Abstract
Frailty is a major cause of disability and loss of independence in the elderly. Using clinically relevant criteria from our previously established mouse frailty index, we investigated the effects of aerobic exercise on frailty in male C57BL/6 mice. In order to measure the effect of treatment on the individual animals, we constructed a composite score, the Frailty Intervention Assessment Value. We hypothesized voluntary aerobic exercise would improve individual criteria and reverse or prevent frailty in the old mice. Five adult and 11 old mice (6 and 28+ months, respectively) were housed individually in cages with running wheels for 4 weeks. Controls (adult, n = 5 and old, n = 17) were housed without wheels. Inverted cling grip and rotarod tests were performed pre- and postintervention. Hind limb muscles were used for biochemical analysis and contractility experiments. We conclude that the exercise stimulus reversed frailty and was sufficient to maintain or improve functional performance in old mice, as well as to produce measurable morphological changes. In addition, the Frailty Intervention Assessment Value proved to be a valuable tool with increased power to detect treatment effects and to examine the intervention efficacy at the level of the individual mouse.
Key Words: Aerobic, Frailty, Exercise, Voluntary wheel running, Mice
It is well known that frailty commonly occurs with advancing age and is a major cause of disability and loss of independence in the elderly. Frailty is also associated with poor health outcomes such as falls, hospitalization, long-term care admission, and mortality (1–3). Many investigations have examined the efficacy of exercise interventions aimed at improving functional measures that are associated with frailty, such as muscular strength and muscle mass. The ability of resistance exercise to improve strength, mass, and physical function is well documented (4–6). However, the ability of aerobic exercise to elicit significant functional and morphological improvements in old skeletal muscle is not as well characterized. Furthermore, few if any studies have used aging mouse models to investigate the effects of aerobic exercise on specific frailty criteria that are commonly used in clinical practice.
Aging mouse models have become an important research tool through which to characterize the etiology of sarcopenia and frailty, as well as to test potential interventions. While various definitions of frailty and its criteria exist (2,3,7) we chose to apply an operationalized frailty definition, as described in our previous work (8) which used criterion measures as set forth by Fried and colleagues (1) and validated by Bandeen-Roche and colleagues (9). In this definition, frailty is conceptualized as a syndrome, characterized by loss of muscle mass and strength, decreased mobility, increased weakness, and low energy expenditure (1,8,9). Then, we sought to use old mice as a model of aging, and use aerobic exercise as a possible intervention for the prevention and treatment of age-related frailty using mouse-specific analogs of the criteria specified as clinical human measurements of frailty (8). The Liu and colleagues mouse frailty index (FI) (8) uses the maximum rotarod speed (in revolutions per minute [RPM]) in place of walking speed, the inverted cling grip test (in seconds) instead of the hand/grip dynamometer reading for strength, an endurance score (mean of the time on the rotarod and grip test in seconds) to substitute for the exhaustion questionnaire, and the average kilometers run per day on a voluntary wheel running (VWR) for activity level. The cutoff points for the determination of frailty were 1.5 SD lower than the mean value of the criteria. An animal having three or more criteria below the cutoff point was considered frail, those with two were considered mildly frail.
While there have been other attempts to quantify frailty in the mouse model (7,10,11), ours (8) is the only adaptation of a clinical frailty phenotype as described in humans and assessed in a common mouse model of aging (C57BL/6). Walston and colleagues (10) did an admirable job with identifying a rodent model that mimicked human frailty, the IL-10 knockout mouse, with the presence of chronic inflammation. The other two published studies (10,11) focused on quantifying frailty are based on deficit accumulation, not phenotype, and include numerous invasive measures and/or highly specialized equipment and techniques. In Liu and colleagues (8), we sought to use simple, noninvasive outcome measures employing commonly available and affordable equipment to quantify a frailty phenotype and called it a FI. Thus, we might well refer to it as a clinically relevant frailty phenotype index to distinguish it from deficit accumulation indices.
In order to assess whether or not an intervention has a significant and clinically relevant effect, it is common to create a composite assessment tool, which provides greater sensitivity to detect change that is not possible with individual determinant factors alone. For instance, the Barthel index is used as a measurement of activities of daily living to assess functional independence in the field of long-term rehabilitation (12). Therefore, in the present study, we aimed to develop a composite score for mice—the Frailty Intervention Assessment Value (FIAV), which measures the combined distance in standard deviations from the mean of all the criteria by summing the standard values, and defined it as the difference from baseline (FIAV1) to postintervention (FIAV2). Thus, the overall effect of an intervention can objectively be determined. Of course, the standard scores of individual criteria, or the means of groups, can also be examined separately to determine what effect the intervention had on each determinate or group.
There were three main goals in this study. The primary goal was to determine if the exercise intervention reversed frailty, as measured by our FI and its criteria. The second major goal was to develop an assessment score (the FIAV) to describe improvement at the individual mouse level. The third major goal was to determine if the exercise stimulus was sufficient to stimulate contractile, morphological, and metabolic changes in the hind limb muscles of the old mice.
We hypothesized that the voluntary aerobic exercise intervention would improve the functional capabilities representing frailty indicators in elderly humans, of the old mice, and that this improvement would translate into a measurable improvement on our FI. In addition, we further hypothesized that: (i) most animals would improve on an individual level, as measured by the FIAV, (ii) there would be a greater response overall to the intervention in the younger animals, and (iii) the exercised mice would demonstrate greater skeletal muscle contractile function and positive morphological and metabolic changes postintervention than control mice.
Methods
Animals
Thirty-eight C57BL/6 male mice (10 adult, 6–8 months and 28 old, 28–30 months, NIA Aging Colony) were housed under a 12-hour light/dark cycle at 20°C in specific pathogen-free facilities. The mice had ad libitum access to Purina Rodent Chow and water, and were acclimated for 7 days prior to initiating the experimental protocol. The control animals were group housed without access to running wheels for the duration of the experiment and the exercise mice were individually housed with a running wheel over the 1-month intervention period. The body mass of the mice did not change over the course of the intervention and there was no statistical difference between groups either before or after the training period (Table 1). The baseline frailty indices and criteria scores of the older exercise mice were reported in a previous publication (8). All procedures conformed to the University’s guidelines on experimental animal research.
Table 1.
