Significance
Methane from enteric fermentation in the ruminant digestive system is a major contributor to anthropogenic greenhouse gas emissions in the United States and worldwide. Methane is also a net loss of feed energy to the animal. This study was undertaken to investigate the effect of a methane inhibitor on enteric methane emissions from lactating dairy cows. The experiment demonstrated that, under industry-relevant conditions, the inhibitor persistently decreased by 30% enteric methane emissions, without negatively affecting animal productivity. The spared methane energy was partially used for tissue synthesis, which led to a greater body weight gain by the inhibitor-treated cows. If adopted, this mitigation practice could lead to a substantial reduction of greenhouse gas emissions from the ruminant livestock sector.
Keywords: enteric methane, hydrogen, 3-nitrooxypropanol, livestock, dairy cattle
Abstract
A quarter of all anthropogenic methane emissions in the United States are from enteric fermentation, primarily from ruminant livestock. This study was undertaken to test the effect of a methane inhibitor, 3-nitrooxypropanol (3NOP), on enteric methane emission in lactating Holstein cows. An experiment was conducted using 48 cows in a randomized block design with a 2-wk covariate period and a 12-wk data collection period. Feed intake, milk production, and fiber digestibility were not affected by the inhibitor. Milk protein and lactose yields were increased by 3NOP. Rumen methane emission was linearly decreased by 3NOP, averaging about 30% lower than the control. Methane emission per unit of feed dry matter intake or per unit of energy-corrected milk were also about 30% less for the 3NOP-treated cows. On average, the body weight gain of 3NOP-treated cows was 80% greater than control cows during the 12-wk experiment. The experiment demonstrated that the methane inhibitor 3NOP, applied at 40 to 80 mg/kg feed dry matter, decreased methane emissions from high-producing dairy cows by 30% and increased body weight gain without negatively affecting feed intake or milk production and composition. The inhibitory effect persisted over 12 wk of treatment, thus offering an effective methane mitigation practice for the livestock industries.
The livestock sector is a significant source of greenhouse gas (GHG) emissions in the United States and globally (1, 2). In the United States, enteric fermentation of feed by ruminants is the largest source of anthropogenic methane emissions (0.14 Gt of CO2 Eq. in 2012; or 25% of the total methane emissions; ref. 3). Globally, according to the most recent Intergovernmental Panel on Climate Change (IPCC) report, GHG emissions from agriculture represent around 10–12% (5.0–5.8 Gt CO2 Eq/yr) of the total anthropogenic GHG emissions (1). In this report, livestock contribution to the global anthropogenic GHG emissions was estimated at 6.3%, with GHG emissions from enteric fermentation accounting for 2.1 Gt CO2 Eq/yr and manure management accounting for 0.99 Gt CO2 Eq/yr (1). The relative contribution of emissions from enteric fermentation to the total agricultural GHG emissions will vary by region depending on the structure of agricultural production and type of livestock production systems. For example, GHG from enteric fermentation were estimated at 57% for New Zealand, a country with a large, pasture-based livestock sector (4). Extensive research in recent years has provided a number of viable enteric methane mitigation practices, such as alternative electron receptors, methane inhibitors, dietary lipids, and increased animal productive efficiency (5). Methane emission in the reticulo-rumen is an evolutionary adaptation that enables the rumen ecosystem to dispose of hydrogen, a fermentation product and an important energy substrate for the methanogenic archaea (6), which may otherwise accumulate and inhibit carbohydrate fermentation and fiber degradation (7, 8). Some compounds may be effective in decreasing methane emission, but they may also decrease feed intake, fiber degradability, and animal productivity (5), or the rumen archaea may adapt to them (9). Therefore, it is important to evaluate methane mitigation strategies in long-term experiments, which for livestock experimentation requires treatment periods considerably longer than the 21–28 d, common for crossover designs. In addition, due to a variety of constraints and confounding factors of batch or continuous culture in vitro systems (5, 10), mitigation compounds, including methane inhibitors, have to be tested in vivo using animals with similar productivity to those on commercial farms. An example of the limitations of in vitro systems is a series of experiments with garlic oil. In continuous rumen culture, garlic oil was very effective in inhibiting rumen methane emission (11), but it failed to produce an effect in sheep (12). The nutrient requirements of high-producing dairy cows are much greater than those of nonlactating or low-producing cows (13) and hence any reduction in feed intake caused by a methane mitigation compound or practice would likely result in decreased productivity, which may not be evident in low-producing cows.
Methane inhibitors are chemical compounds with inhibitory effects on rumen archaea. Compounds such as bromochloromethane, 2-bromoethane sulfonate, chloroform, and cyclodextrin have been tested, some successfully, in various ruminant species (5). Inhibition of methanogenesis by these compounds in vivo can be up to 60% with the effect of bromochloromethane shown to persist in long-term experiments (5, 14). However, the viability of these compounds as mitigation agents has been questioned due to concerns for animal health, food safety, or environmental impact. Bromochloromethane, for example, is an ozone-depleting agent and is banned in many countries.
