Significance
Mammalian barrier surfaces are exposed to environmental stimuli that can result in tissue damage. Interleukin (IL)-33–dependent group 2 innate lymphoid cells (ILC2s) are enriched at barrier sites, but the mechanisms underlying the tissue-protective roles of IL-33 or ILC2s in the intestine remain poorly defined. Here we use a model of murine intestinal inflammation and reveal a previously unrecognized pathway of innate immune cell-mediated tissue protection in which IL-33 ameliorated disease through induction of ILC2s and the growth factor amphiregulin (AREG). Collectively, these data highlight a critical dialogue between damaged epithelia and innate immune cells and indicate that manipulation of the IL-33–ILC2–AREG pathway could provide therapeutic benefit in treatment of intestinal inflammatory diseases.
Keywords: innate immunity, innate lymphoid cell, interleukin-33, inflammatory bowel disease
Abstract
The barrier surfaces of the skin, lung, and intestine are constantly exposed to environmental stimuli that can result in inflammation and tissue damage. Interleukin (IL)-33–dependent group 2 innate lymphoid cells (ILC2s) are enriched at barrier surfaces and have been implicated in promoting inflammation; however, the mechanisms underlying the tissue-protective roles of IL-33 or ILC2s at surfaces such as the intestine remain poorly defined. Here we demonstrate that, following activation with IL-33, expression of the growth factor amphiregulin (AREG) is a dominant functional signature of gut-associated ILC2s. In the context of a murine model of intestinal damage and inflammation, the frequency and number of AREG-expressing ILC2s increases following intestinal injury and genetic disruption of the endogenous AREG–epidermal growth factor receptor (EGFR) pathway exacerbated disease. Administration of exogenous AREG limited intestinal inflammation and decreased disease severity in both lymphocyte-sufficient and lymphocyte-deficient mice, revealing a previously unrecognized innate immune mechanism of intestinal tissue protection. Furthermore, treatment with IL-33 or transfer of ILC2s ameliorated intestinal disease severity in an AREG-dependent manner. Collectively, these data reveal a critical feedback loop in which cytokine cues from damaged epithelia activate innate immune cells to express growth factors essential for ILC-dependent restoration of epithelial barrier function and maintenance of tissue homeostasis.
The mammalian barrier surfaces of the skin, lung, and intestinal tract are continually exposed to microbial, physical, and environmental insults, which can lead to tissue injury and inflammation (1–3). In the context of chronic diseases like inflammatory bowel disease (IBD), the failure to initiate or resolve tissue-protective repair responses can have detrimental effects on the host, resulting in loss of tissue function and promotion of chronic inflammation and fibrosis (4, 5). Therefore, delineating the cellular and molecular mechanisms that direct tissue protection and remodeling could identify new therapeutic targets to improve treatment of multiple chronic inflammatory diseases.
Crosstalk exists between the epithelial barrier and the mammalian immune system in which damaged epithelial cells release cytokine signals such as interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin that activate sentinel immune cell populations (3, 6). Reciprocally, the immune system is integral to orchestrating epithelial repair and maintenance of tissue homeostasis at these barrier sites, producing key cytokines and growth factors that modulate epithelial barrier function. Recently, amphiregulin (AREG), a ligand of the epidermal growth factor receptor (EGFR), has emerged as a component of the type 2 inflammatory response (7–9). In the context of IBD, dysregulated expression of IL-33, EGFR family members, and associated ligands have been reported in patients (10–15) and in murine models of intestinal inflammation (16, 17). Despite these advances, however, the cellular sources and functional significance of specific EGFR ligands during disease remain poorly defined.
We recently identified that a subset of the innate lymphoid cell (ILC) family (called group 2 ILCs or “ILC2”) were a previously unrecognized cellular source of AREG in the respiratory tract and that lung ILC2s were critical for restoration of airway epithelial reparative responses following influenza virus-induced damage (7). However, the majority of studies have implicated classical type 2 cytokines IL-5 and IL-13 in ILC2 function in the lung (18–21), skin (22–24), and intestine (25–28) whereas a role for ILC2-derived growth factors such as AREG has been described only within the lung. Whether ILC2s at other barrier sites such as the intestine can express AREG and what functional significance this may have in regulating intestinal inflammation and tissue homeostasis remains unknown.
In this study, we demonstrate that AREG production is a dominant functional signature of gut-associated ILC2s in response to IL-33. Using the dextran sodium sulfate (DSS) model of intestinal damage and inflammation, we demonstrate that numbers of AREG-expressing ILC2s are increased in response to intestinal injury and genetic disruption of the endogenous AREG–EGFR pathway was associated with exacerbated disease. Administration of exogenous AREG limited intestinal inflammation and decreased disease severity in both lymphocyte-sufficient and lymphocyte-deficient mice, revealing a previously unrecognized innate immune mechanism of intestinal tissue protection. Furthermore, therapeutic treatment with IL-33 or transfer of ILC2s ameliorated intestinal disease severity in an AREG-dependent manner. These data reveal a mechanism through which cytokine cues from damaged epithelia orchestrate immune cells to express growth factors essential for tissue protection and the restoration of intestinal homeostasis.
Results
IL-33–Elicited ILC2s Express AREG in Gut-Associated Lymphoid Tissue.
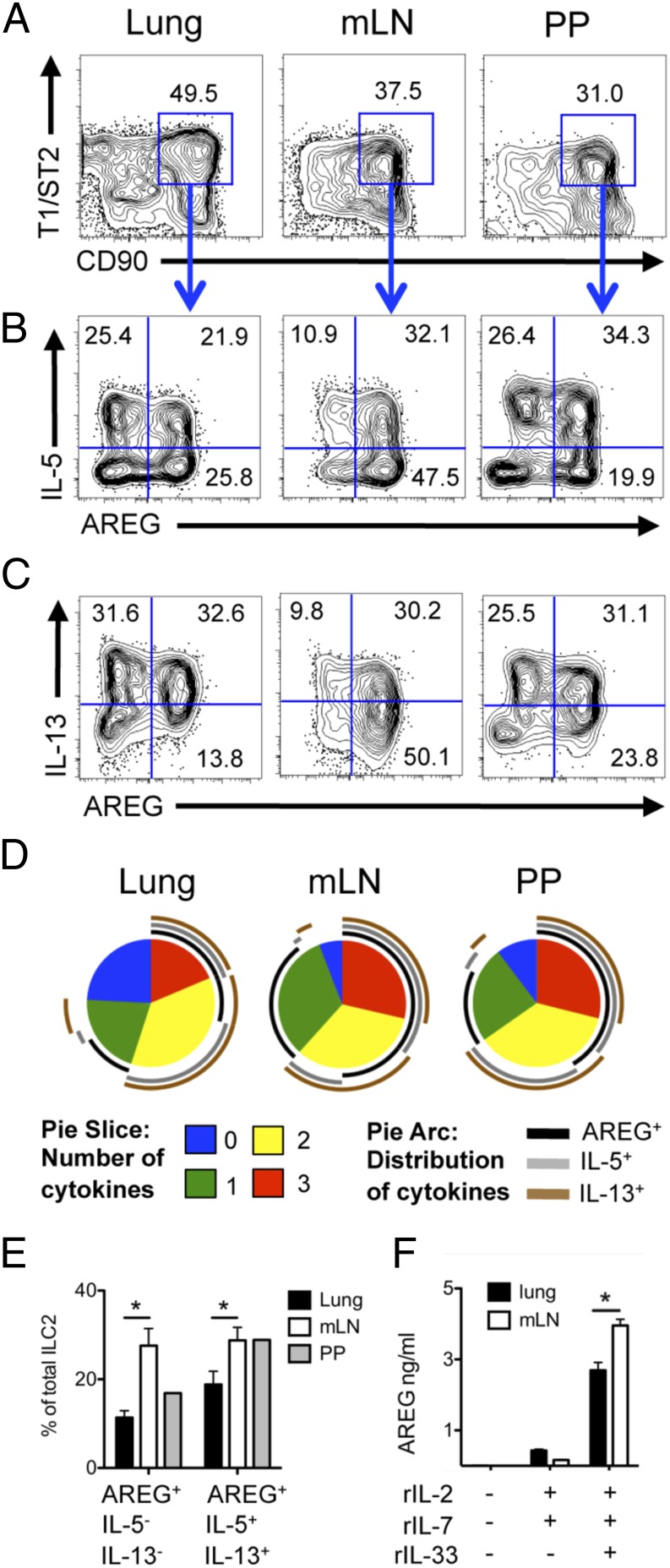
Previous studies investigating the roles of ILC2s have implicated classical type 2 cytokines IL-5 and IL-13 in ILC2 function, whereas the role of ILC2-derived growth factors such as AREG remains poorly characterized. Furthermore, it is unclear if AREG-producing ILC2s represent a distinct population of ILC2s from the classical IL-5– and IL-13–expressing cells. To address these questions, we performed single-cell analysis of intracellular AREG expression in ILC2s. Wild-type (WT) mice were treated in vivo with recombinant (r)IL-33 to activate the ILC2s and assessed for production of AREG, IL-5, and IL-13. In all tissue sites examined, IL-33 elicited distinct populations of polyfunctional ILC2s that expressed IL-5, IL-13, and/or AREG, revealing a previously unrecognized degree of potential functional heterogeneity in ILC2s (Fig. 1 A–C).