Body Mass and Frailty Criteria Data and Post-hoc Results
| Adult (A) | Old (O) | Adult vs Old | ||||||
|---|---|---|---|---|---|---|---|---|
| Control (C) | Exercise (E) | AC vs AE | Control | Exercise | OC vs OE | AC vs OC | AE vs OE | |
| Mean ± SD | Mean ± SD* | p Value | Mean ± SD* | Mean ± SD* | p Value | p Value | p Value | |
| Mass* | - | 32.8±0.423 | - | 32.5 ± 2.38 | 34.9 ± 3.0 | .074 | - | .153 |
| Mass† | 31.7±2.84 | 30.8±1.36 | .584 | 32.8±2.14 | 32.2±3.6 | .552 | .43 | .338 |
| Mass dif. | - | −2.02±1.92 | - | −0.2113±1.23 | −1.41±1.97 | .189 | - | .546 |
| Grip* (s) | 99.8±51.3 | 64.2±30.9 | .162 | 83.9±49.4 | 98.5±45.2 | .526 | .671 | .095 |
| Grip† (s) | 197.2±94.4 | 161±72.0 | .246 | 65.1±39.8 | 122.6±61.5 | .022 | <.001 | .368 |
| %Dif grip | 220.4±140.6 | 330.7±299.4 | .184 | 97.0±56.4 | 150.9±101.4 | .281 | .069 | .015 |
| Rota* (rpm) | 18.0±4.5 | 12.3±1.8 | <.001 | 11.1±2.2 | 9.6±1.8 | .064 | <.001 | .084 |
| Rota† (rpm) | 14.3±4.6 | 18.8±1.9 | .070 | 12.1±4.3 | 12.8±2.1 | .775 | .355 | .007 |
| %Dif rota | 80.5±20.0 | 156.1±30.0 | .001 | 110±32.4 | 137.8±30.9 | .029 | .069 | .298 |
| Endurance* (s) | 108.7±14.8 | 67.3±17.7 | .005 | 72.6±28.8 | 73.5±27.2 | .857 | .014 | .36 |
| Endurance† (s) | 50.0±20.9 | 142.7±37.5 | <.001 | 40.7±19.7 | 99.2±34.7 | <.001 | .808 | .008 |
| %Dif endurance | 46.1±17.3 | 224.3±83.5 | <.001 | 61.2±30.1 | 152.6±75.4 | <.001 | .627 | .021 |
| Activity* (km/d) | - | 3.8±0.733 | - | - | 1.53±0.234 | - | - | <.001 |
| Activity† (km/d) | - | 4.89±2.42 | - | - | 1.55±1.333 | - | - | .006 |
| %Dif activity | - | 137.3±90.2 | - | - | 91±42.7 | - | - | .246 |
| FIAV* (SD) | 0.002±1.52 | −0.002±2.32 | .759 | 0.0±2.59 | 0.3836±3.28 | .935 | .663 | .412 |
| FIAV† (SD) | −1.416±3.62 | 12.49±5.42 | <.001 | −1.04±2.90 | 3.41±3.49 | .001 | .43 | <.001 |
| FIAV | −1.42±3.45 | 12.49±4.72 | <.001 | −1.037±1.97 | 3.026±2.23 | .001 | .625 | <.001 |
Notes: Post-hoc results are from two-way ANCOVA (least significant difference, adjusted for body mass postintervention) for the criteria (rota = rotarod, grip = inverted cling grip test) and FIAV (Frailty Intervention Assessment Value), and from ANOVA for body mass. AC = adult control; AE = adult exercise; ANCOVA = analysis of covariance; ANOVA = analysis of variance; %Dif = percent change from baseline at postintervention; N/A = not applicable (not collected); OC = old control; OE = old exercise; rpm = revolutions per min; - = not collected or not applicable.
*Baseline measurement.
†Postintervention measurement.
General Experimental Protocol and Exercise Intervention of VWR
The study design is shown in Figure 1. All mice underwent baseline testing before being randomized into exercise or control groups by age. Five adult and 11 old mice (exercise groups) were housed individually in Lafayette Activity Wheel Cages (#80820F/ 86060; Lafayette Instruments, Lafayette, IN) outfitted with running wheels and optical sensors that counted the wheel revolutions. Mice in the wheel cages ran ad libitum for 4 weeks. The total wheel revolutions for the exercise animals were recorded daily and converted to kilometers/d (1 rev = 0.4 m), and then the mean daily distance per week was calculated. The 4-week time period was chosen because 4 weeks of exercise have been shown to be sufficient for detecting significant physiological and morphological adaptations in the skeletal muscles of adult mice (10,11). Five adult and 17 old mice served as age-matched controls and were housed without wheels for 4 weeks.
Figure 1.
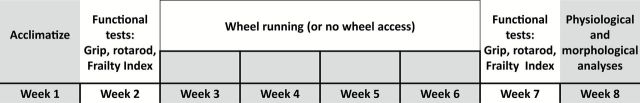
Experimental protocol. After acclimatizing for 1wk, functional performance testing of inverted cling grip and rotarod was performed. Mice were then randomized into exercise or control groups by age. Each mouse was evaluated for frailty using the frailty index, using the four criteria (grip strength, walking speed, physical activity, and endurance score). Mice in the exercise groups were housed individually in running wheel cages for 4wk, while age-matched controls were housed in standard cages without wheels. Wheel revolutions per 24-h period were recorded daily. The same functional tests and frailty index were evaluated in each mouse after the 4-wk period. In wk 8, the mice were sacrificed and hind limb muscles were harvested for biochemical and histological analysis and whole-muscle contractility experiments.
Evaluation of Frailty—FI
To determine the frail animals in the old group, we used the FI as described in our previous work (8). Briefly, the mean score of each frailty criterion was calculated before the VWR intervention, and a cutoff point was determined in each criterion (grip, rotarod, activity level, endurance). The cutoff point was defined as 1.5 SD below the mean (approximately the bottom 7%) for each criterion. After intervention, each animal had a new score for each criterion but used the same cutoff point as before training. Frailty was defined if three or more of the criteria measures were below the cutoff point, whereas mild frailty was designated if two criteria fell below the cutoff.
Evaluation of Frailty—Frailty Criteria
In order to evaluate the degree of frailty before and after the exercise intervention, four measures were used as criteria for generating the FI in each mouse. The criteria included: (i) inverted cling grip test (overall muscle strength and endurance), rotarod rpm (overall neuromotor function, walking speed), physical activity (mean km/wk of VWR), and a derived endurance score (mean of maximum time on rotarod and grip test). Each test was performed both before and after the training intervention. The Supplementary Methods section and our previous work provide further details (8,13).
Inverted Cling Grip Test (Grip Strength)
A custom-built device was used to measure this criterion. The mouse was placed on a grid, which was gently inverted so that the mouse had to cling to the grid while upside down to keep from falling to a pad below. The amount of time before the mouse fell was recorded and the mean of two trials was used as the outcome measurement.
Rotarod Test (Walking Speed)
Each mouse was placed on the cylinder of a Rotarod LSI testing device (LSi Letica Rota-Rod R/S), which was set at a speed of 4 RPM and accelerated to 40 RPM over 5 minutes. The speed (in RPM) at which the mouse fell off the rotating cylinder was recorded, and the mean of three trials was used as the outcome measurement.
Endurance Score
The inverted cling grip and rotarod also evaluate endurance in mice. The endurance score was calculated from the mean time (seconds) of the grip and rotarod test (endurance score [seconds] = [grip time + rotarod time]/2). See the Discussion section for more details.
Physical Activity Level
The mice (exercise groups) were individually housed in the Lafayette Activity Wheel Cages. The mean daily running distance (km/d) during the first and the last week was used as the measure of physical activity levels.
Determination of the Efficacy of the Training Intervention at the Individual Animal Level—FIAV
The FIAV is a single number composite score that is used to compare the effects of treatments on the frailty criteria at an individual animal level (see Figure 2). FIAV condenses the information from the individual frailty criteria into one value. The FIAV was calculated by summing the standardized value for each parameter. For each criterion, both before and after the intervention, the distance in standard deviations from the mean of the baseline of the sample animals was calculated. The FIAV was calculated before the intervention (FIAV1) and afterward (FIAV2). FIAV2 represents the distance in standard deviations from the mean of the baseline score of all the criteria, after treatment. For example, a FIAV2 of four would mean that the mouse criteria scores were 4 total SD from the baseline mean of his cohort after treatment. Whereas, a FIAV2 score of −2.5 would indicate that the animal scored 2.5 SD below the mean after treatment. In this way, the overall treatment effect was calculated for an individual mouse. The control animals were assessed using a score of zero for the activity level criterion because we did not expose the control animals to the running wheel. See the Discussion section for more details.
Figure 2.
