Abstract
Background.
Skeletal muscle fat infiltration (myosteatosis) increases with aging, and has been associated with poor metabolic and musculoskeletal health, independent of overall adiposity. Studies examining the relationship of myosteatosis and mortality among older individuals recruited without regard to their health status are sparse.
Methods.
We evaluated the association of peripheral computed tomography measured calf myosteatosis (intermuscular fat and muscle density as a measure of intramuscular fat) with mortality in 1,063 community-dwelling older men. Cox proportional hazards models were used to estimate the risk of mortality independent of potential confounders.
Results.
During a mean follow-up of 7.2 years, 317 participants died. After adjustment for potential covariates and additional adjustment for whole body fat, lower skeletal muscle density was associated with increased all-cause mortality and cardiovascular disease mortality (hazard ratio [95% confidence interval] per standard deviation lower skeletal muscle density: 1.24 [1.09–1.41] and 1.46 [1.15–1.86], respectively), and to some extent with noncardiovascular disease mortality (1.18 [1.0–1.38], p = .053). After adjusting for trunk fat in a separate multivariable model, the association between skeletal muscle density and all-cause and cardiovascular disease mortality remained significant (both p < .01), while its association with noncardiovascular disease mortality became of borderline significance (p = .085). No other measures of adiposity, including calf intermuscular fat, were associated with mortality.
Conclusion.
Our study reveals an independent association between skeletal muscle density and mortality in a community-based sample of older, predominantly Caucasian men. Further studies are needed to establish if this association is independent of other ectopic fat depots, and to identify the biological mechanisms underlying this relationship.
Key Words: Skeletal muscle fat infiltration, Muscle density, Mortality, Aging.
Increased accumulation of fat around and within nonadipose tissue organs that normally contain only small amounts of fat, such as in liver, heart, and skeletal muscle, can impair the normal physiological function of those organs (1). This “ectopic fat” storage is now recognized as a risk factor for several chronic diseases, in particular type 2 diabetes (T2D) and cardiovascular disease (CVD), independent of general obesity (2–9). Some propose that the tendency to accumulate adipose tissue in ectopic depots may be explained by inadequate function and capacity of subcutaneous depot to store excess fat (10,11), increased fatty acid transport, uptake and storage, and reduced fatty acid oxidation (2,12,13), and/or increased macrophage infiltration which inhibits adipocyte differentiation (14).
Ectopic fat within and around skeletal muscle (known as myosteatosis) is a unique ectopic fat depot that is associated with poor metabolic and musculoskeletal health, and with accelerated aging (1,5,9,15–22). Specifically, myosteatosis has been identified as a risk factor for insulin resistance and T2D (16,22), increased risk of osteoporotic fractures (18,21), decreased muscle strength and mobility loss (15,19), reduced physical performance (23), and impaired longevity (20). Previous studies showed that skeletal muscle fat infiltration increases with advancing age (24), and that elderly individuals have greater myosteatosis (25,26) compared to younger individuals. Despite the emerging role of myosteatosis as an independent risk factor for metabolic dysfunction and other aging-related disorders, no previous studies have examined its association with mortality among older populations recruited without regard to their health status. Thus, in the current study, we evaluated the relationship between myosteatosis and mortality in a cohort of community-dwelling older men. We tested if this relationship was independent of total body and trunk fat, muscle size, lifestyle factors, comorbidities, frailty, and medications that may influence skeletal muscle metabolism. Given its important role in cardio-metabolic health, we hypothesized that increased myosteatosis would be associated with a greater risk of all-cause and CVD mortality.
Methods
Study Population
The Osteoporotic Fractures in Men (MrOS) Study is a prospective multicenter study that enrolled 5,994 men aged 65 years and older from six geographic regions (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA) in the United States between March 2000 and April 2002. Details of the study have been described (27). The parent study was designed to determine risk factors for bone loss, fractures, and other conditions of aging among elderly men. To participate in MrOS, men had to be able to walk without assistance from another person, and could not have had a bilateral hip replacement surgery. Men were recruited primarily through community-based mailing lists. Advertisements in local and senior newspapers and presentations to community groups were used to supplement recruitment efforts. The study was approved by the Institutional Review Boards at all study sites, and written informed consent was obtained from participants prior to data collection.
After an average of 4.5 years, participants at the Minneapolis and Pittsburgh Study Centers returned for a second visit for assessments of body composition with dual-energy X-ray absorptiometry (DXA) and calf skeletal muscle composition with peripheral quantitative computed tomography (CT). Participants also completed questionnaires regarding medical history, medications, physical activity, alcohol intake, and smoking. The present analysis is thus limited to MrOS participants from the Pittsburgh and Minneapolis study sites, and who have complete data on general obesity, whole body and trunk fat, and skeletal muscle composition at Visit 2 (N = 1,063 MrOS men: 489 from Minneapolis and 583 from Pittsburgh).
