Lawson R, Slowinski JB, Crother BI, Burbrink FT. Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Mol Phylogenet Evol. 2005;37:581–601. doi: 10.1016/j.ympev.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Lee MSY, Hugall AF, Lawson R, Scanlon JD. Phylogeny of snakes (Serpentes): combining morphological and molecular data in likelihood, Bayesian and parsimony analyses. System Biodivers. 2007;5:371–89. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.01. 2007. ( http://mesquiteproject.org)
- Maddison WP, Midford PE, Otto SP. Estimating a binary character's effect on speciation and extinction. Syst Biol. 2007;56:701–10. doi: 10.1080/10635150701607033. [DOI] [PubMed] [Google Scholar]
- Marjanović D, Laurin M. Fossils, molecules, divergence times, and the origin of lissamphibians. Syst Biol. 2007;56:369–88. doi: 10.1080/10635150701397635. [DOI] [PubMed] [Google Scholar]
- Masly JP, Presgraves DC. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biol. 2007;5:1890–8. doi: 10.1371/journal.pbio.0050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Tarui H, Toriba M, Yamada K, Nishida-Umehara C, Agata K, Matsuda Y. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc Natl Acad Sci USA. 2006;103:18190–5. doi: 10.1073/pnas.0605274103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi WS, Crews D. Sex chromosomes and sex determination in reptiles. Curr Opin Genet Dev. 2005;15:660–5. doi: 10.1016/j.gde.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Near TJ, Meylan PA, Shaffer HB. Assessing concordance of fossil calibration points in molecular clock studies: an example using turtles. Am Nat. 2005;165:137–46. doi: 10.1086/427734. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Mapping mutations on phylogenies. Syst Biol. 2002;51:729–39. doi: 10.1080/10635150290102393. [DOI] [PubMed] [Google Scholar]
- Ohno S. Sex chromosomes and sex-linked genes. New York: Springer; 1967. [Google Scholar]
- Olmo E. A Reptilia. In: John B, editor. Gebruder Borntraeger. Stuttgart, Berlin: Gebruder Borntraeger; 1986. [Google Scholar]
- Olmo E. Rate of chromosome changes and speciation in reptiles. Genetica. 2005;125:185–203. doi: 10.1007/s10709-005-8008-2. [DOI] [PubMed] [Google Scholar]
- Pagel MD, Meade AB. Bayesian estimation of ancestral character states on phylogenies. Syst Biol. 2004;53:673–84. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol Lett. 2008;4:176–8. doi: 10.1098/rsbl.2007.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest JS, Ast JC, Austin CC, Waddell PJ, Tibbetts EA, Hay JM, Mindell DP. Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome. Mol Phylogenet Evol. 2003;29:289–97. doi: 10.1016/s1055-7903(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Sarre SD, Georges A, Quinn A. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays. 2004;26:639–45. doi: 10.1002/bies.20050. [DOI] [PubMed] [Google Scholar]
- SAS_Institute. JMP, Version 6.03. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- Shedlock AM, Botka CW, Zhao SY, Shetty J, Zhang TT, Liu JS, Deschavanne PJ, Edward SV. Phylogenomics of nonavian reptiles and the structure of the ancestral amniote genorne. Proc Natl Acad Sci USA. 2007;104:2767–72. doi: 10.1073/pnas.0606204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MS, Stanhope MJ, Madsen O, de Jong WW. Molecules consolidate the placental mammal tree. Trends Ecol Evol. 2004;19:430–8. doi: 10.1016/j.tree.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Uller T, Pen I, Wapstra E, Beukeboom LW, Komdeur J. The evolution of sex ratios and sex-determining systems. Trends Ecol Evol. 2007;22:292–7. doi: 10.1016/j.tree.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Van Doorn GS, Kirkpatrick M. Turnover of sex chromosomes induced by sexual conflict. Nature. 2007;449:909–12. doi: 10.1038/nature06178. [DOI] [PubMed] [Google Scholar]
- Vidal N, Delmas AS, David P, Cruaud C, Coujoux A, Hedges SB. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C R Biol. 2007;330:182–7. doi: 10.1016/j.crvi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Vidal N, Hedges SB. The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C R Biol. 2005;328:1000–8. doi: 10.1016/j.crvi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Viets BE, Ewert MA, Talent LG, Nelson CE. Sex-determining mechanisms in squamate reptiles. J Exp Zool. 1994;270:45–56. [Google Scholar]
- Warner DA, Shine R. The adaptive significance of temperature-dependent sex determination: experimental tests with a short-lived lizard. Evolution. 2005;59:2209–21. [PubMed] [Google Scholar]
- Warner DA, Shine R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature. 2008;451:566–8. doi: 10.1038/nature06519. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Brandley MC, Reeder TW. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution. 2006;60:123–41. [PubMed] [Google Scholar]