Significance
Many have argued that major developments in mammalian (including human) evolution were timed with large and sudden changes to Earth’s climate. Our new analyses of the eastern African Plio-Pleistocene mammalian fossil record indicate that most species originations and extinctions took place continuously and gradually. This means that evolution was not clustered in short intervals, nor were sudden global climatic changes the main cause of species extinction in the past. Global climate may have influenced longer-term (million year) evolutionary trends, but local environmental changes and species interactions were more important at shorter (100,000 y) time scales.
Keywords: turnover, Plio-Pleistocene, mammals, Bovidae, Africa
Abstract
Much debate has revolved around the question of whether the mode of evolutionary and ecological turnover in the fossil record of African mammals was continuous or pulsed, and the degree to which faunal turnover tracked changes in global climate. Here, we assembled and analyzed large specimen databases of the fossil record of eastern African Bovidae (antelopes) and Turkana Basin large mammals. Our results indicate that speciation and extinction proceeded continuously throughout the Pliocene and Pleistocene, as did increases in the relative abundance of arid-adapted bovids, and in bovid body mass. Species durations were similar among clades with different ecological attributes. Occupancy patterns were unimodal, with long and nearly symmetrical origination and extinction phases. A single origination pulse may be present at 2.0–1.75 Ma, but besides this, there is no evidence that evolutionary or ecological changes in the eastern African record tracked rapid, 100,000-y-scale changes in global climate. Rather, eastern African large mammal evolution tracked global or regional climatic trends at long (million year) time scales, while local, basin-scale changes (e.g., tectonic or hydrographic) and biotic interactions ruled at shorter timescales.
Modern faunal communities are the products of millions of years of evolutionary and ecological turnover, shaped by species origination, extinction, and migration. The primary driver of turnover at geological time scales is generally taken to be physical environmental change, but the importance of biotic interactions in modulating turnover is increasingly evident (1–5). Related to this has been a debate on whether faunal turnover is continuous or pulsed in mode. Building on Mayr’s (6) allopatric speciation model, and Eldredge and Gould’s (7) hypothesis of punctuated equilibrium, Vrba (8–11) proposed the turnover pulse hypothesis, which predicts that most faunal turnover is concentrated in pulses that are correlated with rapid environmental changes (see ref. 1 for a review of similar ideas). Outside of mass extinctions (these may be seen as extreme but uncommon turnover pulse events), several studies have found support for the turnover pulse hypothesis as a dominant mode of turnover (12–14). Other studies—including many of the African record—have concluded that the dominant mode of turnover was continuous, with limited or no evidence for turnover pulses (15–20). Some of these studies argued for an important role for biotic interactions (e.g., competition, predation) in shaping the often unpredictable responses of faunal communities to environmental change. More-direct evidence for the long-term influence of biotic interactions on turnover in the fossil record has been presented in the form of constant extinction likelihoods (21, 22) and symmetric wax−wane occupancy curves at several taxonomic levels (23–26). These findings suggest that biotic interactions can strongly determine the outcomes of the turnover process, even if physical environmental changes are important in initiating turnover under the allopatric speciation model (8, 10).
We here revisit the tempo and mode of evolutionary and ecological change in the eastern African Plio-Pleistocene large-mammal fossil record using updated data and approaches. Eastern Africa (here including Eritrea, Ethiopia, Kenya, Tanzania, and Uganda) today encompasses primarily a single vegetational zone [Somalia-Masai (27)], and constitutes a reasonable subcontinental scale at which to examine turnover (1). We examine the fossil record for the occurrence of pulsed evolutionary events (here large-scale and rapid changes in turnover rates, relative abundances, or body size) based on expectations from changes in global climate. In particular, there are two temporal intervals during which elevated mammalian speciation rates have been reported in close timing with major global climatic changes, at 3–2.5 Ma (28, 29) and 2–1.5 Ma (reviewed in ref. 30). The first putative peak coincides with rapid global cooling and African aridification, and the second coincides with the inception of Walker Circulation above the Pacific (28–32). Further attention is drawn to these temporal windows given major developments in the hominid clade, including the appearance of Homo, Paranthropus, and Oldowan stone tools during the first, and the development of Achulean technology and the dispersal of Homo erectus from Africa in the second (30, 33).
We compiled and analyzed updated fossil specimen datasets of eastern African bovids (antelopes and relatives) and of Turkana Basin large mammals (see SI Text for full details). Older datasets have been used to investigate modes of turnover in African mammals, with often contradictory results (17, 29).