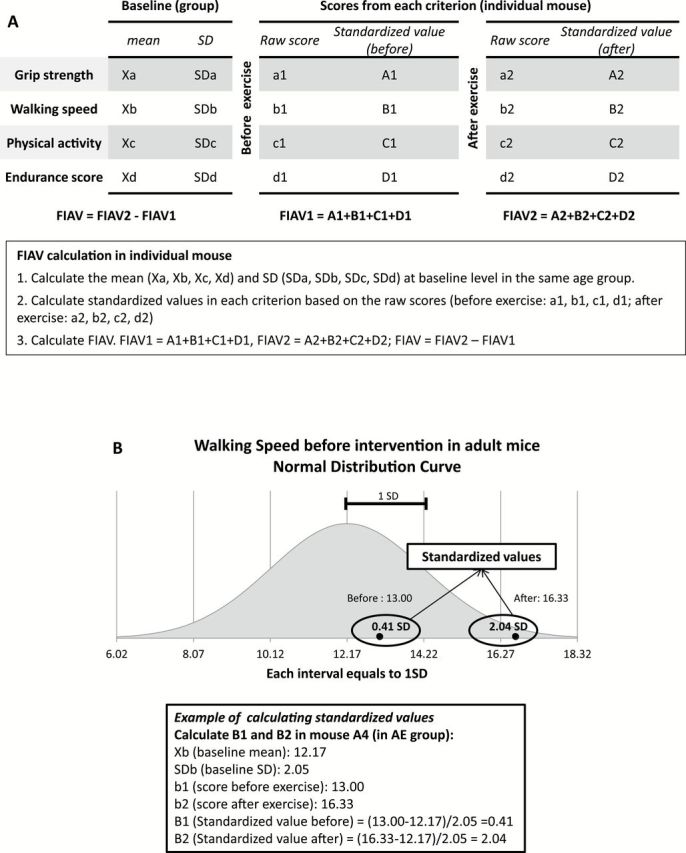
Calculation of Frailty Intervention Assessment Value (FIAV) in individual mice. The FIAV is a composite score that is used to compare the effects of treatments on the frailty criteria at an individual animal level. (A) General process of FIAV calculation. To evaluate the FIAV, three steps are needed. Firstly, the mean (Xa, Xb, Xc, Xd) and the standard deviation (SDa, SDb, SDc, SDd) of each criterion (grip strength, walking speed, physical activity, and endurance score) in the same age group (AE or OE) are calculated. The second step is to calculate the standardized values using the raw scores from each mouse for each criterion, a1, b1, c1, and d1 (before exercise) and a2, b2, c2, and d2 (after exercise). Specifically, the standardized value equals to the difference between raw score and the baseline mean in standard deviations. Lastly, the FIAV1 is the sum of all the standardized values before exercise (FIAV1 = A1+B1+C1+D1) and the FIAV2 is the sum of the standardized values after exercise. The FIAV is the difference between these two values (FIAV = FIAV2 − FIAV1). (B) Example of calculation of standardized values in walking speed of mouse A4. The baseline mean (Xb = 12.17) and standard deviation (SDb = 2.05) were calculated in the AE group before intervention. The before and after walking speed scores (b1 and b2) of mouse A4 were converted into standardized values (B1 and B2) using the following equations: B1 = (b1 − Xb)/SDb and B2 = (b2 − Xb)/SDb. A normal distribution curve in the AE group was generated based on the baseline mean and standard deviation. The standardized values (0.41 and 2.04) represent the distance from the baseline cohort mean in standard deviations, which is shown in the normal distribution curve. AE = adult exercise; OE = old exercise.
Muscle Physiology, Morphology, and Biochemistry
After behavioral testing, the mice were sacrificed and hind limb muscles were used for biochemical and histological analysis and for whole-muscle contractility experiments. The purpose of the measurements was twofold: (i) to ascertain whether a 1-month period of voluntary aerobic conditioning was a sufficient stimulus to alter the parameters, and (ii) if so, to determine to what extent the muscles, and not the nervous system, were affected. The soleus (SOL) and extensor digitorum longus (EDL) were used for isolated whole-muscle physiology to determine whether the exercise increased contractile ability at the tissue level, and subsequent myosin heavy chain (MHC) analyses to determine if there was a change in MHC isoform content. The tibialis anterior (TA) was used for histochemical analyses of fiber types and fiber cross-sectional area (CSA) to assess whether there was an increase in muscle fiber size or a change in the muscle’s fiber type composition. The plantaris was also used for measurement of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), an indicator of mitochondrial biogenesis. A brief description of each procedure follows, with further details available in the Supplementary Methods section, or in prior publications as noted.
Physiological Analysis—Muscle Contractility
The maximum isometric contractile force (P 0), maximum velocity (V max), and maximal power (P max) were analyzed as described previously (13). Briefly, the muscles were kept viable in oxygenated Krebs buffer, tied to a force transducer, and suspended between platinum electrodes at optimal length. The muscles were then electrically stimulated to examine P 0 and velocity at percentages of P 0, which was then used to derive P max.
Morphological Analysis—Fiber Size Identification
CSA of individual fibers were analyzed by using ImageJ analysis software (National Institutes of Health, http://rsb.info.nih.gov/ij/) to measure the circumference of hematoxylin and eosin-stained cryoslices of TA muscle.
Morphological Analysis—Fiber Type Composition and MHC Analysis
To identify the fiber type composition transformation after intervention in the adult and old groups, MHC isoforms were analyzed by silver stain and immunohistochemistry. After homogenization, MHC isoform expression of the SOL and EDL were determined using sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 5% acrylamide gels and silver staining as previously described (13). Additional cross-sectional slices of the TA muscles were immunostained for type I (slow), fast, IIa, and IIb fibers, detailed in previous work (14).
Biomarker of Mitochondrial Biogenesis—PGC-1α
Total relative content of PGC-1α, was measured in the plantaris muscles via western immunoblotting (15).
Statistical Analyses
Differences were considered significant at p < .05 and a trend at p < .010. Data are expressed as mean ± SE, unless otherwise indicated. Statistical analyses were performed using SPSS version 16.0 and 21.0 (IBM, Chicago, IL). Differences in means were examined using one-way analysis of covariance (ANCOVA; for the activity criterion), two-way analysis of variance (for physiological and morphological measurements), and 2×2 ANCOVA, for all other measurements. Differences between control and exercised old plantaris PGC-1α content, fiber type, and CSA were analyzed using independent Student’s t tests. In order to examine how the intervention changed within subjects we used 2×2 × 2 repeated measures ANCOVA. All ANCOVA were adjusted for body mass. A more detailed discussion of the statistics used is found in the Supplementary Methods section. Skew, kurtosis, and the results of Kolmogorov–Smirnov and Shapiro–Wilk goodness-of-fit tests for relevant data are reported in Supplementary Table S1.
Results
Evaluation of Frailty—FI
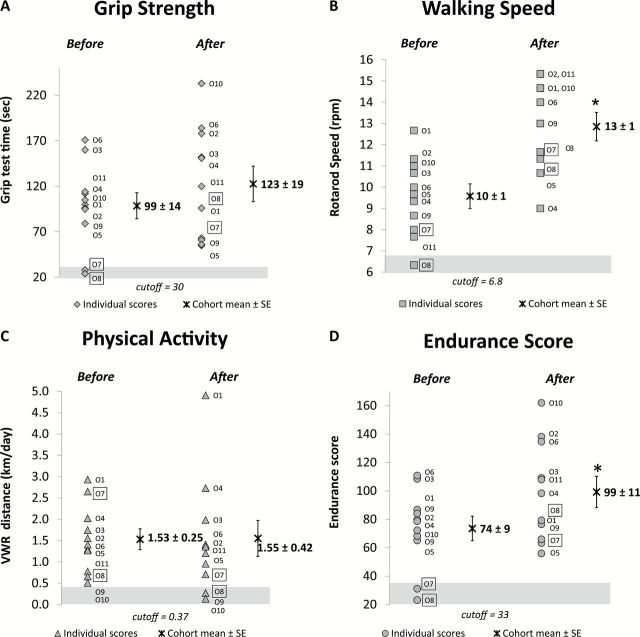
One mouse of the older age group was designated as frail at baseline. This mouse had three of the frailty criteria (rotarod, grip test, and endurance) below the cutoff point of 1.5 SD. A second mouse was identified as mildly frail with two criteria (rotarod and endurance) below the cutoff points. After the exercise intervention, no animals were identified as frail (Table 2, and Figure 3, old mice; and Supplementary Figure S1, adult mice).
Table 2.