CT Quantification of Myosteatosis
Studies that utilize CT scans for measures of myosteatosis examine either intermuscular fat (visible fat beneath the fascia lata) and/or muscle density (fat between muscle fibers and fat within muscle cell). Lower skeletal muscle density is indicative of greater intramuscular fat content (28,29). In our study, measures of myosteatosis were obtained by peripheral quantitative CT (pQCT) scan of the calf, which was performed using the Stratec XCT-2000 (Pittsburgh site) and the XCT-3000 (Minneapolis site) scanners (Orthometrix, Inc., White Plains, NY). The only difference between the 2000 and 3000 scanners is the gantry size. The 3000 model has twice the gantry size opening (15 vs 30cm), which permits the upper limbs to be scanned. Both scanners are calibrated to the same factory set standard. This calibration is for a single energy (~58 kVp) and to hydoxyapatite. Specifically, each Stratec XCT 2000 and 3000 scanner is calibrated to the European Forearm Phantom which contains four volumetric density bone mineral inserts of 0, 50, 100, and 200mg/cc. Each XCT 2000 and 3000 scanner operated at a fixed kVp, and were calibrated to the European Forearm Phantom daily. Quality assurance phantom, supplied by the manufacturer, provided an accurate check of machine stability. This quality assurance phantom contains density inserts of a known density and cross-sectional area that must be correctly determined before clinical scans can be acquired. The same acquisition and analysis software was used to analyze scans at both sites.
At a second study visit, conducted after an average of 4.5 years from the baseline visit, 2.2-mm cross-sectional images of the calf skeletal muscle composition were obtained at 66% of the tibia length, proximal to the terminal end of the tibia, because this is the region of the calf with the largest circumference of the calf with less variability between individuals (30). The length of each tibia was measured (in millimeters) and men were seated comfortably with their lower leg positioned in the pQCT gantry such that the tibia was parallel to the floor with the limb supported at the distal thigh and foot. Standardized procedures for participant positioning and data analysis are used for all scans (31). A scout view was obtained prior to the tomographic scan to define an anatomic reference line, to which the relative location of the subsequent tomographic slice is automatically adjusted. The reference line was set according to the recommendations outlined in the manual supplied by the manufacturer (31). It is recommended that the reference line is placed through the flat portion of the distal tibia endplate. The reference line location was used between the two study sites and confirmed for each image before analysis. Different tissues in the analyses were separated according to different density thresholds, appropriate for soft tissue segmentation, using the manufacturer’s suggested analysis parameters (31). Based on calibration of the XCT scanners (2000 and 3000), fat, muscle or lean tissue, and cortical bone were measured with mineral equivalent densities of 0, 80, and 1,200mg/cm3, respectively. Therefore, muscle, fat, and bone with an image can be separated using appropriate fixed and gradient thresholds to separate the tissue compartments. Changes in muscle tissue to fat tissue were detected as a shift in mineral equivalent density of the muscle from 80 to 0mg/cm3. Automatic threshold-based iterative edge detection-guided segmentation of muscle from bone was performed using a density threshold of 280mg/cm3 with contour mode 1 and peel mode 2 (bone area and mass). Segmentation of muscle from subcutaneous fat followed a threshold of 40mg/cm3 with contour mode 3 and peel mode 1 (total muscle + bone mass/content). The threshold of 40mg/cm3 was selected as it is halfway between the densities for fat and water or muscle-like tissues and represents a standard image processing threshold selection for defining the gradient (or edge) between tissues. The units of bone mass/content (or bone mineral content) are mg/cm. To determine muscle cross-sectional area, bone area was subtracted from total bone mass/content + muscle area. Similarly, bone mass/content was subtracted from total bone + muscle mass/content to derive muscle mass/content. After subtraction of intermuscular adipose tissue cross-sectional area, muscle density was computed by dividing total muscle mass (mg/cm) by muscle cross-sectional area (cm2).
Image processing and quality control procedures were completed by a single investigator blinded to outcome status using the Stratec analysis software (Version 5.5). In addition to measures of myosteatosis (intermuscular adipose tissue cross-sectional area [mm2] and skeletal muscle density [mg/cm3]), we also obtained measures of total adipose tissue cross-sectional area (mm2) and subcutaneous adipose tissue cross-sectional area (mm2). The coefficients of variation were determined by repeat pQCT scanning in 15 individuals. The coefficients of variations for total, subcutaneous, and intermuscular fat, and muscle density were 0.98%, 1.5%, 7.6%, and 1.1%, respectively.
DXA Measured Total Body and Trunk Fat
Percent whole body fat and trunk fat (calculated as [trunk fat/total fat] × 100) were measured from DXA scans using Hologic QDR 4500 scanners (Hologic, Inc., Bedford, MA). Scans were analyzed with QDR software version 8.26a. Reproducibility of DXA measurements was ensured by use of a central quality control laboratory (San Francisco Coordinating Center, San Francisco, CA), standardized scanning procedures, and certification of DXA operators.