Turnover in Eastern African Plio-Pleistocene Mammals
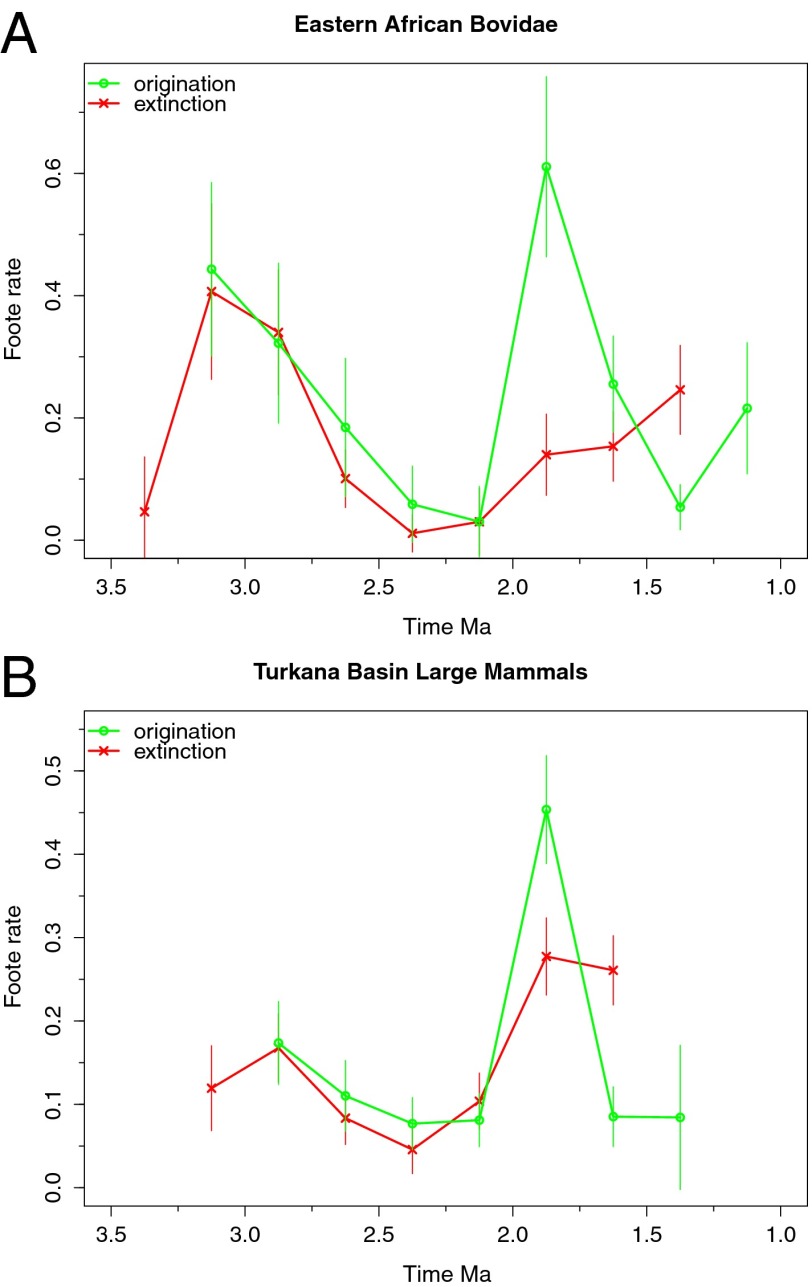
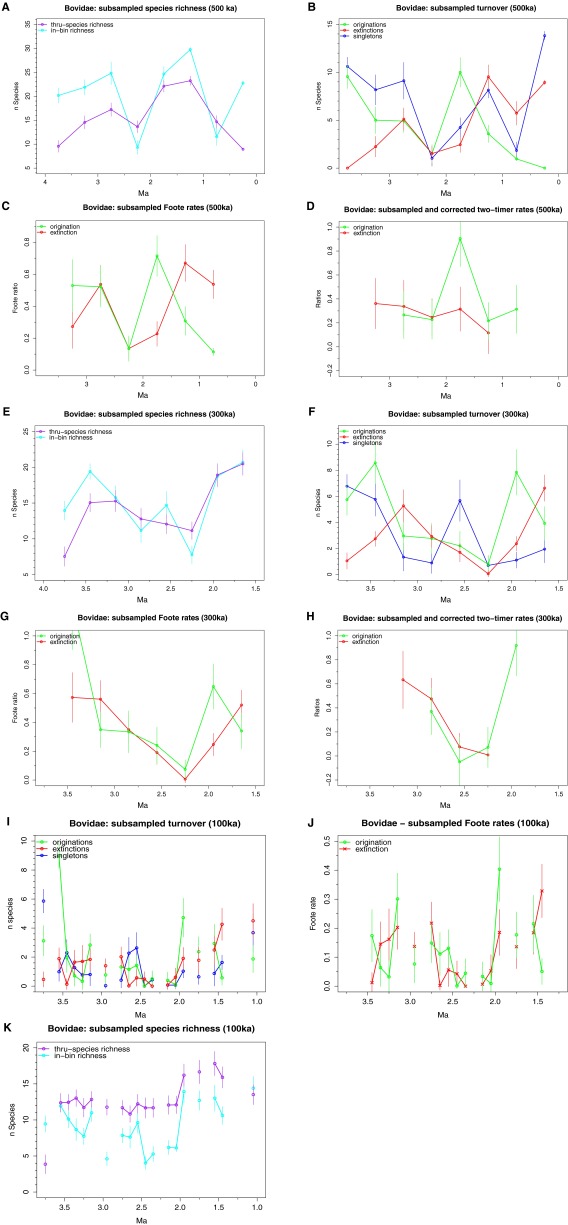
Although we included sites from late Miocene to Holocene age in our data compilation, we focus our turnover analyses on the period between 3.75 Ma and 1.25 Ma (late Pliocene−early Pleistocene) because sample sizes and sampling completeness are too poor outside this interval and edge effects become prevalent when including older and younger bins (see SI Text and Figs. S1 and S2). In both the bovid and Turkana datasets, we find that speciation and extinction rates were highly variable through time (Fig. 1). We used 250-ka time bins, but results for 100-, 300-, and 500-ka bins are similar (Fig. S3). Elevated turnover rates are recorded at 3.25–2.75 Ma in the bovid data, and at 2.0–1.75 Ma in both the bovid and Turkana data. Only the origination peak in the Turkana data at 2.0–1.75 Ma is of statistically significant magnitude (more than 1.5 times outside the interquartile range). Two-timer counts (34) provide similar results, and subsampled species richness also indicates increases at 2.0–1.75 Ma in both datasets (Figs. S1 and S2).
