Abstract
Aims:
This study was done with the aim to compare the clinical outcome and patients’ quality of life between early versus delayed surgically treated patients of acute subaxial cervical spinal cord injury. The current study was based on the hypothesis that early surgical decompression and fixations in acute subaxial cervical spinal cord trauma is safe and is associated with improved outcome as compared to delayed surgical decompression.
Materials and Methods:
A total of 69 patients were recruited and divided into early decompression surgery Group A (operated within 48 h of trauma; n = 23) and late/delayed decompression surgery Group B (operated between 48 h and 7 days of trauma; n = 46). The patients in both groups were followed up, and comparative differences noted in the neurological outcome, quality of life, and bony fusion.
Results:
The early surgery group spent lesser days in the intensive care unit and hospital (Group A 28.6 vs. Group B 35 days) had lesser postoperative complications (Group A 43% vs. Group B 61%) and a reduced mortality (Group A 30% vs. Group B 45%). In Group A, 38% patients had 1 American Spinal Injury Association (ASIA) grade improvement while 31% experienced >2 ASIA grade improvement. In Group B, the neurological improvement was 27% and 32%, respectively (P = 0.7). There was a significant improvement in the postoperative quality of life scores in both groups.
Conclusion:
Early surgery in patients with acute subaxial cervical spine injury should be considered strongly in view of the lesser complications, early discharge, and reduced mortality.
Keywords: Acute cervical spine injury, delayed surgery, early surgery, neurological outcome
Introduction
The role and timing of surgical decompression after an acute spinal cord injury (SCI) remain controversial. Despite an enormous amount of interest and research in SCI, the prognosis for neurologic recovery in patients with severe SCI remains poor. Prevalence of traumatic SCI worldwide is approximately 750/million with an annual incidence that appears to be rising.[1] Majority of patients with SCI are young,[2] making immense economic and social impact. The primary mechanism is rapid spinal cord compression caused by bone displacement from a fracture-dislocation or burst fracture, and is irreversible. It also initiates a cascade of secondary injury mechanisms, including ischemia, and lipid peroxidation.[3] The increased understanding of the pathophysiology of acute SCI has led to clinically relevant neuroprotective therapies to attenuate the effects of the secondary injury. The development of these secondary injury events, which lead to tissue destruction during the first few hours after injury, is of relevance to the surgical treatment of SCI as well. There is experimental evidence suggesting that persistent compression of the spinal cord is a potentially reversible form of secondary injury.[4,5,6,7,8,9,10,11,12,13] However, despite its widespread use in patients with acute SCI at most of the hospitals in developed countries, the role of surgery in improving neurologic recovery remains controversial because of the absence of well-designed randomized controlled trials.[14] The presence and duration of a therapeutic window, during which surgical decompression could mitigate the secondary mechanisms of SCI, remain unclear.[14] Moreover, the practical and logistical challenges related to early decompression of patients with an acute SCI remain important issues for clinical caregivers.
Materials and Methods
The study was conducted to compare clinical outcomes and patient's quality of life between surgically treated patients in early and late surgery group. The study was conducted at Department of Neurosurgery, Jai Prakash Narayan Apex Trauma Center, All India Institute of Medical Sciences, New Delhi. This prospective observational study included patients admitted with acute subaxial cervical spinal cord trauma in the Trauma Center between November 2011 and April 2013 and was carried out after taking the necessary clearance from the Institutional Ethics Committee. The possibility of randomized analysis to compare outcome was not considered because of ethical concerns about allocating a deteriorating patient to delayed decompression.
The inclusion criteria were: (1) Cervical SCI of American Spinal Injury Association (ASIA) Grade A, B, C or D; (2) admitted within 7 days of injury, (3) spinal cord compression determined by magnetic resonance imaging (MRI)/computed tomography (CT) and (4) age 16–75 years. The exclusion criteria were: (1) Penetrating or gunshot injury, (2) cognitive impairment preventing neurologic assessment, (3) multiple life-threatening injuries, (4) medically unstable patients including those with myocardial infarction within 3 months, (5) previous neurological injury/cerebrovascular disease with neurological deficits and (6) cancer/AIDS/ankylosing spondylitis/uncertain follow-up.
Neurological assessment was done using ASIA[15] Impairment Scale (AIS), preoperatively, postoperatively and during follow-up. All patients underwent radiological evaluation including noncontrast CT (NCCT) scan and MRI of the cervical spine to determine level of spine injury, spinal cord compression, signal changes, associated cord edema and cord contusions, canal compromise, alignment of spine curvature, and antero-posterior diameter at the level of maximum compression.