Among the efficacious methane inhibitors identified is 3-nitrooxypropanol (3NOP; ref. 15). This compound was part of a developmental program designing specific small molecule inhibitors for methyl coenzyme-M (CoM) reductase, the enzyme that catalyzes the last step of methanogenesis, the reduction of methyl CoM and coenzyme-B (CoB) into methane and a CoM–CoB complex (16). A continuous in vitro culture study (11) was followed by in vivo experiments in sheep (17), beef (18), and dairy cattle (19, 20), which demonstrated that 3NOP is an effective methane inhibitor. However, these experiments were conducted using nonlactating animals (17), or were short-term (<35 d; refs. 19 and 20). The rumen microorganisms have the ability to adapt to foreign agents or changes in the feeding regimen and, therefore, short-term responses are not representative of the effect of a given mitigation compound or practice in real farm conditions. McIntosh et al. (21), for example, showed that the MIC50 of essential oils doubled or tripled for a number of important rumen bacteria (Butyrivibrio fibrisolvens, Prevotella bryantii, Ruminococcus albus, Ruminobacter amylophilus), if they were adapted to the treatment for a period of 10 d. Thus, it is critically important for the success of GHG mitigation efforts to substantiate the mitigation potential of a given compound in long-term animal experiments before considering it for adoption by the livestock industries.
Results
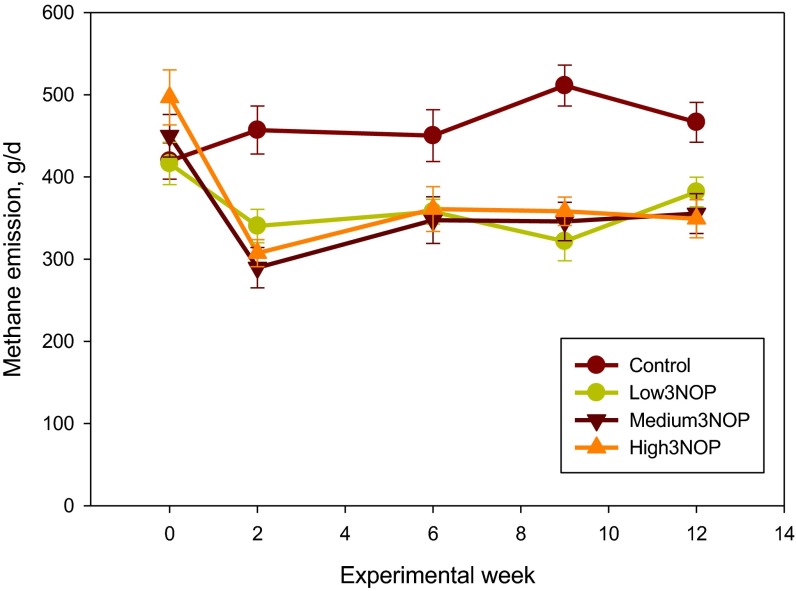
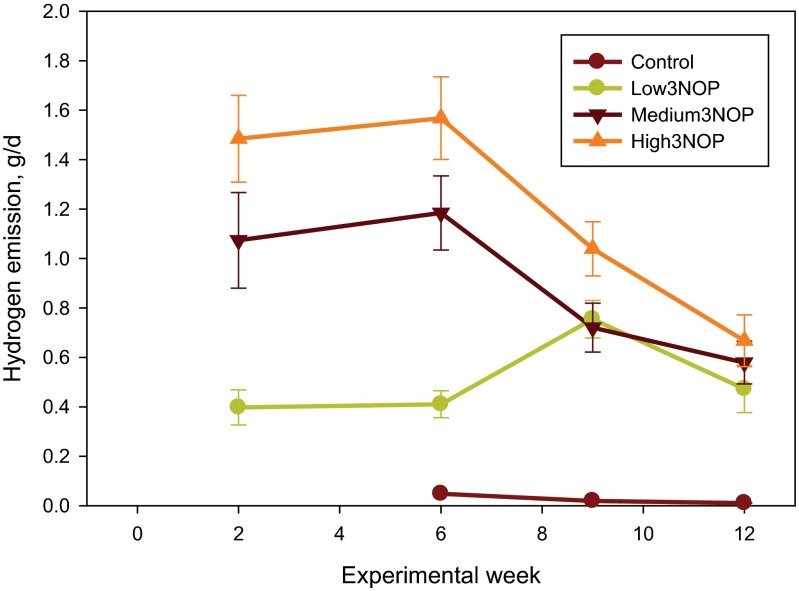
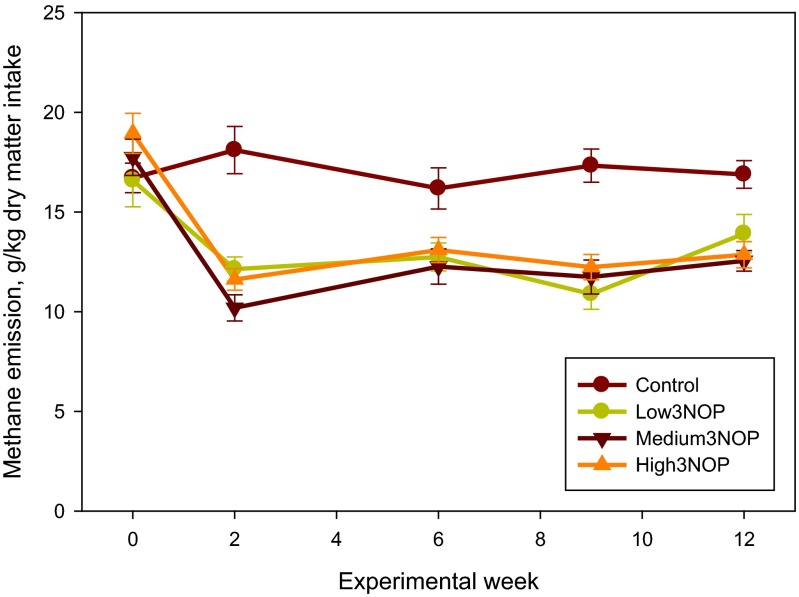
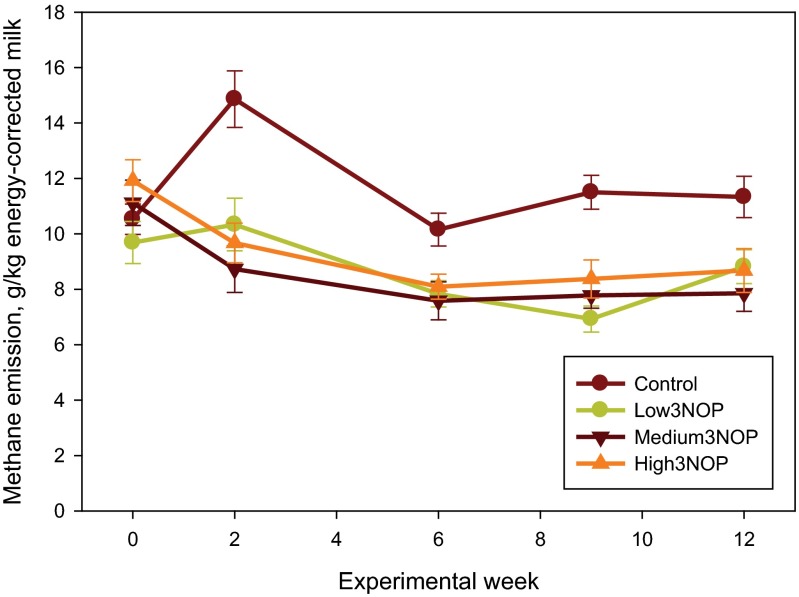
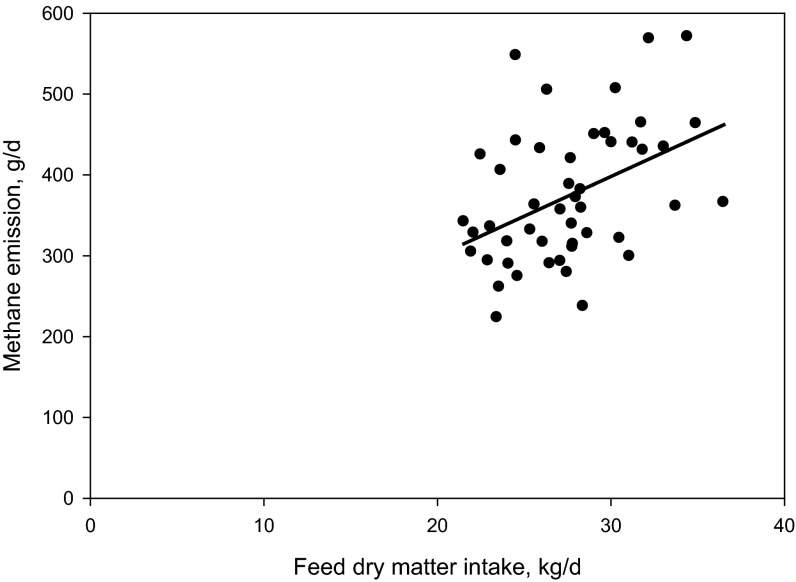
Additional results are presented in SI Results. The diet fed in this experiment was based on corn silage and alfalfa haylage and supplied net energy of lactation and metabolizable protein slightly (−0.2 to −3.0%) below National Research Council (13) requirements (Table S1). As illustrated in Fig. 1, methane emission in cows receiving all levels of 3NOP decreased within 2 wk of initiating the treatment and the difference persisted throughout the 12-wk experiment. Average methane emission values for the control and the 3NOP treatments, 40, 60, and 80 mg/kg feed dry matter, determined using the GreenFeed system (C-Lock, Inc., Rapid City, SD) were: 481, 363, 333, and 329 g per cow per day (SEM = 15.9; P < 0.001, linear and P = 0.05, quadratic effects of 3NOP). Compared with the control, 3NOP decreased the average methane emission by 25%, 31%, and 32%, respectively. A similar decrease in methane emission by 3NOP was also observed when determined using the sulfur hexafluoride (SF6) technique: 485, 390, 365, and 345 g per cow per day (SEM = 29.8; P < 0.001, linear effect of 3NOP). Compared with the control, 3NOP decreased average methane emission by 20%, 25%, and 29%, respectively, when the SF6 method was used. Methane emission measured using the GreenFeed system was similar to that determined using the SF6 technique for the control cows, but was on average 8% lower for the 3NOP treatments. The difference between the two methods was slightly greater when methane emission was expressed per unit of dry matter intake or energy-corrected milk (10% and 14%, respectively). Carbon dioxide emissions (measured using the GreenFeed system) were not different between the control and the 3NOP treatments (P = 0.26; Table S2). Hydrogen emission from the control cows was negligible throughout the experiment (on average 0.02 g per cow per day), but increased considerably for the 3NOP treatments (0.48, 0.96, and 1.27 g per cow per day, respectively; SEM = 0.116, P < 0.001, linear effect) (Fig. 2). Hydrogen emissions were greater for the medium and high 3NOP treatments, compared with Low3NOP, steadily decreased throughout the experiment, and were similar among 3NOP treatments by week 12. This decrease occurred despite the persistent inhibitory effect of 3NOP on methane emission. Expressed per unit of dry matter intake (Fig. S1) or energy-corrected milk (Fig. S2), methane emission was on average about 29 and 31%, respectively, lower for the 3NOP treatments compared with the control (see also Table S2). When expressed as a percent of gross energy intake, methane energy was about 28% lower for the 3NOP treatments (average of 3.8%) vs. the control (5.3%) (P = 0.001, quadratic effect) when methane emission was measured using the GreenFeed system. Similar trends were observed when the SF6 technique was used and when methane emission was expressed as percent of digestible energy intake (Table S2). At the end of the experiment (week 12), the difference in methane emission per kg of dry matter intake between the 3NOP treatments and the control was on average 25% (Fig. S1). The relationship between feed dry matter intake and methane emission determined using the GreenFeed system for all cows was weak (R2 = 0.19, n = 48; Fig. S3). The relationship for the control (i.e., not treated with 3NOP) cows was considerably better (R2 = 0.47, n = 12; data not shown). There was no relationship between dry matter intake and methane emission determined using the SF6 technique (R2 = 0.08, n = 48; data not shown).
Table S1.
Ingredients and chemical composition of the diet fed in the experiment
| Diet* | ||
| Item | Phase 1 | Phase 2 |
| Ingredient, % of dry matter | ||
| Corn silage† | 42.2 | 43.2 |
| Alfalfa haylage‡ | 18.0 | 18.0 |
| Soybean seeds, whole, heated§ | 8.0 | 8.0 |
| Corn grain, ground | 7.0 | 7.0 |
| Candy by-product meal¶ | 6.6 | 6.6 |
| Canola meal, mechanically extracted# | 6.0 | 6.0 |
| Molasses|| | 5.2 | 5.2 |
| SoyPLUS** | 4.0 | 3.0 |
| Mineral/vitamin premix†† | 3.0 | 3.0 |
| Composition, % of dry matter | ||
| Crude protein‡‡ | 16.6 | 16.1 |
| Neutral-detergent fiber‡‡ | 27.3 | 27.9 |
| Acid-detergent fiber‡‡ | 18.0 | 19.2 |
| Starch‡‡ | 24.4 | 23.9 |
| Gross energy, MJ/kg | 18.0 | 18.1 |
| Net energy of lactation, MJ/kg§§ | 6.65 | 6.53 |
| Nonfiber carbohydrates§§ | 46.3 | 46.1 |
| Ash‡‡ | 6.93 | 7.33 |
| Ca‡‡ | 0.97 | 0.98 |
| P‡‡ | 0.37 | 0.38 |
The experiment was conducted in two phases (for details, see SI Methods).
Corn silage was 36.1% and 33.9% dry matter and contained (dry matter basis): 7.2% and 6.2% crude protein, 43.8% and 41.6% starch, and 30.8% and 33.9% neutral-detergent fiber (phase 1 and 2, respectively).
Alfalfa haylage was 37.0% and 33.1% dry matter and contained (dry matter basis): 20.0% and 21.9% crude protein, and 46.0% and 41.4% neutral-detergent fiber (phase 1 and 2, respectively).
Soybean seeds contained (dry matter basis) 39.9% crude protein (average of phase 1 and 2).
Candy by-product meal (Graybill Processing) contained (dry matter basis) 17.3% crude protein (average of phase 1 and 2).
Canola meal contained (dry matter basis) 42.7% crude protein (average of phase 1 and 2).