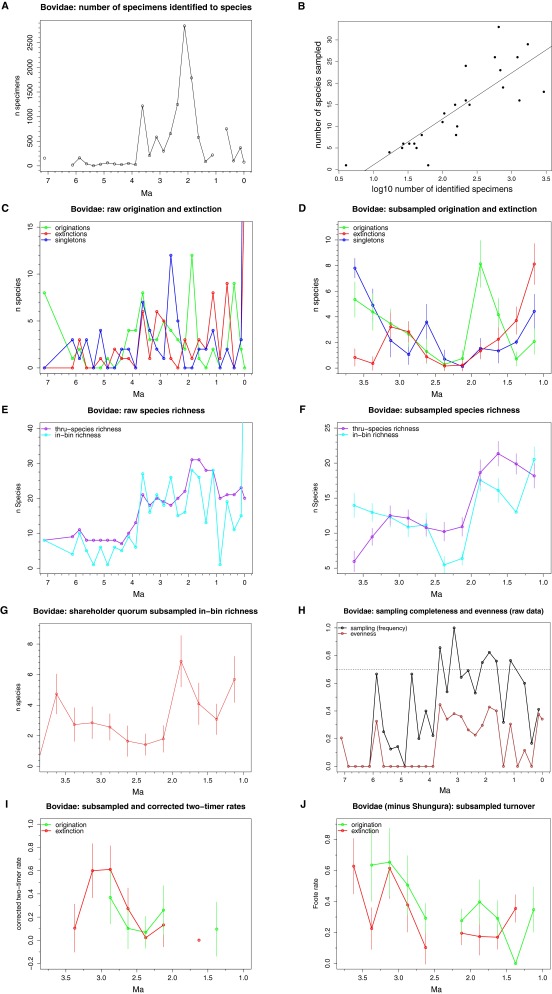
Fig. S1.
Eastern African bovid dataset. (A) Number of specimens identified to species by time bin. (B) Relationship between number of specimens and in-bin species richness in the raw data (R2 = 0.68). (C) Raw and (D) subsampled origination, extinction, and singleton values. Note the large singleton peaks at 3.75–3.5 Ma and 2.75–2.5 Ma, a main sources of Vrba’s (29) bovid origination pulses. (E) Raw species richness based on sampled-in-bin and range-through counting methods. (F) Subsampled mean (±SD) species richness. (G) In-bin mean (±SD) species richness subsampled using the shareholder quorum method. (H) Sampling completeness and evenness in the raw data. At an arbitrary 70% cutoff (dashed line), only the 3.75–1.5 Ma series approaches sufficient sampling quality for analysis. (I) Subsampled and corrected two-timer rates. (J) Subsampled Foote origination and extinction rates calculated with the Shungura Formation excluded. Note significant attenuation of the 2.0–1.75 Ma origination peak.
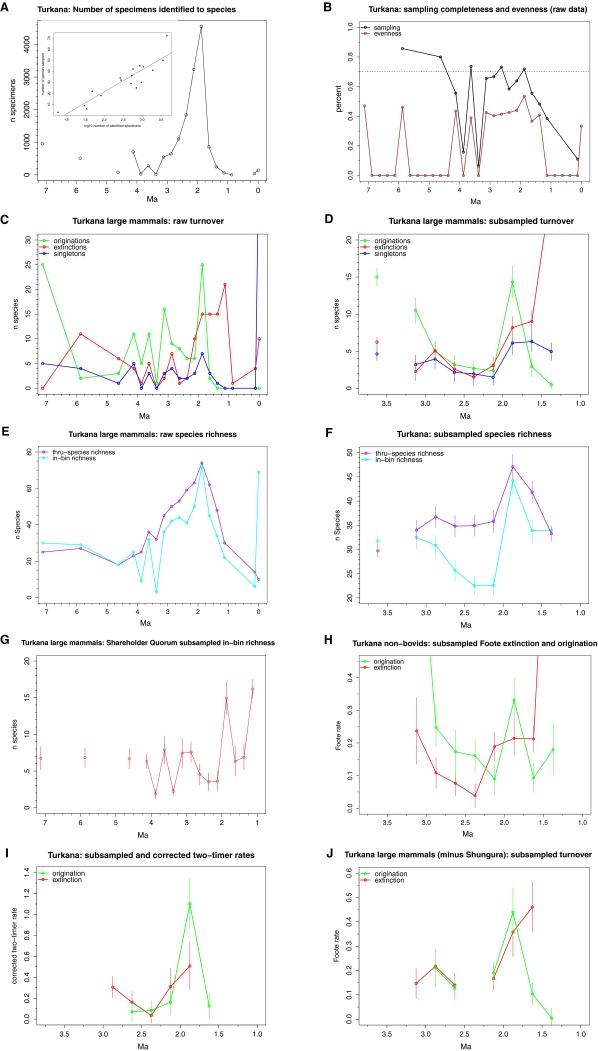
Fig. S2.
Turkana Basin large mammal dataset. (A) Distribution of specimens identified to species, and the relationship between number of specimens and in-bin species richness in the raw data (Inset, R2 = 0.60). (B) Raw sampling completeness and evenness. At an arbitrary 70% cutoff (dashed line), only the 3.25–1.5 Ma series approaches sufficient sampling quality for analysis. (C) Raw origination, singleton, and extinction values. (D) Subsampled origination, singleton, and extinction values. (E) Raw species richness. (F) Subsampled mean (±SD) species richness. (G) In-bin species richness as subsampled using the shareholder quorum method. (H) Subsampled Foote origination and extinction rates calculated with bovids excluded. (I) Subsampled and corrected two-timer rates. (J) Subsampled Foote origination and extinction rates calculated with Shungura Formation excluded.
Fig. 1.
Sampling-standardized origination and extinction rates in (A) eastern African Bovidae and (B) Turkana Basin large mammals. Older origination and younger extinction rates are increased by edge effects, and the oldest and youngest points, respectively, are omitted. Elevated turnover is recorded at 3.25–3.0 Ma (bovids) and 2.0–1.75 Ma (both). Turnover is low between 3 Ma and 2 Ma. (Error bars, 1 SD of 100 subsampling trials in each direction.)
Fig. S3.
(A) Subsampled (±SD) species richness for Bovidae using 500 ka bins. (B) Subsampled turnover using 500 ka bins. (C) Subsampled Foote rates using 500 ka bins. (D) Subsampled and corrected two-timer rates using 500 ka bins. (E) Subsampled (±SD) species richness for Bovidae using 300 ka bins. (F) Subsampled turnover using 300 ka bins. (G) Subsampled Foote rates using 300 ka bins. (H) Subsampled and corrected two-timer rates using 300 ka bins. (I) Subsampled turnover using 100 ka bins. (J) Subsampled Foote rates using 100 ka bins. (K) Subsampled species richness using 100 ka bins.
