Significance
Antibodies are important therapeutic agents and have been used for the treatment of many diseases, including infectious and inflammatory diseases, and cancer. The therapeutic efficacy of an antibody is usually determined not only by the selectivity and affinity toward the target but also by the Fc-glycan structure interacting with the Fc receptors on immune cells. This study describes the preparation of various antibodies with different Fc-glycan structures as homogeneous glycoforms for the investigation of their effector activities. During this study, it was discovered that the biantennary N-glycan structure with two terminal alpha-2,6-linked sialic acids is a common and optimal structure that is able to enhance the activities of antibodies against cancer, influenza, and inflammatory diseases.
Keywords: endoglycosidase, Fc glycosylation, glycoengineered antibodies, homogeneous antibodies, sugar oxazoline
Abstract
Antibodies have been developed as therapeutic agents for the treatment of cancer, infection, and inflammation. In addition to binding activity toward the target, antibodies also exhibit effector-mediated activities through the interaction of the Fc glycan and the Fc receptors on immune cells. To identify the optimal glycan structures for individual antibodies with desired activity, we have developed an effective method to modify the Fc-glycan structures to a homogeneous glycoform. In this study, it was found that the biantennary N-glycan structure with two terminal alpha-2,6-linked sialic acids is a common and optimized structure for the enhancement of antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antiinflammatory activities.
Antibody-based therapies have been effectively used to treat many diseases, including inflammatory disorders, cancers, infectious diseases, and organ transplant rejection. Currently, more than 40 therapeutic monoclonal antibodies (mAbs) have been approved for clinical use in the United States, European Union, and other countries, including antibodies targeting CD20, Her2, EGFR, CD40, TNFα, CTLA-4, and PD-1.
Most therapeutic antibodies are monoclonal and prepared by hybridoma technology (1) as humanized antibodies to avoid undesired immunological responses derived from species difference. Recently, development of human antibodies through the screening of phage display libraries from human B cells or from single B-cell clones has become a major trend (2–4).
Like many other mammalian proteins, antibodies are heterogeneously glycosylated, and the glycosylation in the Fc region, specifically at position 297, has been an important issue in the development of therapeutic monoclonal antibodies, because the glycan moiety can significantly affect the activities of antibodies through interaction with the Fc receptors on immune cells, including natural killer cells, macrophages, dendritic cells, neutrophils, etc. Therefore, there is a need for development of homogeneous monoclonal antibodies with well-defined Fc glycans to examine these interactions and improve their safety and efficacy. Toward this goal, it has been reported that removal of the core fucose residue enhances the antibody-dependent cellular cytotoxicity (ADCC) activity of immunoglobulin Gs (IgGs) (5, 6) due to the increased Fc-glycan interaction with FcγRIIIa receptor (5, 7, 8). Two FDA-approved glycoengineered antibodies, mogamulizumab (POTELLIGENT) and obinutuzuman (GA101), are defucosylated antibodies in which POTELLIGENT was produced by the fucosyltransferase 8 (FUT8) knockout CHO cell line and GA101 was from the N-acetylglucosamine transferase III (GnT-III) overexpression system. In addition, it has been reported that more FcγIIIa was expressed on the monocytes of long-term rheumatoid arthritis (RA), and that more fucosylation was also found in the IgG heavy chain of RA patients (9, 10), indicating the possibility of RA treatment with afucosylated antibodies to neutralize proinflammatory cytokines and compete with autoantibodies for FcγIIIa.
It was also observed that the 2,6-linked sialic acid of the biantennary glycan increases the antiinflammatory activity (11), although its effect on ADCC was not clear (11–13), and that the remission of RA was often accompanied by an increase of IgG sialylation during pregnancy, and its relapse coincided with the decrease of IgG sialylation after delivery (14). Inflammation and cytotoxicity are two sides of the immune response, and the occurrence of cancer or infection is normally accompanied by inflammation. However, all of the antibodies described above were still heterogeneous even when a specific glycan structure was enriched through pathway engineering and cell culture preparation. To understand the effect of Fc glycans on ADCC, complement-dependent cytotoxicity (CDC), and antiinflammatory activities, homogeneous antibodies with well-defined glycan structures are needed.
Many methods have been developed for the preparation of homogeneous glycoproteins with well-defined glycans, including native chemical ligation (NCL) (15, 16), expressed protein ligation (EPL) (17), Staudinger ligation (18–20), sugar-assisted ligation (21), and glycoprotein remodeling in vitro using endoglycosidases and glycosyltransfer enzymes (22). Similarly, glycosylation pathway engineering has been developed to improve the biological function and reduce the heterogenecity of therapeutic antibodies (23, 24). Of these methods, the most practical way to acquire homogeneous glycoproteins is based on the strategy of glycoprotein remodeling, a strategy first reported in 1997 (22) and later applied to antibody glycoengineering (25–27). The strategy starts with the use of exoglycosidases or endoglycosidases to cleave most of the N-glycans to form a homogeneous glycoform containing a well-defined glycan, followed by extension of the glycan using glycosyltransfer enzymes. Compared with the other endoglycosidases of the Glycoside Hydrolase Family 18 (GH18), like endo H, endo F1, F2, and F3, endoS showed the best specific hydrolytic activity for the asparagine-linked biantennary glycan of human IgG to give mono-GlcNAc antibody. In addition, some endoglycosidases of GH18 can also be used as glycosynthases with glycan oxazolines as substrates to form the chitobiose linkage, a strategy first demonstrated by Shoda and coworkers using chitinases (28, 29) and further elaborated by Wang with endoglycosidase mutants developed using Wither's approach, including endoS D233Q and endoM N175Q (26, 30, 31).