Patients were divided into two groups based on the duration from injury to surgery, within 48 h (Group A) and between 48 h and 7 days (Group B). Surgical approach (anterior or posterior or combined 360° approach (anterior and posterior), and levels of decompression and type of instrumentation were decided by operating surgeon [Table 3].
Table 3.
Duration of surgery and blood loss
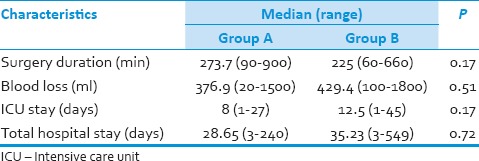
The endpoints for this study were neurological outcome (ASIA scale) assessed at 3, 6, and 12 months, quality of life assessed by the Short Form-36 (SF-36) questionnaire at 3 and 6 months and bony fusion assessed by NCCT scan at 3 months using the Bridwell grading system.[16] Good fusion was defined when Grade I and II fusion was noted on CT scans while, pseudoarthrosis was defined when Grade III and IV changes were noted on CT scan of cervical spine postoperatively.
Statistical analysis
Statistical analysis was done using? SPSS software (Version 17.0). For qualitative variables, Chi-square test and Fisher's test were done. For quantitative variables, Student's t-test and Mann–Whitney test were done. Univariate and multivariate regression analysis were done for the predictors of outcome such as age, gender, preoperative AIS scale, associated injury, comorbidities, administration of steroid, preoperative closed reduction, level of injury, associated cord contusion, and postoperative complications.
Results
A total of 206 patients were operated for cervical spine injury during the study period. Of them, 69 patients met the inclusion criteria and were divided into two groups according to duration from injury to surgery. Twenty-three patients (33%) were included in the early surgery group and 46 patients (67%) in late/delayed surgery group [Figure 1].
Figure 1.
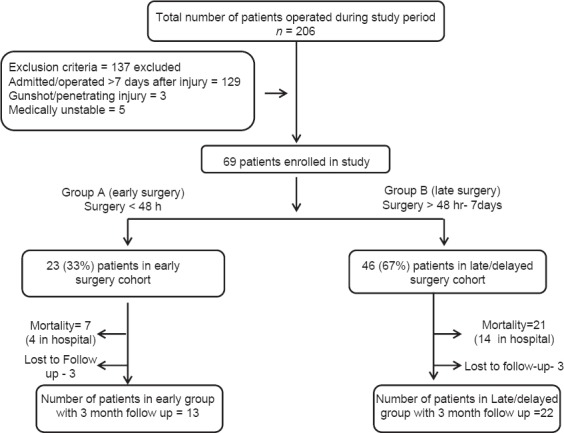
Patient selection flowchart
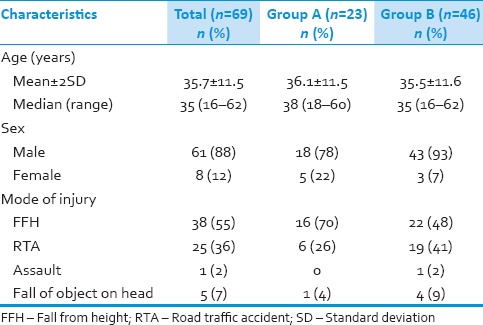
Demographic data
Mean age in Group A was 36.1 ± 11.5 and ranged from 18 to 60 years compared to 35.5 ± 11.6 in Group B with a range of 16–60 years (P = 0.82). In Group A, 18 (78%) were male and 5 (22%) were females as compared to Group B in which 43 (93%) male and 3 (7%) female (P = 0.06). In both the groups, fall was the most common mode of injury [Table 1]. In Group A, 4 patients had other associated injury, 2 (8%) patients had associated minor head injury, and other 2 (8%) patients had a long bone fracture. While in Group B, 6 patients (13%) had associated head injury, 2 (4%) patients had associated with long bone fracture and 5 (11%) patients with poly-trauma (chest and abdominal injury) (P = 0.36).
Table 1.