Vrba (29) found evidence for statistically significant origination (and possibly extinction) pulses at 3.6 Ma, 2.7–2.5 Ma, and 1.8 Ma. A highly discontinuous fossil record before 3.75 Ma means we are unable to properly assess the record older than 3.5 Ma. However, we find no evidence for origination or extinction pulses anytime between 3.0 Ma and 2.5 Ma. Two main differences between our study and Vrba’s are the exclusion of the North and South African records and the exclusion of single-interval species in our study. The North and South African records are sparse but include a large number of sites dated between 3 Ma and 2.5 Ma (29), potentially inflating turnover counts at this time. Additionally, Vrba’s (29) original first appearance datum (FAD) pulse at 2.7–2.5 Ma included a large number of single-interval species (also found here; Fig. S1). Vrba (35) argued that single-interval taxa are important because environmental perturbations are expected to produce a large number of rare and short-lived species. However, simulations show that single-interval taxa are especially sensitive to preservational biases and lead to spurious correlations between origination and extinction rates (16, 36). No similar peak in single-interval taxa is seen in the Turkana Basin large-mammal record (Fig. S2; see also refs. 17, 37), nor in range compilations that retain singletons such as for suids (15, 38), carnivores (19, 39), or cercopithecids (20). This suggests that the single-interval taxon peak at 2.75–2.5 Ma, if real, is restricted to the bovid fossil record.
Subsampled species richness is also more or less stable at 3.25–2.0 Ma in both the bovid and Turkana datasets (Figs. S1 and S2). Global cooling and drying between 3 Ma and 2.5 Ma may have promoted the development of more open habitats and associated faunal communities (28, 33, 37, 40, 41), but our results indicate that such evolutionary and ecological changes took place gradually and without significant increases in turnover rates (42). This would fit with paleoclimate reconstructions indicating that global cooling and the end of the Pliocene warm period were not confined to between 3 Ma and 2.5 Ma but rather occurred gradually over a protracted time between about 4 Ma and 1.5 Ma (31).
The origination peak recovered here at 2.0–1.75 Ma does match Vrba’s 1.8 Ma pulse. Elevated origination rates and associated increases in sampling-standardized species richness shortly after 2 Ma (cf. refs. 43 and 44) coincide with the expansion of C4 grasslands as recorded in Turkana paleosol isotopes (45), Gulf of Aden pollen and plant biomarker records (40, 46), greater regional aridity through increased wind-borne dust in the Arabian Sea (32), and increases in open habitat faunal communities in both eastern and southern Africa around this time (47). These environmental and faunal changes may be related to the development of Walker Circulation above the Pacific Ocean around 1.9 Ma and resulting precipitation decreases over eastern Africa (31, 46, 48). Alternately, the origination pulse at 2.0–1.75 Ma may be an artifact of the record, because very few sites are known from the preceding interval, and the eastern African 2.5–2.0 Ma record is almost entirely dominated by the Shungura Formation. Removal of the Shungura Formation sharply reduces the origination pulse in the eastern African bovid data, but not the Turkana large mammals, suggesting the pulse may be even less prominent outside the Turkana Basin (Figs. S1 and S2). Further testing will require the improvement of the 2.5–1.5 Ma fossil record from outside the Turkana Basin (e.g., in the Afar).
Besides a possible origination pulse at 2.0–1.75 Ma, we find that most faunal change in the late Pliocene and early Pleistocene took place under variable turnover without unequivocal pulses. Although differing in taxonomic scope and geographic scale, the bovid and Turkana large-mammal datasets provide very similar results, and these furthermore match those of numerous studies cited above. All these studies, however, are heavily dependent on the Turkana Basin record, and further fossil data outside this basin is needed to be more certain that the pattern seen reflects a regional (if not continental) scale.
Species Duration and Occupancy Patterns
We examined whether the probability of extinction, measured through mean species durations (or residence times), varied among clades of varying ecological attributes. In particular, mammalian resource-use specialists, i.e., species dependent on a narrow range of environments (8, 49, 50), and herbivores (51) have been shown to have higher diversification rates than resource-use generalists and carnivores, respectively. Specialists and herbivores might therefore be expected to have shorter species durations if their ecological requirements left them more susceptible to abrupt environmental changes.