Other Measures
Height was measured with wall-mounted Harpenden stadiometers, and body weight was measured on standard balance beam using standard protocols. Body mass index was calculated from height and weight (kg/m2). Information on lifestyle habits (smoking, alcohol use, sedentary lifestyle, and physical activity), demographic information, and medical conditions were assessed using self-administered questionnaires. Self-reported smoking behavior was recorded as never smoked, past smoker, and current smoker. Alcohol drinking frequency was self-reported in predefined categories. Physical activity was measured by the Physical Activity Scale for the Elderly (PASE) (a unit less measure of relative physical activity) which measures total, occupational, household, and leisure physical activities over the previous 7 days. Sedentary activity was described as > 4 hours sitting per day. Participants were instructed to bring in all prescription medications taken in the past 30 days to their clinic visit, and study coordinators recorded the medications and data were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). The Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) was used to identify ingredient(s) in the medications (32). Antidiabetic drugs were defined as hypoglycemic medications and insulin. Antihypertensive drugs were defined as thiazide diuretics, potassium-sparing diuretics, loop diuretics, angiotensin-converting-enzyme (ACE) inhibitors, α-adrenergic blockers, hypotensive agents-angiotensin II, calcium channel blockers, β-blockers, and nitrates. Lipid-lowering drugs were defined as gemfibrozil and HMG-CoA reductase inhibitors (statin).
Frailty is a clinical syndrome in older adults characterized by decreased physiologic reserve and weakness that causes an increased vulnerability to stressors, and was defined using criteria similar to those initially proposed in the Cardiovascular Health Study (33). A man was considered frail if three or more of the following five criteria were present: (i) Shrinking or sarcopenia (34): appendicular lean mass (adjusted for height and whole body fat) in the lowest quintile; (ii) Weakness: grip strength in the lowest quintile stratified according to BMI (quartiles); (iii) Exhaustion: answer of “a little or none” to the question “How much of the time during the past four weeks did you have a lot of energy?”; (iv) Slowness: walk speed in the lowest quintile stratified according to standing height (median); and (v) Low physical activity: PASE score in the lowest quintile.
Mortality Assessment
During a mean follow-up of 7.2 years, 317 participants died (Table 1). A single physician adjudicator at the Coordinating Center verified all deaths using death certificates and hospital discharge summaries when available, and a prespecified adjudication protocol. The International Classification of Diseases, Ninth Revision(ICD9) code was used for the underlying cause of death. The primary outcomes of interest were all-cause, cardiovascular, and non-CVD mortality, which included all causes of mortality except for CVD. Codes 394.9, 396.9, 398.9, 401.1, 401.9–442.0, 443.9, 785.51, and 996.71 were classified as CVD deaths.
Table 1.
Characteristics by Vital Status in 1,063 MrOS Men (Follow-up March 2005–Feb 2014)
| Characteristics | All (n = 1,063) | Survivors (n = 746) | Deceased (n = 317) | p Value* |
|---|---|---|---|---|
| Socio-demographic | ||||
| Age (years) | 77.2±5.2 | 76.2±4.7 | 79.4±5.4 | <.0001 |
| Site, n (%) | <.0001 | |||
| Minneapolis | 480 (45.2) | 369 (49.5) | 111 (35.0) | |
| Pittsburgh | 583 (54.8) | 377 (50.5) | 206 (65.0) | |
| Race, n (%) | .3058 | |||
| White | 1045 (98.3) | 730 (97.9) | 315 (99.4) | |
| Non-white | 18 (1.7) | 16 (2.1) | 2 (0.6) | |
| Physical measures | ||||
| Height (cm) | 173.0±6.8 | 173.4±6.6 | 171.9±7.0 | .0006 |
| Weight (kg) | 83.7±13.3 | 84.4±12.8 | 82.0±14.3 | .0021 |
| Weight change since baseline visit (kg) | -1.5±4.9 | -1.0±4.6 | -2.6±5.4 | <.0001 |
| BMI (kg/m2) | 27.9±3.8 | 28.0±3.6 | 27.7±4.3 | .0616 |
| Lifestyle, physical activity, and health status | ||||
| Smoker at Visit 2, n (%) | 32 (3.0) | 19 (2.6) | 13 (4.1) | .1749 |
| Drinking frequency at Visit 2, n (%) | .2599 | |||
| None | 417 (39.2) | 276 (37.0) | 141 (44.5) | |
| <1 drink/wk | 167 (15.7) | 121 (16.2) | 26 (14.5) | |
| 1–2 drinks/wk | 121 (11.4) | 86 (11.5) | 35 (11.0) | |