Demographic and injury characteristics of patients
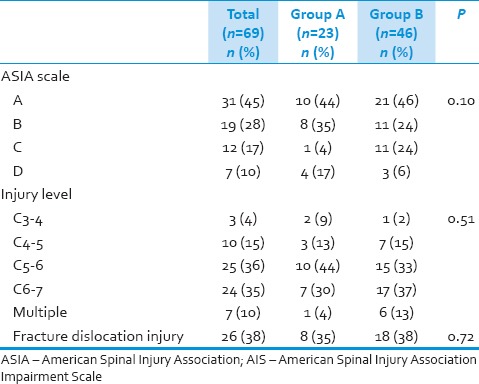
Preoperative American Spinal Injury Association Impairment Scale and level of injury
In group preoperative injuries were of ASIA scale A in 10 (43%) patients, ASIA B in 8 (35%), while in Group B, 21 (46%) patients were in ASIA scale A, 11 (24%) patients in ASIA scale B [Table 2]. In Group A, most commonly affected level in subaxial cervical spine injury was C5–6 level with 10 (44%) patients, followed by C6–7 in 7 (30%) patients. In Group B, C6–7 level was most commonly affected in 17 (37%) patients followed by C5–6 level involved in 15 (33%) patients. Facet/fracture dislocation type injury were present in 26 (38%) patients, 8 (35%) in Group A and 18 (38%) patients in Group B [Table 2].
Table 2.
Preoperative AIS and level of injury
Magnetic resonance imaging findings
Magnetic resonance imaging of cervical spine was done in 55 (80%) patients. In Group A, MRI was done in 12 (52%) patients while in Group B, it was done in 43 (94%) of patients. Cord edema and contusion were seen in 10 (83%) and 3 (25%) in Group A and 41 (95%) and 18 (42%) in Group B, respectively. The differences in findings were due to lesser patients in Group A in whom MRI was performed.
Surgery
Patients in Group A were operated at a mean 30.5 ± 14.1 (range: 6–48) h after injury as compared to 111.2 ± 40.7 (range: 52–168) h in Group B patients.
In Group A, 21 (92%) patients underwent surgery through anterior approach, 1 (4%) with posterior approach and 1 (4%) with combined approach. In Group B, 39 (85%) were operated by anterior approach, 3 (6%) by posterior approach and 4 (9%) operated by combined approach [Figure 2].
Figure 2.
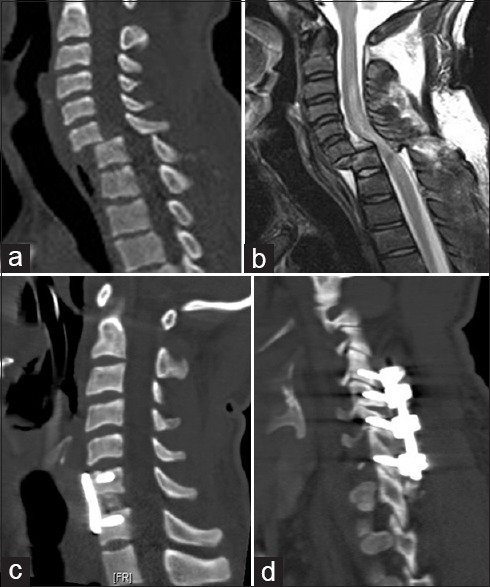
(a) Noncontrast computed tomography (NCCT) of cervical spine with C6–7 listhesis and bilateral locked facets; (b) Magnetic resonance imaging of cervical spine showing severe cord compression at C6–7 level with cord edema from CVJ to D1. (c and d) Postoperative NCCT scan showing good lordosis of cervical spine achieved with C6–7 discectomy, iliac crest graft with plating and posterior fixation
Postoperative events
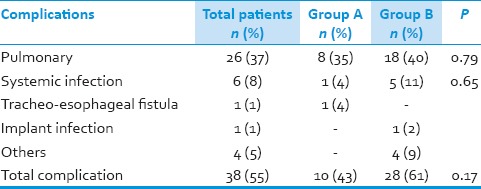
Postoperative ventilator support was required in 46 (61%) patients. Prolonged ventilator support was required in 9 (39%) patients in Group A and 26 (57%) in Group B (P - 0.20). Pulmonary complications were noted in 8 (35%) patients in Group A as compared to 18 (40%) patients in Group B, which included infection, effusions, and ventilation-related complications. Systemic infections developed in 1 (4%) patient in Group A as compared to 5 (11%) in Group B. One patient in Group A developed trachea-esophageal fistula in postoperative period, which was managed conservatively. One patient in Group B developed implant infection with an epidural collection with subsequent deterioration in power for which urgent implant removal was done, and collection drained. Fixation was done at a later date through posterior approach. In the postoperative period, one patient developed massive pulmonary embolism and died while another had an enteric perforation, underwent exploratory laparotomy, developed sepsis and died. Over all the complication rate was 43% in Group A as compared to 61% in Group B (P = 0.17). Mortality rate in Group A was 30% as compared to 45% in Group B (P - 0.30). In hospital mortality was 4 (17%) in Group A and 14 (30%) in Group B (P - 0.38) [Table 4].