Molasses (Westway Feed Products) contained (dry matter basis) 3.9% crude protein and 66% total sugar.
SoyPLUS (West Central Cooperative) contained (dry matter basis) 47.2% crude protein (average of phase 1 and 2).
The mineral/vitamin premix (Cargill Animal Nutrition, Cargill Inc.) contained (%, as-is basis) trace mineral mix, 0.86; MgO (56% Mg), 8.0; NaCl, 6.4; vitamin ADE premix (Cargill Animal Nutrition), 0.48; limestone, 37.2; selenium premix (Cargill Animal Nutrition), 0.07; and dry corn distillers grains with solubles, 46.7. Ca, 14.1%; P, 0.39%; Mg, 4.59%; K, 0.44%; S, 0.39%; Se, 6.91 mg/kg; Cu, 362 mg/kg; Zn, 1,085 mg/kg; Fe, 186 mg/kg; vitamin A, 276,717 IU/kg; vitamin D, 75,000 IU/kg; and vitamin E, 1,983 IU/kg.
Values calculated using the chemical analysis (Cumberland Valley Analytical Services) of individual feed ingredients of the diet.
Estimated by NRC (13).
Fig. 1.
Methane emission of dairy cows treated with 3-nitrooxypropanol (3NOP). Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Methane emission was measured using the GreenFeed system (C-Lock, Inc.). Data are treatment means and bars represent SE; n = 12 (number of independent data points for each mean value).
Table S2.
Effect of 3-nitrooxypropanol on rumen carbon dioxide (CO2), methane (CH4), and hydrogen (H2) emissions and total tract digestibility of dietary nutrients in dairy cows
| Treatment* | P value† | |||||||
| Item | Control | Low3NOP | Medium3NOP | High3NOP | SEM‡ | C vs. Trt. | L | Q |
| Rumen gas emission data: GreenFeed§ | ||||||||
| CO2, g/d | 14,861 | 14,003 | 14,116 | 14,881 | 407.1 | 0.26 | 0.80 | 0.05 |
| CH4, g/d | 481 | 363 | 333 | 319 | 15.9 | <0.001 | <0.001 | 0.05 |
| CH4, g/kg of DMI¶ | 17.2 | 12.5 | 11.8 | 12.2 | 0.60 | <0.001 | <0.001 | 0.002 |
| CH4, g/kg of ECM milk¶ | 12.0 | 8.7 | 7.9 | 8.3 | 0.48 | <0.001 | <0.001 | 0.008 |
| CH4 energy, % of GEI (Ym)# | 5.3 | 3.9 | 3.7 | 3.9 | 0.18 | <0.001 | <0.001 | 0.001 |
| CH4 energy, % of DEI# | 7.8 | 5.7 | 5.4 | 5.7 | 0.26 | <0.001 | <0.001 | 0.001 |
| H2, g/d | 0.02 | 0.48 | 0.96 | 1.27 | 0.116 | <0.001 | <0.001 | 0.35 |
| Rumen gas emission data: SF6 technique§ | ||||||||
| CH4, g/d | 485 | 390 | 365 | 345 | 29.8 | 0.001 | <0.001 | 0.47 |
| CH4 g/kg of DMI¶ | 19.0 | 14.0 | 13.1 | 12.9 | 1.09 | <0.001 | <0.001 | 0.14 |
| CH4, g/kg of ECM milk¶ | 14.4 | 10.1 | 9.3 | 9.1 | 0.78 | <0.001 | <0.001 | 0.08 |
| Total tract apparent digestibility of nutrients, %|| | ||||||||
| Dry matter | 68.5 | 70.8 | 69.8 | 68.9 | 0.62 | 0.06 | 0.58 | 0.006 |
| Organic matter | 69.8 | 71.3 | 71.0 | 69.9 | 0.71 | 0.21 | 0.66 | 0.06 |
| Crude protein | 64.1 | 66.4 | 65.1 | 63.1 | 0.82 | 0.42 | 0.52 | 0.006 |
| Neutral-detergent fiber | 46.6 | 48.8 | 47.2 | 45.9 | 1.35 | 0.64 | 0.76 | 0.14 |
| Acid-detergent fiber | 43.7 | 47.2 | 44.5 | 43.6 | 1.43 | 0.23 | 0.87 | 0.01 |
| Starch | 97.4 | 97.5 | 97.1 | 97.1 | 0.20 | 0.58 | 0.25 | 0.44 |
Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). GreenFeed gas emission data are covariate-adjusted means.
Contrasts: C vs. Trt., Control vs. all 3NOP treatments; L, linear effect of 3NOP; Q, quadratic effect of 3NOP.
Largest SEM published in table. GreenFeed data: CO2 and CH4, n = 183–187; H2, n = 119; SF6 data, n = 141; digestibility data, n = 48 (n represents number of observations used in the statistical analysis).
GreenFeed (C-Lock Technology Inc., Rapid City, SD; 43) gas emission data were collected at eight different time points in 3 d during wks 2, 6, 9, and 12 of the experiment and averaged per wk. Sulfur hexafluoride (SF6) technique (47) data were collected for an average of 3 d during experimental weeks 2, 9, and 12 (phase 1) and 2, 6, and 12 (phase 2) and averaged per wk.
Based on energy-corrected milk (ECM) yield and dry matter intake (DMI) data during the gas measurement periods. Energy-corrected milk (kg/d) = kg of milk × ((38.3 × % fat x 10 + 24.2 × % true protein x 10 + 16.54 × % lactose × 10 + 20.7) ÷ 3,140) (48).
Gross (GEI) or digestible (DEI) energy intake were: GEI = 498 ± 9.5 MJ/d and DEI = 341 ± 6.5 MJ/d.
Digestibility was estimated using indigestible neutral-detergent fiber as a marker.
Fig. 2.
Hydrogen emission of dairy cows treated with 3-nitrooxypropanol (3NOP). Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Hydrogen emission was measured using the GreenFeed system (C-Lock, Inc.). Data are treatment means and bars represent SE; n = 12 (number of independent data points for each mean value). Where not visible, error bars are smaller than the symbols. Hydrogen data were not collected during the covariate period for all treatments and during experimental week 2 for the control cows.
Fig. S1.
Methane emission per unit of feed dry matter intake of dairy cows treated with 3-nitrooxypropanol (3NOP). Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Methane emission was measured using the GreenFeed system (C-Lock, Inc.). Data are treatment means and bars represent SE; n = 12 (number of independent data points for each mean value).
Fig. S2.
Methane emission per unit of energy-corrected milk of dairy cows treated with 3-nitrooxypropanol (3NOP). Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Methane emission was measured using the GreenFeed system (C-Lock, Inc.). Data are treatment means and bars represent SE; n = 12 (number of independent data points for each mean value).
Fig. S3.
Relationship between feed dry matter intake and enteric methane emission in all cows (i.e., cows not treated and treated with 3-nitrooxypropanol): Methane, g/d = 103.2 (SE = 84.59) + 9.8 (SE = 3.04) × dry matter intake, kg/d (n = 48; R2 = 0.19; P = 0.002). Methane emission was measured using the GreenFeed system (C-Lock, Inc.).
Milk production data are shown in Table 1. Overall, 3NOP had no effect on dry matter intake, milk yield, and feed efficiency of the cows. There was a treatment × experimental week interaction for feed efficiency (P < 0.001). However, examination of the data revealed no clear trends for variable effect of 3NOP during the course of the experiment; feed efficiency decreased with increasing days in milk for cows of all treatments due to decreased milk production. Compared with the control, concentrations of fat, true protein, and lactose in milk and fat yield were not affected by treatment, but yield of protein and lactose were quadratically increased (P ≤ 0.05) by 3NOP. Although the average body weight of the cows did not differ among treatments, body weight gain during the experiment was greater (P = 0.05) for the 3NOP treated cows compared with control cows. On average, cows treated with 3NOP had about 168 g/d greater daily body weight gain, or 80% greater gain during the 12-wk experiment. Apparent total tract digestibility of dry and organic matter, crude protein, and acid-detergent fiber were quadratically increased (P ≤ 0.06) by 3NOP compared with the control (Table S2). The methane inhibitor had marked effects on milk fatty acid composition (Table S3). Concentrations of most of the shortchain fatty acids (C6:0 through C12:0) were increased (P ≤ 0.03) by 3NOP and there was on average a 23% increase in the concentration of C15:0 (P < 0.01, quadratic effect) and a 6% increase in C17:0 (P = 0.03, quadratic effect) for the 3NOP treatments compared with the control.
Table 1.
Effect of 3-nitrooxypropanol (3NOP) on feed dry matter intake, lactation performance, and body weight change of Holstein dairy cows
| Treatment* | P value† | |||||||
| Item | Control | Low3NOP | Medium3NOP | High3NOP | SEM‡ | C vs. Trt. | L | Q |
| Dry matter intake, kg/d | 28.0 | 28.0 | 27.7 | 27.5 | 0.45 | 0.58 | 0.38 | 0.69 |
| Milk yield, kg/d | 46.1 | 46.4 | 45.9 | 43.6 | 1.21 | 0.59 | 0.21 | 0.19 |
| ECM yield, kg/d§ | 44.9 | 45.2 | 46.2 | 43.9 | 1.59 | 0.91 | 0.84 | 0.44 |
| Feed efficiency kg/kg¶ | 1.64 | 1.65 | 1.67 | 1.62 | 0.033 | 0.94 | 0.80 | 0.41 |
| Milk fat, % | 4.08 | 3.98 | 4.02 | 4.25 | 0.123 | 0.98 | 0.43 | 0.15 |
| Milk fat yield, kg/d | 1.85 | 1.81 | 1.87 | 1.85 | 0.086 | 0.98 | 0.90 | 0.85 |
| Milk true protein, % | 3.06 | 3.14 | 3.12 | 3.13 | 0.033 | 0.07 | 0.14 | 0.31 |
| Milk true protein yield, kg/d | 1.37 | 1.46 | 1.45 | 1.33 | 0.042 | 0.42 | 0.75 | 0.02 |
| Milk lactose, % | 4.78 | 4.79 | 4.81 | 4.77 | 0.026 | 0.69 | 0.95 | 0.32 |
| Milk lactose yield, kg/d | 2.16 | 2.22 | 2.25 | 2.04 | 0.069 | 0.90 | 0.43 | 0.05 |
| Body weight, kg | 664 | 672 | 672 | 664 | 5.0 | 0.38 | 0.83 | 0.13 |
| Body weight change, g/d# | 210 | 353 | 451 | 330 | 71.2 | 0.05 | 0.09 | 0.16 |
Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Data, except body weight change, are presented as covariate-adjusted means.