| 3–5 drinks/wk | 152 (14.3) | 116 (15.6) | 36 (11.4) | |
| 6–13 drinks/wk | 157 (14.8) | 112 (15.0) | 45 (14.2) | |
| ≥14 drinks/wk | 49 (4.6) | 35 (4.7) | 14 (4.2) | |
| Physical Activity Scale for the Elderly score | 141.1±65.0 | 145.7±63.2 | 130.1±68.1 | <.0001 |
| Sedentary lifestyle (> 4 hours sitting activity per day), n (%) | 331 (31.1) | 222 (29.8) | 109 (34.4) | .1362 |
| Excellent/good health status, n (%) | 921 (86.6) | 674 (90.4) | 247 (77.9) | <.0001 |
| Frailty status†, n (%) | < 0.0001 | |||
| Robust | 318 (29.9) | 258 (34.6) | 60 (18.9) | |
| Intermediate | 573 (53.9) | 414 (55.5) | 159 (50.2) | |
| Frail | 172 (16.2) | 74 (9.9) | 98 (30.9) | |
| Obesity ‡, n (%) | 272 (25.6) | 193 (25.9) | 79 (24.9) | .7453 |
| Comorbidities | ||||
| Prevalent diabetes at Visit 2, n (%) | 199 (18.7) | 131 (17.6) | 68 (21.5) | .1368 |
| Hypertension, n (%) | 557 (52.4) | 380 (50.9) | 177 (55.5) | .1436 |
| Myocardial infarction, n (%) | 178 (16.8) | 112 (15.0) | 66 (20.8) | .0204 |
| Stroke, n (%) | 72 (6.9) | 37 (5.0) | 36 (11.4) | .0002 |
| Cancer, n (%) | 319 (30.0) | 199 (26.7) | 120 (37.9) | .0003 |
| Renal disease, n (%) | 16 (1.5) | 6 (0.8) | 10 (3.2) | .0040 |
| Medication use | ||||
| Antihypertensive drugs, n (%) | 733 (69.0) | 497 (66.6) | 236 (74.5) | .0116 |
| Lipid lowering drugs, n (%) | 493 (46.4) | 358 (48.0) | 135 (42.6) | .1061 |
| Anti-diabetic drugs, n (%) | 127 (12.0) | 81 (10.9) | 46 (14.5) | .0930 |
| Biochemical measures | ||||
| HDL-C (mg/dL) | 46.7±12.5 | 47.0±12.6 | 46.2±12.3 | .2491 |
| LDL-C (mg/dL) | 116.1±31.3 | 116.8±30.9 | 114.3±32.3 | .2429 |
| Triglycerides (mg/dL) | 153.2±79.0 | 151.5±77.5 | 157.1±82.6 | .3671 |
| Total Cholesterol (mg/dL) | 193.4±35.2 | 194.1±34.8 | 191.9±36.2 | .3654 |
Notes: Values are unadjusted mean ± SD, unless indicated otherwise. BMI = body mass index; CHS = Cardiovascular Health Study; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
*p Values for comparisons between survivors and decedents from two-sample t-tests, Wilcoxon two-sample test, or chi-square tests.
†Frailty status: Robust (none of 5 CHS frailties), Intermediate (one or two of five CHS frailties), frail (at least three of five CHS frailties).
‡Obesity = BMI ≥ 30.
Statistical Methods
We described the participant characteristics at the visit when pQCT measures were obtained (MrOS Visit 2) using means (standard deviation [SD]) or prevalence. We first inspected the distributions of the skeletal muscle composition measures for outliers using histograms and scatter plots. An outlier was defined as any observation outside the interval (Q1−3 × IQR, Q3 + 3 × IQR), IQR = Interquartile range, and was excluded from subsequent analyses. We used two-sample t-tests and/or Wilcoxon rank sum tests (continuous variables) or chi-square tests (categorical variables) to compare characteristics between survivors and deceased participants. We assessed the association between continuous measures of myosteatosis (per 1 SD greater intermuscular fat and per 1 SD lower muscle density) and all-cause, CVD, and non-CVD mortality using semiparametric Cox proportional hazard models. We also calculated hazard ratios and 95% risk limits for all-cause, CVD, and non-CVD mortality across quartiles of muscle density.
Covariates of interest included age, race, study site, height, weight change since baseline visit, lifestyle variables (smoking status, alcohol intake, PASE score, sedentary activity ≥4 hours sitting activity, excellent/good health status), lipids and lipoproteins (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol), comorbidities (T2D, coronary heart disease, hypertension, stroke, cancer, renal disease), frailty status, and medications that influence metabolism (antidiabetic, antihypertensive, and lipid lowering). T2D was defined by self-report, use of hypoglycemic medications at Visit 2, or having a baseline fasting glucose level ≥126mg/dL. All multivariable models were adjusted for the covariates listed above regardless of statistical significance within the model. Models of calf skeletal muscle fat measures also included adjustment for calf muscle cross sectional area. The proportional hazards assumption was confirmed graphically and formally using Schoenfeld residuals. The model fit was verified by plotting the Nelson-Aalen cumulative hazard of the Cox-Snell residuals. All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.3; SAS Institute, Cary, NC).