Table 4.
Complication rate in patient groups
Follow-up
Thirteen patients completed mean follow of 6.9 ± 4.8 months in Group A and 22 patients in Group B with mean follow-up of 9.9 ± 5.9 months (P - 0.12). Six patients, 3 (13%) in Group A and 3 (7%) in Group B were lost to follow-up. This difference in follow-up was statistically not significant.
Neurological status
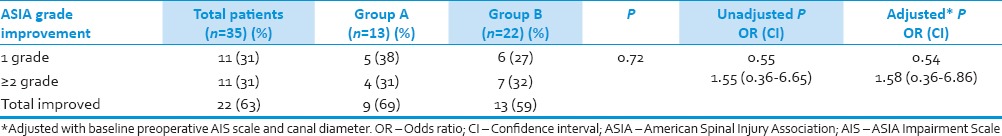
At last follow-up 22 (63%, of the 35 patients with long follow-up patients) had improvement in ASIA grade in both groups, 9 (69%, out of 13 followed up) patients in Group A as compared to 13 (59%, out of 22 followed up) patients in Group B (P - 0.72). More than 2 ASIA grade improvement was noted in 4 (31%) patients in Group A and in 7 (32%) patients in Group B [Table 5]. On multivariate logistic regression after removing significant baseline factors, difference in improvement in ASIA scale between the two groups were not significant (P - 0.54, odds ratio: 1.58; confidence interval: 0.36–6.86). The factors like administration of methylprednisolone (2 patients [9%] were given methylprednisolone in Group A as compared to 11 [24%] in Group B [P = 0.31]) and operating surgeon did not have any effect on improvement of neurological recovery (P - 0.99).
Table 5.
Improvement in both group
Quality of life related to general health
Short Form-36 score
Preoperative and postoperative quality of life scores improved significantly, but intergroup difference in improvement was not significant between two groups on statistical analysis. There was marked improvement in GH (General Perception of Health), SF (Social Functioning), role emotional, and Mental Composed Score in both groups, this difference was statistically not significant.
Fusion rate in both patient groups
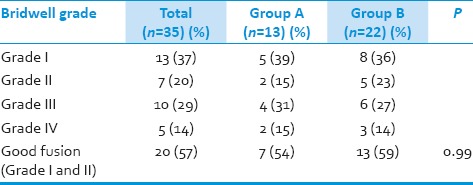
Twenty patients (60%) showed a bony fusion of Bridwell Grade I/II at last follow-up. In Group A, good fusion rate was 54% as compared to 59% in Group B (P - 0.99) [Table 6].
Table 6.
Fusion rate in patients groups according to Bridwell criteria
Discussion
Experimental studies of decompression in acute spinal cord injury in animal models
There is convincing evidence from laboratory studies in various animal models that persistent compression of the spinal cord is a potentially reversible form of secondary injury.[4,5,6,7,8,9,10,17,18,19] These studies have consistently shown that neurologic recovery is enhanced by early decompression. Using a canine model, Delamarter et al.[6] demonstrated a statistically significant correlation between the duration of spinal cord compression and the degree of neurological recovery. Dimar et al.[7] provided the most compelling experimental evidence using a weight drop model in rat that showed spinal cord decompression after SCI is beneficial. The neurologic recovery was inversely related to the duration of compression, with statistically significant differences seen in all experimental groups. Carlson et al.[10] also reported similar results in dogs.