Fig. 1.
IL-33–elicited ILC2s express AREG in gut-associated lymphoid tissue. (A–E) C57BL/6 WT mice were treated i.p. with 12 μg/kg rIL-33 daily for 6 d. Lung, mLN, and Peyer’s Patches (PP) cells were stimulated ex vivo with phorbol 12-myristate 13-acetate (PMA) and Ionomycin plus brefeldin A for 4 h and examined for intracellular cytokine expression of IL-5, IL-13 and AREG via flow cytometry. (A) Frequency of CD90+ T1/ST2+ ILC2s after rIL-33 treatment, gated on Lin− CD45+ cells. Lin mixture includes CD3, CD5, CD11b, CD11c, FcεR1, B220, and NK1.1. Frequency of Lin− CD45+ CD90+ T1/ST2+ ILC2s expressing IL-5 and AREG (B) or IL-13 and AREG (C) in rIL-33–treated WT mice. (D) Multivariate analysis of flow cytometric data from B and C using SPICE software. Pie-slice color indicates number of cytokines (IL-5, IL-13, or AREG) produced by lung ILC2s (blue, 0; green, 1; yellow, 2; red, 3). Pie arcs illustrate the distribution of IL-5–, IL-13–, and/or AREG-expressing cells within each population of single (green pie slice), double (yellow pie slice), or triple (red pie slice) cytokine-producing ILC2s. IL-5 (gray arc), IL-13 (brown arc), and AREG (black arc). (E) Frequency of total ILC2s expressing AREG alone or expressing AREG, IL-5, and IL-13 simultaneously. (F) Sort-purified ILC2s from the lung or mLN cultured in vitro for 72 h with IL-2, IL-7, and IL-33. AREG was measured in culture supernatant via ELISA. Data are representative of at least three independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05.
Examination of the patterns of effector molecule expression in ILC2s revealed that the majority of IL-33–elicited ILC2s expressed AREG (black arc surrounding the pie graph) compared with IL-5 (gray arc) or IL-13 (brown arc), indicating that AREG production is a dominant functional signature of ILC2s following IL-33 stimulation (Fig. 1D). Notably, multivariate analysis revealed that a greater proportion of ILC2s in gut-associated lymphoid tissues exhibited triple effector molecule producing-capacity compared with their lung counterparts (Fig. 1D, red pie slices). Specifically, the mesenteric lymph node (mLN) contained a significantly higher proportion of ILC2s that expressed AREG alone and a higher percentage of triple cytokine-producing cells capable of expressing AREG, IL-5, and IL-13 simultaneously compared with the lung (Fig. 1D, black arcs, and Fig. 1E). This tissue-specific functionality was not a result of differential diffusion of rIL-33 across tissues, as direct in vitro stimulation of sort-purified ILC2s with rIL-33 also demonstrated enhanced AREG production in gut-associated ILC2s compared with their lung counterparts (Fig. 1F). Collectively, these data reveal unexpected functional heterogeneity in IL-33–elicited ILC2s and demonstrate that gut-associated ILC2s express high levels of AREG.
Epithelial Remodeling During DSS-Induced Intestinal Damage Requires Endogenous AREG–EGFR Interactions and Is Associated with Enhanced ILC2 Responses.
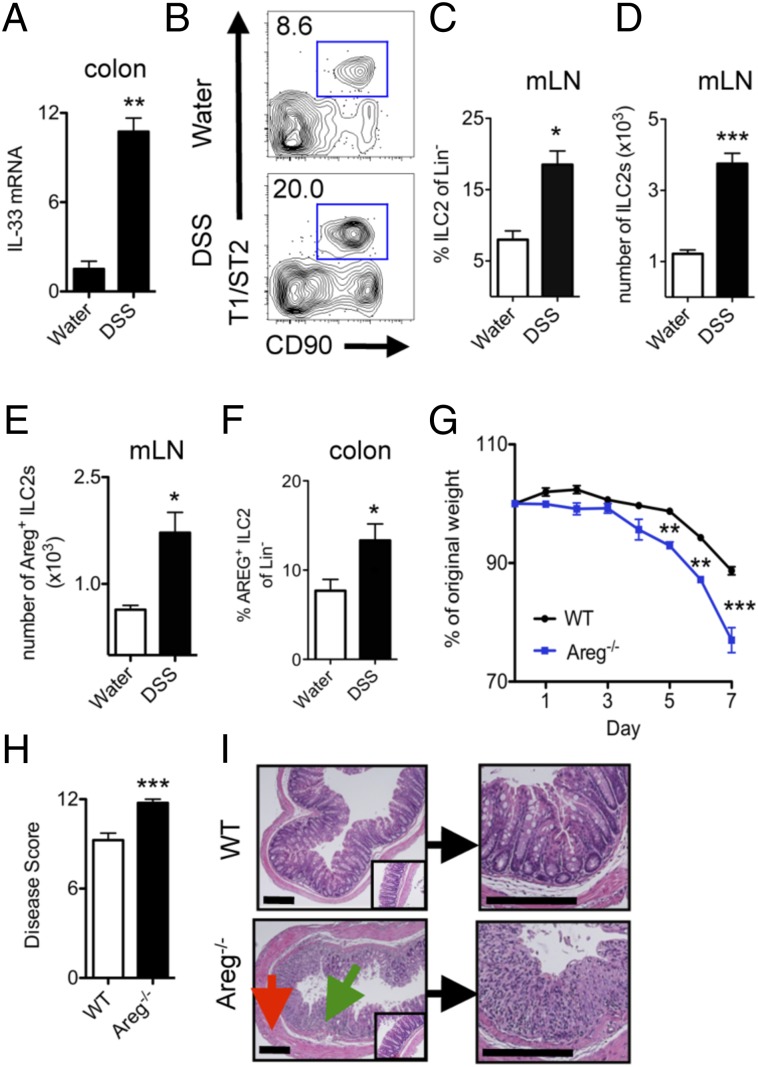
To test the potential contribution of the ILC2–AREG pathway to intestinal inflammation and tissue protection, we used the dextran sodium sulfate (DSS)-induced model of intestinal damage and inflammation (29). Consistent with previous reports (30, 31), IL-33 mRNA expression was increased in the colon of WT mice in response to DSS-induced intestinal damage (Fig. 2A). The increase in IL-33 expression was associated with an elevation in ILC2s expressing AREG in the mLN (Fig. 2 B–E) and colonic lamina propria (Fig. 2F), provoking the hypothesis that the ILC2–AREG pathway may influence inflammation or tissue-protective repair responses following intestinal damage.
Fig. 2.