Contrasts: C vs. Trt., Control vs. all 3NOP treatments; L, linear effect of 3NOP; Q, quadratic effect of 3NOP. Treatment × experimental week interactions for dry matter intake, milk yield, feed efficiency, and body weight: P = 0.05, 0.97, < 0.001, and 0.93, respectively; milk composition and ECM yield data, P ≥ 0.17.
Largest SEM published in table. Production data were averaged per experimental week and the average values were used in the statistical analysis. Dry matter intake, milk yield, feed efficiency, and body weight data, n = 460, 480, 460, and 474, respectively; body weight change, n = 48; milk composition and ECM yield data, n = 140 (n represents number of observations used in the statistical analysis).
Energy-corrected milk (kg/d) = kg of milk × ((38.3 × % fat x 10 + 24.2 × % true protein x 10 + 16.54 × % lactose × 10 + 20.7) ÷ 3,140) (48). Based on milk yield during the weeks of milk sampling (i.e., experimental weeks 6, 9, and 12).
Feed efficiency = milk production ÷ feed dry matter intake.
Body weight change = (average body weight, final week of experiment – average body weight, second week of covariate period) ÷ days on experiment.
Table S3.
Effect of 3-nitrooxypropanol on fatty acid composition of milk fat (g/100 g of total fatty acids) in dairy cows
| Diet* | P value† | |||||||
| Fatty acid | Control | High3NP | Low3NP | Medium3NP | SEM‡ | C vs. Trt. | L | Q |
| 4:0 | 4.10 | 4.17 | 4.13 | 4.03 | 0.083 | 0.96 | 0.60 | 0.27 |
| 6:0 | 2.12 | 2.32 | 2.23 | 2.21 | 0.041 | <0.01 | 0.11 | <0.01 |
| 8:0 | 1.21 | 1.40 | 1.34 | 1.33 | 0.028 | <0.001 | <0.01 | <0.01 |
| 10:0 | 2.82 | 3.35 | 3.20 | 3.21 | 0.083 | <0.001 | <0.01 | <0.01 |
| 12:0 | 3.28 | 3.89 | 3.72 | 3.79 | 0.112 | <0.001 | <0.01 | 0.03 |
| 14:0 | 10.1 | 10.2 | 10.3 | 10.3 | 0.14 | 0.18 | 0.17 | 0.79 |
| 14:1 | 0.90 | 0.88 | 0.90 | 1.04 | 0.051 | 0.58 | 0.12 | 0.06 |
| 15:0 | 0.93 | 1.24 | 1.08 | 1.10 | 0.059 | <0.01 | 0.07 | <0.01 |
| 16:0 | 25.6 | 26.0 | 24.9 | 25.7 | 0.43 | 0.87 | 0.66 | 0.97 |
| 16:1 | 1.33 | 1.31 | 1.20 | 1.29 | 0.07 | 0.40 | 0.40 | 0.63 |
| 17:0 | 0.47 | 0.52 | 0.49 | 0.49 | 0.013 | 0.03 | 0.31 | 0.03 |
| 18:0 | 10.2 | 10.1 | 10.2 | 9.41 | 0.414 | 0.48 | 0.25 | 0.38 |
| 18:1, trans-4 | 0.031 | 0.030 | 0.030 | 0.028 | 0.0011 | 0.19 | 0.08 | 0.44 |
| 18:1, trans-5 | 0.029 | 0.034 | 0.026 | 0.037 | 0.0072 | 0.66 | 0.62 | 0.78 |
| 18:1, trans-6–8 | 0.45 | 0.40 | 0.43 | 0.41 | 0.013 | 0.04 | 0.11 | 0.28 |
| 18:1, trans-9 | 0.42 | 0.35 | 0.35 | 0.35 | 0.037 | 0.09 | 0.15 | 0.42 |
| 18:1, trans-10 | 0.84 | 0.72 | 0.81 | 0.77 | 0.069 | 0.36 | 0.56 | 0.54 |
| 18:1, trans-11 | 1.17 | 0.98 | 1.14 | 0.93 | 0.060 | 0.03 | 0.03 | 0.95 |
| 18:1, trans-12 | 1.35 | 0.94 | 0.60 | 1.32 | 0.555 | 0.53 | 0.75 | 0.37 |
| 18:1, cis-9 | 23.6 | 22.1 | 23.5 | 22.8 | 0.758 | 0.35 | 0.59 | 0.49 |
| 18:1, cis-11 | 1.48 | 1.38 | 1.53 | 1.51 | 0.043 | 0.81 | 0.41 | 0.12 |
| 18:2, cis-9,cis-12 | 4.23 | 4.03 | 4.25 | 4.31 | 0.080 | 0.73 | 0.38 | 0.04 |
| 20:0 | 0.10 | 0.11 | 0.11 | 0.10 | 0.004 | 0.81 | 0.31 | 0.13 |
| 18:3 | 0.46 | 0.45 | 0.47 | 0.47 | 0.007 | 0.80 | 0.35 | 0.36 |
| CLA-cis-9,trans-11 | 0.55 | 0.43 | 0.51 | 0.46 | 0.021 | <0.01 | 0.02 | 0.06 |
| CLA-trans-10,cis-12 | 0.005 | <0.001 | <0.001 | 0.001 | 0.0014 | 0.01 | 0.04 | 0.16 |
| Others | 2.46 | 2.52 | 2.57 | 2.59 | 0.062 | 0.17 | 0.12 | 0.98 |
| Total trans fatty acids | 4.20 | 3.51 | 3.35 | 3.92 | 0.602 | 0.37 | 0.58 | 0.35 |
| ΣSFA | 60.9 | 63.3 | 61.7 | 61.7 | 0.52 | 0.02 | 0.35 | <0.01 |
| ΣMUFA | 29.2 | 27.1 | 28.3 | 28.2 | 0.51 | 0.02 | 0.19 | 0.03 |
| ΣPUFA | 5.23 | 4.91 | 5.23 | 5.25 | 0.088 | 0.34 | 0.64 | 0.02 |
CLA, conjugated linoleic acid; SFA, saturated fatty acids, MUFA, monounsaturated fatty acids; PUFA, poly-unsaturated fatty acids.
Control = 0 mg/kg of 3NOP, Low3NOP = 40 mg/kg of 3NOP, Medium3NOP = 60 mg/kg 3NOP, and High3NOP = 80 mg/kg 3NOP (dietary dry matter basis). Data are presented as least squares means.
Contrasts: C vs. Trt., Control vs. all 3NOP treatments; L, linear effect of 3NOP; Q, quadratic effect of 3NOP.
n = 140 (n represents number of observations used in the statistical analysis).
Discussion
Ruminant animals contribute to anthropogenic methane emissions through microbial anaerobic fermentation of feed in the reticulo-rumen, or through anaerobic decomposition of organic matter in manure during storage (3). Anaerobic fermentation of ingested plant material in the reticulo-rumen is a symbiotic process between the ruminant host and microorganisms supplying energy and protein to the host while providing optimal growth conditions and nutrients for the microorganisms. This symbiosis provides ruminants with an evolutionary advantage enabling them to efficiently use otherwise indigestible fibrous feeds despite the loss of a small portion of the plant gross energy as methane gas. In the United States, the contribution of livestock to total anthropogenic methane emissions is estimated at 25% (or 6.7 Tg/yr) from enteric fermentation and 9% (2.5 Tg/yr) from manure management (3). These estimates have been recently challenged by “top-down” approaches suggesting livestock methane emissions are in the range of 12–17 Tg/yr (22, 23), which is roughly 30% and 85% greater than EPA’s estimate for 2012 (3). In turn, top-down estimates have been questioned by simple but reliable “bottom-up” calculations based on animal inventories, feed intake, and methane emissions factors from enteric fermentation or anaerobic manure decomposition (24). Despite these discrepancies, it is apparent that animal agriculture is an important source of GHG and, globally, research efforts and funding have been directed toward mitigation of ruminant methane emissions. Apart from its GHG effect, the emission of enteric methane is a net loss of feed energy (25), which cannot be used by the ruminant animal for productive purposes.