Results
Baseline Characteristics
Characteristics of the 1,063 study participants are presented in Table 1. The mean age of the study sample was 77.2 years. Most participants were Caucasian (98%), only 3.0% were current smokers, and the majority reported excellent or good health (86.6%). Participants who died during the follow-up (N = 317) were older, more likely from the Pittsburgh site, lost more weight since baseline, were less physically active, less likely to report excellent or good health status, and more likely to use antihypertensive drugs. Furthermore, men who died during the follow-up also had a higher prevalence of several chronic diseases (myocardial infarction, stroke, cancer, and renal disease) and were more likely to be frail. Participants who died during the follow-up also had lower calf muscle area, lower trunk fat percent, greater intermuscular fat, and lower muscle density (indicative of greater intramuscular fat) compared with those who survived (Table 2).
Table 2.
Body Composition and Skeletal Muscle Characteristics by Vital Status in 1,063 MrOS Men (follow-up March 2005–Feb 2014)
| Characteristics | All (N = 1,063) | Survivors (n = 746) | Deceased (n = 317) | p value† |
|---|---|---|---|---|
| DXA whole body fat % | 27.3±5.2 | 27.3±5.1 | 27.2±5.5 | .6913 |
| DXA trunk fat % | 55.1±5.3 | 55.4±5.3 | 54.4±5.3 | .0046 |
| Calf total fat (mm2) | 2,269.5±901.1 | 2,264.9±876.0 | 2,280.4±959.3 | .7708 |
| Calf muscle area (mm2) | 7,502.3±1,197.9 | 7,642.9±1,194.1 | 7,171.3±1,142.0 | <.0001 |
| Calf subcutaneous fat (mm2) | 1,772.5±755.4 | 1,788.8±731.3 | 1,734.1±809.4 | .0863 |
| Calf intermuscular fat (mm2) | 304.6±271.6 | 280.8±256.7 | 361.5±296.9 | <.0001 |
| Calf skeletal muscle density (mg/cm3) | 74.1±3.5 | 74.5±3.4 | 73.1±3.6 | <.0001 |
Notes: Values are unadjusted mean ± SD.
† p Values for comparisons between survivors and decedents from two-sample t-tests or Wilcoxon two-sample test.
Myosteatosis and All-Cause Mortality
Cox proportional hazard models exploring the association of intermuscular fat with mortality revealed a significant association of intermuscular fat with all-cause mortality in unadjusted analyses (hazard ratio [HR] [95% confidence interval (CI)] per SD greater intermuscular fat: 1.23 [1.11–1.35]). However, after multivariable adjustments for potential confounders (HR [95% CI]: 1.03, [0.93–1.16]), or after the additional adjustment for whole body fat percent (HR [95% CI]: 1.04, [0.93–1.17]) or trunk fat percent (HR [95% CI]: 1.03, [0.92–1.15]), we found no significant association between intermuscular fat and all-cause mortality.
In addition, we found an 18% increase in the multivariable-adjusted risk of all-cause mortality per SD lower muscle density (HR [95% CI]: 1.18, 1.06–1.33; Table 3). This association remained statistically significant after additional adjustments for whole body fat or trunk fat percent (results presented in Table 3).
Table 3.
Hazard Ratios (95% CI) for Mortality Per SD Increase in Skeletal Muscle Density in 1,063 MrOS Men
| Unadjusted model | Adjusted for age, race, study site | Multivariable model | MV model* + DXA whole body fat (%) | MV model* + DXA % trunk fat | |
|---|---|---|---|---|---|
| All-cause mortality | 1.39 (1.25–1.55)‡ | 1.20 (1.07–1.35)† | 1.18 (1.06, 1.33)† | 1.24 (1.09, 1.41)† | 1.18 (1.05, 1.33)† |
| CVD mortality | 1.66 (1.37–2.01)‡ | 1.34 (1.09–1.65)† | 1.34 (1.08, 1.66)† | 1.46 (1.15, 1.86)† | 1.34 (1.09, 1.66)† |
| Non-CVD mortality | 1.29 (1.12–1.49)‡ | 1.15 (0.99–1.33)§ | 1.14 (0.99, 1.33)‖ | 1.18 (1.00, 1.38)¶ | 1.14 (0.98, 1.32)# |
Notes: *All multivariable (MV) models were adjusted for age, race, study site, height, weight change since baseline, smoking, drinking, sedentary lifestyle, Physical Activity Scale for the Elderly score, health status, frailty status, diabetes, hypertension, myocardial infarction, stroke, cancer, renal disease, antihypertensive drugs use, lipid-lowering drugs, antidiabetic drugs use, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and calf muscle cross sectional area. CVD = cardiovascular disease.
† p < .01, ‡ p < .001, § p = .073, ‖p = .079, ¶ p = .053, # p = .085.
No other adiposity measures studied were associated with mortality, with the exception of trunk fat percent, which was inversely associated with the risk of all-cause mortality in unadjusted analysis (HR [95% CI] per SD greater percent trunk fat: 0.85 [0.76–0.95]), but not after the inclusion of age, race, and study site or after the inclusion of other potential confounders (Supplementary Table 1).