Frailty Identification Using the Frailty Index (Liu and colleagues, 2013)
| Frailty Criteria, Identify the Mouse Below the Cutoff | Frailty Identification | ||||||
|---|---|---|---|---|---|---|---|
| Grip Strength | Walking Speed | Physical Activity | Endurance Score | Frail | Mildly Frail | ||
| Adult mouse ID | Before | — | A2 | — | — | None | None |
| After | — | — | — | — | None | None | |
| Old mouse ID | Before | O8,O7 | O8 | — | O8,O7 | O8 | O7 |
| After | — | — | O10,O9 | — | None | None | |
Notes: The frailty index was used to identify frail mice. The frailty index is composed of four frailty criteria: grip strength, walking speed, physical activity, and endurance score. Cutoff point is set at 1.5 SD below the cohort mean in each criterion. A mouse is identified as frail if three or more criteria fall below the cutoff points. Mildly frail is defined as if two criteria are below the cutoff. Values in italics are criteria scores below the cutoff by individual animals.
Figure 3.
Effect of exercise (VWR) on frailty criteria in old mice. (A–D) Show the performance of individual mice in each criterion (grip strength, walking speed, physical activity, and endurance score) before and after exercise. In each panel, the scores of individual mice and the group mean ± SE before and after exercise intervention are presented. The data points within the shaded regions represent the mice that fell below the cutoff points (1.5 SD below the baseline mean). *Indicates significant difference from the group mean before intervention (paired t test, p < .05). The two frail mice are framed (O7 and O8). VWR = voluntary wheel running.
Evaluation of Frailty—Frailty Criteria
The effect of the exercise intervention on the individual frailty criteria is summarized in Tables 3 and 4. Table 3 describes the main effects/interactions and Table 4 details both post-hoc analyses and the results of repeated measure ANCOVAs. A detailed analysis of the individual criteria, describing the statistical analysis of multiple factors of each criterion is found in the Supplementary Results section. The values for baseline and postintervention testing are detailed on Table 1. Overall, a close examination of the data showed that exercise improved three of the four frailty criteria outcome measurements (rotarod, grip, and endurance). Physical activity levels did not change significantly with exercise. A brief overview of the main effects and the post-hoc testing from the 2×2 ANCOVA (adjusted for body mass) comparing the means of the three criteria that changed follows.
Table 3.
p Values for Main Effects of Age, Exercise, and the Age × Exercise Interaction on Percent Difference
| Criteria | 2×2 ANCOVA | 2×2 × 2 Repeated Measures ANCOVA | ||||
|---|---|---|---|---|---|---|
| Age | Exercise | Age × Exercise | Age | Exercise | Age × Exercise | |
| Grip | .004* | .094† | .561 | - | - | .026* |
| Rotarod | - | <.001* | .045* | - | <.001* | <.001* |
| Endurance | - | <.001* | .040* | - | <.001* | .001* |
| Activity‡ | N/A | N/A | ||||
Notes: p Values given for the repeated measures ANCOVA were from the within-subject test. Main effects reported with the caveat that with a significant age × exercise interaction, the main effect is tempered by and depends upon the interaction. ANCOVA = analysis of covariance; - = no difference.
*Significant at p < .05.
† p = .051–.100.
‡Activity level was only tested in exercise groups; but the ANCOVA was not significant.
Table 4.
Post-hoc Tests: Effect of Intervention (VWR) on the Frailty Criteria (percent of change)
| Adult (A) | Old (O) | Exercise | Control | |||||
|---|---|---|---|---|---|---|---|---|
| Control (C) | Exercise (E) | AC vs AE | Control | Exercise | OC vs OE | AE vs OE | AC vs OC | |
| (% Difference Mean ± SE) | (p Value) | (% Difference mean ± SE) | (p Value) | (p Value) | (p Value) | |||
| Grip | 220±63 | 331±134 | .184 | 97±14 | 151±31 | .281 | .015* | .069† |
| Rotarod | 81±9 | 156±13 | <.001* | 110±8 | 138±9 | .029* | - | .069† |
| Endurance‡ | 46±8 | 224±37 | <.001* | 58±8 | 153±23 | <.001* | .021* | - |
| Activity§ | N/A | 137±28 | N/A | N/A | 91±19 | N/A | - | N/A |
| n | 5 | 5 | 17 | 11 | ||||
Notes: Statistical analysis from ANCOVA adjusted for body mass with least significant difference post-hoc testing. AC = adult control; AE = adult exercise; ANCOVA = analysis of covariance; OC = old control; OE = old exercise; VWR = voluntary wheel running; - = no difference. n = number of animals per group.
*Significant at p < .05.
† p = .051–.100.
‡Endurance post-hoc was conducted using Holm–Bonferroni correction (less Type II error).
§Activity level was only tested in exercise groups; but the ANCOVA was not significant, therefore no post-hoc tests were conducted.
Main Effects From 2×2 ANCOVA Comparisons of Means of Criteria Measurements
For grip test, the adult mice performed better than the older mice (main effect of age was significant). Exercised groups performed significantly better than the control mice, overall, in both the rotarod and endurance criteria and had a trend in the grip test (main effect of exercise). The interaction between age and exercise was also significant, between groups, in rotarod and endurance, indicating the effect of exercise was modulated by age and vice versa. When each mouse was examined using a 2×2 × 2 repeated measures ANCOVA, exercise was a significant factor in rotarod and grip test, and the age × exercise interaction was significant in all three criteria (rotarod, grip, endurance). Main effects reported with the caveat that with a significant age × exercise interaction, the main effect is tempered by and depends upon the interaction. (Table 3)
Post-hoc Results From 2×2 ANCOVA Comparisons of Means of Criteria Measurements
In the grip test, there was no significant difference between the adult and old exercise groups at baseline (95% confidence interval, 64±27 and 99±27 seconds, respectively) (Table 1). The adult exercise group improved their grip time significantly (331%), whereas there was no significant difference in either of the old groups. It should be noted, however, that the exercise old group did not decline in their grip time (mean increase of 24 seconds), whereas the age-matched control group did (mean decline of 19 seconds). In the rotarod scores, the old mice improved (95% confidence interval: 138% ± 18%) to the same relative extent as the adults (95% confidence interval: 156% ± 26%). The endurance score changed significantly after the intervention with improvement seen in both the adult and old groups. Again, the adult animals improved much more than the old (95% confidence interval: 224±73% vs 153±45%, respectively). (Table 4)
Evaluation of Treatment—FIAV
Table 5 summarizes the FIAV and the mean criterion standardized scores (standardized scores being the distance in standard deviations from the mean of each animal’s criterion value). Figure 4 highlights the individual standardized criterion, FIAV per animal, and the means. The control animals were not tested on the activity measurements; thus, we calculated the control FIAV scores with a score of zero for activity, as if all animals performed at the mean ability level. This is discussed further in the Discussion section. Once again, main effects reported with the caveat that with a significant age × exercise interaction, the main effect is tempered by and depends upon the interaction.
Table 5.
Effect of Intervention (VWR) on the Frailty Criteria (differences from the standardized scores) and FIAV
| Adult (A) | Old (O) | ANCOVA | |||||
|---|---|---|---|---|---|---|---|
| Control (C) | Exercise (E) | AC vs AE | Control | Exercise | OC vs OE | AE vs OE | |
| Mean ± SE | p Value | Mean ± SE | p Value | p Value | |||
| Grip | 1.9±0.7 | 3.1±1.2 | .402 | −0.4±0.2 | 0.5±0.4 | .020* | .026 |
| Rotarod | −0.8±0.4 | 3.6±0.7 | <.001* | 0.5±0.4 | 1.4±0.5 | .152 | .001 |
| Activity§ | N/A | 1.49±1.69 | N/A | N/A | 0.15±0.33 | N/A | - |
| Endurance | −2.5±1.7 | 4.3±1.0 | .009* | −1.1±0.2 | 0.9±0.3 | <.001* | .003 |
| FIAV | −1.4±1.5 | 12.5±2.1 | .001* | −1.0±0.5 | 3.0±0.7 | <.001* | <.001 |
| n | 5 | 5 | 17 | 11 | |||
Notes: The standardized scores represent the differences between post- and preintervention values in SDs. n: sample size. AC = adult control; AE = adult exercise; ANCOVA = analysis of variance; FIAV = Frailty Intervention Assessment Value; OC = old control; OE = old exercise; VWR = voluntary wheel running; - = no difference.