In this study, we used the endoS from Streptococcus pyogenes (32) and the fucosidase from Bacteroides fragilis (BtFucH) in combination to treat a mixture of antibody glycoforms to obtain a homogeneous antibody containing the mono-GlcNAc residue at position 297, which was then further extended with various glycans containing a leaving group catalyzed by an endoglycosidase mutant to give homogeneous antibodies with a well-defined glycan at the Fc region for functional study. The endoS was to hydrolyze the chitobiose core of the asparagine-linked glycan, and the fucosidase was to cleave the fucose attached to the remaining innermost GlcNAc to form an antibody with mono-GlcNAc. During this process, it was found that the biantennary N-glycan structure with two terminal alpha-2,6-linked sialic acids at position 297 of the Fc region is a common and optimized structure for the enhancement of ADCC, CDC, and antiinflammatory activities.
Results and Discussion
Glycoengineering of IgG1 Antibody.
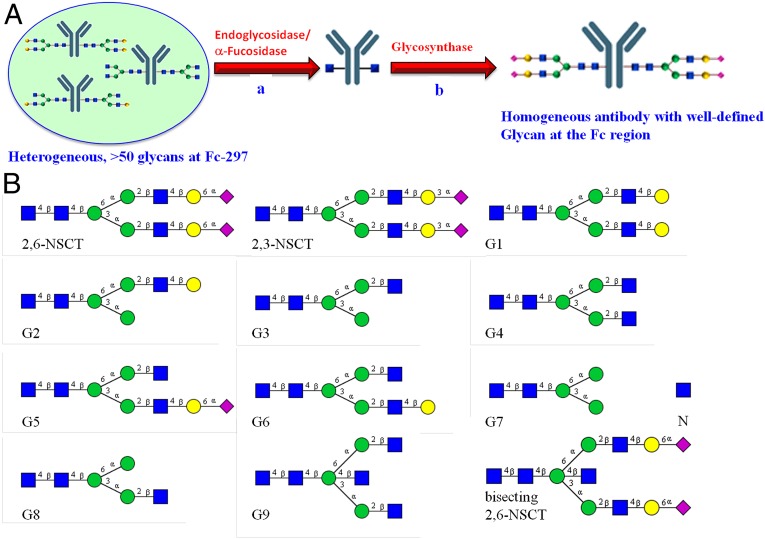
The goal of this study was to prepare homogenous antibodies with optimized anticancer and antiinflammatory functions. The commercially available Rituximab IgG1 was selected as a model because it has been used for the treatment of both cancer and autoimmune diseases. We used the combined endoglycosidase/fucosidase system to treat the antibody glycoforms to first obtain a homogeneous antibody with mono-GlcNAc at the Fc region, and then a pure synthetic glycan oxazoline was ligated with the GlcNAc residue under the catalysis of an endoglycosidase mutant to obtain the homogeneous antibody for activity assay (Fig. 1A). The fucosidase expressed from Bacteroides fragilis (BfFucH) was used in combination with either the EndoS from Streptococcus pyogene alone or mixtures of Endo F1/S, to prepare the homogeneous mono-GlcNAc antibody in one pot within 1 d. This fucosidase is more efficient than the one from bovine kidney, which requires 20 d of incubation (26). We also found that incubation of the antibody at 37 °C for 1 wk would cause a significant loss of binding affinity toward its antigen. Then, by using an EndoS mutant, a series of synthetic glycan oxazolines were successfully transferred to GlcNAc-Rituximab to form homogeneous antibodies with different glycans at the Fc region for the subsequent binding and functional assays (Fig. 1 and SI Appendix, Fig. S3).
Fig. 1.
(A) A general strategy for the preparation of homogeneous antibodies with a well-defined glycan structure through in vitro enzymatic remodeling of a mixture of antibody glycoforms. The mixture was first treated with a combination of endoS and the fucosidase from Bacteroides fragilis to generate mono-GlcNAc antibody, followed by ligation with a synthetic glycan oxazoline catalyzed by an endoS mutant. (B) The glycan structures on the homogeneous antibody prepared for the study. G9 can be further extended by glycosyltransferases to form the bisecting 2,3-NSCT-antibody and 2,6-NSCT-antibody.
Characteristics of 2,3- and 2,6-Sialylated Rituximab Glycoforms.