Epithelial remodeling during DSS-induced intestinal inflammation requires AREG and is associated with enhanced ILC2 responses. (A–F) C57BL/6 WT mice were exposed to 3% (wt/vol) DSS in the drinking water for 7 d. (A) mRNA expression levels of IL-33 in colonic tissue, normalized to β-actin, and shown relative to expression levels in mice on normal water. Frequency (B and C) and cell number (D) of CD90+ T1/ST2+ ILC2 in the colonic draining mLNs, gated on Lin− CD45+ cells. (E and F) mLN (E) and colon lamina propria (F) cells were briefly stimulated ex vivo with PMA and Ionomycin plus brefeldin A for 4 h and assessed for AREG-expressing ILC2s. (G–I) WT or Areg−/− mice were administered 3% DSS and assessed on day 7 for weight loss (G) and clinical disease score (H). (I) H&E staining of colon sections from WT or Areg−/- mice at day 7. (Insets) Images are from water controls. Green arrow denotes regions of cellular infiltrate, and red arrow denotes thickening and hyperplasia of the smooth muscle lining. (Scale bar, 200 μm.) Data are representative of at least three independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
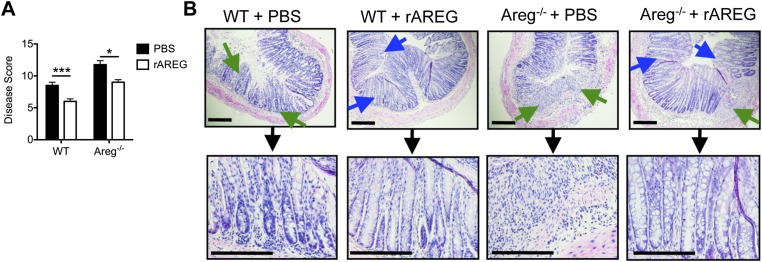
To test this, WT and Areg−/− mice were exposed to DSS in the drinking water for 7 d, and parameters of clinical disease were monitored. In comparison with DSS-treated WT mice, Areg−/− mice exhibited increased weight loss (Fig. 2G) and a more severe disease pathology score (Fig. 2H). Furthermore, histological examination of the colon tissue revealed that Areg−/− mice exhibited more severe pathological changes within the intestinal mucosa, including increased smooth muscle hyperplasia (red arrow) and greatly enhanced immune cell infiltration (green arrow) that resulted in loss of crypt architecture (Fig. 2I). Together, these data provide, to our knowledge, the first evidence that a single EGFR ligand, AREG, is a critical component of a tissue-protective pathway in response to DSS-induced intestinal damage.
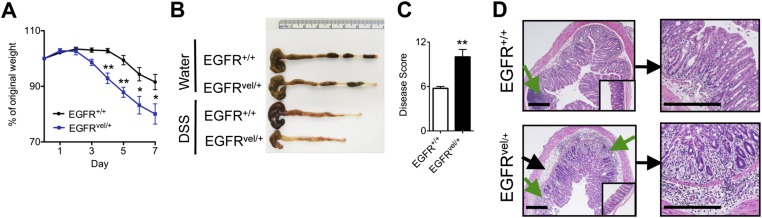
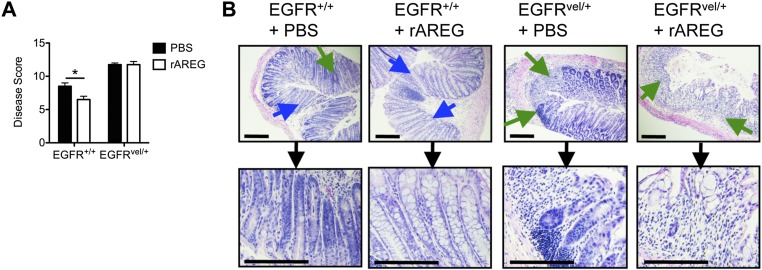
To test the role of downstream EGFR signaling in regulating intestinal inflammation, we used the EGFRvel/+ mouse model that exhibits reduced EGFR tyrosine kinase activity (32). Upon exposure to DSS, EGFRvel/+ mice exhibited increased weight loss (Fig. S1A), profound colonic shortening (Fig. S1B), and a more severe clinical disease score (Fig. S1C) compared with littermate controls. Strikingly, histological examination revealed that EGFRvel/+ mice exhibited increased edema (black arrows) and greater immune cell infiltration (green arrows) that resulted in near complete loss of crypt architecture throughout the colon (Fig. S1D). Taken together, these results demonstrate a critical role for AREG–EGFR signaling in limiting inflammation and/or in regulating epithelial remodeling responses during DSS-induced intestinal injury.
Fig. S1.
Genetic disruption in EGFR signaling results in exacerbated intestinal damage and inflammation. (A–D) EGFRvel/+ and EGFR+/+ littermates were exposed to 3% DSS in the drinking water for 7 d and assessed for weight loss (A), colon length (B), and pathological disease score (C). (D) H&E staining of colon sections from EGFRvel/+ or EGFR+/+ mice at day 7. (Insets) Images are from water controls. Green arrows denote regions of cellular infiltrate and black arrows denote areas of edema. (Scale bar, 200 μm.) Data are representative of at least three independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, **P < 0.01.
Exogenous AREG Ameliorates DSS-Induced Intestinal Inflammation in the Presence or Absence of the Adaptive Immune System.
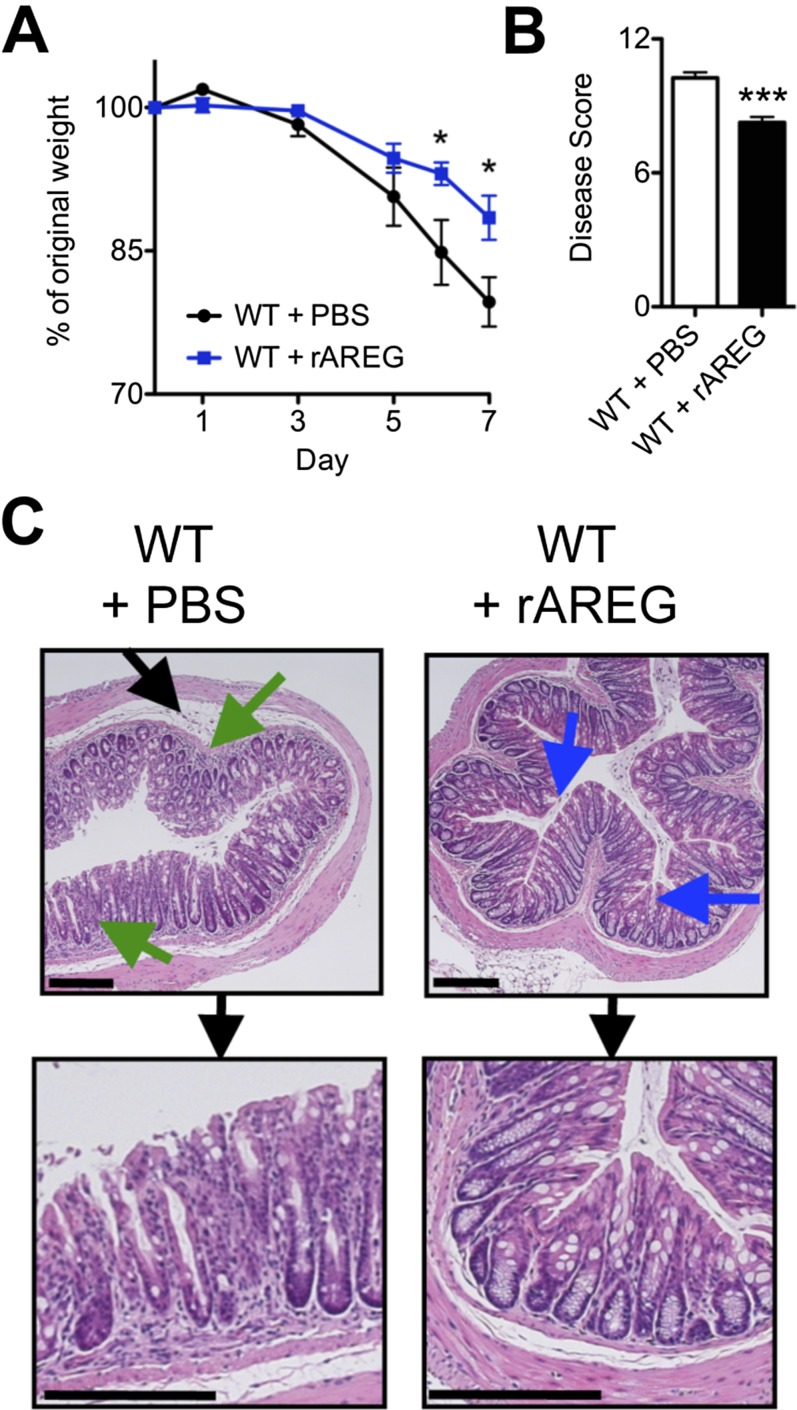
To test whether therapeutic manipulation of the AREG–EGFR pathway could limit inflammation or promote tissue protection, WT mice were treated with exogenous recombinant AREG (rAREG) over the course of DSS exposure. Administration of rAREG resulted in reduced weight loss (Fig. S2A) and improved clinical disease score (Fig. S2B) compared with PBS-treated controls. Furthermore, histological examination of colonic tissue revealed reduced inflammatory infiltrates (green arrows) and improved restoration of epithelial architecture with goblet-cell responses (blue arrows) (Fig. S2C), suggesting a role for AREG in limiting disease severity in response to DSS-induced intestinal damage. The ability of rAREG to ameliorate disease severity and promote tissue protection was dependent on downstream EGFR signaling, as treatment with rAREG was capable of ameliorating colonic inflammation in WT and Areg−/− mice (Fig. S3) but not in EGFRvel/+ mice that exhibit impaired EGFR signaling (Fig. S4).