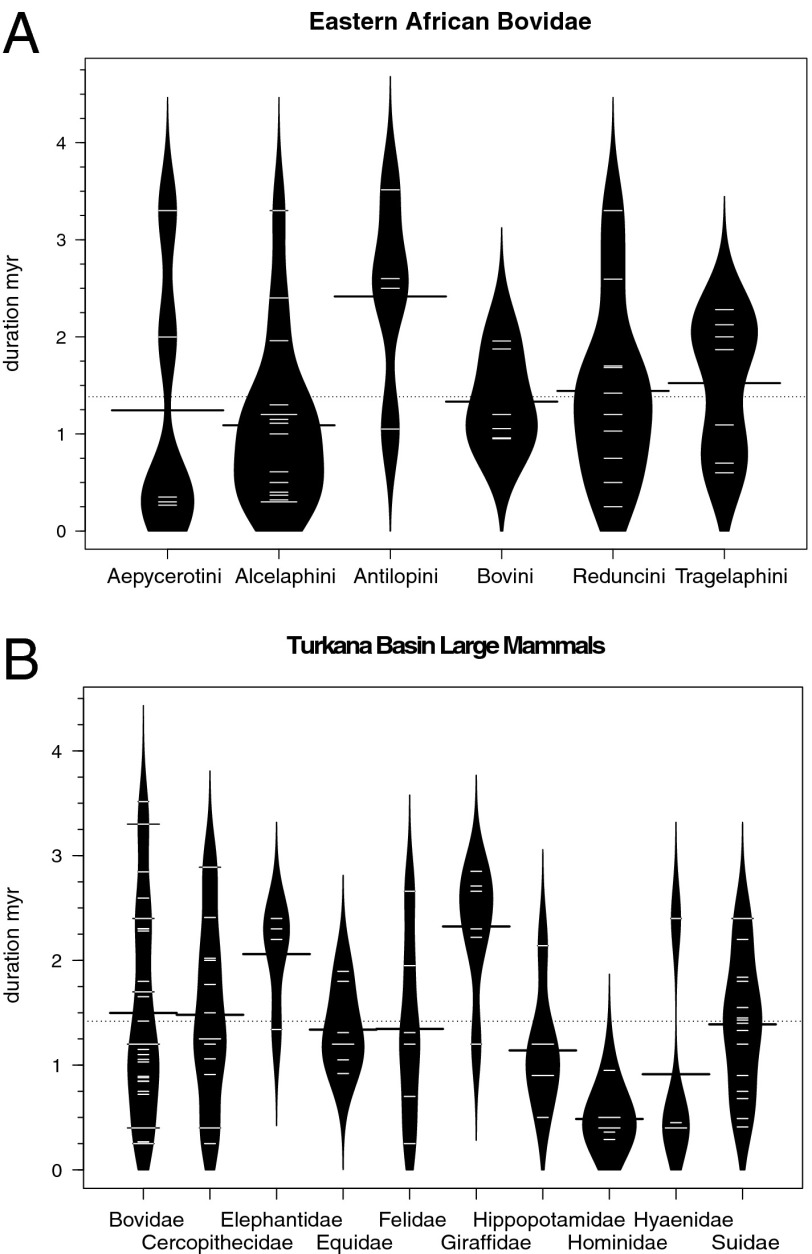
We find mean species duration of 1.4 ± 0.9 my (median = 1.2 My) for both eastern African bovids and Turkana Basin large mammals (Fig. 2). This is similar to the 1.3-My mean species duration of Neogene African large mammals (52) and the 1.5-My median species duration for North American Cenozoic mammals (16). Pairwise comparisons indicate that species durations are statistically indistinguishable among aepycerotin, alcelaphin, bovin, reduncin, and tragelaphin bovids (pairwise Wilcoxon, P > 0.1). Among Turkana large mammal families, species durations are statistically indistinguishable among bovids, cercopithecids, equids, felids, hyaenids, suids, and hippopotamids (P > 0.09). This implies that similar probabilities of species persistence and extinction apply across large mammal communities, regardless of habitat (woodland vs. grassland) or dietary (herbivory vs. carnivory) preferences, and fits expectations for continuous turnover in interconnected biotic networks (e.g., food webs).
Fig. 2.
Beanplots of species durations, arranged by tribe for eastern African bovid species (A) and by family for Turkana Basin large mammals (B). Durations among most bovid tribes and most Turkana families are statistically indistinguishable. Black lines indicate means for each clade, and dotted lines indicate the mean for the whole plot.
Significantly (P < 0.05) longer species durations were found for antilopins, elephantids, and giraffids and shorter durations in hominids, but these signals are likely to be artifacts of taxonomic practices rather than true biological differences. In particular, hominid species are highly susceptible to taxonomic splitting (53), especially in the current dataset. The inverse is probably true of antilopins, giraffids, and elephantids, which are rare and/or not well studied in the current datasets.
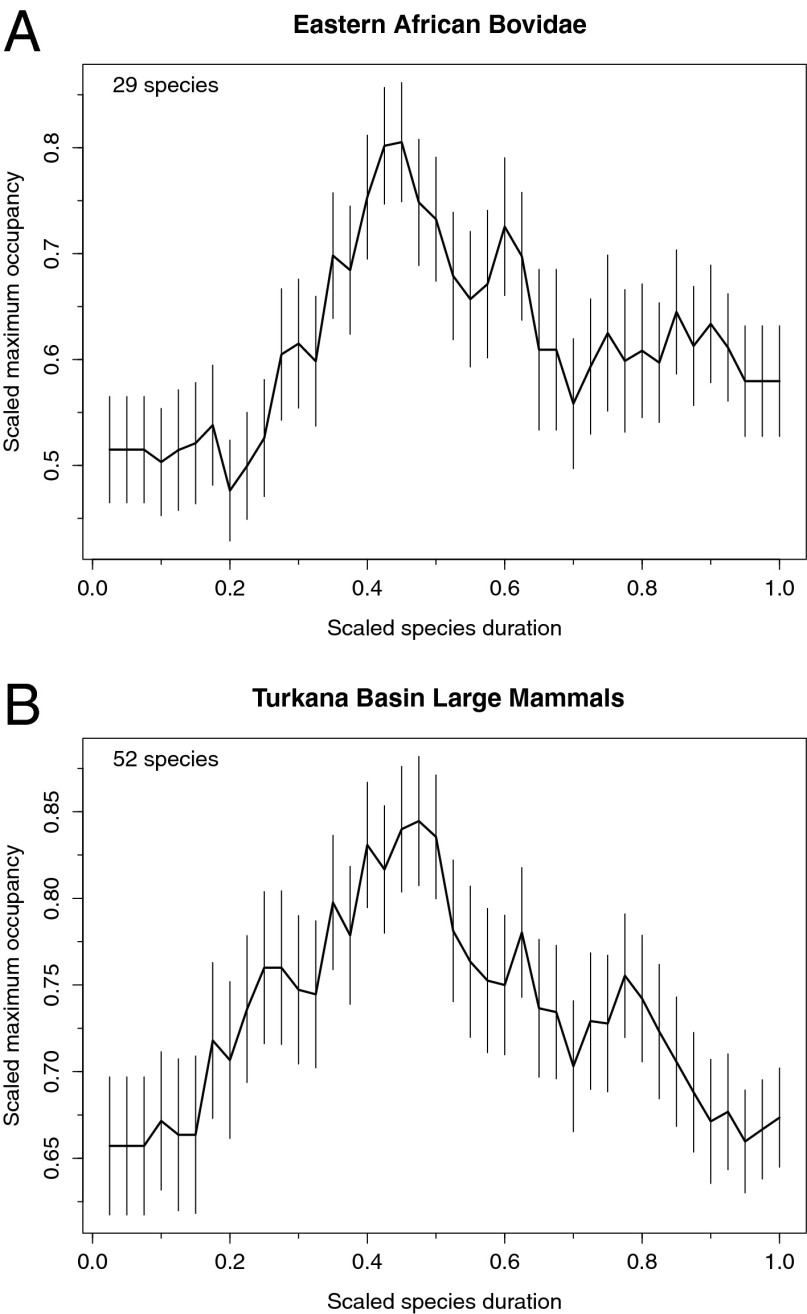
Additionally, we examined species occupancy, the number of sites at which a fossil species is recorded over its duration (e.g., Hadar, Omo, Kanapoi, Laetoli; see SI Text). Under continuous origination and extinction, species occupancy should show roughly symmetrical increase and decline phases (24–26, 54, 55). Pulsed extinction dynamics would be expected to produce asymmetrical patterns, with rapid truncations of species increase, equilibrial, or decline phases (25).