The Ravetch group has demonstrated the effect of Fc glycans on the effector-mediated activities of antibodies using highly enriched antibody glycoforms (11, 13). The FcγRI-mediated effector function of antibodies with truncated glycans prepared by treatment with glycosidases has also been reported (33). Although the 2,6-sialylated IVIG rather than the 2,3-linked isomer was found to be the major structure responsible for the antiinflammation activity, the detailed interactions of different homogeneous glycoforms with different receptors (FcγRs) have not been studied (11). Moreover, it has been shown that high levels of sialylation reduce ADCC activity (12), but it is not clear whether both 2,6- and 2,3-sialylated antibodies would have a similar effect on cytotoxicity. To study the differences in these sialylation linkages, we prepared 2,6- and 2,3-sialylated homogeneous antibodies (denoted as 2,6-NSCT-Rituximab and 2,3-NSCT-Rituximab) from mono-GlcNAc Rituximab. Compared with the nonmodified Rituximab, the mono-GlcNAc Rituximab showed a complete loss or substantially reduced binding affinity toward FcγRIIIa, FcγRIIa, FcγRI, and C1q but not toward FcγRIIb. However, after elongation of the glycan to form the structure of 2,6-NSCT-Rituximab, its binding affinity toward FcγRIIa, FcγRIIb, and, especially, FcγRIIIa increased whereas no significant change was observed toward C1q (Table 1). On the other hand, for 2,3-NSCT-Rituximab, only the interaction with FcγRIIIa was partially increased, whereas its interaction with FcγRIIa was significantly decreased and that with FcγRIIb was unchanged (Table 1).
Table 1.
Fcγ-receptor binding of the commercial Rituximab and the glycoengineered 2,3-NSCT- and 2,6-NSCT-Rituxmab: binding experiments of the mono-GlcNAc, 2,3-NSCT- and 2,6-NSCT-Rituxmab toward Fcγ receptors and C1q performed in ELISA
| Fc receptor | EC50, nM (95% CI) | |||
| Roche-Rituximab | 2,6-NSCT-Rituximab | 2,3-NSCT-Rituximab | N-Rituximab | |
| FcγRIIIa | 6.179 (3.303–9.054) | 0.239 (0.209–0.269) | N.D. | 36.27 (−31.56–104.1) |
| 3.375 (1.785–4.964) | 0.298 (0.242–0.353) | 1.216 (0.350–2.081) | N.D. | |
| FcγRIIa | 5.445 (3.656–7.234) | 2.983 (1.953–4.013) | 16.45 (1.013–31.89) | N.M.* |
| FcγRI | 0.188 (0.162–0.215) | 0.228 (0.206–0.251) | N.D. | 0.854 (0.627–1.080) |
| FcγRIIb | 0.512 (0.205–0.819) | 0.280 (0.235–0.325) | 0.529 (0.404–0.655) | 0.315 (0.261–0.370) |
| C1q | 6.471 (5.211–7.731) | 8.069 (6.439–9.699) | N.D. | not fitted |
CI, confidence interval; N.D., no data, was not examined; N.M., not measured.
Data were out of range examined. Deglycosylation rendered mono-GlcNAc Rituximab to lose its binding affinity towards FcγRIIIa, FcγRIIa, FcγRI, and C1q, whereas the 2,3- and 2,6-sialylated antibodies restored their affinity, and the 2,6-sialylated Rituximab showed enhanced interactions with FcγRIIa, FcγRIIb, and FcγRIIIa.
Recently, it was reported that hFcγRIIa is engaged in the antitumor vaccinal effect of antitumor monoclonal antibody (34), suggesting that monoclonal antibodies may have another medicinal aspect. The increased affinity of the 2,6-NSCT-Rituximab toward FcγRIIa in this study implies a possible enhancement of the vaccinal effect compared with the 2,3-NSCT-Rituximab and the nonmodified Rituximab. It was also reported that hFcγRIIIa on macrophages is vital for the antitumor cytotoxicity in a humanized mouse model (34) and that defucosylation of IgG1 was found to enhance the ADCC effect via increasing the interaction between the afucosylated Fc glycans and FcγRIIIa (5, 35). In the binding experiments, we found that both the defucosylated 2,6-NSCT-Rituximab and 2,3-NSCT-Rituximab indeed showed a stronger interaction with FcγRIIIa than the nonmodified Rituximab; however, the 2,6-NSCT-Rituximab has much higher affinity than does the 2,3-NSCT-Rituximab (Table 1). Using three different B lymphoma cell lines, Raji, Ramos, and SKW6.4, we monitored the luminescence caused by the released proteases of dead cells to evaluate the peripheral blood mononuclear cell (PBMC)-mediated cytotoxicity induced by Rituximab, 2,3-NSCT-Rituximab, and 2,6-NSCT-Rituximab. Indeed, compared with the nonmodified Rituximab, both the 2,6-NSCT-Rituximab and 2,3-NSCT-Rituximab showed a stronger interaction with FcγRIIIa (Tables 1 and 2), and the 2,6-sialyl linkage showed excellent affinity toward FcγRIIIa and a strong ADCC to the three lymphoma cell lines, whereas the 2,3 linkage had weaker activities.
Table 2.