Fig. S2.
Administration of exogenous AREG ameliorates intestinal disease severity in lymphocyte-sufficient mice. (A–C) C57BL/6 WT mice were exposed to 3% DSS in the drinking water for 7 d and treated with PBS or 400 μg/kg rAREG i.p. daily starting on the initial day of DSS exposure. Mice were examined for weight loss (A) over the course of treatment, and pathological disease score (B) was assessed on day 7. (C) H&E staining of colon tissue from PBS or rAREG-treated mice on day 7. Blue arrows indicated goblet-cell hyperplasia, green arrows denote regions of cellular infiltrate, and black arrows denote areas of edema. (Scale bar, 200 μm.) Data are representative of two to three independent experiments; n = 4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, ***P < 0.001.
Fig. S3.
Administration of exogenous AREG ameliorates intestinal disease severity in AREG-deficient mice. (A and B) C57BL/6 WT and Areg−/− mice were exposed to 3% DSS in the drinking water for 7 d and treated with PBS or 400 μg/kg rAREG i.p. daily starting on the initial day of DSS exposure. (A) Mice were assessed for pathological disease score over the course of treatment. (B) H&E staining of colon tissue from PBS or rAREG-treated mice on day 7. Blue arrows indicated goblet-cell hyperplasia, and green arrows denote regions of cellular infiltrate. (Scale bar, 200 μm.) Data are representative of two independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, ***P < 0.001.
Fig. S4.
AREG-mediated amelioration of intestinal disease severity is dependent on EGFR. (A and B) EGFR+/+ and EGFRvel/+ mice were exposed to 3% DSS in the drinking water for 7 d and treated with PBS or 400 μg/kg rAREG i.p. daily starting on the initial day of DSS exposure. (A) Mice were assessed for pathological disease score over the course of treatment. (B) H&E staining of colon tissue from PBS or rAREG-treated mice on day 7. Blue arrows indicated goblet-cell hyperplasia; green arrows denote regions of cellular infiltrate. (Scale bar, 200 μm.) Data are representative of two independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05.
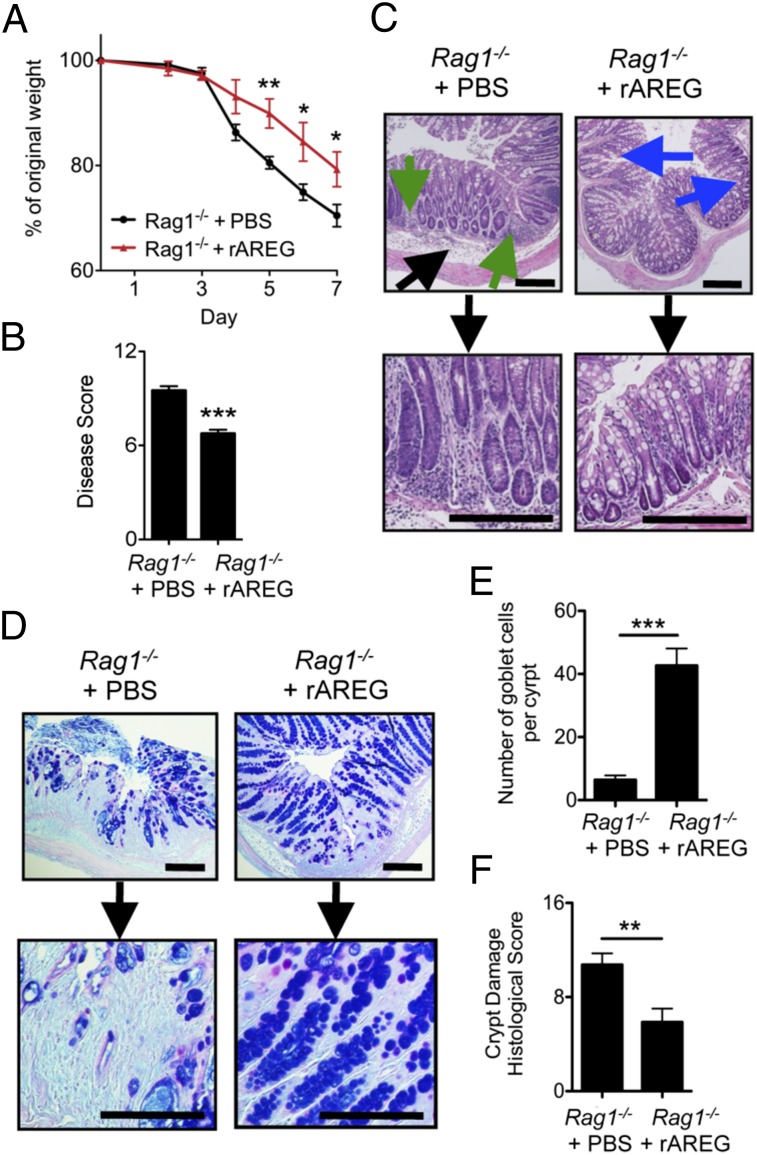
In the intestine, EGFR is primarily expressed on epithelial cells (16) although recent evidence suggests that it can also be expressed on some hematopoietic cells, including T regulatory (Treg) cells (33). To test whether the ability of rAREG to ameliorate intestinal inflammation was dependent or independent of the adaptive immune system, Rag1−/− mice were exposed to DSS and treated daily with rAREG. Similar to the results observed with wild-type mice (Fig. S2 A–C), rAREG treatment of DSS-exposed Rag1−/− mice resulted in decreased weight loss (Fig. 3A), a significant improvement in disease score (Fig. 3B), reduced inflammation in the colon (green arrows), and enhanced Periodic Acid Schiff (PAS+) Alcian Blue+ goblet-cell mucin production in the crypts (Fig. 3C, blue arrows, and Fig. 3 D and E). Furthermore, quantification of the degree of crypt damage revealed a significant improvement upon rAREG treatment (Fig. 3F). Taken together, these results demonstrate that administration of exogenous AREG can promote tissue protection and ameliorate disease severity independently of the adaptive immune system.
Fig. 3.