Myosteatosis and Cause-Specific Mortality
There were 100 CVD deaths during 7.2 years of follow-up. We found a significant association between intermuscular fat and CVD mortality in an unadjusted model (HR [95% CI] per SD greater intermuscular fat: 1.44 [1.22–1.68]), which was no longer significant after adjustment for potential confounders (HR [95% CI]: 1.17 [0.97–1.41]), after the additional adjustment for whole body fat percent (HR [95% CI]: 1.18 [0.98–1.42]), or after the additional adjustment for trunk fat percent (HR [95% CI]: 1.15 [0.95–1.38]). Lower skeletal muscle density was associated with an increase in the multivariable-adjusted risk of CVD mortality (HR [95% CI] per SD lower muscle density 1.34, [1.08–1.66]) and remained significant after additional adjustment for whole body fat or trunk fat percent (Table 3).
There were 199 non-CVD deaths during 7.2 years of follow-up. We found a significant association between intermuscular fat and non-CVD mortality in an unadjusted model (HR [95% CI] per SD greater intermuscular fat: 1.14 [1.00–1.30]), which was no longer significant after adjustment for potential confounders (HR [95% CI]: 1.00 [0.86–1.16]). One SD lower muscle density (Table 3) was associated with non-CVD death in an unadjusted model (HR [95% CI] 1.29 [1.12–1.49]), but this association became weaker and borderline significant (Table 3) after adjusting for potentially important confounders or whole body fat and trunk fat percent (p = .079 in multivariable-adjusted model, p = .053 in multivariable-adjusted model with whole body fat percent, and p = .085 in multivariable-adjusted model with trunk fat percent).
Quartiles of Myosteatosis and Mortality Risk
There was a significant trend for decreasing risk of all-cause mortality with lowering muscle density quartile in an unadjusted model (p trend < .0001, data not shown) and after adjustment for significant covariates and whole body fat percent (Figure 1, p trend = .0072). Similarly, in unadjusted models, the hazard ratios for CVD (p trend = .0001, data not shown) and for non-CVD mortality decreased from lowest to highest quartile (p trend = .0043, data not shown). The trend for decreasing risk of CVD mortality with greater muscle density quartile remained significant after adjustment for significant covariates as well as whole body fat (Figure 1, p trend = .0048), but was not significant for non-CVD mortality (Figure 1, p trend = .2262). Moreover, there appeared to be a threshold at a muscle density value of approximately 71.99mg/cm3 below which a lower muscle density was associated with a greater risk of CVD mortality.
Figure 1.
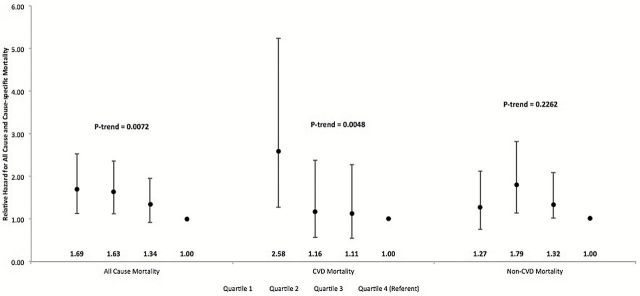
Adjusted hazard ratios for mortality across quartiles of skeletal muscle density in 1,063 MrOS men. All multivariable models were adjusted for age, race, study site, height, weight change since baseline, smoking, drinking, sedentary lifestyle, Physical Activity Scale for the Elderly score, health status, frailty status, diabetes, hypertension, myocardial infarction, stroke, cancer, renal disease, antihypertensive drugs use, lipid-lowering drugs, antidiabetic drugs use, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, calf muscle cross sectional area, and dual-energy X-ray absorptiometry total body fat.
Discussion
The current study found an independent association between calf skeletal muscle density and increased all-cause and CVD mortality risk among older men, which persisted even after adjustment for potentially important lifestyle and medical covariates, and general and central adiposity. With the exception of trunk fat percent, which was inversely associated with the risk of all-cause mortality in unadjusted analysis, no other measures of adiposity were associated with mortality. This is not surprising as increasing evidence suggests that the association between general adiposity and mortality may be attenuated with aging (35–37). Despite the growing appreciation of a potential role for ectopic fat in metabolic health and aging independent from overall adiposity (9,18–23), few studies have examined the association between ectopic fat and mortality, particularly for skeletal muscle fat infiltration. Existing studies of ectopic fat have focused exclusively on visceral, liver, and pericardial fat (9), or on highly selected individuals rather than the general population (24,25). Visceral fat has been the most extensively studied ectopic fat depot and is associated with mortality in some (38–40), but not all studies (41). Likewise, despite a strong link between increased liver fat infiltration and cardio-metabolic disturbances (42), few studies have examined the association of liver fat with mortality. In the Third National Health and Nutrition Examination Survey, no association was observed between fatty liver and mortality among 1,371 participants aged 20–74 (43). Although, pericardial fat has been associated with both prevalent (8) and incident (44) CVD, the Framingham Heart Study (41) found no association between pericardial fat and mortality. Moreover, very few data exist on the potential relationship between skeletal muscle fat infiltration and mortality in community dwelling populations, and if the associations are independent of general adiposity and other potentially important covariates. The InCHIANTI study reported no significant association between calf muscle density and mortality over a mean of 5.1 years among 934 community-dwelling women and men aged 65+ years (45). Although in this study lower calf muscle density was associated with higher mortality in unadjusted analyses, none of the associations remained statistically significant after additional adjustment for age and sex. Thus, the current study reports new results in a larger sample of older men and if the associations of muscle density and mortality are independent of general and central adiposity. Similar to our findings, the Walking and Leg Circulation Study (46) reported that lower calf skeletal muscle density is associated with greater all-cause and CVD mortality among 434 older women and men with peripheral arterial disease. Our study extends these observations to the general population who were recruited without regard to their health status.