The occupancy trajectories we calculated (Fig. 3) show that, on average, species display a unimodal wax-and-wane pattern with long growth and decline phases, and no signs of long-term equilibrium or stasis (bovids do not differ from normal, Shapiro−Wilk test P = 0.23, but Turkana data do, P = 0.046). The decline phase is slightly longer than the growth phase, as also found for marine microfossils (55). Vrba and DeGusta (56) showed that newly originating African mammal species gradually increased their occupancy (over about 1 Ma), but they did not examine the mode of species decline. Our findings establish the presence of unimodal occupancy curves with long and gradual origination and extinction phases. Even a model in which the initiating causes of origination and extinction require physical environmental change (8, 10, 28) would then have to allow for turnover under conditions of incremental and continuous (background-level) landscape change. Most turnover in the eastern African Plio-Pleistocene would have occurred at these background rates. Such wax−wane patterns also implicate biotic interactions in generating diversity-dependent turnover (23–25). Combining biotic interactions with continuous landscape change provides a powerful mechanism for species turnover, because even small environmental changes could have profound ecological consequences when amplified through food webs (57).
Fig. 3.
Geographic occupancy curves of extinct species scaled from origination (0) to extinction (1) for (A) bovids and (B) Turkana Basin large mammals. Occupancy was measured as the proportion of sites where a species has been encountered and is scaled to the maximum occupancy for each species. (Error bars, 1 SD in each direction for 1,000 bootstrap replicates.)
Relative Abundance of Arid-Adapted Bovids
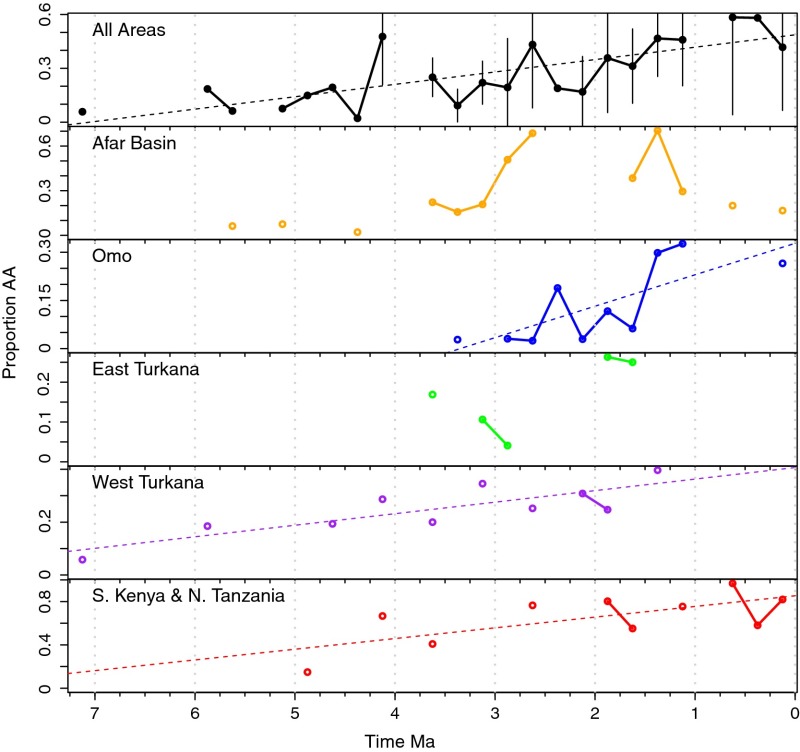
In modern African ecosystems, the relative abundances of monophyletic bovid tribes vary according to habitat (58), reflecting primary climatic variables such as temperature and precipitation (59). In particular, the relative abundance of specimens of Alcelaphini plus Antilopini (AA) among all bovids serves as a relative measure of aridity or open habitats at African fossil sites (58, 60). Vrba found that the proportions of AA bovids in the African record increased significantly after ∼2.5 Ma, signaling a shift to more arid and open conditions (28, 58, 61). Later studies indicated that this trend extended to between 3.5 Ma and 1 Ma, with changes at different times in different areas (44, 47, 62).
An expanded compilation of AA data (Fig. 4) indicates a significant long-term increase across eastern Africa since at least the late Miocene (P < 0.001). When broken down by site, the trend is significant in the Omo (P = 0.01), West Turkana (P = 0.002), and southern Kenya and northern Tanzania (P = 0.02). Except perhaps for the Afar, there is no evidence for a pulsed increase in AA associated with rapid global cooling between 3 Ma and 2.5 Ma. Rather, increases in AA bovids are part of a long-term trend that may be correlated with global or regional climatic changes such as decreasing mean global temperatures (63) or regional drying through continental uplift/rifting (64) since the late Miocene. Differences in the rate and directionality of change in AA from one area to another, however, implicate local environmental factors (e.g., tectonic−hydrological) in modulating the timing and rate of local landscape change at any point in time (65, 66). Additionally, a consistently higher proportion of AA in southern Kenyan and northern Tanzanian sites reflects a long-term lack of perennial water sources there (e.g., ref. 67), as opposed to the more stable Omo and Awash rivers to the north. The same might be said of the predominantly karstic South African record as well, where greater than 50% of the bovid assemblages at sites like Langebaanweg (∼5 Ma) and Sterkforntein (2.5 Ma) are made up of alcelaphins and antilopins (60, 68, 69). The most interesting AA record comes from the Afar Basin. In contrast to all other areas, there is a rapid increase between 3 Ma and 2.5 Ma that is followed by a reversal to lower values (better watered habitats) in the early and mid Pleistocene. In the Afar Basin at least, local hydrological changes appear to have been more important than global climate in modulating the timing and rate of local landscape change (65).