*Significant at p < .05.
§Activity level was only tested in exercise groups.
Figure 4.
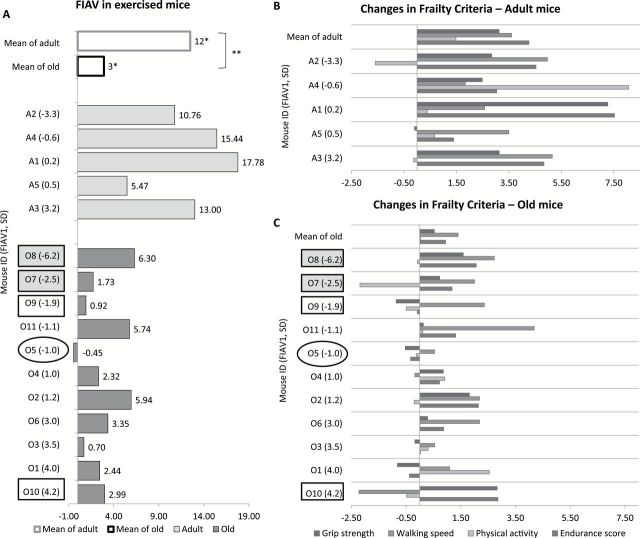
Effect of exercise on Frailty Intervention Assessment Value (FIAV). (A) FIAV in exercised mice. White bars represent the mean FIAV in adult and old mice. Mice from exercise groups are ranked based on the FIAV before exercise (FIAV1) from low to high within each age group. Light gray bars: adult; dark gray bars: old. Each bar indicates the FIAV difference between pre- and postexercise from each mouse, with the FIAV presented at the end. *Indicates significant improvement in FIAV within group. **Indicates significant difference between age groups. (B and C). Effect of exercise on standardized values of each criterion in adult (B) and old (C) mice. Mice from adult and old groups are ranked based on the FIAV1 (FIAV before exercise) from low to high. Each bar represents the change (standard deviation from the baseline cohort mean) in each frailty criterion (grip test, walking speed, physical activity, and endurance score) following intervention. The top row in each panel shows the mean changes in age group. (B) After 1 mo of exercise (VWR), grip strength, walking speed, physical activity, and endurance score improved 3.13, 3.61, 1.48, and 4.27 (SD) in adult mice, respectively (top row). (C) In old mice, the four criteria improved 0.53, 1.40, 0.03, and 0.94 (SD) after exercise, respectively (top row). Five mice are highlighted for case studies. The two frail mice (O7 and O8) identified as frail before the intervention are highlighted in the gray boxes. Mouse O5, which is circled, did not show improvement in the FIAV. Mice O9 and O10, highlighted in white boxes, had activity levels below the cutoff point after the exercise intervention yet showed improvement in the FIAV. VWR = voluntary wheel running.
An examination of the main effects showed difference with age, improvement with exercise, and an interaction between the two. Age was a significant factor on FIAV (defined as FIAV2 − FIAV1), adult mice perform 1000% better than old mice (main effect, change of 4.5±1 SD; p < .001). FIAV showed an improvement of 300% following the exercise intervention (main effect, percentage change, mean difference of 9±1 SD; p < .001). There was an interaction between age and the exercise intervention with the FIAV (F = 24.159, p < .001). The mean FIAV2 (representing the sum of the change after treatment in standard deviations from the mean of all of the criteria measured from the established baseline values) showed 410% greater change in the adult exercised mice versus the old (12.5±2.4 and 3.0±0.7 SD, respectively, p = .009).
A pairwise comparison of the differences between the groups showed FIAV improved substantially after treatment (3.0±0.7 and 12.5±2.1 SD for old and adult, respectively; p = .001), whereas the control mice declined (−1.0±0.5 and −1.4±1.5 SD for old and adult, respectively; p = .001). Notably, the range of the FIAV for adult and old exercise groups was 5.5–17.8 and 0.7–6.3 (except for one animal: −0.5), respectively (above statistics from 2×2 ANCOVA, adjusted for body mass: F = 35.4, p < .001).
The above results for FIAV were confirmed when examining each mouse (within-subject age, treatment, and age × treatment interaction were significant, main effect age [F = 20.58, p < .001], main effect treatment [F = 80.5, p < .001], and the interaction of age × treatment [F = 24.2, p < .001], repeated measures general linear model 2×2 × 2 ANCOVA, adjusted for body mass). In response to the exercise intervention, the FIAV showed a clinically relevant increase as demonstrated by effect size index and the standardized response mean. The data demonstrated the adult mice had an increased response to treatment in comparison to old mice (effect size index [5.4, adult; 0.9, old] and standardized response mean [2.7, adult; 1.4 old]). We noted that the FIAV of the adult mice had less variation (coefficient of variation: 43%, adult; 102%, old); thus, the mice uniformly improved more in response to the exercise. In addition, 68% of the variability in the FIAV is explained by treatment, the age group, and body mass of the animal (multivariable linear regression; R = .82, r 2 = .68, p = .001).
Case Studies of the FIAV (Description of Five Mice)
Two mice were identified as frail or mildly frail before the exercise intervention (O8, O7), Figure 4A and C. The FIAV1 of the frail mouse (O8) was −6.0 and following the intervention the FIAV2 was 0.1 (FIAV of 6.1). Notably, this mouse (O8) was not identified as frail postexercise intervention. The mildly frail mouse (O7) started with a FIAV1 of −2.5 and after exercising improved to a FIAV2 of −0.9 (FIAV of 1.60), also was not identified as frail after the intervention.
One mouse (O5) showed no improvement (FIAV of −0.5, Figure 4A). Evaluation of the individual standardized scores showed improvement in the rotarod (0.6) only (the grip test, −0.5; endurance, −0.3, Figure 5C). Mouse O5 was not frail as defined by our FI and had no criteria below the cutoff points.
Figure 5.
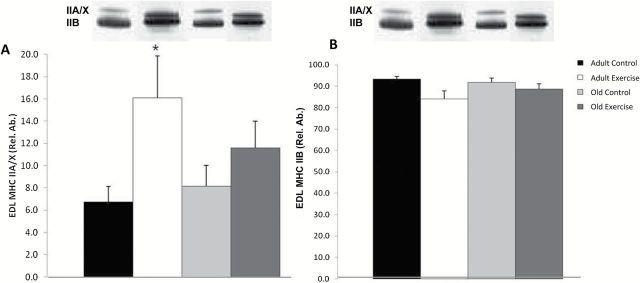
Myosin heavy chain IIA/X and IIB isoform protein expression in the EDL. (A) EDL MHC IIA/X. *Adult exercise vs adult control (p ≤ .05). (B) No significant differences were found in EDL MHC IIB isoform expression (there were no detectable MHC I isoforms). Main effect analyses were determined by two-way ANOVA and post-hoc analysis was performed with the t test. Representative bands from silver-stained gel. ANOVA = analysis of variance; EDL = extensor digitorum longus; MHC = myosin heavy chain; Rel. Ab. = relative abundance.
There were two mice (O10 and O9) with activity levels below the cutoff point (0.37) after intervention, yet their FIAV improved (Figure 4A). In fact one mouse (O10) had an overall improvement of 3.0 (from FIAV1 of 4.2 to FIAV2 of 7.2) and this mouse had the best FIAV2 among the old mice. In contrast, the other mouse that was below the threshold (O9) had an overall improvement of 0.9 (started with a FIAV1 of −1.9 and finished with a FIAV2 of −1.0) and this was the third lowest level of improvement of all of the old animals. See Figure 4A and C.