Some investigators have indicated that neither spinal surgery nor anatomic realignment of the spinal column improved neurologic outcome in patients with acute SCI, with the possible exception of bilateral locked facets.[20,21,22]
Role of Timing of surgery in the treatment of acute spinal cord injury
There are few published studies in literature, which are prospective, controlled studies of surgical decompression in acute SCI[23,24,25,26,27,28] Papadopoulos.[20] evaluated 91 patients with acute cervical SCI. Thirty-nine out of 66 patients in the protocol group (MRI ± surgery) had improvement, including some presenting with a complete SCI, compared to 6 out of 25 in the control group. La Rosa et al.[17] in systemic review of all studies published between 1966 and 2000 concluded that early (<24 h) surgical decompression in patients with incomplete injuries resulted in better neurologic outcomes than patients treated with either delayed decompression (>24 h) or nonoperative treatment.[29] A number of investigators have advocated early reduction (4–10 h) and operative fixation of spinal fractures in patients with acute SCI.[23,24,25,30,31] These studies suggest that early decompression may enhance neurologic recovery in selected patients with SCI. However, most of these studies lack randomization or appropriate controls and thus, represent Class III evidence only. The clinical benefits of early surgery for fracture dislocations of the spine are difficult to assess in the absence of Class I data.[26,29,30,31,32,33,34,35,36,37,38,39,40,41,42]
In contrast, several prospective studies.[24,27] have failed to document a beneficial effect of early surgical decompression. In the prospective trial by Vaccaro et al.,[19] they compared patients who had early surgery (<72 h after injury) with those who had late surgery (>5 days after injury). They concluded that there is no significant neurologic benefit when cervical spinal cord decompression is performed <72 h after injury as opposed to waiting longer than 5 days. In their retrospective study of 412 patients, Pollard and Apple[43] concluded that early surgery (<24 h) was not associated with improved neurologic outcome, but these conclusions must be interpreted very cautiously since only 49% of patients (202) had baseline neurologic assessments, and 168 were lost to follow-up. Larson et al.[44] advocated operating a week or more after SCI to allow medical and neurologic stabilization of the injured patient. This remains the practice in many institutions, particularly in light of early reports suggesting an increased rate of medical complications with early surgery (<5 days after SCI).[42] Interestingly, a number of investigators have documented recovery of neurologic function after delayed decompression of the spinal cord (months to years) after the injury.[45,46,47,48] Although these studies are retrospective in design (Class III evidence), the improvement in neurologic function with delayed decompression in patients with cervical SCI who have had a plateau in their recovery is noteworthy and suggests that compression of the cord is an important contributing cause of neurologic dysfunction.
Although reports of significant neurologic improvement in some cervical cases decompressed by early traction are encouraging, they do not provide sufficient evidence to support standards or guidelines.[24,30,35,38] Cotler et al.[28] in their prospective study examined the safety and efficacy of early reduction by traction in 24 patients and found no neurologic deterioration in any of the patients, most of whom had successful reduction with closed techniques within 24 h of injury. But, Tator et al.[37] documented an 8.1% rate of neurologic deterioration (majority were transient radicular deficits cleared with a reduction in weight) with attempts at the closed reduction in 585 cases.
These data and a number of other Class II studies[20,25,29,35,36,37,38,39,40] support a recommendation for urgent reduction of bilateral locked facets in a patient with incomplete tetraplegia or in a patient with neurologic deterioration. Importantly, no study has associated adverse neurologic outcomes with early surgical intervention, regardless of a specific time cut-off.[49]
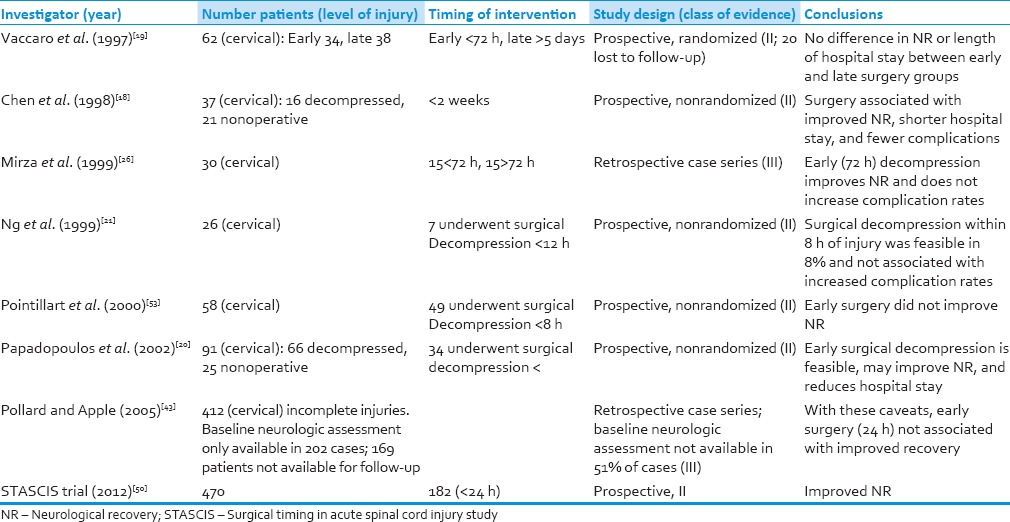
Fehlings et al.[50] published the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) trial and recommend decompression before 24 h after SCI is associated with improved neurologic outcome and less complication rate at 6 months follow-up. In our study, neurological improvement (at least 1 ASIA grade) at mean follow-up of 8.7 months was 69% patients in the early group compared to 59% in the late group. Two or more AIS grade improvement was seen in 31% in the early group and 32% patients in the late surgical group. Preoperative ASIA Grade A injury patients showed improvement in 26% of patients in our study, which is comparable to historical rate of 15–25%[51] Table 7 tabulates the important studies of surgical decompression in cervical spine injury.