The mechanisms linking increased skeletal muscle fat infiltration and mortality are not clear. A possible mechanism by which inter- and intramuscular fat may influence risk of mortality may be through an association with cardio-metabolic risk factor levels, and particularly through effects on insulin sensitivity (9). However, the mechanisms linking inter- and intramuscular fat with insulin resistance and T2D and other metabolic diseases may differ, which could explain the observed differences in their association with mortality. Increased accumulation of intermuscular fat may induce changes in muscle metabolism and insulin sensitivity via increasing local secretion of proinflammatory adipokines from adipocytes surrounding muscle fibers (3). Intermuscular fat may also impair nutritive blood flow to muscle and thus contribute to insulin resistance by impairing insulin action and insulin diffusion capacity (47). As for intramuscular fat, it is still controversial whether it is a marker or a mediator of insulin resistance. Some propose that accumulation of intramuscular fat may impair the insulin receptor substrate 1 (IRS1)/phosphatidylinositol 3-kinase (PI3K) pathway and growth-factor–regulated protein kinase B (Akt/PKB) pathway of insulin signaling (48). In our study, an association between muscle density, a measure of intramuscular fat, and mortality persisted even after adjusting for a wide range of cardio-metabolic comorbidities, including diabetes, suggesting that this fat depot might be an independent marker of aging. In fact, middle-aged individuals predisposed to increased familial longevity have less intramuscular fat compared to matched controls with similar body composition (20). The authors postulated that lower intramuscular fat found in the offspring of long-lived individuals might be associated with better mitochondrial capacity as greater accumulation of intramuscular fat can cause mitochondrial dysfunction (49). Indeed, mitochondrial dysfunction has been previously linked to greater intramuscular fat, and may play a role in increased mortality risk for adults with higher amounts of intramuscular fat (49). Another possible link between intramuscular fat and increased risk of mortality may be via increased oxidative stress, as visceral fat has been associated with increased oxidative stress (50). Increased lipid content in skeletal muscle cells affects their metabolism, thereby causing an increased expression in oxidative metabolism genes (51). Finally, recent evidence in animal models shows that intramuscular fatty-acid metabolism plays a critical role in mediating the protective effects of dietary restriction in flies (52). Thus, insulin resistance, increased inflammation, greater oxidative stress, and poorer mitochondrial function are potential mechanisms underlying an association between greater intramuscular fat and mortality.
The present study has several potential limitations. First, our study is observational, and causality cannot be definitely determined. Second, our sample consists of predominately Caucasian males, and thus, our findings may not apply to women or to other ethnicities. Third, although the observed association between intramuscular fat and mortality was independent of whole body and trunk body fat, ectopic depots in other nonadipose tissues and organs, such as the visceral compartment, liver and heart, were not assessed, and thus, we were not able to test if the observed associations are independent of these other ectopic fat depots. Fourth, due to the lack of data on oxidative stress, mitochondrial function, and markers of insulin resistance, we were unable to test for mechanisms that may link intramuscular fat with an increased risk of mortality. Thus, the potential mechanisms underlying an association between greater intramuscular fat and mortality remain to be determined. However, our study also has notable strengths including its longitudinal design, community-based sampling design, relatively long follow-up period, and extensive measures of potential confounding lifestyle and medical variables.
In conclusion, our study reveals a previously unreported, independent association between skeletal muscle density, a measure of intramuscular fat, and mortality in a community-based sample of older, predominantly Caucasian men. Further studies are needed to establish if the association of intramuscular fat with mortality is independent of other ectopic fat depots, and to identify the possible biological mechanisms underlying this relationship.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128. Dr. Miljkovic was supported by the Mentored Research Scientist Development Award from the National Institute of T2DM and Digestive and Kidney Diseases (grant DK083029).