Direct inhibition of methanogenesis in the rumen is just one of the proposed and tested approaches from a suite of mitigation options for reducing enteric methane emissions from ruminant animals (5). A number of inorganic or organic molecules, such as halomethanes and ionophoric antibiotics, have been found to inhibit methane emissions in various experimental systems, including animal studies (5). However, issues related to toxicity, adaptation, or environmental regulations render many of these compounds unlikely candidates for widespread, on-farm application as methane mitigants. The compound tested in this experiment, 3NOP, is the result of a small molecule inhibitor development program at DSM Nutritional Products (Basel, Switzerland), that started with an in silico virtual screening approach specifically targeting the active site of the methyl CoM reductase (MCR) of Methanobrevibacter ruminantium, one of the most represented methanogenic Archaea in the rumen of domesticated and wild ruminants. Indeed, St-Pierre and Wright (26) reported that although variation in the level of representation of Methanobrevibacter spp. could be observed, depending on ruminant species investigated, diet, and geographical location, the prevalence of these archaeal species in a diverse range of host herbivores worldwide was quite remarkable. The targeted enzyme (methyl CoM reductase) was discussed as one of the most promising candidates for chemical inhibition (27) because all biologically generated methane on earth derives from the catalytic activity of MCR in methanogenic microbes (28). Moreover X-ray cocrystal structures were available (16). The central catalytic element of the active site in MCR is the nickel porphinoid coenzyme F430 and it is established that the nickel center must be in the nickel-(I) oxidation state for the enzyme to be active and able to catalyze the reduction of methyl CoM and CoB into CoM CoB complex + methane (29). Nickel sites in enzymes were studied in detail for their catalytic mechanisms (30) including coordination and redox chemistry (31). Furthermore, it was proven by Leahy et al. (27) that Methanobrevibacter ruminantium was unable to produce its own Coenzyme M as three genes needed in the CoM biosynthetic pathway were not encoded in its genome. This dependence on environmental CoM was the reason why structural analogs of this particular molecule were thought to have a high probability of success. Finally, the physico-chemical properties of the inhibitor molecule were rendered such that it will most likely not rely on an active transport mechanism to cross the archaeal cell membrane to enhance the chances of reaching its target. Several structural analogs of methyl-CoM were identified, synthesized and tested first in vitro (11) and, later on, in vivo using sheep (17) followed by beef (18,32) and dairy cattle (19,20). Thus far, all observations made in vitro and in vivo appear to be consistent with the above hypothesis, particularly the accumulation of hydrogen reported in this manuscript and the expected change in acetate and propionate proportions in ruminal fluid (33) reported elsewhere (18–20, 32).
The studies with dairy cows by Haisan et al. (19) and Reynolds et al. (20) were relatively short-term (up to 35 d) and with cows producing up to 30–35 kg/d milk. As indicated earlier and in our review (5), the effect of methane mitigation practices should be ultimately determined in long-term experiments to address adaptation to treatment by the rumen ecosystem under industry-relevant conditions (i.e., high-producing cows fed diets typical for a given region or country). The study we report here is, to our knowledge, the first investigating 3NOP in a long-term experiment with cows fed a typical North American diet and with milk production comparable to high-producing dairy herds in the US. Under these conditions, 3NOP reduced methane emission by up to 30%, a remarkable effect that was maintained throughout the 12-wk experiment. Reynolds et al. (20) reported a much smaller methane reduction effect (7–10%) when 3NOP, applied at a daily dose of 500 or 2,500 mg per cow (25–125 mg/kg feed dry matter), was administered directly into the rumen through a rumen cannula. Interestingly, these authors reported a sharp decline in methane emission, measured in respiration chambers, following the afternoon 3NOP treatment, but not during the morning treatment, which was at the end of the feeding cycle. They concluded that, being water-soluble, 3NOP is likely rapidly washed out of the rumen and its effect would be greater if the compound were to be ingested continuously during the feeding cycle. In contrast, the other published 3NOP study with lactating dairy cows (19), reported a dramatic 60% decrease in methane emission per kg of dry matter intake at a dose of 2,500 mg/d when 3NOP was mixed with the feed (i.e., continuous intake throughout the day). Based on these studies and the current experiment, it can be concluded that 3NOP is effective in decreasing daily enteric methane emissions from dairy cows under industry-relevant feeding and management conditions, provided the compound is continuously delivered into the rumen. In practice, this means mixing it with the daily allotment of feed. If delivered as a pulse-dose, the inhibitory effect will likely be transient.
The 64-fold increase in hydrogen emission by 3NOP treated cows observed in the current experiment was remarkable and this is one of the few experiments which have documented a substantial reduction in enteric methane emissions and a concatenated increase in the emission of hydrogen by ruminants. Similar effects were reported by Trei et al. (14) using a diet containing a hemiacetal of choral and starch and by Mitsumori et al. (34) using bromochloromethane. The present experiment is, to our knowledge, the first to document this effect using a methane inhibitor with potential for widespread use in the livestock industries. Hydrogen is a critical intermediate of the fermentation process and an energy substrate for the rumen archaea (6). Its central role in the thermodynamics of rumen fermentation has been covered in a review by Janssen (35). Hydrogen exists in the rumen in two forms, gaseous and dissolved in the rumen liquor (35) and in vitro work has shown that accumulation of hydrogen may result in production of fermentation end products, such as propionate and butyrate, that are net sinks of hydrogen (36). Concentrations of volatile fatty acids in ruminal contents were not analyzed in the current experiment because the cows were not rumen cannulated and, therefore, information on hydrogen accumulation in the rumen liquor, or its effects on the fermentation process were not determined. The increase in hydrogen emissions, along with the lack of effect on fiber digestibility or feed intake, may be indicative of the capacity of the rumen to cope with excess hydrogen. The amount of hydrogen emitted from the rumen was only a fraction of the amount potentially available from the decrease in methane synthesis. For example, the difference in methane emissions between the control cows and those treated with the highest level of 3NOP was 162 g/d (481–319 g/d). On a molar basis, this would represent about 40.7 g of hydrogen not being used for methane synthesis (4 mol of dihydrogen required for 1 mol of methane), which is a far greater amount than the 1.27 g/d emitted on average by the High3NOP cows. We did not measure hydrogen dissolved in the rumen liquor in this experiment and cannot speculate on the fate of the excess hydrogen from the above stoichiometric calculations. It is obvious, however, that this hydrogen was not emitted with the eructation gases. The relatively small amount of emitted hydrogen and the trend for decreasing hydrogen emissions from week 6 to the end of the experiment also suggest possible adaptation and decreased hydrogen production or redirection to alternative hydrogen sinks. It is well known that when halogenated methane analogs and some other halogenated hydrocarbons are administered to ruminants, the increase in hydrogen production is generally of a similar order of magnitude to the decrease in methane production (14, 37). The fact that in the current experiment, the increase in the measured emissions of hydrogen was only about 3% of that expected due to the decrease in methane production, suggests that the modes of action of 3NOP may be different to those of the halogenated methane analogs and halogenated hydrocarbons.
In a recent metaanalysis of in vitro data, rumen stoichiometry could not completely explain hydrogen balance when methanogenesis was inhibited, and it was suggested that hydrogen may have been increasingly incorporated into formate, microbial biomass, or reductive acetogenesis (33). In a related study, we observed no effect on rumen propionate concentration (but decreased acetate concentration) in cows treated with 60 mg 3NOP/kg feed dry matter (38). In that study, the isotopic composition of methane was similar between the control (δ13CCH4 = −20.91 ± 0.32‰, δDCH4 = −266.92 ± 0.14‰, and Δ13CH3D = −1.96 ± 1.78‰) and 3NOP (δ13CCH4 = −24.91 ± 1.72‰, δDCH4 = −266.94 ± 0.27‰, and Δ13CH3D = −1.72 ± 2.97‰). The relative abundance (as percentage of the total sequences analyzed within the sample) of methanogenic archaea, Methanobrevibacter, Methanosphaera, and Methanomicrobium spp., was also not affected by 3NOP. Collectively, these data support the conclusion that there was no change in the metabolic strategy of rumen archaea associated with the 3NOP treatment. Decreased rumen acetate concentration in 3NOP-treated cows was reported by others (19, 20) and is in line with the observed decreased in fibrolytic species such as Ruminococcus spp. in our related experiment (38).
The quadratic increase in dietary dry and organic matter, crude protein, and acid-detergent fiber total tract apparent digestibility as a result of 3NOP supplementation was surprising. The increased digestibility was primarily with the Low and Medium3NOP treatments vs. the control. Whereas the increased digestibility with Medium3NOP could be partially explained with the slightly lower dry matter intake (compared with the control), cows on the Low3NOP and control treatments had the same dry matter intake. Another possible explanation could be more efficient ruminal fermentation with 3NOP. Others have reported increased propionate concentration in the rumen fluid of dairy cows treated with 3NOP (18–20, 32). Such a shift in the pattern of ruminal fermentation would likely result in a more efficient utilization of feed energy and may improve nutrient digestibility, provided there is no negative effect on fiber degradability due to hydrogen accumulation. It has to be pointed out that digestibility in the current experiment was analyzed using an intrinsic digestibility marker, indigestible neutral-detergent fiber. This marker has been tested and proven to deliver reliable total tract digestibility estimates compared with the “gold standard”, total fecal collection (39, 40). Occasionally, the use of this marker produces large variability in digestibility due to variability in indigestible neutral-detergent fiber concentration of the diet. In the current experiment, however, this variability was low (average concentration of indigestible neutral-detergent fiber of 11.4%, SD = 0.32%, and min and max values of 11.1% and 11.8%, respectively).
The analyzed gross energy concentration of the basal diet fed in this experiment was similar to that of typical US dairy diets and the estimated digestible energy, as proportion of dietary gross energy, was close to that assumed for dairy cows by EPA (3, Annex 3.9; 68.5% vs. 66.7%, respectively). Overall, methane energy as proportion of gross (i.e., the Ym factor) or digestible energy intake was relatively low for all cows in this experiment. For example, the Ym used by the most recent EPA GHG emissions inventory ranges from 5.9% to 6.9% (3). The control cows in the current experiment had an average Ym of 5.3 (SE = 0.22; min = 4.5 and max = 6.9%), which is about 16% lower than the 6.3% assumed by EPA for the Northeastern U.S. As feed dry matter intake and animal productivity increase, Ym is expected to decrease, mostly as a result of decreased feed digestibility (5, 41, 42), although the relationship of Ym with feed dry matter intake was very weak in this experiment (R2 = 0.03 for all cows, or R2 = 0.09 for the control cows only).