Table 7.
Various published clinical studies of surgical decompression in acute cervical spinal cord injury
Effect of surgery on complications and length of stay after spinal cord injury
Duh et al.[22] showed that patients operated in the first 24 h had a lower rate of complications than those undergoing surgery later. In the prospective, randomized trial by Vaccaro et al.,[19] there was no significant difference in length of acute postoperative intensive care stay or length of inpatient rehabilitation between the early and late groups, which has been reiterated by others as well.[23,32] In the present study also, we confirmed that there is no statistically significant difference in length of intensive care unit stay or in hospital stay between two groups. McKinley et al.[52] have reported that early surgery is associated with shorter hospitalization, reduced pulmonary complications, and equivalent neurologic outcomes as delayed surgery. In STASCIS trial, there were no significant differences in complication in between two groups. In present study rate of complications among early surgery group were less than late group (43% vs. 61%), but not statistically significant.[49]
Improvement in quality of life-related to general health was also comparable in both the groups without significant change. However, intra-group improvement was significant. Quality of life is not studied well after cervical spine injury in the context of timing of surgery from injury. Very few studies reported in the literature show improvement in quality of life after surgery as compared to nonoperative treatment.[50,53]
Limitation of our study is that the number of patients included in the study was less. Timing of surgical intervention in the early surgery group was 48 h, which may need to be lessened as in literature early surgery were defined as <24 h or <8 h in some studies. Surgeon's selection bias was another drawback of this study as there were 8 operating surgeons with different surgical experience. However, there was no significant difference in improvement between operating surgeon. Therefore, further well-designed studies with long follow-ups are required to evaluate the role of early surgery accurately in acute subaxial cervical spine injury.
Conclusions
Early surgical decompression in acute cervical spinal cord trauma is safe and associated with improvement in quality of life/neurological status/shorter hospital stay. There was a significant improvement in the quality of life in all patients. The early surgery group had less complications and mortality rate as compared to late/delayed surgical decompression in acute subaxial cervical spinal cord trauma patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kraus JF, Franti CE, Riggins RS, Richards D, Borhani NO. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471–92. doi: 10.1016/0021-9681(75)90057-0. [DOI] [PubMed] [Google Scholar]
- 2.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 3.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–39. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 4.Bohlman HH, Bahniuk E, Raskulinecz G, Field G. Mechanical factors affecting recovery from incomplete cervical spinal cord injury: A preliminary report. Johns Hopkins Med J. 1979;145:115–25. [PubMed] [Google Scholar]
- 5.Carlson GD, Minato Y, Okada A, Gorden CD, Warden KE, Barbeau JM, et al. Early time dependent decompression for spinal cord injury: Vascular mechanisms of recovery. J Neurotrauma. 1997;14:951–62. doi: 10.1089/neu.1997.14.951. [DOI] [PubMed] [Google Scholar]
- 6.Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042–9. doi: 10.2106/00004623-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dimar JR, 2nd, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24:1623–33. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749–55. doi: 10.3171/jns.1980.53.6.0749. [DOI] [PubMed] [Google Scholar]
- 9.Guha A, Tator CH, Endrenyi L, Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324–39. doi: 10.1038/sc.1987.61. [DOI] [PubMed] [Google Scholar]
- 10.Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: Part I: Time dependent effect on long term pathophysiology. J Bone Joint Surg Am. 2003;85 A:86–94. [PubMed] [Google Scholar]