AREG ameliorates DSS-induced intestinal inflammation and tissue damage independently of adaptive immunity. Rag1−/− mice were exposed to 3% DSS for 7 d and treated with PBS or 400 μg/kg rAREG i.p. daily. Mice were examined for weight loss (A) over the course of treatment, and clinical disease score (B) was assessed on day 7. H&E (C) or PAS Alcian Blue mucin (D) staining of colon tissue from PBS or rAREG-treated mice on day 7. Blue arrows indicated goblet-cell hyperplasia, green arrows denote regions of cellular infiltrate, and black arrows denote areas of edema. (E) Quantification of the number of PAS+ Alcian Blue+ goblet cells per colon crypt. (F) Histological score of colonic crypt damage. (Scale bar, 200 μm.) Data are representative of two to three independent experiments; n = 4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Administration of Exogenous IL-33 Elicits ILC2s and Ameliorates Intestinal Disease Severity in an AREG-Dependent Manner.
We sought to test whether treatment with recombinant IL-33 could ameliorate inflammation or promote tissue protection through induction of the ILC2–AREG pathway. Treatment with rIL-33 elicited a robust 16- to 20-fold expansion of a lineage negative (Lin−) cell population in both WT and Areg−/− mice (Fig. S5 A and B) compared with PBS-treated controls. Further examination of this Lin− population revealed induction of CD90+ T1/ST2+ ILC2s at comparable frequencies in WT and Areg−/− mice (Fig. S5 C and D).
Fig. S5.
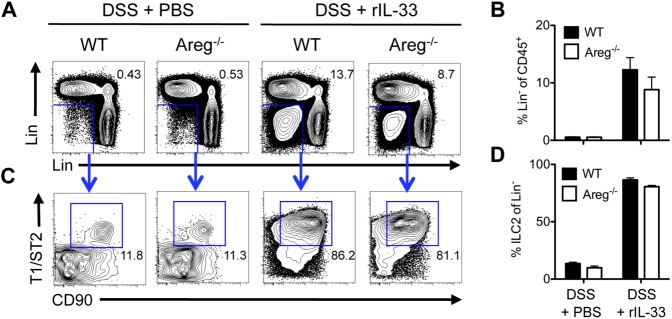
IL-33 administration elicits expansion of lineage-negative ILC2s. (A–D) C57BL/6 WT or Areg−/− mice were exposed to 3% DSS in the drinking water for 7 d and administered PBS or 16 μg/kg rIL-33 i.p. daily. Frequency of CD45+ Lin− cells (A and B) and CD45+ Lin− CD90+ T1/ST2+ ILC2s (C and D) in the colonic draining mLNs of WT and Areg−/− mice at day 7 following PBS or rIL-33 treatment. Lineage antibodies include NK1.1, CD11b, CD11c, FcεR1, CD3, CD5, TCRβ, and B220. Data are representative of two independent experiments; n = 4 mice per group per experiment. Data shown are the mean ± SEM.
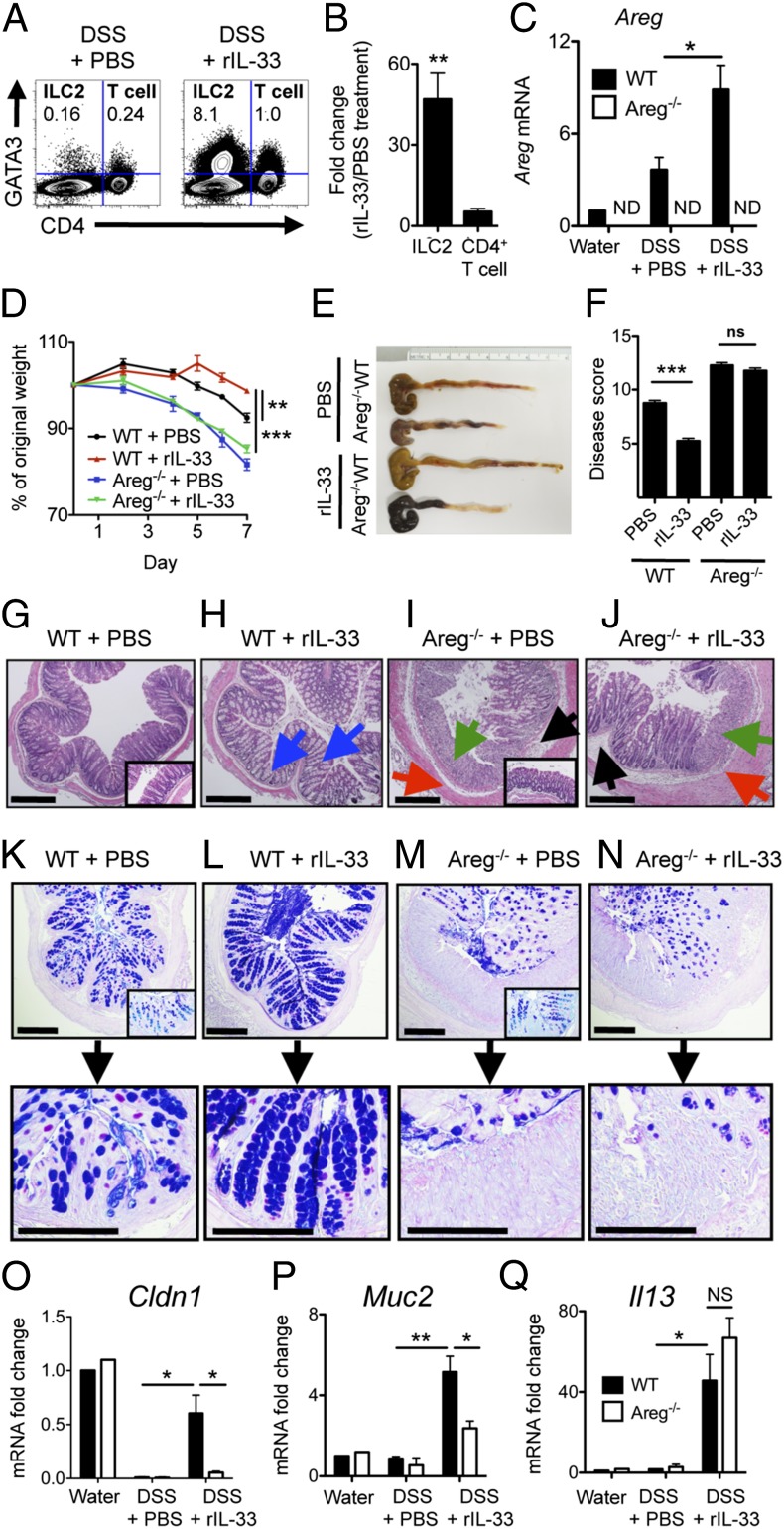
CD4+ Th2 cells and Tregs can also express IL-33R (33, 34) and can express AREG mRNA in the context of anti-helminth intestinal immunity (8) and muscle injury (9), raising the possibility that IL-33 may induce T-cell responses during intestinal inflammation. However, total CD4+ T-cell frequencies were unaffected by this acute 6-d rIL-33 treatment (Fig. S6A). Additionally, comparative analysis of GATA3+ CD4− ILC2 and GATA3+ CD4+ T-cell induction with DSS revealed that rIL-33 preferentially induced ILC2s while having a minimal effect on T-cell responses (Fig. 4A), corresponding to an average 47-fold increase in the frequencies of ILC2s versus a 4-fold change in CD4+ T cells at this time point (Fig. 4B). The IL-33–mediated induction of ILC2 responses was associated with a significant increase in colonic AREG mRNA expression (Fig. 4C), demonstrating that rIL-33 preferentially induces a robust ILC2 response and results in enhanced colonic expression of AREG.
Fig. S6.
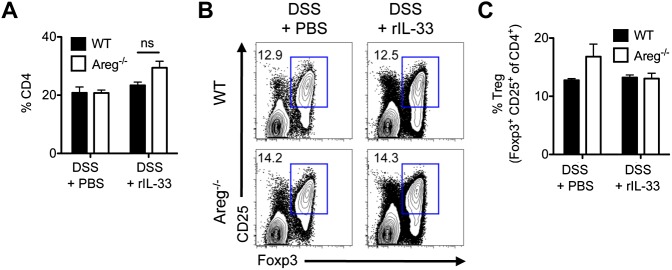
Regulatory T-cell frequency is unaltered following rIL-33 treatment. C57BL/6 WT or Areg−/− mice were exposed to 3% DSS in the drinking water for 7 d and administered PBS or 16 μg/kg rIL-33 i.p. daily starting on the day of initial DSS treatment. (A) Frequency of total CD4+ T cells in the colonic draining mLN, gated on CD45+ cells. Flow cytometry plots (B) and frequency (C) of CD45+ CD4+ CD25+ Foxp3+ Tregs in the mLN. Data are representative of two independent experiments; n = 4 mice per group per experiment. Data shown are the mean ± SEM.