Fresh PBMC-mediated ADCC assays of the commercial Rituximab and the glycoengineered 2,3-NSCT- and 2,6-NSCT-Rituxmab: Experiments were conducted with three different B-cell lines, Raji, Ramos, and SKW6.4
| Antibody | EC50, ng/mL (95% CI) | ||
| Raji | Ramos | SKW6.4 | |
| Rituximab | 1.981 (−0.589–4.552) | 30.19 (−14.10–74.48) | 14.26 (−54.14–82.65) |
| 2,3-NSCT-Rituximab | 0.573 (−0.05–1.197) | 9.953 (−5.76e+7–5.76e+7) | 8.022 (0.881–15.16) |
| 2,6-NSCT-Rituximab | 0.159 (0.014–0.304) | 2.553 (−0.347–5.453) | 3.130 (−0.254–6.514) |
The activity measured by EC50 was significantly increased from the unmodified Rituximab to the glycoengineered afucosylated 2,3-NSCT-Rituximab, and to the most active 2,6-NSCT-Rituximab.
In addition to ADCC, CDC is also important in antibody therapy. Although Rituximab and 2,6-NSCT-Rituximab had similar activities in CDC and C1q binding, the cell-based ELISA data showed that the 2,3-NSCT-Rituximab had a weaker CDC (Table 3).
Table 3.
CDC assays of the commercial Rituximab and the glycoengineered 2,3-NSCT- and 2,6-NSCT-Rituxmab towards target cells, Ramos
| Antibody | EC50, μg/mL (95% CI) |
| Rituximab | 0.230 (0.103–0.356) |
| 2,3-NSCT-Rituximab | 0.587 (0.256–0.918) |
| 2,6-NSCT-Rituximab | 0.270 (0.110–0.431) |
In the CDC assay performed with FACS, 2,6-NSCT-Rituximab showed similar CDC activity to the nontreated antibody, but the 2,3-NSCT-Rituximab showed reduced CDC efficacy.
Binding Affinity and the B-Cell Depletion Activity of Various Afucosylated Rituximab Glycoforms.
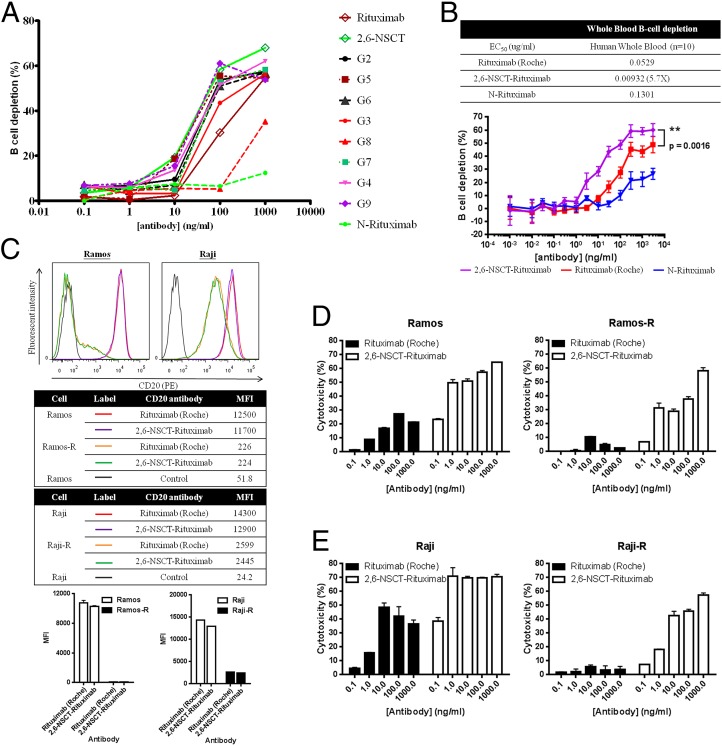
To study whether the cytotoxicity was affected by the 2,6-sialylation, we prepared other homogeneous afucosylated Rituximab glycoforms for comparison. In surface plasma resonance (SPR) analysis, none of the modified afucosylated Rituximab glycoforms displayed a stronger binding affinity toward FcγRIIIa than 2,6-NSCT-Rituximab, although some Kd variations among different glycoforms were observed (SI Appendix, Table S5). We further performed a cytotoxicity study of PBMC-mediated depletion of human B cells by analyzing CD19+ CD3− B cells with flow cytometry. Consistent with the SPR data, the B-cell depletion efficacy of the 2,6-NSCT-Rituximab was superb when the antibody concentration was 10 ng/mL or higher (Fig. 2A). Moreover, the activity of the 2,6-NSCT-Rituximab was also significantly higher than the nonmodified Rituximab, with a P value of 0.0016 in the whole blood B-cell depletion tests of 10 donors, whereas the mono-GlcNAc Rituximab showed the lowest activity (Fig. 2B). These data indicated that the 2,3- and 2,6-sialylation on IgG1 had different activities, and the 2,6-NSCT-Rituximab was the best for B-cell depletion. These results could not have been obtained from the samples prepared from CHO cells directly, which expressed proteins with various glycans, and the sialic acid residues are mainly in the 2,3 linkage instead of the 2,6 linkage (36).