The lack of a strong relationship between methane emission and feed dry matter intake in the current experiment was in contrast to our meta-analysis data (5) and reports by others (42–44). The discrepancy is perhaps a result of the known effect of sample size on R2. For example, our meta-analysis data (5) encompassed dry matter intakes ranging from 1–25 kg/d and the relationship between methane emission and dry matter intake was strong (R2 = 0.86). If only experiments, in which dry matter intake ranged from 15 to 25 kg/d are considered, however, the relationship becomes much weaker (R2 = 0.18) and similar to that observed in the current experiment. Thus, it can be concluded that feed dry matter intake alone is not a reliable predictor of enteric methane emission from individual animals.
In the two previously published experiments with lactating cows (19, 20) and the current study, feed intake and milk production were not affected by 3NOP. There was no effect on cow’s body weight in the Reynolds et al. (20) study, but remarkably, body weight gain of the treated cows was much greater than that of the control cows (1.06 vs. 0.39 kg/d) in the study by Haisan et al. (19). Although the latter experiment (19) was a crossover design with 28-d periods, the body weight increase is similar to the weight gain of the 3NOP cows observed in the current 84-d experiment. Cows lose body weight and body condition during the early stages of lactation but compensate for this loss during late lactation and the dry period (13). Thus, an increased rate of body weight gain during midlactation (when milk production is not an energetic priority for the cow), as observed in the current experiment, is beneficial for the energy balance and overall performance of a dairy cow. The current experiment illustrates how the reduced loss of gross feed energy as methane can result in greater energy availability for productive purposes, i.e., increased milk lactose and protein synthesis, or recovery of body weight lost in early lactation. Assuming that 24.4 MJ net energy of lactation is required for 1 kg of body weight gain (13) and that energy in methane is converted to net energy for lactation with efficiency similar to that of dietary digestible energy, it can be calculated that the reduction in energy loss as methane with the 3NOP treatments (on average 3.8 MJ/d) would account for 38 (Medium3NOP) to 71% (High3NOP) of the increased daily body weight gain in the 3NOP cows. Haisan et al. (19) estimated that the reduction in energy loss as methane with 3NOP accounted for 80% of the increased body weight gain in their experiment. In the current experiment, digestibility of most dietary nutrients was increased by the 3NOP treatment, which may have also contributed to the greater body weight gain of the treated cows, compared with the control cows.
Methods
Detailed methods are provided in SI Methods. Animals involved in this experiment were cared for according to the guidelines of the Pennsylvania State University Animal Care and Use Committee. The committee reviewed and approved the experiment and all procedures carried out in the study. The experiment was a randomized block design with a 2-wk covariate and a 12-wk data collection periods, involved 48 Holstein cows, and was conducted in two phases. Cows were blocked into 12 blocks and cows within block were randomly allocated to one of the following treatments: control (no additive) and 3NOP applied at 40 (Low3NOP), 60 (Medium3NOP), or 80 (High3NOP) mg/kg feed dry matter. The 3NOP supplement contained 8.85% 3NOP on SiO2 and propylene glycol and was mixed with the total mixed ration of the treatment cows to deliver the final 3NOP concentrations indicated above. The control diet was supplemented with a placebo premix containing SiO2 and propylene glycol only. Methane, carbon dioxide, and hydrogen gas emissions from the cows were measured using the GreenFeed system (C-Lock Technology; ref. 45). Methane emissions were also measured using the SF6 tracer method (46) as modified by Deighton et al. (47). Gas emissions using the GreenFeed system were measured during the covariate period and experimental weeks 2, 6, 9, and 12. During each measurement period, gas emission data were collected in 3 d as follows: starting at 0900, 1500, and 2100 h (sampling day 1), 0300, 1200, and 1700 h (sampling day 2), and 0000 and 0500 h (sampling day 3). Breath gas samples were collected for 5 min followed by a 2-min background gas sample collection. Data using the SF6 technique were collected during experimental weeks 2, 9, and 12 (phase 1) and 2, 6, and 12 (phase 2). Permeation tubes containing SF6 were placed in the reticulum of each cow at the beginning of the experiment, one week before the first measurement occurred. The mean ± SD rate of SF6 release from permeation tubes used in the experiment was 4.38 ± 0.261 mg/d. All data were analyzed using the MIXED procedure of SAS (version 9.4; SAS Institute Inc.). Dry matter intake, milk yield, feed efficiency (milk production ÷ feed dry matter intake), and body weight data were averaged per week, and the average values were used in the statistical analysis. GreenFeed and SF6 gas emission data were averaged per cow and gas measurement period and the averaged data were used in the statistical analysis. Orthogonal contrasts were used to evaluate 3NOP treatments vs. control, linear, and quadratic effects of 3NOP. Data are presented as least squares means, or covariate-adjusted least squares means. Significant differences among treatments were declared at P ≤ 0.05. Differences at 0.05 < P ≤ 0.10 were considered a trend toward significance.
SI Methods
This experiment was a randomized block design with a 2-wk covariate and a 12-wk data collection periods and involved 48 Holstein cows (17 primi- and 31 multiparous). The average days in milk, lactation number, and body weight of the cows at the beginning of the experiment were (mean ± SE): 77 ± 3.9 d, 2.2 ± 0.15 lactations, and 653 ± 11.8 kg. Cows were blocked by days in milk, parity, and milk production into 12 blocks of 4 cows each. Cows within block were randomly allocated to one of the following treatments: control (no additive) and 3NOP applied at 40 (Low3NOP), 60 (Medium3NOP), or 80 (High3NOP) mg/kg feed dry matter. The experiment was conducted in 2 phases: blocks 1–6, in phase 1 and blocks 7–12, in phase 2 (phase 2 began immediately following phase 1). Cows were fed a total mixed ration (Table S1) that was formulated to meet or exceed the net energy of lactation and metabolizable protein requirements (13) of a cow producing 45 kg/d milk with 3.60% milk fat and 3.10% milk true protein and consuming 27 kg/d feed dry matter. On average during the experiment, the diet supplied net energy of lactation and metabolizable protein slightly (−0.2 to −3.0%) below National Research Council (NRC) requirements. The 3NOP supplement contained 8.85% 3NOP on SiO2 and propylene glycol and was mixed with the total mixed ration of the treatment cows to deliver the final 3NOP concentrations indicated above. The control diet was supplemented with a placebo premix containing SiO2 and propylene glycol only. Cows were offered feed and water ad libitum. Feed intake and refusals, milk production, and cow body weight data were collected daily throughout the experiment. Feed intake and milk production data for the first 2 wk of the experiment were omitted from the statistical analysis. Milk samples were collected during experimental weeks 6, 9, and 12 and a composite sample (am and pm milkings) was analyzed for fat, true protein, lactose, and fatty acids as described elsewhere (49). Fecal samples were collected during weeks 8 and 12 and composited samples were analyzed to estimate apparent total tract digestibility of dry and organic matter, crude protein, fiber fractions (neutral- and acid-detergent fiber), and starch using indigestible neutral-detergent fiber as an internal marker (49). Fecal samples were analyzed for chemical composition as described elsewhere (50). Feed samples were analyzed for chemical composition using wet chemistry procedures (Cumberland Valley Analytical Services; details at: www.foragelab.com/Resources/Lab-Procedures; accessed January 26, 2015). The basal diet was analyzed for gross energy content using an adiabatic bomb calorimeter (Dairy One). Digestible energy concentration of the diet was calculated from dietary metabolizable energy estimated by the NRC Dairy model using NRC equations based on the actual feed composition and intake, cow body weight, and milk production and composition (13).
Methane, carbon dioxide, and hydrogen gas emissions from cows were measured using the GreenFeed system (C-Lock, Inc., Rapid City, SD; ref. 45). Methane emissions were also measured using the SF6 tracer method (46) as modified by Deighton et al. (47). Gas emissions using the GreenFeed system were measured during the covariate period and experimental weeks 2, 6, 9, and 12. During each measurement period, gas emission data were collected in 3 d as follows: starting at 0900, 1500, and 2100 h (sampling day 1), 0300, 1200, and 1700 h (sampling day 2), and 0000, and 0500 h (sampling day 3). Three GreenFeed units were used and all cows were sampled in about 50 min. Breath gas samples were collected for 5 min followed by a 2-min background gas sample collection. Each GreenFeed unit was calibrated 3–4 times before and 3–4 times after each sampling period with a standard gas mixture containing (mol %): carbon dioxide, 0.98, methane, 0.151, with the balance being nitrogen gas (Air Liquide America Specialty Gases). Dihydrogen calibration was carried out using a calibration gas standard containing 5% dihydrogen and the balance being nitrogen (GTS-Welco). The calibration was performed at least once before every gas measurement period. Bait feed was offered at each sampling event for a total of 4,000 g over 3 d, which was ∼5% of the total dry matter intake during each sampling period. The bait feed used was a premix containing (as-is basis): 70% ground corn grain, 28% dried molasses, and 2% soybean oil. A detailed description of the gas sampling procedure using GreenFeed is provided elsewhere (51). It is noted that recent comparative experiments have concluded that methane emission rates estimated by means of the GreenFeed system are comparable to those estimated using respiration chambers or the SF6 technique. For example, a study with growing dairy heifers concluded that estimates of methane emission generated by GreenFeed were comparable to values obtained by respiration chambers (52). These authors pointed out, however, that deployment of the GreenFeed units and replication must be carefully considered to ensure sufficient numbers of measurements are obtained. In a study with lactating dairy cows, methane emissions measured by the GreenFeed system were similar to values derived from respiration chambers and between animal variability was also within the range observed in respiration chambers (53). Analyzing the data from the current experiment, we concluded that the SF6 technique produced larger variability in methane yield than the GreenFeed system (54). The difference between the two methods was not consistent over time, perhaps influenced by barn ventilation and background methane and SF6 concentrations (54).