Fig. 4.
Administration of exogenous IL-33 elicits ILC2 responses and promotes tissue protection in an AREG-dependent manner. (A–Q) C57BL/6 WT or Areg−/− mice were exposed to 3% DSS for 7 d and administered PBS or 16 μg/kg rIL-33 i.p. daily. (A) Frequency of GATA3+ CD4− ILC2s and GATA3+ CD4+ T cells in the mLN of WT mice on 3% DSS; plots are gated on CD45+ cells. (B) Fold increase in relative frequency of GATA3+ CD4− ILC2s and GATA3+ CD4− T cells following rIL-33 treatment during DSS. (C) mRNA expression levels of AREG in the colon, normalized to β-actin and shown relative to expression levels in WT mice on normal water. ND, not detected. Weight loss (D), colon length (E), and clinical disease score (F). (G–J) H&E staining of colon tissue at day 7. (Insets) Images are from water controls. Blue arrows denote goblet-cell hyperplasia, green arrows indicate regions of severe cellular infiltrate, red arrows indicate hyperplasia of the smooth muscle lining, and black arrows denote areas of edema. (K–N) PAS and Alcian Blue staining of colon tissue at day 7. (O–Q) mRNA expression levels of claudin 1 (Cldn1) (O), mucin 2 (Muc2) (P), and IL-13 (Il13) (Q) in colon tissue at day 7, normalized to β-actin and shown relative to expression levels in WT mice on normal water. (Scale bar, 200 μm.) Data are representative of two independent experiments; n = 4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Strikingly, administration of rIL-33 to WT mice resulted in decreased weight loss (Fig. 4D), reduced colonic shortening (Fig. 4E), and improved clinical disease score (Fig. 4F) compared with PBS-treated mice. In contrast, severe weight loss, profound colonic shortening, and elevated disease score (Fig. 4 D–F) was still evident in IL-33–treated Areg−/− mice, indicating that IL-33–mediated disease amelioration was dependent on AREG. Amelioration of disease did not appear to be associated with enhanced Treg responses, as PBS- and rIL-33–treated WT and Areg−/− mice had equivalent frequencies of Tregs (Fig. S6 B and C), further supporting a role for IL-33 in eliciting an innate AREG–EGFR-dependent tissue-protective pathway in the intestine.
IL-33–Induced AREG Enhances Colonic Mucin Responses.
To investigate the mechanisms by which IL-33 and AREG signaling influences tissue protection, we first examined the colonic crypts by histology. In contrast to PBS-treated animals (Fig. 4 G and K), WT mice given rIL-33 exhibited enhanced goblet-cell mucin responses in the crypts (Fig. 4 H, blue arrows, and L) and restoration of normal colonic architecture without excessive immune cell infiltration. The restoration of crypt architecture and goblet-cell responses were dependent upon AREG, as Areg−/− mice treated with rIL-33 exhibited severe disruption of colonic crypt architecture with poor goblet-cell responses, robust immune cell infiltration (green arrow), edema (black arrow), and smooth muscle hyperplasia (red arrow) (Fig. 4 J and N), similar to Areg−/− mice treated with PBS alone (Fig. 4 I and M). Additionally, gene expression analysis revealed that administration of rIL-33 to WT mice resulted in increased colonic expression of the tight junction protein Claudin 1 (Cldn1) (Fig. 4O) and the secretory mucin gene Muc2 (Fig. 4P) that did not occur in the absence of AREG. Taken together with the histological evidence of AREG-associated goblet-cell hyperplasia, these data suggest one mechanism by which IL-33–induced AREG can influence host tissue protection is by promoting mucus production and tight junctions that aid in limiting inflammation and/or restoring epithelial barrier integrity. The failure to generate mucin responses in the absence of AREG occurred despite heightened IL-13 mRNA levels in the colons of both IL-33–treated WT and Areg−/− mice (Fig. 4Q). Together, these data highlight a role for IL-33–AREG signaling in promoting tissue protection that is associated with enhanced mucin responses.
ILC2-Intrinsic AREG Is Sufficient to Ameliorate Intestinal Disease and Promote Enhanced Mucin Production.
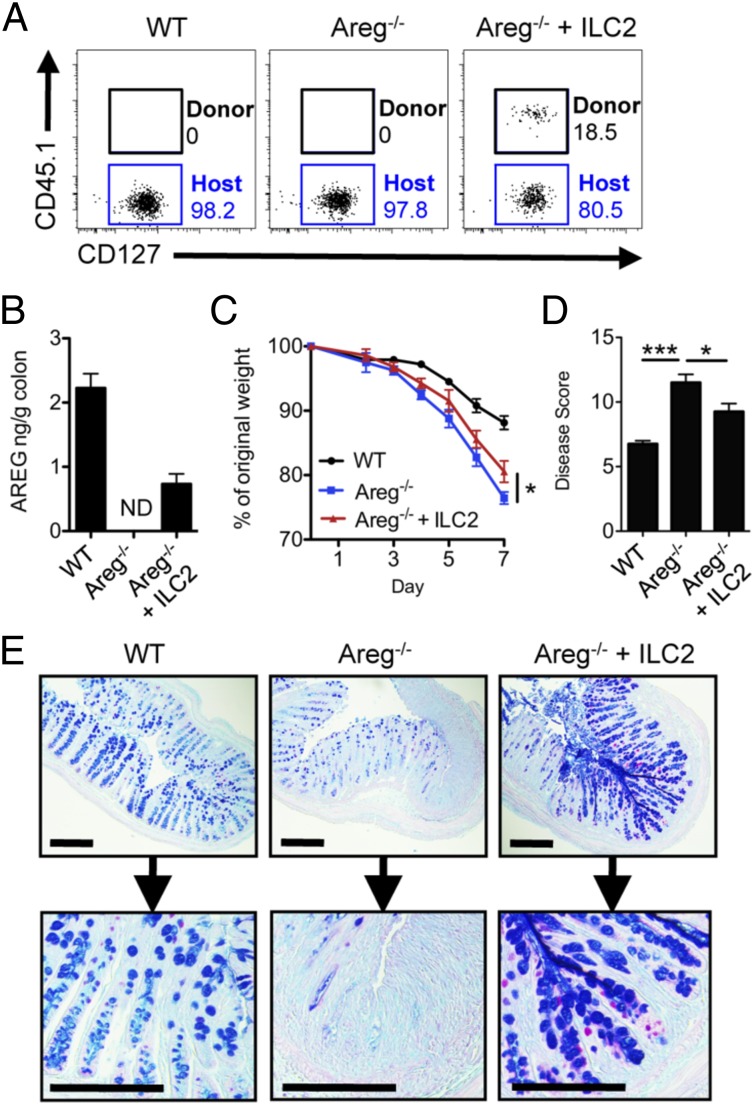
To directly test whether ILC2-intrinsic AREG is sufficient to promote tissue protection, IL-33–elicited ILC2s were transferred into Areg−/− mice during DSS-induced intestinal inflammation. Donor AREG-sufficient ILC2s could be identified in the mLN (Fig. 5A), and production of AREG protein was detected in the colonic tissue of Areg−/− mice receiving transferred ILC2s (Fig. 5B). Transfer of AREG-sufficient ILC2s to Areg−/− mice resulted in moderately reduced weight loss (Fig. 5C), improved clinical disease scores (Fig. 5D), and strongly enhanced goblet-cell mucin production (Fig. 5E) compared with Areg−/− control mice. Collectively, these results demonstrate that expression of ILC2-intrinsic AREG alone can ameliorate intestinal damage and inflammation and is associated with enhanced mucin responses.
Fig. 5.