Fig. 2.
Antibody-dependent B-cell depletion of various Rituximab glycoforms. The depletion of human B cells was conducted using freshly prepared human PBMC cells and analyzed with FACS, based on the CD19+ CD3− B cells. (A) Compared with a series of different Rituximab glycoforms, the 2,6-NSCT-Rituximab showed higher depletion activity. (B) In the whole blood B-cell depletion activity of 10 donors, the 2,6-sialylated Rituximab was significantly more active than the nontreated Rituximab, with a P value of 0.0016, whereas the mono-GlcNAc Rituximab showed the lowest activity. (C) The Rituximab-resistant cells of Ramos (Ramos-R) and Raji (Raji-R) express a lower level of CD20 on the cell surface; MFI, medium fluorescence intensity. (D) Ramos and Ramos-R and (E) Raji and Raji-R: The 2,6-NSCT-Rituximab showed remarkable ADCC efficacy toward both normal and resistant cells, whereas the nontreated antibody dramatically lost its activity toward resistant strains.
The ADCC of 2,6-NSCT-Rituximab Toward Resistant Cell Lines.
Like many pharmaceuticals, some patients are resistant to Rituximab due to high dosage and long-term use (37, 38). To understand whether the 2,6-NSCT-Rituximad is effective against drug-resistant cells, we prepared Ramos and Raji Rituximab-resistant cell lines to evaluate their PBMC-mediated ADCC under different concentrations of 2,6-NSCT-Rituximab (Fig. 2 C−E). After coculturing with Rituximab, both Ramos and Raji B cells evolved to become Rituximab resistant with low CD20 expression on the cell surface (Fig. 2C). As a result, the nonmodified Rituximab dramatically lost its activity against resistant strains (Fig. 2 D and E), but the 2,6-NSCT Rituximab showed significant ADCC activity against both nonresistant and resistant cells.
FcγRIIIa Binding Affinity of Various Afucosylated Herceptin Glycoforms.
To further evaluate the impressive cytotoxicity derived from the 2,6-NSCT glycan modification, Herceptin, an antibody for the treatment of her2+ breast cancer, was selected and modified with different glycan structures and evaluated.
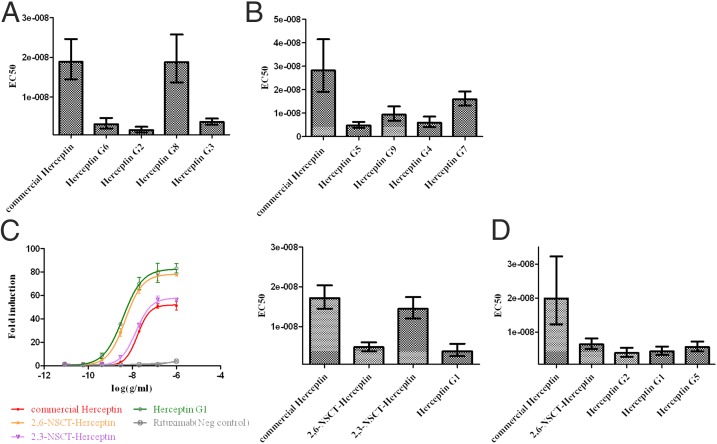
The binding analysis of various homogeneous Herceptin glycoforms interacting with FcγRIIIa is shown in SI Appendix, Table S6. Similar to the affinity difference of the 2,3- and 2,6-NSCT-Rituximab in ELISA analysis, the 2,6-NSCT-Herceptin showed a stronger interaction with FcγRIIIa, whereas a detrimental effect was observed with the 2,3-NSCT-Herceptin, and the effect of Fc afucosylation was more significant than the sialylation effect. Moreover, the corresponding Kd of all Herceptin glycoforms showed a similar tendency to those of the Rituximab glycoforms (SI Appendix, Table S6). Antibodies, such as G1, G2, and 2,6-NSCT, had a more than ninefold increase in affinity for FcγRIIIa, compared with the other derivatives like G3, G4, G5, G6, G7, G9, and 2,3-NSCT. Particularly, in both Rituximab and Herceptin, the afucosylayed glycoform G8 almost lost its defucosylation advantage for the ADCC activity. The antibody with bisecting glycan, G9, showed a slight but not significant increase in affinity toward FcγRIIIa in both Rituximab and Herceptin when it was compared with the nonbisecting analog, G4. Likewise, the bisecting 2,6-NSCT-Herceptin also showed no significant enhancement in affinity toward FcγRIIIa compared with its nonbisecting analog, 2,6NSCT-Herceptin. Overall, the 2,6-NSCT-Herceptin showed a superb FcγRIIIa binding affinity among the afucosylated analogs in the SPR analysis.