Evaluation of Physiological, Morphological, and Cellular Metabolic Changes
Overall, aerobic training for 4 weeks was able to produce significant physiological and morphological changes.
Whole-Muscle Contractility and CSA—Benefits of Exercise Intervention
Peak tetanic force (P o) in EDL muscle was higher in the adult exercise group compared with the old exercise group (382±46 vs 303±20 mN, p < .05), and the effect of age was significant (p < .05) (Supplementary Figure S2A). Isolated whole-muscle physiological CSA was greater in adult mice compared with old mice (1.1±0.12 vs 0.85±0.06 and 0.86±0.16mm2, respectively; p < .05) (Supplementary Figure S2B). No significant differences between groups were found for maximal power (P max) in the EDL (Supplementary Figure S2C).
In SOL, P o was higher in the adult exercise group compared with adult and old control groups (243±14 vs 190±20 and 163±9 mN, respectively; p < .05) (Supplementary Figure S2D). Main effects of age as well as exercise were significant (p < .05). Consistent with the greater force generation, isolated whole-muscle physiological CSA of SOL was also greater in adult exercise compared with control (1.1±0.15 vs 0.9±0.05mm2, p < .05), and the main effect of exercise was significant (p < .05) (Supplementary Figure S2E). P max was significantly lower in old mice compared with adult mice, with significant main effects of age as well as the age × exercise interaction (p < .05) (Supplementary Figure S2F).
MHC Isoform Expression in Isolated SOL and EDL Muscles
In SOL, no significant differences were found in MHC isoform expression for type I, IIa/x, or IIb isoforms, although there was a trend for an age effect in the type IIb (p = .07; data not shown). In EDL, a significant increase in IIa/x isoform was found in adult exercise group compared with adult control group (p < .05) (Figure 5A and B).
Fiber Type Percentages and Fiber CSA in the TA
In the old exercise group, the type IIb percentage is decreased while the type IIx is increased (p < .05) (Figure 6A). Age × exercise interaction was also significant (p < .05). The percentages of type IIa and hybrid fibers were not different between groups.
Figure 6.
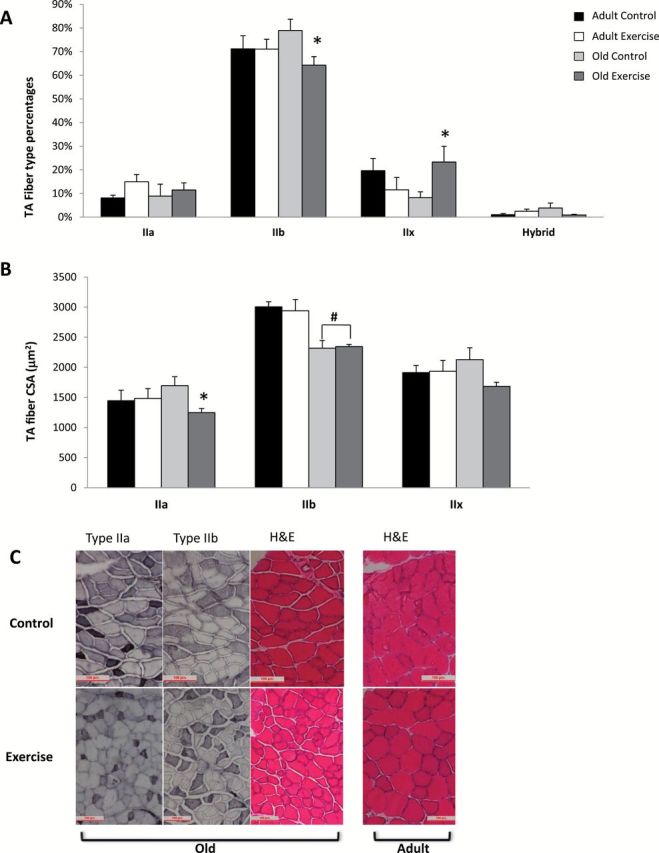
(A) Fiber type percentages in the TA. *Old control vs old exercise, (p ≤ .05) with a significant effect of age × exercise (p < .05). (B) Cross-sectional area of type IIa, IIb, and IIx fibers in the TA. *Old control vs old exercise (p ≤ .05). #Adult control and adult exercise vs old control and old exercise, with a significant effect of age (p < .05); (trend for significance between old control and old exercise in CSA of the type IIx fibers, p = .061). (C) Sections of TA muscles showing type IIa, IIb, and H&E. Top: left three sections from old control, right H&E stain from adult control; bottom: left three sections from old exercise, right H&E stain from adult exercise (type IIa and IIb for adult control and adult exercise not shown). CSA = cross-sectional area; H&E = hematoxylin and eosin; TA = tibialis anterior.
The CSA of the type IIa fibers was significantly smaller in the old exercise group compared with the control group (p < .05) (Figure 6B and C). In the type IIb fibers, CSA was significantly larger in both adult groups (control and exercise) compared with the old groups (control and exercise), and the effect of age was significant (p < .05).
PGC-1α Content
Total PGC-1α relative content in the plantaris was 61% significantly higher in the old exercise group compared with the control group (p = .016), relative abundance 116 to 71, respectively (Supplementary Figure S3).
Discussion
This study was designed to evaluate the efficacy of voluntary aerobic exercise in preventing or reversing frailty, and to construct a composite outcome measure (FIAV) to determine the treatment effect at the level of the individual. This study also investigated whether VWR for 1 month was sufficient to stimulate contractile, morphological, and metabolic adaptations in the hind limb muscles of mice. We hypothesized that VWR would improve indicators of frailty (FI, FIAV, individual frailty criterion) in the old mice, there would be improvement in muscle contractility, and finally the treatment would result in muscle morphological changes. Therefore, voluntary aerobic exercise would be considered a valid intervention to reverse frailty.
The first key finding of this study was that 4 weeks of VWR was sufficient to reverse frailty as defined by the FI of Liu and colleagues (8). The second key finding was that the FIAV is a valuable assessment tool for identifying the efficacy of an intervention at the individual animal level. Third, aerobic exercise maintained or improved four key functional indicators of frailty: grip strength (grip test), walking speed (rotarod), exhaustion (endurance), activity level (VWR), and the composite FIAV. Finally, we also demonstrated that significant morphological and metabolic changes occurred in response to exercise in the mice.
Frailty Index
While human frailty classifications exist, the ability to assess functional measures of frailty in the C57BL/6 aging mouse model is an important step in testing interventions that aim to improve or prevent frailty in the elderly. Recently, Liu and colleagues (8) created a FI based on human clinical assessment criteria (1). The strength of the FI lies in its ability to assess functional ability quickly, easily, and noninvasively. Using this index, prior to the intervention 9% of the mice were frail, which is consistent with the 9.5% human frailty prevalence as reported by Fried and colleagues (1) in 75–79 years old subjects (this is the equivalent age in human years of our mice—at 28 months or 50% survival) (13). After 4 weeks of voluntary aerobic activity, the frail mice had their functional ability rescued (nonfrail).
In humans, previous studies have shown that functional ability can be restored through exercise. The seminal study by Fiatarone and colleagues (4) demonstrated that resistance training in the oldest old and frail can restore physical function; individuals who utilized mobility assistance devices had remarkable transformations (eg, putting aside walkers and canes at the study’s conclusion). Numerous studies have since confirmed the ability of resistance training to restore function and reduce symptoms of sarcopenia by increasing muscle mass and strength in older adults (16–19). There has been less exploration into the ability of aerobic training to restore function (16,18,20). The current study shows that even the weakest and most frail mice have the potential to improve markedly with aerobic exercise.