ILC2-intrinsic AREG is sufficient to ameliorate intestinal disease and is associated with enhanced mucin production. (A–E) IL-33–elicited CD45.1+ ILC2s were transferred into Areg−/− mice during oral exposure to 3% DSS. (A) Frequency of donor CD45.1+ ILC2s in the mLN at day 7 of DSS exposure, gated on Lin− CD45+ CD90+ T1/ST2+ CD25+ cells. (B) AREG protein in colonic tissue at day 7, measured by ELISA. ND, not detected. Weight loss (C) and disease pathology score (D) at day 7. (E) PAS and Alcian Blue staining of colon tissue at day 7. (Scale bar, 200 μm.) Data are representative of three independent experiments; n = 3–4 mice per group per experiment. Data shown are the mean ± SEM; *P < 0.05, ***P < 0.001.
Discussion
Results presented here provide, to our knowledge, the first evidence for a tissue-protective function for ILC2s in the intestinal tract and implicate the IL-33–AREG–EGFR signaling pathway as a critical mechanism by which ILC2s mediate tissue protection during intestinal injury by limiting inflammation and/or promoting epithelial repair. These data identify a critical feedback loop in which cytokine signals from damaged epithelia orchestrate innate immune cell responses and promote expression of growth factors essential for restoration of epithelial barrier function and tissue homeostasis. Although the contribution of epithelial cell-derived EGFR ligands to intestinal tissue repair is well-appreciated (2, 16, 17, 35), our data identifying a novel innate immune component to this growth factor pathway suggest that intestinal tissue protection requires a cooperative response from both hematopoietic and stromal cell lineages that is initiated by rapid damage signals such as IL-33. Notably, AREG-mediated protection was operational in lymphocyte-sufficient and lymphocyte-deficient mice and was not associated with enhanced Treg frequencies, highlighting a previously unappreciated role for the innate immune system in regulating tissue protection in the intestine. However, given the known influence of IL-33 on Treg induction (36) and the ability of Treg-derived AREG to promote muscle regeneration (9), the question of whether ILC2s and Tregs may act cooperatively to regulate AREG-dependent tissue homeostasis remains an open area of investigation.
IL-33 acts a cytokine “alarmin” that is released rapidly upon epithelial cell damage to activate resident immune cell populations of the lung and intestine (11, 34). Our data demonstrate that IL-33 is a potent in vivo stimulus for inducing AREG expression in ILC2s, particularly in gut-associated tissue sites, suggesting that ILC2 functionality may be differentially “licensed” by the local tissue microenvironment. Single-cell analysis revealed that IL-33 elicited distinct populations of polyfunctional ILC2s that expressed IL-5, IL-13, and/or AREG simultaneously, identifying a degree of functional heterogeneity in ILC2s that may reflect their differential roles in regulating proinflammatory versus tissue-protective responses at barrier surfaces. Consistent with this, a previous study using an oxazolone-induced intestinal injury model where type 2 cytokines are known to be pathologic observed that IL-25 elicited IL-13+ ILC2s correlated with disease severity (28), suggesting that under some circumstances ILC2s may serve proinflammatory functions during intestinal injury.
Expression of IL-33 and its receptor ST2 has been shown to be dysregulated in IBD patients (10, 12, 13, 37). However, whether IL-33 plays a tissue-protective or pathologic role in the colonic intestinal mucosa during disease remains controversial (10, 11, 30, 31, 37–39), and the cellular and molecular mechanisms acting downstream of IL-33 to regulate disease are poorly defined. Here we identify the ILC2–AREG–EGFR axis as one mechanism by which IL-33 orchestrates intestinal tissue protection, associated with promotion of AREG-dependent mucin production. IL-33 is a well-appreciated regulator of mucin responses in multiple inflammatory settings (10, 11, 37), but in colitis this process is largely thought to act through Notch- and IL-13–dependent signaling (31, 39). Our data indicate that ILC2-intrinsic AREG is an additional contributor to regulation of mucin responses. AREG signaling in the injured lung has recently been shown to promote epithelial cell differentiation into mucin-producing secretory lineages (40), a paradigm similar to what we observe in the intestine, thereby illustrating the evolutionarily conserved nature of this AREG-dependent tissue-protective pathway at multiple barrier surfaces. The identification of ILC2s as a previously unrecognized hematopoietic cellular source of AREG in the intestine indicates that manipulation of the ILC2–AREG–EGFR pathway could provide therapeutic benefit in the treatment of intestinal inflammatory diseases.
Materials and Methods
Mice.
C57BL/6 WT and CD45.1 mice were purchased from the Jackson Laboratory. Rag1−/− and EGFRvel/+ mice were also purchased from the Jackson Laboratory and bred in-house at the University of Pennsylvania or Weill Cornell Medical College. Areg−/− mice on C57BL/6 background were provided by D.Z. and were then bred in-house. For all DSS experiments, mice either were cohoused littermates (EGFRvel/+ and EGFR+/+ mice) or had shared soiled bedding at least 2 wk before DSS exposure (Areg−/−, C57BL/6 WT, and Rag1−/− mice). Mice used throughout the research were between the ages of 8 and 16 wk, and within each individual experiment, all animals were sex- and age-matched. All mice were maintained in specific pathogen-free facilities at the University of Pennsylvania or Weill Cornell Medical College and all protocols were approved by both institutions’ animal care and use committees.
DSS Administration and Clinical Scoring.
Mice were given 3% (wt/vol) DSS in the drinking water (molecular weight 36,000–50,000, MP Biomedicals). Mice were monitored daily for morbidity (piloerection, lethargy), weight loss, and rectal bleeding. Severity of disease was scored as follows: weight loss (no change, 0; <5%, 1; 6–10%, 2; 11–20%, 3; >20%, 4); feces (normal, 0; pasty, semiformed, 1; sticky, 2; sticky with some blood, 3; completely liquid, bloody, or unable to defecate after 10 min, 4); rectal bleeding (no blood, 0; visible blood in rectum, 1; visible blood on fur, 2); general appearance (normal, 0; piloerect, 1; lethargic and piloerect, 2; lethargic and hunched, 3; motionless and sickly, 4).
In Vivo Cytokine Treatments.
For examination of intracellular cytokine production, 12 μg/kg of recombinant murine IL-33 (carrier-free, R&D Systems) in sterile PBS was administered intraperitoneally (i.p.) into C57BL/6 wild-type mice daily for 6 d, and cytokine expression was assessed on day 7. For treatment during DSS-induced intestinal inflammation, 400 μg/kg of recombinant murine AREG or 12–16 μg/kg recombinant murine IL-33 (carrier-free, R&D Systems) was administered i.p. daily for the duration of the 7 d on 3% DSS. Endotoxin levels were reported to be ≤0.00669 EU/μm.
Additional methods information can be found in SI Materials and Methods.
SI Materials and Methods
Flow Cytometry.
Isolation of cells from mouse lymphoid, lung, and intestinal tissues were performed as previously described (7, 39). Single-cell suspensions were stained with a combination of the following monoclonal fluorescently conjugated antibodies: FITC-conjugated anti-FcεR1; PE-conjugated anti-CD127; PerCP-Cy5.5–conjugated anti-CD3, anti-CD5, anti-NK1.1, anti-CD11b; biotinylated T1/ST2 (IL-33R) (MD Bioproducts); APC streptavidin; eFluor-450–conjugated anti-CD127; PE-Cy7–conjugated anti-CD25; Alexa 700-conjugated anti-CD90.2 (Biolegend); e-Fluor-780–conjugated anti-CD11c, anti-TCRβ, anti-B220; PE-Texas Red-conjugated anti-CD4, anti-CD11b (Invitrogen); eFluor 605NC anti-CD45; eFluor 650NC anti-CD45, and anti-CD4. All antibodies were purchased from eBioscience unless specified otherwise. For measurement of intracellular cytokine expression, cells were isolated ex vivo and stimulated with PMA and ionomycin for 4 h in the presence of brefeldin A as previously described (7). Cells were subsequently surface-stained with a combination of the antibodies listed above, fixed, permeabilized using a commercially available kit (BD Cytofix/Cytoperm, BD Biosciences), and stained with anti-IL-13 FITC (eBioscience), anti-IL-5 PE (eBioscience), and anti-AREG Alexa Fluor 647 [AREG rat anti-mouse monoclonal mAb (clone 206220, R&D Systems) conjugated to Alexa Fluor 647 according to the manufacturer’s instructions (Molecular Probes)]. For measurement of intracellular transcription factor expression, cells were isolated directly ex vivo, stained with antibodies against surface antigens, fixed and permeabilized according to manufacturer’s instructions (Foxp3/Transcription Factor Staining Buffer Set, eBioscience), and stained with anti-GATA3 PE (eBioscience) and anti-Foxp3 eFluor 450 (eBioscience). For all stains, dead cells were excluded from analysis by means of a viability stain (Live/Dead Fixable Aqua stain, Invitrogen). Samples were acquired on a BD LSRII or BD Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (v9.2, Tree Star).