Data using the SF6 technique were collected during experimental weeks 2, 9, and 12 (phase 1) and 2, 6, and 12 (phase 2). Permeation tubes containing SF6 were placed in the reticulum of each cow at the beginning of the experiment, one week before the first measurement occurred. The mean ± SD rate of SF6 release from permeation tubes used in the experiment was 4.38 ± 0.261 mg/d. The SF6 equipment, sample and canister processing, and analysis of the gas samples for methane and SF6 were as described in Deighton et al. (47). Briefly, evacuated canisters were secured on the back of each cow using a harness. An aliquot of the breath gas was continuously collected through tubing extending to the nostrils of the cow. Background gas collection canisters were placed on each other cow. Canisters were replaced every 24 h for an average sampling duration of 3.2 ± 0.10 d per cow. An aliquot of the collected gas sample was extracted and analyzed for methane using a gas chromatograph (Varian CP-3800, Agilent Technologies) as described in Williams et al. (55) with the following modifications. Ultra-high-purity He (999.99 g/kg He) and ultra-high-purity N (999.99 g/kg N2) were used as carrier gases for methane and SF6 analysis, respectively, operating at 80 °C. Separation of methane was achieved using a Hayesep QS 80–100 mesh column (2.0 m × 2.0 mm, Agilent Technologies) operated at a pressure of 117 kPa. The temperature of the flame ionization detector set was at 300 °C. Separation of SF6 was on a Molecular Sieve 5A 80–100 mesh column (1.8 m × 3 mm stainless steel, Grace Davison Discovery Sciences) using a flow rate of 40 mL/min. Samples were drawn from the canisters using syringes, transferred into 12 mL glass vials (Exetainer, Labco) and infused into the GC by an auto-sampler (CombiPAL, CTC Analytics). Interpretation of the GC results was via three standards (Air Liquide) consisting of a low standard of 20.5 mg/kg methane and 18.8 ng/kg SF6, a medium standard of 66.6 mg/kg methane and 59.1 ng/kg SF6, and a high standard of 252.4 mg/kg methane and 230.9 ng/kg SF6.
All data were analyzed using the MIXED procedure of SAS (version 9.4; SAS Institute Inc.). Dry matter intake, milk yield, feed efficiency (milk production ÷ feed dry matter intake), and body weight data were averaged per week and the average values were used in the statistical analysis. GreenFeed and SF6 gas emission data were averaged per cow and gas measurement period and the averaged data were used in the statistical analysis. Digestibility data were averaged between the 2 sampling periods and the average values were used in the statistical analysis. The weekly feed intake, milk production and composition, and gas emission data were analyzed as repeated measure assuming an ar (1) covariance structure. The statistical model included treatment, week, phase, treatment × week and treatment × phase interactions, and the covariate term, with the error term assumed to be normally distributed with mean = 0 and constant variance. Block and block × treatment effects were random, whereas all others were fixed. Orthogonal contrasts were used to evaluate 3NOP treatments vs. control, linear, and quadratic effects of 3NOP. All data are presented as least squares means, or covariate-adjusted least squares means. Significant differences among treatments were declared at P ≤ 0.05. Differences at 0.05 < P ≤ 0.10 were considered a trend toward significance.
SI Results
The methane inhibitor had some marked effects on milk fatty acid composition (Table S3). Concentrations of most of the short-chain fatty acids (C6:0 through C12:0) were increased (P ≤ 0.03) by 3NOP, compared with the control. The increase ranged from an average of 6% for C6:0 to about 15% for C10:0 and C12:0. There was on average a 23% increase in the concentration of C15:0 (P < 0.01, quadratic effect) and an average of 6% increase in C17:0 (P = 0.03, quadratic effect) for the 3NOP treatments, compared with the control. Concentrations of the long-chain fatty acids in milk were generally not clearly affected by 3NOP, except for a linear decrease (P = 0.02) in the concentration of cis-9,trans-11 (and trans-10,cis-12) conjugated linoleic acid.
SI Discussion
The possibility of using milk fatty acid profiles to predict methane emission in lactating ruminants is attractive because milk samples can be relatively easily collected from a large number of animals and in commercial settings. The consistent increase in the concentration of short-chain milk fatty acids (C6:0 through C12:0) with 3NOP in the current experiment is intriguing and was not observed by Reynolds et al. (20). The origin of these fatty acids is predominantly from de novo synthesis in the mammary gland (56), although C12:0 can be quantitatively transferred from the diet (57), and are indicative of the energy status of the animal (58). The latter is in agreement with the increased availability of digestible energy and the increased milk lactose yield with the 3NOP treatments compared with the control. The lack of effect of 3NOP on the short-chain milk fatty acids in the experiment by Reynolds et al. (20) is likely a reflection of the lack of effect of the inhibitor on methane emission in that experiment. Some short-chain milk fatty acids (C8:0, C10:0, and C11:0) have been positively correlated (r = 0.30–0.42) with methane emissions in dairy cows in a meta-analysis (59) and similar trends were reported in more recent meta-analyses (60, 61). Another fatty acid, which concentration was markedly increased by 3NOP in the current experiment, C15:0, has been associated with ruminal synthesis and duodenal flow of microbial protein (62, 63). In several meta-analyses, however, there was no consistent relationship between C15:0 and enteric methane emissions (59, 61).
Footnotes
Conflict of interest statement: M.K. and S.D. are employees of DSM Nutritional Products. The study was partially funded by DSM Nutritional Products.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504124112/-/DCSupplemental.
References
- 1.IPCC, Intergovernmental Panel on Climate Change 2014. Working Group III – Mitigation of Climate Change. Chapter 11, Agriculture, Forestry and Other Land Use (AFOLU) (Cambridge Univ Press, Cambridge, UK)
- 2.Opio C, et al. Greenhouse Gas Emissions from Ruminant Supply Chains – A Global Life Cycle Assessment. Food and Agriculture Organization of the United Nations. FAO; Rome: 2013. [Google Scholar]
- 3.EPA, United States Environmental Protection Agency . Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2012. U.S. Environmental Protection Agency; Washington, DC: 2014. [Google Scholar]
- 4.FAOSTAT . Statistical Database. Food and Agriculture Organization of the United Nations; Rome, Italy: 2015. [Google Scholar]
- 5.Hristov AN, et al. 2013. Mitigation of greenhouse gas emissions in livestock production – A review of technical options for non-CO2 emissions. Gerber P, Henderson B, Makkar H, editors. FAO Animal Production and Health Paper No. 177. FAO, Rome, Italy. [Google Scholar]
- 6.Hungate RE. Hydrogen as an intermediate in the rumen fermentation. Arch Mikrobiol. 1967;59(1):158–164. doi: 10.1007/BF00406327. [DOI] [PubMed] [Google Scholar]
- 7.Wolin MJ. The rumen fermentation: a model for microbial interactions in anaerobic ecosystems. Adv Microb Ecol. 1979;3:49–77. [Google Scholar]
- 8.McAllister TA, Newbold CJ. Redirecting rumen methane to reduce methanogenesis. Aust J Exp Agric. 2008;48(2):7–13. [Google Scholar]
- 9.Knight T, et al. Chloroform decreases rumen methanogenesis and methanogen populations without altering rumen function in cattle. Anim Feed Sci Technol. 2011;166-167(special issue):101–112. [Google Scholar]
- 10.Hristov AN, Lee C, Hristova R, Huhtanen P, Firkins JL. A meta-analysis of variability in continuous-culture ruminal fermentation and digestibility data. J Dairy Sci. 2012;95(9):5299–5307. doi: 10.3168/jds.2012-5533. [DOI] [PubMed] [Google Scholar]
- 11.Soliva CR, Amelchanka SL, Duval SM, Kreuzer M. Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec) Br J Nutr. 2011;106(1):114–122. doi: 10.1017/S0007114510005684. [DOI] [PubMed] [Google Scholar]
- 12.Klevenhusen F, Zeitz JO, Duval S, Kreuzera M, Soliva CR. Garlic oil and its principal component diallyl disulfide fail to mitigate methane, but improve digestibility in sheep. Anim Feed Sci Technol. 2011;166–167(special issue):356–363. [Google Scholar]
- 13. National Research Council (2001) Nutrient Requirements of Dairy Cattle. 7th Revised Edition (National Academy of Sciences Press, Washington, DC)
- 14.Trei JE, Scott GC, Parish RC. Influence of methane inhibition on energetic efficiency of lambs. J Anim Sci. 1972;34(3):510–515. doi: 10.2527/jas1972.343510x. [DOI] [PubMed] [Google Scholar]
- 15.Duval S, Kindermann M. 2012. Use of nitrooxy organic molecules in feed for reducing enteric methane emissions in ruminants, and/or to improve ruminant performance. World Intellectual Property Organization. International Patent Application WO 2012/084629 A1.