Fig. 4.
Relative abundance of AA among eastern African areas and for time bins with at least 40 specimens identified to bovid tribe. Significant (P < 0.05) long-term increasing trends are shown by regression lines.
Changes in Bovid Body Mass
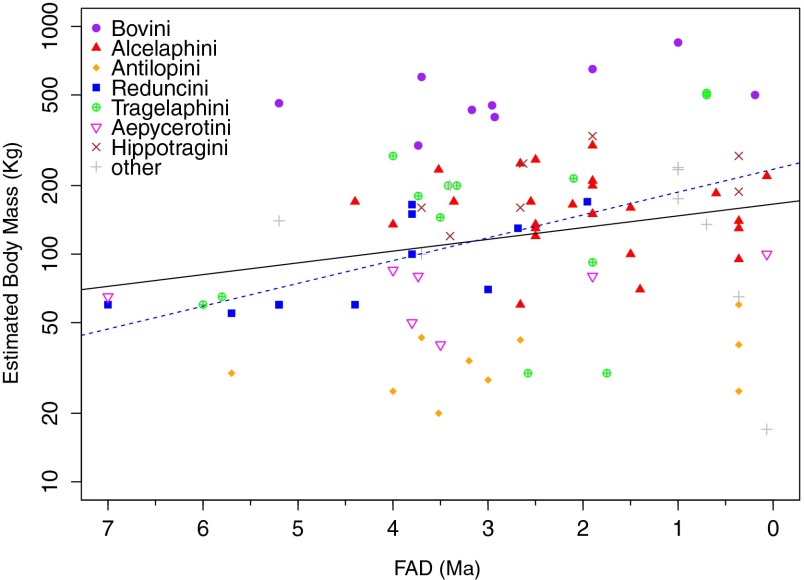
Citing Bergmann’s Rule, Vrba (29, 70, 71) proposed that a significant number of large-bodied bovid species appeared in Africa between 3 Ma and 2.5 Ma in response to pronounced global cooling. We find that the mean body mass of eastern African Plio-Pleistocene bovid species increased gradually through time (P = 0.048) but with large scatter (Fig. 5). Comparisons of median body size across a moving breakpoint indicates that species originating earlier than 3.75 Ma, 3.5 Ma, 3.25 Ma, or 3.0 Ma are significantly (P < 0.05) smaller than those appearing later, but the comparison is no longer significant at or after 2.75 Ma (up to 1.25 Ma, P > 0.05). Therefore, whereas our methods differ from those of Vrba (71) and do not consider within-species increases, we do not see a disproportionate number of first appearances of large-bodied bovid species between 3 Ma and 2.5 Ma.
Fig. 5.
Estimated body mass of extinct eastern African bovid species, plotted by FAD. There is a significant trend to body size increase when all bovids are considered together (black solid line). By tribe, only Reduncini (blue dashed line) show a significant (increasing) trend. Note log scale of y axis.
Furthermore, when broken down by tribe, only Reduncini show an increasing trend (P < 0.01, R = 0.15). Removal of Reduncini removes the trend for all bovids as well. Large body size confers dietary, not to mention defensive, advantages to open grassland ungulates (72), so one might have expected Plio-Pleistocene increases in body size to have been greatest among inhabitants of open grasslands (e.g., Alcelaphini, Antilopini). Observed increases were therefore lineage specific, were not determined by the expansion of open habitats or rapid global temperature changes, and are probably indistinguishable from broader phenomena grouped under Cope’s Rule, which has many possible explanations (e.g., ref. 73).
Conclusions
We originally set out to test whether faunal change in eastern Africa was pulsed and timed with major global climate change events. Instead, our results show that species turnover was mainly continuous. The only exception may be an origination pulse shortly after 2.0 Ma (29), which might be associated with the inception of Walker Circulation above the southern Pacific (30, 31). This should be further tested through the recovery of more fossil assemblages in the 2.5–1.5 Ma interval, particularly from outside the Turkana Basin.
In the paleontological record, continuous turnover, consistent species durations, and nearly symmetrical wax−wane patterns of geographic occupancy most likely reflect a continuum of physical and biotic processes acting at multiple scales (1, 5, 55). The initiation of turnover might always require changes in the physical environment (10), but it seems that continuous (background-level) environmental changes may have been sufficient for this. Physical changes probably acted through biotic networks, and even small changes to a taxon’s physical environment may have been amplified or attenuated through the responses of the other species around it (4, 5). The Rift Valley is a tectonically dynamic environment, and this serves to produce a diverse patchwork of habitats within close proximity. From the point of view of the Red Queen’s hypothesis, which predicts the constant deterioration of an organism’s effective environment (21), environmental dynamics within the African Rift should promote constant taxonomic shifts, and increase the potential for population fragmentation leading to allopatric or peripatric speciation.
We conclude that global climate drove large mammal evolution at the million-year timescale, whereas local environmental changes and biotic interactions ruled at 100-ka and smaller scales. Much work during the last decades has (rightly) focused on the initiating role of physical drivers on the evolution of African mammals. As the fossil record continues to improve in both taxonomic and chronological resolution, the modulating role of biotic drivers should be increasingly investigated and integrated into the broader picture of community turnover.