Frailty Criteria
The FI was composed of four individual criteria, which were selected to match human clinical measurements as we discuss extensively in our previous work (8). A brief discussion of the criteria follows:
Inverted cling grip test
This is a well-documented assessment of strength and endurance that in practice, is very similar to the human assessment of the bent-arm hang (a physical fitness measurement use by the U.S. Marine Corps) in which a marine holds their bodyweight for as long as they can while suspended from a bar with arms bent to 90° at the elbow (13,21).
Rotarod
The rotarod test has been used for decades to evaluate overall motor function (13,22). It is a measurement of co-ordination, balance, endurance, walking speed, and power production. The mouse must continually increase the velocity that it runs (produce more power to increase gait speed) without falling from the spinning rod (balance and coordination).
Endurance
For the endurance score we combined the time element of two distinct criteria, each having elements of endurance: the inverted cling grip test and the rotarod measurement. In our previous work, we showed that there is no correlation between the grip test and rotarod (nonsignificant, R = .33, p = .323; also no significance in the current study, R = .147, p = .378). Thus, combining the two scores creates a relatively independent third measurement (13).
Physical activity level
The measurement of VWR to describe activity level is apt because this criterion measures the distance the mice move, in kilometers, beyond activities of daily living (ie, moving around the cage). The mice have the option to simply ignore the wheel and remain sedentary. There was a significant difference in wheel running volume between the adult and old groups, suggesting a reduction in activity level with age. This is in agreement with the literature and gives further validation to the measurement (23,24).
While each of the criteria has its own merit and measures different components of frailty in its own way, individually each criterion is not sufficient to diagnose frailty in mice, which is consistent with the human literature (1,2,9). This is the rationale supporting our FI (8), which detects the presence or absence of frailty, using multiple assessments. Because our long-term goal is to develop interventions to alleviate, prevent, or reverse frailty, there exists a need for an assessment tool, most realistically a composite score, to detect treatment efficacy. A frailty intervention assessment score, as a composite scoring system, has increased power to detect changes resulting from the intervention because it takes into consideration the totality of change in each mouse, not just changes in an individual parameter.
Frailty Intervention Assessment Value
In the current study, the FIAV was shown to be a valuable tool with which to assess intervention efficacy. While the FI can show whether an animal is frail or not, the FIAV, as a composite score, can more powerfully describe the degree of efficacy of an intervention, both to an individual animal or to a cohort overall. Composite scoring systems are commonly in use as a tool for many clinical or experimental assessments. For example, in the fields of stroke rehabilitation and in geriatric medicine, the Instrumental Activities of Daily Living scale is routinely used to assess disability level (25,26). In physical therapy rehabilitation, another popular composite scale used is the timed movement battery to assess mobility (27).
Using the FIAV we were able to determine that, while both exercised cohorts improved, the adult animals received more benefit (mean FIAV: adult, 12.5±2.4 SD; old, 2.9±0.7 SD). The control animals in both groups actually declined in ability (mean FIAV: adult −1.4±1.6 SD; old −1.0±0.5 SD). In agreement with this, the effect size index and the standardized response mean were also higher in the adult group, leading us to conclude that the adult animals received far more benefit from the intervention than the older mice. This conclusion is consistent with the literature concerning exercise response in older subjects (28). Indeed, more investigation is necessary to evaluate if the older animals would benefit by running more (volume increase) since they ran much less than the adults.
The FIAV was sensitive to detecting change between groups. Similar to how there is increased power in a repeated measure analysis of variance (because the change within each animal is examined in the within-subject comparison), the FIAV examines the change in each mouse and compiles the totality of change of the criteria. In addition, an advantage is gained because both extreme and nonresponders can easily be documented—opening up additional avenues for exploration (ie, mechanisms of response).
Overall, there are three main advantages to using the composite FIAV developed here: (i) is a more powerful detection tool than the individual criteria alone, (ii) gives a snapshot of how the intervention affected the individual animal and to what degree, and (iii) shows what effect the treatment had overall to the experimental cohort. Furthermore, we believe that the approach used to construct the FIAV is easily adapted to any sort of index measurement by carefully considering the validity of each measurement to be included and the overall validity of the constructed composite score.
Physiology and Morphology
Although it is well known that resistance exercise elicits significant improvements in strength, muscle mass, and physiological function in elderly individuals, even into advanced age (4,16–19,28,29), aerobic exercise such as brisk walking or jogging is often a preferred mode of exercise due to its simplicity and practicality. In elderly humans, moderate aerobic exercise training has been shown to increase the percentage and CSA of type IIa fibers in the gastrocnemius in response to 10 months of walking or jogging exercise (30). Previous studies established that 4 weeks of VWR exercise is sufficient to induce positive physiological and morphological adaptations in mice (31–34) and are consistent with the findings of the current study. Specifically, the SOL showed improved contractile function and evidence of hypertrophy after the exercise intervention, though the EDL did not. This was not surprising because the SOL, a plantar flexor, received direct stimulation from the exercise (training specificity), whereas the EDL was only indirectly affected. The moderate correlation between the force generating capacity of the SOL muscle and the frailty measurements of rotarod, endurance, and FIAV (R = .43, .50, and .59, respectively) demonstrates the significance of this muscle in functional movements.
We found evidence of age-related atrophy at the whole muscle and single fiber level, which was not rescued by the intervention. This was also not surprising because aerobic exercise is not known to result in vast hypertrophy, particularly in type IIb muscle fibers. However, we did find a phenotype shift in the EDL myosin expression and TA fiber type composition, indicating the exercise stimulus resulted in changes consistent with the literature (34–37).
Cellular Metabolism: PGC-1α Content as a Biomarker
Aging is associated with not only loss of muscle mass and strength, but, also, with a decline in metabolic quality (38). PGC-1α functions as a transcriptional activator that mediates cellular respiratory function and serves as a biomarker of mitochondrial biogenesis (34,39,40). Our finding that the old exercised mice had 61% greater total PGC-1α content suggests that the metabolic quality of the muscle in the old exercised mice may have improved, as would be an expected result of aerobic exercise. Thus, overall, this intervention may improve not only functional parameters associated with frailty in old mice, but also morphological and metabolic outcomes as well.
Caveats to Consider
In the current study, there are three caveats worth discussing centered on the research design. First, the FI and body mass were not determined in all animals: The control mice were not tested for activity rate, which is one of the frailty criteria, because of the possibility of a training effect resulting from the activity test itself. Therefore, it was not possible to assess the control mice for frailty. As a result, we designated the control animals to have a score of zero for activity on the FIAV, indicating no change. In future investigations, using a 3-day testing period, rather than the 1 week to determine the activity score (8), may provide less chance of a detectable exercise effect, and allow for control mice to be scored for activity; therefore, subsequently, receiving a frailty evaluation. In respect to body mass, we did not collect initial body mass for five control young, nine control old, and four exercise old. Although, based upon analysis of the mice for which we have body mass measures, (eight control old, five exercise young, and seven exercise old), there is no statistical difference in mass either between groups or between baseline mass and final mass (Supplementary Table S1). In future studies, especially if the training intervention is of longer duration, it would be advantageous to have these measures.
Second, the use of VWR in both the FI (as a criterion) and as the exercise intervention has potential to weaken the study design. Foremost, VWR is an easy way to determine activity levels and is part of the published FI (8). Because the goal of the current study was to test a voluntary aerobic exercise intervention, wheel running was the logical choice. Hence, the rationale for the selection is logical; however, a stronger research design would have identified a different means for aerobic exercise (eg, treadmill running, or swimming).
The last caveat in the current study involves the selection of the outcome measure for testing the criteria of endurance in our FI. Currently, the outcome measure for the endurance criteria is a combination of two independent measurements (latency to fall of rotarod and grip test). Endurance score is moderately correlated to both rotarod and grip, R = .66 and .69, respectively. Thus, we suggest that another possible outcome measure for endurance might be treadmill running to exhaustion. Treadmill running to exhaustion (time) meets the proposed index criteria of being noninvasive and easy to apply.