To further understand the Fc glycosylation effect on the FcγRIIIa-mediated ADCC of Herceptin glycoforms, we conducted an ADCC reporter bioassay using the signaling nuclear factor of the activated T-cell (NFAT) pathway of V158 FcγRIIIa engineered Jurkat effector cells and SKBR3 target cells with an E/T ratio of 6. Consistent with the kinetic data, the EC50 of the afucosylated G8 Herceptin showed a loss of FcγRIIIa activity and displayed a similar ADCC effect to the fucosylated Herceptin (Fig. 3A). Interestingly, a previous study showed that more bisecting glycans on the antibody, prepared by the increased expression of β (1, 4)-N-acetylglucosaminyltransferase III, correlate with its stronger ADCC (39). On the contrary, our study showed that no significant Kd difference was observed between the bisecting and nonbisecting glycoforms of Herceptin and Rituximab, G9 and G4, and the EC50 values of the Herceptin glycoforms showed a similar cytotoxicity profile in FcγRIIIa cell-mediated assay (Fig. 3B). A comparison of the bisecting and nonbisecting 2,6-NSCT-Herceptin showed a similar result, suggesting that the bisecting IgG1 does not perform better in the FcγRIIIa-mediated ADCC activity.
Fig. 3.
EC50 of homogeneous Herceptin glycoforms in the V158 FcγRIIIa mediated ADCC reporter assay. Experiments were performed under the E/T ratio of 6–1 with SKBR3 target cells and the V158 FcγRIIIa engineered effector Jurkat cells. All data shown in the same graph were experiments done in the same microplate and the same batch of effector cells; bars of 95% confidence interval were plotted. (A) The afucosylated Herceptin G8 and the commercial Herceptin showed a similar ADCC effect, indicating that the defucosylation advantage of anti-FcγRIIIa is lost in the afucosylated Herceptin G8. (B) The bisecting and nonbisecting Herceptin analogs G9 and G4 showed similar EC50 values, indicating that the bisecting GlcNAc effect was not observed in this assay. (C) Compared with the glycoengineered Herceptin G1 with two galactose terminals, no significant EC50 change in the 2,6-sialylated antibody was observed, whereas the apparent EC50 increase was shown in the 2,3-sialylated Herceptin. The curves of fold induction were the results of induced luminescence divided by the induction of no antibody control. (D) Samples with the lowest EC50 in A−C were chosen and compared with the commercial Herceptin. All samples demonstrated better activity in this ADCC reporter bioassay.
In addition, compared with the nonsialylated G1-Herceptin, the ADCC of 2,3-sialylated Herceptin was obviously reduced, whereas the 2,6-NSCT-Herceptin still maintained its activity (Fig. 3C), indicating that sialylation-mediated ADCC reduction is caused by the 2,3 linkage. To explore the potential utility of 2,6-NSCT in antibody medication, we selected glycoengineed afucosylated Herceptin samples with lower EC50 than the commercial Herceptin for further activity studies (Fig. 3D), and it was found that all samples exhibited better cytotoxicity and were capable of killing cancer cells under low concentrations.
The ADCC Effect of the 2,6-NSCT Glycan Modification on Antiviral Antibodies.
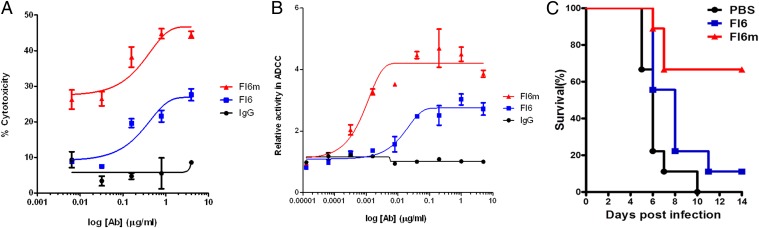
We evaluated whether the homogenous 2,6-NSCT glycan modification of antibodies can also increase the ADCC effect of antiviral antibodies to remove virus-infected cells. We prepared an antiinfluenza broadly neutralizing antibody, FI6, which was known to recognize the stem region of hemagglutinin (HA) of various subtypes of influenza, and its neutralizing activity was linked to ADCC (40). The antibody was modified to the homogeneous 2,6-NSCT-glycoform (F16m) and mixed with human HEK293T cells, which express HA on the cell surface to mimic influenza-infected cells; then, the ADCC effects were measured by both the PBMC-mediated cytotoxicity in target cells and the activation of ADCC by effector cells. The cytotoxicity results showed that the homogeneous antibody (FI6m) indeed exhibits a significantly higher (twofold to threefold increase) ADCC activity than the unmodified antibody F16 (Fig. 4A). In addition, the activation of the ADCC signaling NFAT pathway of the effector NK cells was also observed to have a twofold enhancement when the homogeneous FI6m was used (Fig. 4B). Thus, our observation indicates that the homogeneous 2,6-NSCT glycan modification of antiviral antibodies can be a general strategy to enhance the ADCC activity on virus-infected cells.
Fig. 4.