- 11.Fehlings MG, Sekhon LH, Tator C. The role and timing of decompression in acute spinal cord injury: What do we know? What should we do. Spine (Phila Pa 1976) 2001;26:S101–10. doi: 10.1097/00007632-200112151-00017. [DOI] [PubMed] [Google Scholar]
- 12.Fehlings MG, Tator CH. An evidence based review of decompressive surgery in acute spinal cord injury: Rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91:1–11. doi: 10.3171/spi.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 13.Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: Update with a review of recent clinical evidence. Injury. 2005;36(Suppl 2):B13–26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: A systematic review of recent clinical evidence. Spine (Phila Pa 1976) 2006;31(11 Suppl):S28–35. doi: 10.1097/01.brs.0000217973.11402.7f. [DOI] [PubMed] [Google Scholar]
- 15.Chicago, IL: American Spinal Injury Association; 2000. American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, Revised 2000. [Google Scholar]
- 16.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410–8. [PubMed] [Google Scholar]
- 17.La Rosa G, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta analytical approach. Spinal Cord. 2004;42:503–12. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 18.Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976) 1998;23:2398–403. doi: 10.1097/00007632-199811150-00007. [DOI] [PubMed] [Google Scholar]
- 19.Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976) 1997;22:2609–13. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulos SM, Selden NR, Quint DJ, Patel N, Gillespie B, Grube S. Immediate spinal cord decompression for cervical spinal cord injury: Feasibility and outcome. J Trauma. 2002;52:323–32. doi: 10.1097/00005373-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Ng WP, Fehlings MG, Cuddy B, Dickman C, Fazl M, Green B, et al. Surgical treatment for acute spinal cord injury study pilot study #2: Evaluation of protocol for decompressive surgery within 8 hours of injury. Neurosurg Focus. 1999;6:e3. doi: 10.3171/foc.1999.6.1.4. [DOI] [PubMed] [Google Scholar]
- 22.Duh MS, Shepard MJ, Wilberger JE, Bracken MB. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240–8. doi: 10.1227/00006123-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Aebi M, Mohler J, Zäch GA, Morscher E. Indication, surgical technique and results of 100 surgically treated fractures and fracture dislocations of the cervical spine. Clin Orthop Relat Res. 1986;203:244–57. [PubMed] [Google Scholar]
- 24.Wolf A, Levi L, Mirvis S, Ragheb J, Huhn S, Rigamonti D, et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75:883–90. doi: 10.3171/jns.1991.75.6.0883. [DOI] [PubMed] [Google Scholar]
- 25.Hadley MN, Fitzpatrick BC, Sonntag VK, Browner CM. Facet fracture dislocation injuries of the cervical spine. Neurosurgery. 1992;30:661–6. [PubMed] [Google Scholar]
- 26.Mirza SK, Krengel WF, 3rd, Chapman JR, Anderson PA, Bailey JC, Grady MS, et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop Relat Res. 1999;359:104–14. doi: 10.1097/00003086-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Weinshel SS, Maiman DJ, Baek P, Scales L. Neurologic recovery in quadriplegia following operative treatment. J Spinal Disord. 1990;3:244–9. [PubMed] [Google Scholar]
- 28.Cotler JM, Herbison GJ, Nasuti JF, Ditunno JF, Jr, An H, Wolff BE. Closed reduction of traumatic cervical spine dislocation using traction weights up to 140 pounds. Spine (Phila Pa 1976) 1993;18:386–90. doi: 10.1097/00007632-199303000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Burke DC, Berryman D. The place of closed manipulation in the management of flexion rotation dislocations of the cervical spine. J Bone Joint Surg Br. 1971;53:165–82. [PubMed] [Google Scholar]
- 30.Brunette DD, Rockswold GL. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445–7. doi: 10.1097/00005373-198704000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Sonntag VK. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1981;8:150–2. doi: 10.1227/00006123-198102000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Star AM, Jones AA, Cotler JM, Balderston RA, Sinha R. Immediate closed reduction of cervical spine dislocations using traction. Spine (Phila Pa 1976) 1990;15:1068–72. doi: 10.1097/00007632-199015100-00016. [DOI] [PubMed] [Google Scholar]