Supplementary Material
References
- 1. Carobbio S, Rodriguez-Cuenca S, Vidal-Puig A. Origins of metabolic complications in obesity: ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Curr Opin Clin Nutr Metab Care. 2011;14:520–526. 10.1097/MCO.0b013e32834ad966 [DOI] [PubMed] [Google Scholar]
- 2. Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24(5):933–41. [DOI] [PubMed] [Google Scholar]
- 3. Vettor R, Milan G, Franzin C, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–E998. 10.1152/ajpendo.00229.2009 [DOI] [PubMed] [Google Scholar]
- 4. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. 10.1053/j.gastro.2008.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–264. 10.1097/MCO.0b013e328337d826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Raalte DH, van der Zijl NJ, Diamant M. Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care. 2010;13(4):478–485. 10.1097/MCO.0b013e32833aa1ef [DOI] [PubMed] [Google Scholar]
- 7. Ding J, Kritchevsky SB, Hsu FC, et al. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;88:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. 10.1093/eurheartj/ehn573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arsenault BJ, Beaumont EP, Despres J-P, Larose E. Mapping body fat distribution: A key step towards the identification of the vulnerable patient? Ann Med. 2012;44(8):758–772. 10.3109/07853890.2011.605387 [DOI] [PubMed] [Google Scholar]
- 10. Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26:13. [DOI] [PubMed] [Google Scholar]
- 11. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. [DOI] [PubMed] [Google Scholar]
- 13. Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002;51:1477–1484. [DOI] [PubMed] [Google Scholar]
- 14. Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14(12):1225–1230. [DOI] [PubMed] [Google Scholar]
- 15. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 16. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. [DOI] [PubMed] [Google Scholar]
- 17. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 18. Lang T, Koyama A, Li C, et al. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone. 2008;42:798–805. 10.1016/j.bone.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 19. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 20. Wijsman CA, van Opstal AM, Kan HE, et al. Proton magnetic resonance spectroscopy shows lower intramyocellular lipid accumulation in middle-aged subjects predisposed to familial longevity. Am J Physiol Endocrinol Metab. 2012;302:E344–E348. 10.1152/ajpendo.00455.2011 [DOI] [PubMed] [Google Scholar]
- 21. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–519. 10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miljkovic I, Cauley JA, Wang PY, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring). 2013;21:2118–2125. 10.1002/oby.20346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manini TM, Buford TW, Lott DJ, et al. Effect of dietary restriction and exercise on lower extremity tissue compartments in obese, older women: a pilot study. J Gerontol A Biol Sci Med Sci. 2014;69:101–108. 10.1093/gerona/gls337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johannsen DL, Conley KE, Bajpeyi S, et al. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab. 2012;97:242–250. 10.1210/jc.2011-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. [DOI] [PubMed] [Google Scholar]
- 26. Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–128. 10.1093/gerona/glp179 [DOI] [PubMed] [Google Scholar]
- 27. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. [DOI] [PubMed] [Google Scholar]
- 28. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–110. [DOI] [PubMed] [Google Scholar]
- 29. Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring). 2006;14:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simonsick EM, Maffeo CE, Rogers SK, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M264–M274. [DOI] [PubMed] [Google Scholar]
- 31. Stratec. XCT 2000 Manual Version 6.66. Medizintechnik GmbH: Pforzheim, Germany; 2005. [Google Scholar]
- 32. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. [DOI] [PubMed] [Google Scholar]
- 33. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 34. Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. [DOI] [PubMed] [Google Scholar]
- 35. Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–1735. [DOI] [PubMed] [Google Scholar]
- 36. Park HS, Song YM, Cho SI. Obesity has a greater impact on cardiovascular mortality in younger men than in older men among non-smoking Koreans. Int J Epidemiol. 2006;35:181–187. [DOI] [PubMed] [Google Scholar]
- 37. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. [DOI] [PubMed] [Google Scholar]
- 38. Katzmarzyk PT, Mire E, Bouchard C. Abdominal obesity and mortality: The Pennington Center Longitudinal Study. Nutr Diabetes. 2012;2:e42. 10.1038/nutd.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–341. [DOI] [PubMed] [Google Scholar]
- 40. McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations among visceral fat, all-cause mortality, and obesity-related mortality in Japanese Americans. Diabetes Care. 2012;35:296–298. 10.2337/dc11-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- 43. Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. 10.1136/bmj.d6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. 10.3945/ajcn.2008.27358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. 10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDermott MM, Liu K, Tian L, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol. 2012;59:1159–1167. 10.1016/j.jacc.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS. Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E1210–E1229. 10.1152/ajpendo.00015.2009 [DOI] [PubMed] [Google Scholar]
- 48. Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med, 2006. 119(5 Suppl 1):S10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schrauwen P. High-fat diet, muscular lipotoxicity and insulin resistance. Proc Nutr Soc. 2007;66:33–41. [DOI] [PubMed] [Google Scholar]
- 50. Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- 51. Kovalik JP, Slentz D, Stevens RD, et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes. 2011;60:1882–1893. 10.2337/db10-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Katewa SD, Demontis F, Kolipinski M, et al. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012;16:97–103. 10.1016/j.cmet.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