Antiinfluenza antibody FI6 with 2,6-NSCT glycan attached to its Fc Asn297 (FI6m) significantly enhances its ADCC activity and prophylactically protects mice from a lethal dose of H1N1 virus challenge. (A) Cytotoxicity is represented as the percentage of lysed HEK293T cells (target cells) expressed with influenza H1 HA (A/California/07/09) when incubated with PBMCs (effector cells) and various concentrations of antibodies. (B) The ADCC activity is shown as fold increases of bioluminescence from a luciferase reporter assay that gave signals when ADCC NFAT pathway was activated. HA-expressed HEK293T cells (target cells) were incubated with NK cells with the aforementioned luciferase reporter (effector cells) and various amounts of antiinfluenza antibody FI6 and FI6m. Curve fitting was conducted with software GraphPad Prism in 4PL nonlinear regression. (C) Survival of mice was monitored upon lethal dose (10 MLD50) infection of influenza virus A/California/07/09 (H1N1). Two hours before infection, each group of mice (n = 9) was administered 2.5 mg/kg of FI6, FI6m, or PBS intraperitoneally. The FI6 and FI6m groups had significant survival difference (P < 0.01).
Next we tested whether the in vitro ADCC enhancement of FI6 can be translated into protection in a mouse model that was given a lethal dose infection of influenza H1N1. The passive transfer of FI6 monoclonal antibody has been shown to protect H1N1 infection previously (41). Indeed, antibody FI6m showed a significantly better protection when mice were challenged with A/California/07/2009 H1N1 virus (Fig. 4C). The survival rate was 66% for FI6m versus 11% for F16, demonstrating that the in vitro ADCC enhancement by the homogeneous F16m is consistent with the in vivo protection from viral infection.
Conclusion
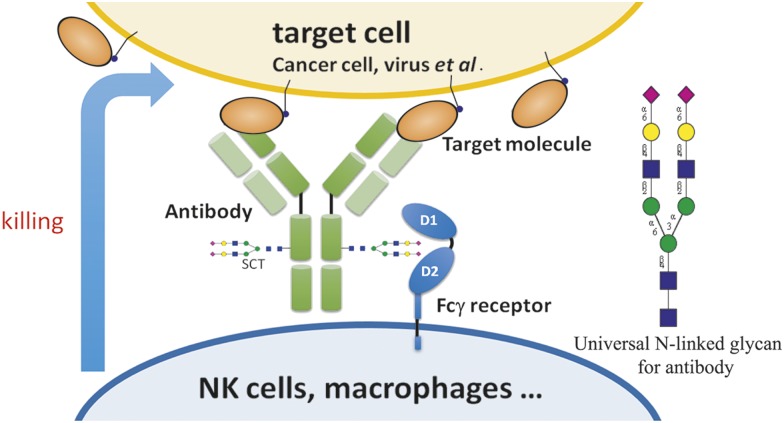
The therapeutic properties of antibodies depend on the target-binding specificity and the Fc glycan-mediated effector functions. In this study, we have shown that homogeneous antibodies with well-defined glycan structure in the Fc region can be prepared to optimize the effector-mediated ADCC, CDC, and antiinflammatory activities. Although all existing therapeutic antibodies and more than 20 glycoengineered antibodies currently in clinical studies are still mixtures of different glycofoms, our results show that the biantennary N-linked glycan structure with two terminal alpha-2,6-linked sialic acids at the Fc glycosylation site Asn-297 is a common optimal structure for the enhancement of effector functions (Fig. 5). The activity enhancement is mainly due to the increased interaction between the Fc glycan and Fc receptors as shown by the SPR analysis of the Fc glycan and Fc receptor interaction in this study and by the previous structural studies on the defucosylated antibodies interacting with the FcγRIIIa receptor (8). Our preliminary study based on the X-ray structure also showed that the 2,6-sialylated glycan had a stronger interaction with the FcγRIIIa receptor than did the other glycoforms. In conclusion, this study provides a previously unidentified strategy for the development of therapeutic antibodies in the future.
Fig. 5.
A common and optimal N-linked glycan on the Fc region of a therapeutic antibody for the enhancement of antibody activities against infectious and inflammatory diseases, as well as cancer.
Materials and Methods
Rituximab (2.5 mg) in a sodium phosphate buffer (50 mM, pH 7.0, 1.25 mL) was incubated with EndoS (125 μg) and BfFucH (2.5 mg) at 37 °C for 22 h. The reaction mixture was subjected to affinity chromatography on a column of protein A-agarose resin (1 mL) preequilibrated with a sodium phosphate buffer (20 mM, pH 7.0). After washing, the bound IgG was released with glycine HCl (50 mM, pH 3.0, 10 mL), and the elution fractions were immediately neutralized with Tris-Cl buffer (1.0 M, pH 8.3) and concentrated by centrifugal filtration (Amicon Ultra centrifugal filter) to give mono-GlcNAc Rituximab (1.93 mg). Detailed materials and methods are in SI Appendix, including the synthesis and analysis of pertinent glycans, glycan oxazolines and homogeneous antibodies, functional assays and binding analysis of various homogeneous antibodies, and H1N1 virus challenge in mice.