- 33.Hadley MN, Fitzpatrick BC, Sonntag VK, Browner CM. Facet fracture dislocation injuries of the cervical spine. Neurosurgery. 1992;30:661–6. [PubMed] [Google Scholar]
- 34.Grant GA, Mirza SK, Chapman JR, Winn HR, Newell DW, Jones DT, et al. Risk of early closed reduction in cervical spine subluxation injuries. J Neurosurg. 1999;90:13–8. doi: 10.3171/spi.1999.90.1.0013. [DOI] [PubMed] [Google Scholar]
- 35.Lee AS, MacLean JC, Newton DA. Rapid traction for reduction of cervical spine dislocations. J Bone Joint Surg Br. 1994;76:352–6. [PubMed] [Google Scholar]
- 36.Harris P, Karmi MZ, McClemont E, Matlhoko D, Paul KS. The prognosis of patients sustaining severe cervical spine injury (C2 C7 inclusive) Paraplegia. 1980;18:324–30. doi: 10.1038/sc.1980.59. [DOI] [PubMed] [Google Scholar]
- 37.Tator CH, Fehlings MG, Thorpe K, Taylor W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: Results of a retrospective multicenter study. J Neurosurg. 1999;91:12–8. doi: 10.3171/spi.1999.91.1.0012. [DOI] [PubMed] [Google Scholar]
- 38.Anderson PA, Bohlman HH. Anterior decompression and arthrodesis of the cervical spine: Long term motor improvement. Part II – Improvement in complete traumatic quadriplegia. J Bone Joint Surg Am. 1992;74:683–92. [PubMed] [Google Scholar]
- 39.Bohlman HH, Anderson PA. Anterior decompression and arthrodesis of the cervical spine: Long term motor improvement. Part I – Improvement in incomplete traumatic quadriparesis. J Bone Joint Surg Am. 1992;74:671–82. [PubMed] [Google Scholar]
- 40.Bohlman HH, Kirkpatrick JS, Delamarter RB, Leventhal M. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res. 1994;300:24–9. [PubMed] [Google Scholar]
- 41.Brodkey JS, Miller CF, Jr, Harmody RM. The syndrome of acute central cervical spinal cord injury revisited. Surg Neurol. 1980;14:251–7. [PubMed] [Google Scholar]
- 42.Marshall LF, Knowlton S, Garfin SR, Klauber MR, Eisenberg HM, Kopaniky D, et al. Deterioration following spinal cord injury. A multicenter study. J Neurosurg. 1987;66:400–4. doi: 10.3171/jns.1987.66.3.0400. [DOI] [PubMed] [Google Scholar]
- 43.Pollard ME, Apple DF. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine (Phila Pa 1976) 2003;28:33–9. doi: 10.1097/00007632-200301010-00009. [DOI] [PubMed] [Google Scholar]
- 44.Larson SJ, Holst RA, Hemmy DC, Sances A., Jr Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628–37. doi: 10.3171/jns.1976.45.6.0628. [DOI] [PubMed] [Google Scholar]
- 45.Guttmann L. Initial treatment of traumatic paraplegia and tetraplegia. In: Harris P, editor. Spinal Injuries Symposium. Edinburgh, UK: Royal College of Surgeons; 1963. pp. 80–92. [Google Scholar]
- 46.Guttmann L. 2nd ed. Oxford, UK: Blackwell; 1976. Spinal Cord Injuries: Comprehensive Management and Research. [Google Scholar]
- 47.Bedbrook GM, Sakae T. A review of cervical spine injuries with neurological dysfunction. Paraplegia. 1982;20:321–33. doi: 10.1038/sc.1982.62. [DOI] [PubMed] [Google Scholar]
- 48.Wilmot CB, Hall KM. Evaluation of the acute management of tetraplegia: Conservative versus surgical treatment. Paraplegia. 1986;24:148–53. doi: 10.1038/sc.1986.19. [DOI] [PubMed] [Google Scholar]
- 49.Levi L, Wolf A, Belzberg H. Hemodynamic parameters in patients with acute cervical cord trauma: Description, intervention, and prediction of outcome. Neurosurgery. 1993;33:1007–16. [PubMed] [Google Scholar]
- 50.Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarlov IM. Spinal cord injuries; early treatment. Surg Clin North Am. 1955;35:591–607. [PubMed] [Google Scholar]
- 52.McKinley W, Meade MA, Kirshblum S, Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818–25. doi: 10.1016/j.apmr.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 53.Pointillart V, Petitjean ME, Wiart L, Vital JM, Lassié P, Thicoipé M, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71–6. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]