Overall, the FI may well be called the frailty phenotype index because it is based upon the human frailty phenotype. The four criteria of walking speed, grip strength, physical activity level, and endurance, as published (8), can be tested by numerous outcome measures. One obvious example would be adding dual X-ray absorptiometry to measure lean mass/fat% as a fifth criterion that could fill-in for the unexplained weight loss criterion, as described by Fried and colleagues (1). Importantly, other outcome measures other than the ones we validated here might be used to measure the four criteria. For example, depending on the resources available, a force transducer grip test could measure grip strength, laser motion cages could measure activity or walking speed measured by something like the “air-puff test” (mouse placed on platform leading to home cage, air-puff is used to startle the mouse to begin running to cage, latency to reach cage as outcome measure). New tests would need to be evaluated for validity, of course, but there is definitely some inherent flexibility in how the frailty criteria can be measured.
Conclusion
VWR for 4 weeks was able to reverse symptoms of frailty as measured by our FI (8) and moved the two frailty-assessed mice to nonfrail status. At an individual level, the FIAV proved to be a valuable tool to track intervention-induced changes and is easily adapted to assess other indices and/or interventions. The FIAV concept could easily be adapted to other groups of validated criteria measurements. We found exercise-induced improvement in grip strength, walking speed, and endurance, albeit with some age-modulated responses. At the morphological and physiological level we found tissue, cellular, and biochemical changes consistent with adaptation to increasing aerobic capacity and improving muscle mass and strength. Overall, aerobic training alone proved to be a valuable treatment to frailty in the aging mouse model. Further investigation should be conducted to examine whether combined resistance and aerobic training could act in synergy to improve fitness and function even more. In addition, the FIAV should be applied to such combined intervention models to better elucidate their effects on age-related frailty.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This project was funded by University of Minnesota Center for Excellence in Critical Care and by the National Institutes of Health/National Institute on Aging: L.F.-S. and in part T.G.G. as fellows on T32AG029796 (L.V.T., Ferrington), F31AG044108 (T.G.G.), and R01AG017768 (L.V.T.).
Supplementary Material
Acknowledgments
The authors thank Janice Shoeman, Briana Jones, and Windy Torgerud for technical assistance; as well as Sarah Lojovich, Katelin Ludwig, Lauren Kratzer, Taylor Larson, Jay Loso, Lindsey Legatt, and Melissa Ludescer for assisting in histological data analysis. L F.-S. is now an Assistant Professor of Biology at Hamline University, St. Paul, Minnesota.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. :10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 2. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. :10.1016/S0140-6736(98)04402-X [DOI] [PubMed] [Google Scholar]
- 3. Winograd CH. Targeting strategies: an overview of criteria and outcomes. J Am Geriatr Soc. 1991;39(9 Pt 2):25S–35S. [DOI] [PubMed] [Google Scholar]
- 4. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. :10.1001/jama.263.22.3029 [PubMed] [Google Scholar]
- 5. Foster-Burns SB. Sarcopenia and decreased muscle strength in the elderly woman: resistance training as a safe and effective intervention. J Women Aging. 1999;11:75–85. :10.1300/J074v11n04_06 [DOI] [PubMed] [Google Scholar]
- 6. Reeves ND, Narici MV, Maganaris CN. Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol (1985). 2004;96:885–892. :10.1152/japplphysiol.00688.2003 [DOI] [PubMed] [Google Scholar]
- 7. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227. :10.1093/gerona/glr193 [DOI] [PubMed] [Google Scholar]
- 8. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinical relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2013. 10.1093/gerona/glt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. :10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 10. Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. :10.1093/gerona/63.4.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. :10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahoney FI, Barthel DW. Functional Evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 13. Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68:1326–1336. doi:10.1093/gerona/glt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhong S, Thompson LV. The roles of myosin ATPase activity and myosin light chain relative content in the slowing of type IIB fibers with hindlimb unweighting in rats. Am J Physiol Cell Physiol. 2007;293:C723–C728. :10.1152/ajpcell.00009.2007 [DOI] [PubMed] [Google Scholar]
- 15. Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. :10.2337/diabetes.53.2.330 [DOI] [PubMed] [Google Scholar]
- 16. Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27:101–110. :10.1016/j.cger.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sundell J. Resistance training is an effective tool against metabolic and frailty syndromes. Adv Prev Med. 2011;2011:984683. :10.4061/2011/984683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chin A Paw MJ, van Uffelen JG, Riphagen I, et al. The functional effects of physical exercise training in frail older people: a systematic review. Sports Med. 2008;38(9):781–793. :10.2165/00007256-200838090-00006 [DOI] [PubMed] [Google Scholar]
- 19. Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51:108–115. :10.1159/000082195 [DOI] [PubMed] [Google Scholar]
- 20. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. :10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- 21. United States Marine Corps. (2013) From World Wide Web. http://www.marines.com/becoming-a-marine/how-to-prepare/pft#. Accessed December 12, 2013.
- 22. Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. :10.1038/nrn2652 [DOI] [PubMed] [Google Scholar]
- 23. Marinik EL, Kelleher S, Savla J, Winett RA, Davy BM. The Resist Diabetes trial: rationale, design, and methods of a hybrid efficacy/effectiveness intervention trial for resistance training maintenance to improve glucose homeostasis in older prediabetic adults. Contemp Clin Trials. 2014;37:19–32. 10.1016/j.cct.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging. 1985;6:17–24. :10.1016/0197-4580(85)90066-1 [DOI] [PubMed] [Google Scholar]
- 25. Fieo R, Manly JJ, Schupf N, Stern Y. Functional status in the young-old: establishing a working prototype of an extended-instrumental activities of daily living scale. J Gerontol A Biol Sci Med Sci. 2014;69:766–772. :10.1093/gerona/glt167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tse T, Douglas J, Lentin P, Carey L. Measuring participation after stroke: a review of frequently used tools. Arch Phys Med Rehabil. 2013;94:177–192. :10.1016/j.apmr.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 27. Creel GL, Light KE, Thigpen MT. Concurrent and construct validity of scores on the Timed Movement Battery. Phys Ther. 2001;81:789–798. [DOI] [PubMed] [Google Scholar]
- 28. Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 2011;43:1177–1187. 10.1249/MSS.0b013e318207c15d [DOI] [PubMed] [Google Scholar]
- 29. Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin heavy chain plasticity in aging skeletal muscle with aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2011;66:835–841. :10.1093/gerona/glr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (1985). 1992;72:1780–1786. [DOI] [PubMed] [Google Scholar]
- 31. Connell ML, Durrant JR, Russell MJ, Donato AJ, Seals DR, Lesniewski LA. Voluntary wheel running abolishes vascular inflammation and restores endothelial function in old mice. FASEB J. 2009;23(1_MeetingAbstracts):777. [Google Scholar]
- 32. Lesniewski LA, Durrant JR, Connell ML, et al. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1025–H1032. :10.1152/ajpheart.01276.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM., Jr Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscul Disord. 1995;5:323–332. :10.1016/0960-8966(94)00063-F [DOI] [PubMed] [Google Scholar]
- 34. Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985). 2001;90:1900–1908. [DOI] [PubMed] [Google Scholar]
- 35. Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol (1985). 2005;99:95–102. :10.1152/japplphysiol.00129.2005 [DOI] [PubMed] [Google Scholar]
- 36. Kraemer WJ, Patton JF, Gordon SE, et al. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol (1985). 1995;78:976–989. [DOI] [PubMed] [Google Scholar]
- 37. Jacobs-El J, Ashley W, Russell B. IIx and slow myosin expression follow mitochondrial increases in transforming muscle fibers. Am J Physiol Cell Physiol. 1993;265(1):C79–C84. [DOI] [PubMed] [Google Scholar]
- 38. Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. :10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geng T, Li P, Okutsu M, et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C572–C579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Austin S, St-Pierre J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125(Pt 21):4963–4971. :10.1242/jcs.113662 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.