Supplementary Material
Acknowledgments
We thank Dr. S.-C. Jao of the Biophysics Core Facility of Academia Sinica for providing technical assistance for surface plasma resonance experiments and Dr. B.-S. Kuo of Fountain Biotechnology, and his group for help with the CD20 binding, antibody-dependent cellular cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC) assays. Mass spectrometry analyses were performed by the Genomics Research Center, Academia Sinica. This work was supported by Academia Sinica and CHO Pharma.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513456112/-/DCSupplemental.
References
- 1.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 2.Kang AS, Barbas CF, Janda KD, Benkovic SJ, Lerner RA. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci USA. 1991;88(10):4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 4.Tiller T, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329(1-2):112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 6.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki A, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcγRIIIa. J Mol Biol. 2004;336(5):1239–1249. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara C, et al. Unique carbohydrate−carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108(31):12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DL, et al. YEAR Consortium FcγRIIIa expression on monocytes in rheumatoid arthritis: Role in immune-complex stimulated TNF production and non-response to methotrexate therapy. PLoS One. 2012;7(1):e28918. doi: 10.1371/journal.pone.0028918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gornik I, Maravić G, Dumić J, Flögel M, Lauc G. Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem. 1999;32(8):605–608. doi: 10.1016/s0009-9120(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 11.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320(5874):373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44(7):1524–1534. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 14.van de Geijn FE, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res Ther. 2009;11(6):R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson PE, Kent SBH. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69(1):923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 16.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266(5186):776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 17.Muir TW. Semisynthesis of proteins by expressed protein ligation. Annu Rev Biochem. 2003;72(1):249–289. doi: 10.1146/annurev.biochem.72.121801.161900. [DOI] [PubMed] [Google Scholar]
- 18.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287(5460):2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson BL, Kiessling LL, Raines RT. High-yielding Staudinger ligation of a phosphinothioester and azide to form a peptide. Org Lett. 2001;3(1):9–12. doi: 10.1021/ol006739v. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Hong Z-Y, Wong C-H. Convergent glycopeptide synthesis by traceless Staudinger ligation and enzymatic coupling. ChemBioChem. 2006;7(3):429–432. doi: 10.1002/cbic.200500437. [DOI] [PubMed] [Google Scholar]
- 21.Brik A, Yang Y-Y, Ficht S, Wong C-H. Sugar-assisted glycopeptide ligation. J Am Chem Soc. 2006;128(17):5626–5627. doi: 10.1021/ja061165w. [DOI] [PubMed] [Google Scholar]
- 22.Witte K, Sears P, Martin R, Wong C-H. Enzymatic glycoprotein synthesis: Preparation of ribonuclease glycoforms via enzymatic glycopeptide condensation and glycosylation. J Am Chem Soc. 1997;119(9):2114–2118. [Google Scholar]
- 23.Yamane-Ohnuki N, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87(5):614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 24.Davies J, et al. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC γ RIII. Biotechnol Bioeng. 2001;74(4):288–294. [PubMed] [Google Scholar]
- 25.Zou G, et al. Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J Am Chem Soc. 2011;133(46):18975–18991. doi: 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Giddens J, Fan S-Q, Toonstra C, Wang L-X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc. 2012;134(29):12308–12318. doi: 10.1021/ja3051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodfellow JJ, et al. An endoglycosidase with alternative glycan specificity allows broadened glycoprotein remodelling. J Am Chem Soc. 2012;134(19):8030–8033. doi: 10.1021/ja301334b. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Kiyosada T, Shoda S-i. Synthesis of artificial chitin: Irreversible catalytic behavior of a glycosyl hydrolase through a transition state analogue substrate. J Am Chem Soc. 1996;118(51):13113–13114. [Google Scholar]
- 29.Shoda S-i, et al. Efficient method for the elongation of the N-acetylglucosamine unit by combined use of chitinase and β-galactosidase. Helv Chim Acta. 2002;85(11):3919–3936. [Google Scholar]
- 30.Huang W, Li J, Wang L-X. Unusual transglycosylation activity of Flavobacterium meningosepticum endoglycosidases enables convergent chemoenzymatic synthesis of core fucosylated complex N-glycopeptides. ChemBioChem. 2011;12(6):932–941. doi: 10.1002/cbic.201000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umekawa M, et al. Efficient transfer of sialo-oligosaccharide onto proteins by combined use of a glycosynthase-like mutant of Mucor hiemalis endoglycosidase and synthetic sialo-complex-type sugar oxazoline. Biochim Biophys Acta. 2010;1800(11):1203–1209. doi: 10.1016/j.bbagen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20(12):3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimura Y, et al. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: Properties of a series of truncated glycoforms. Mol Immunol. 2000;37(12-13):697–706. doi: 10.1016/s0161-5890(00)00105-x. [DOI] [PubMed] [Google Scholar]
- 34.DiLillo DJ, Ravetch JV. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161(5):1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niwa R, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcγRIIIa functional polymorphism. Clin Cancer Res. 2004;10(18 Pt 1):6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 36.Raju TS, Lang SE. Diversity in structure and functions of antibody sialylation in the Fc. Curr Opin Biotechnol. 2014;30(0):147–152. doi: 10.1016/j.copbio.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24(2):203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barok M, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6(7):2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 39.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 40.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. 2014;20(2):143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.