RNA Isolation and Real-Time Quantitative PCR.
For measurement of Areg, Cldn1, Muc2, and Il13 transcripts in whole colonic tissue, 1 cm of colonic tissue distal to the cecum was flushed out with PBS and placed in RNAlater (Qiagen). Tissue was homogenized and cells lysed in RLT buffer, and RNA was isolated using RNeasy mini kit according to the manufacturer’s instructions (Qiagen). cDNA was generated using SuperScript reverse transcription (SSRTII) (Invitrogen). Real-time quantitative PCR was performed on cDNA using SYBR green master mix (Applied Biosystems) and commercially available primer sets (Qiagen). Reactions were run on a real-time PCR system (ABI7500; Applied Biosystems). Samples were normalized to β-actin and displayed as a fold induction relative to expression levels in tissue from WT water-treated control mice.
ELISA.
For measurement of AREG protein in the supernatant of ILC2 in vitro cultures, C57BL/6 WT mice were treated i.p. with 12 μg/kg rIL-33 for 6 d, and Lin− CD45+ CD90+ CD127+ CD25+ T1/ST2+ ILC2s were sort-purified from the lung and mLN using a BD FACSAria. A total of 5,000 ILC2s per well were cultured with recombinant mouse IL-2 (20 ng/mL), IL-7 (20 ng/mL), and IL-33 (50 ng/mL) for 72 h, and supernatants were analyzed using a commercially available kit (R&D Systems). For measurement of AREG protein in whole tissue, 1-cm colon pieces from mice exposed to 3% DSS were homogenized and centrifuged to remove debris. Supernatants were analyzed using a commercially available kit (R&D Systems) and normalized to tissue weight.
ILC2 Adoptive Transfer.
CD45.1+ C57BL/6 WT mice were treated with 12 μg/kg rIL-33 i.p. for 6 d, and Lin− CD45+ CD90+ CD127+ CD25+ T1/ST2+ ILC2s were sort-purified from the colon-draining mesenteric and caudal lymph nodes using a BD FACSAria. ILC2s (1–2 × 105) were transferred i.v. into recipient mice at days 1, 3, and 5 of 3% DSS exposure.
Tissue Histological Sections.
One centimeter of colon tissue distal to the cecum was removed and flushed out with PBS. Colon tissue was fixed in 4% paraformaldehyde and embedded in paraffin, and 5-μm sections were used for staining with H&E or PAS combined with Alcian Blue to detect neutral and acidic mucins. Images were acquired using Nikon Eclipse Ti microscope. Fifteen crypts per section, per mouse were scored on a grade of 0–4 (0 = none; 4 = most severe) for each of the following four pathologic parameters for a maximum score of 16: (1) inflammatory cell infiltration; (2) goblet-cell depletion; (3) mucosa thickening/edema; and (4) destruction of crypt architecture.
Statistical Analysis.
Results represent the mean ± SEM. Researchers were not blinded to experimental groups. Statistical significance was determined by unpaired Student’s t test unless indicated otherwise. Statistical analyses were performed using Prism GraphPad software v5.0. (*P < 0.05; **P < 0.01; ***P < 0.001).
Acknowledgments
We thank members of the D.A. and G. F. Sonnenberg laboratories for discussions and critical reading of the manuscript and the Mucosal Immunology Studies Team of the National Institute of Allergy and Infectious Diseases for sharing expertise and resources. This research is supported by the National Institutes of Health (Grants AI061570, AI095608, AI087990, AI074878, AI095466, AI106697, AI102942, and AI097333 to D.A.; Grant T32AI007532 to L.A.M.); the Crohns and Colitis Foundation of America (D.A.); the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (to D.A.), the Edmond J. Safra Foundation/Cancer Research Institute Irvington Fellowship (to L.C.O.) and the Swiss National Foundation Advanced Research Fellowships (to M.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509070112/-/DCSupplemental.
References
- 1.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 3.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 4.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11(4):289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaiss DM, et al. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314(5806):1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- 9.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair. 2012;5(1):18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Miguel M, González MJ, Quera R, Hermoso MA. Innate immunity modulation by the IL-33/ST2 system in intestinal mucosa. BioMed Res Int. 2013;2013:142492. doi: 10.1155/2013/142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori A, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45(10):999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 13.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sipos F, Molnár B, Zágoni T, Berczi L, Tulassay Z. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: Ulcerative colitis shows specific p53- and EGFR expression alterations. Dis Colon Rectum. 2005;48(4):775–786. doi: 10.1007/s10350-004-0831-5. [DOI] [PubMed] [Google Scholar]
- 15.Sipos F, et al. Regeneration associated growth factor receptor and epithelial marker expression in lymphoid aggregates of ulcerative colitis. Scand J Gastroenterol. 2010;45(4):440–448. doi: 10.3109/00365521003624144. [DOI] [PubMed] [Google Scholar]
- 16.Yan F, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121(6):2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandl K, et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc Natl Acad Sci USA. 2010;107(46):19967–19972. doi: 10.1073/pnas.1014669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartemes KR, et al. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlow JL, et al. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 129(1):191–198.
- 20.Halim TY, Krauss RH, Sun AC, Takei F. 2012. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 36(3):451–463. [DOI] [PubMed]
- 21.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BS, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 28.Camelo A, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol. 2012;47(11):1198–1211. doi: 10.1007/s00535-012-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan L, et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3⁺ regulatory T-cell responses in mice. Mol Med. 2012;18:753–761. doi: 10.2119/molmed.2011.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012;18(10):1900–1909. doi: 10.1002/ibd.22900. [DOI] [PubMed] [Google Scholar]
- 32.Du X, et al. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics. 2004;166(1):331–340. doi: 10.1534/genetics.166.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaiss DM, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38(2):275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le H, Kim W, Kim J, Cho HR, Kwon B. Interleukin-33: A mediator of inflammation targeting hematopoietic stem and progenitor cells and their progenies. Front Immunol. 2013;4:104. doi: 10.3389/fimmu.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan K, Arnone B, Buchman A. Intestinal growth factors: Potential use in the treatment of inflammatory bowel disease and their role in mucosal healing. Inflamm Bowel Dis. 2011;17(1):410–422. doi: 10.1002/ibd.21316. [DOI] [PubMed] [Google Scholar]
- 36.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513(7519):564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastorelli L, De Salvo C, Cominelli MA, Vecchi M, Pizarro TT. Novel cytokine signaling pathways in inflammatory bowel disease: Insight into the dichotomous functions of IL-33 during chronic intestinal inflammation. Therap Adv Gastroenterol. 2011;4(5):311–323. doi: 10.1177/1756283X11410770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedhom MA, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62(12):1714–1723. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imaeda H, et al. Interleukin-33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium-induced colitis. Int J Mol Med. 2011;28(4):573–578. doi: 10.3892/ijmm.2011.718. [DOI] [PubMed] [Google Scholar]
- 40.Manzo ND, Foster WM, Stripp BR. Amphiregulin-dependent mucous cell metaplasia in a model of nonallergic lung injury. Am J Respir Cell Mol Biol. 2012;47(3):349–357. doi: 10.1165/rcmb.2011-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]