- 16.Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer RK. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation. Science. 1997;278(5342):1457–1462. doi: 10.1126/science.278.5342.1457. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Fernández G, et al. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J Dairy Sci. 2014;97(6):3790–3799. doi: 10.3168/jds.2013-7398. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Perez A, et al. The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. J Anim Sci. 2014;92(10):4682–4693. doi: 10.2527/jas.2014-7573. [DOI] [PubMed] [Google Scholar]
- 19.Haisan J, et al. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J Dairy Sci. 2014;97(5):3110–3119. doi: 10.3168/jds.2013-7834. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds CK, et al. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J Dairy Sci. 2014;97(6):3777–3789. doi: 10.3168/jds.2013-7397. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh FM, et al. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl Environ Microbiol. 2003;69(8):5011–5014. doi: 10.1128/AEM.69.8.5011-5014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wecht KJ, Jacob DJ, Frankenberg C, Jiang Z, Blake DR. Mapping of North American methane emissions with high spatial resolution by inversion of SCIAMACHY satellite data. J Geophys Res Atmos. 2014;119(12):7741–7756. [Google Scholar]
- 23.Miller SM, et al. Anthropogenic emissions of methane in the United States. Proc Natl Acad Sci USA. 2013;110(50):20018–20022. doi: 10.1073/pnas.1314392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristov AN, Johnson KA, Kebreab E. Livestock methane emissions in the United States. Proc Natl Acad Sci USA. 2014;111(14):E1320. doi: 10.1073/pnas.1401046111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson KA, Johnson DE. Methane emissions from cattle. J Anim Sci. 1995;73(8):2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- 26.St-Pierre B, Wright A-DG. Diversity of gut methanogens in herbivorous animals. Animal. 2013;7(Suppl 1):49–56. doi: 10.1017/S1751731112000912. [DOI] [PubMed] [Google Scholar]
- 27.Leahy SC, et al. The genome sequence of the rumen methanogen Methanobrevibacter ruminantium reveals new possibilities for controlling ruminant methane emissions. PLoS One. 2010;5(1):e8926. doi: 10.1371/journal.pone.0008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragsdale SW. Nickel-based enzyme systems. J Biol Chem. 2009;284(28):18571–18575. doi: 10.1074/jbc.R900020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheller S, Goenrich M, Mayr S, Thauer RK, Jaun B. Intermediates in the catalytic cycle of methyl coenzyme M reductase: isotope exchange is consistent with formation of a σ-alkane-nickel complex. Angew Chem Int Ed Engl. 2010;49(44):8112–8115. doi: 10.1002/anie.201003214. [DOI] [PubMed] [Google Scholar]
- 30.Chen S-L, Blomberg MRA, Siegbahn PEM. An investigation of possible competing mechanisms for Ni-containing methyl-coenzyme M reductase. Phys Chem Chem Phys. 2014;16(27):14029–14035. doi: 10.1039/c4cp01483a. [DOI] [PubMed] [Google Scholar]
- 31.Holliger C, Pierik AJ, Reijerse EJ, Hagen WR. A spectroelectrochemical study of factor F430 Ni(II/I) from methanogenic bacteria in aqueous solution. J Am Chem Soc. 1993;115(13):5651–5656. [Google Scholar]
- 32.Romero-Perez A, et al. Sustained reduction in methane production from long-term addition of 3-nitrooxypropanol to a beef cattle diet. J Anim Sci. 2015;93(4):1780–1791. doi: 10.2527/jas.2014-8726. [DOI] [PubMed] [Google Scholar]
- 33.Ungerfeld EM. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Front Microbiol. 2015;6:37. doi: 10.3389/fmicb.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsumori M, et al. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br J Nutr. 2012;108(3):482–491. doi: 10.1017/S0007114511005794. [DOI] [PubMed] [Google Scholar]
- 35.Janssen PH. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim Feed Sci Technol. 2010;160(1-2):1–22. [Google Scholar]
- 36.Wang M, Sun XZ, Janssen PH, Tang SX, Tan ZL. Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Anim Feed Sci Technol. 2014;194:1–11. [Google Scholar]
- 37.Trei JE, Parish RC, Singh YK. Effect of methane inhibitors on rumen metabolism and feedlot performance of sheep. J Dairy Sci. 1971;54(4):536–540. doi: 10.3168/jds.s0022-0302(71)85882-4. [DOI] [PubMed] [Google Scholar]
- 38.Matos LF, et al. Effect of 3-nitrooxypropanol on ruminal fermentation, methane and hydrogen emissions, and methane isotopic composition in dairy cows. J Dairy Sci. 2015;98(E-Suppl. 2) doi: 10.3168/jds.2015-10832. :246. [DOI] [PubMed] [Google Scholar]
- 39.Huhtanen P, Kaustell K, Jaakkola S. The use of internal markers to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Anim Feed Sci Technol. 1994;48(3-4):211–227. [Google Scholar]
- 40.Lee C, Hristov AN. Short communication: Evaluation of acid-insoluble ash and indigestible neutral detergent fiber as total-tract digestibility markers in dairy cows fed corn silage-based diets. J Dairy Sci. 2013;96(8):5295–5299. doi: 10.3168/jds.2012-6442. [DOI] [PubMed] [Google Scholar]
- 41.Yan T, et al. Mitigation of enteric methane emissions through improving efficiency of energy utilization and productivity in lactating dairy cows. J Dairy Sci. 2010;93(6):2630–2638. doi: 10.3168/jds.2009-2929. [DOI] [PubMed] [Google Scholar]
- 42.Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM. Invited review: Enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. J Dairy Sci. 2014;97(6):3231–3261. doi: 10.3168/jds.2013-7234. [DOI] [PubMed] [Google Scholar]
- 43.Cottle DJ, Nolan JV, Wiedemann SG. Ruminant enteric methane mitigation: A review. Anim Prod Sci. 2011;51(6):491–514. [Google Scholar]
- 44.Ramin M, Huhtanen P. Development of equations for predicting methane emissions from ruminants. J Dairy Sci. 2013;96(4):2476–2493. doi: 10.3168/jds.2012-6095. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman P, Zimmerman S, Utsumi S, Beede D. Development of a user-friendly online system to quantitatively measure metabolic gas fluxes from ruminants. J Dairy Sci. 2011;94(E-Suppl. 1):760. [Google Scholar]
- 46.Johnson K, Huyler M, Westberg H, Lamb B, Zimmerman P. Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ Sci Technol. 1994;28(2):359–362. doi: 10.1021/es00051a025. [DOI] [PubMed] [Google Scholar]
- 47.Deighton MH, et al. A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Anim Feed Sci Technol. 2014;197:47–63. [Google Scholar]
- 48.Sjaunja LO, Baevre L, Junkkarinen L, Pedersen J, Setälä J. 1990. A Nordic proposal for an energy corrected milk (ECM) formula. In 27th Session of the International Commission for Breeding and Productivity of Milk Animals, Paris, France. Wageningen Academic Publishers, Wageningen, the Netherlands.
- 49.Hristov AN, et al. Effect of Origanum vulgare L. leaves on rumen fermentation, production, and milk fatty acid composition in lactating dairy cows. J Dairy Sci. 2013;96(2):1189–1202. doi: 10.3168/jds.2012-5975. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, et al. Effect of 2-hydroxy-4-methylthio-butanoic acid on ruminal fermentation, bacterial distribution, digestibility, and performance of lactating dairy cows. J Dairy Sci. 2015;98(2):1234–1247. doi: 10.3168/jds.2014-8904. [DOI] [PubMed] [Google Scholar]
- 51.Hristov AN, et al. The use of an automated system to monitor enteric methane and carbon dioxide emissions from ruminant animals. J Vis Exp. 2015 doi: 10.3791/52904. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammond KJ, et al. Methane emissions from growing dairy heifers estimated using an automated head chamber (GreenFeed) compared to respiration chambers or SF6 techniques. Adv Anim Biosci. 2013;4(2):391. [Google Scholar]
- 53.Huhtanen P, Krizsan S, Cabezas Garcia EH, Hetta M, Gidlund H. Repeatability and between cow variability of enteric CH4 and total CO2 emissions. Adv Anim Biosci. 2013;4(2):588. [Google Scholar]
- 54.Oh J, et al. Comparison between the GreenFeed system and the sulfur hexafluoride tracer technique for measuring enteric methane emissions from dairy cows. J Dairy Sci. 2015;98(E-Suppl. 2) doi: 10.3168/jds.2016-10897. :601. [DOI] [PubMed] [Google Scholar]
- 55.Williams SRO, et al. Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: A critical evaluation of the SF6 procedure. Anim Feed Sci Technol. 2011;170(3-4):265–276. [Google Scholar]
- 56.Bauman DE, Griinari JM. Nutritional regulation of milk fat synthesis. Annu Rev Nutr. 2003;23:203–227. doi: 10.1146/annurev.nutr.23.011702.073408. [DOI] [PubMed] [Google Scholar]
- 57.Hristov AN, et al. Effects of lauric and myristic acids on ruminal fermentation, production, and milk fatty acid composition in lactating dairy cows. J Dairy Sci. 2011;94(1):382–395. doi: 10.3168/jds.2010-3508. [DOI] [PubMed] [Google Scholar]
- 58.Palmquist DL. Milk fat: origin of fatty acids and influence of nutritional factors thereon. In: Fox PF, McSweeney PLH, editors. Advanced Dairy Chemistry, Volume 2: Lipids, 3rd Edition. Kluwer Academic/Plenum; New York: 2006. pp. 43–92. [Google Scholar]
- 59.Dijkstra J, et al. Relationships between methane production and milk fatty acid profiles in dairy cattle. Anim Feed Sci Technol. 2011;166–167(special issue):590–595. [Google Scholar]
- 60.Williams SRO, Moate PJ, Deighton MH, Hannah MC, Wales WJ. Methane emissions of dairy cows cannot be predicted by the concentrations of C8:0 and total C18 fatty acids in milk. Anim Prod Sci. 2014;54(10):1757–1761. [Google Scholar]
- 61.van Lingen HJ, Crompton LA, Hendriks WH, Reynolds CK, Dijkstra J. Meta-analysis of relationships between enteric methane yield and milk fatty acid profile in dairy cattle. J Dairy Sci. 2014;97(11):7115–7132. doi: 10.3168/jds.2014-8268. [DOI] [PubMed] [Google Scholar]
- 62.Vlaeminck B, Fievez V, Cabrita ARJ, Fonseca AJM, Dewhurst RJ. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim Feed Sci Technol. 2006;131(3-4):389–417. [Google Scholar]
- 63.Fievez V, Colman E, Castro-Montoya JM, Stefanov I, Vlaeminck B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim Feed Sci Technol. 2012;172(1-2):51–65. [Google Scholar]