Materials and Methods
The eastern African bovid and Turkana Basin large-mammal specimen databases were assembled from numerous sources, with the largest contributions from the Turkana Public Database (74), the International Omo Research Expedition and Omo Group Research Expedition databases (both courtesy of J.-R. Boisserie), and Middle Awash (courtesy of T. White), and data from the literature (e.g., Hadar, Laetoli, Olduvai; see SI Text), with many updates based on specimen study by F.B. In total, these comprise 134 bovid species (77 non-single-interval taxa) (Dataset S1) and 172 Turkana large mammal species (130 nonsingletons) (Dataset S2). Turnover analyses used repeated specimen-based subsampling (rarefaction) and equal-coverage shareholder quorum methods (76) to standardize sample sizes among time bins. Calculations of turnover rates followed Foote (36), and those of two-timer and shareholder quorum counts followed Alroy (34, 75, 76). Calculation of species occupancy followed Foote (54). Each species duration was rescaled to 0 (origin) and 1 (extinction) and binned into 40 intervals of equal duration. Occupancy was measured as the proportion of fossiliferous sites in which the species was encountered and also scaled between 0 (minimum occupancy) and 1 (maximum). Species durations and AA counts were calculated on raw data. Body mass estimates were made using molar tooth length regressions of Damuth (77) and Janis (78) with measurement data from the literature or taken by F.B. Full methods are provided in SI Text.
Eastern African Bovidae Dataset
The specimen database of eastern African Bovidae was assembled from the following sources, organized by study area.
East and West Turkana, Kenya.
The Turkana Public Database (74) (www.mnh.si.edu/ete/ETE_Datasets_Turkana.html, version of 21 January 2011, accessed July 2012), including identifications from numerous published sources. Updates were made based on personal observations by F.B. on bovid specimens at the National Museums of Kenya. West Turkana includes the Nachukui Formation, Lothagam, and Kanapoi. East Turkana includes the Koobi Fora Formation.
Lower Omo Valley, Ethiopia (Omo).
The Shungura Formation bovids from collections of the International Omo Research Expedition (IORE, both French and American teams), as identified by Gentry (79) and from the Omo Group Research Expedition as identified by F.B. These data were kindly provided to us by J.-R. Boisserie; the IORE database is the same as that used for direct management of the Omo collections in Addis Ababa from 1992 to 1997, and updated by J.-R. Boisserie since that time.
Mursi bovids (80).
Kibish bovids (81).
Afar Basin (Including Awash River Basin).
Published bovid specimens from the Middle Awash (e.g., refs. 82–84), and collections studied by F.B. from the Lower and Upper Herto members. Data were kindly provided by T. White.
Hadar bovids (85).
Woranso-Mille bovids (86).
Bovids from Ledi Geraru studied by F.B.
Bovids from the Pleistocene Boolihinan, Dahuli, and Dark Paleosol beds at Gona (65).
Melka Kunture bovids (87).
Asbole bovids (88).
Buia (Eritrea) bovids (89).
Northern Tanzania and Southern Kenya.
Olduvai bovids from the Comprehensive Olduvai Database Initiative (Hlusko and Njau, olduvai-paleo.org, accessed August 2012), comprising published bovid specimens (e.g., refs. 90 and 91), many of these studied by F.B.
Manonga bovids (94).
Late Pleistocene bovids of the Wasiriya (Rusinga) and Warware (Mfangano) Beds (e.g., refs. 95–97).
Lainyamok bovids (98).
Other Sites.
Turkana Basin Large Mammal Dataset
The Turkana Basin large-mammal specimen dataset comprises East Turkana, West Turkana, and the Omo. Bovids are as noted above, whereas nonbovids derive from the Turkana Public Database (www.mnh.si.edu/ete/ETE_Datasets_Turkana.html), the Omo Shungura database of the IORE American expeditions (1967−1976, provided to F.B. by J.-R. Boisserie), and the Mursi Formation fauna (80). East Turkana carnivores were updated following Werdelin and Lewis (102, 103) and Werdelin (104). All nonmammals and small mammals (e.g., Herpestidae, Viverridae, Rodentia, Lagomorpha, Chiroptera, Afrosoricida, Macroscelidea, Soricomorpha, Galagidae) were excluded from our analyses.
Processing
All fossil data were examined and corrected for spelling errors. Taxonomy was standardized, especially for indeterminate identifications (cf., aff., ?), and updated for synonyms and reidentifications. Indeterminate species identifications (cf., ?) were not included, whereas taxa identified as aff. genus or species, or species A, B, etc., were retained as separate species (Datasets S1 and S2). Species analyses in both the Turkana and the bovid datasets used only specimens securely identified to species level and with geochronological age assignments (ca. 11,500 in the Bovidae database and ca. 16,000 in the Turkana Basin database, of which ca. 8,500 are nonbovids). Calculations of relative proportions of bovid tribes used all bovid specimens in the Bovidae dataset securely identified to tribal level (ca. 24,000). Extant African large mammals were added to both datasets at one specimen per species and their frequencies multiplied according to their frequency distributions in the fossil data.
Species and Chronospecies
In paleontology, species are defined on the basis of diagnostic morphological characteristics, usually informed by the biological and/or phylogenetic species concepts. Thereby, species origination rates reflect the rate of appearance of phenotypes distinctive enough to be recorded by a paleontologist. Differences in taxonomic approaches among paleontologists will naturally produce varying classifications of specimens, but such discrepancies can be minimized through taxonomic vetting. For the bovid data, at least, one of us (F.B.) has directly examined large portions of this material and updated identifications accordingly. This provides consistency in species recognition and to the taxonomic structure of the bovid data.
We treated chronospecies (i.e., consecutive species on the same phyletically evolving lineage) the same as true species. This means our origination and extinction counts might be slightly elevated compared with an approach that would exclude pseudoextinctions.
Age Assignments and Time Bins
Age assignments were made for each specimen based on the midpoint of the age range of its locality or geological unit. Data were analyzed in 100-, 250-, 300-, and 500-ka bins (Fig. S3). Using 250-ka bins allowed for subsampling with a reasonable quota and without loss of resolution in the 3.75–1.5 Ma period.
Subsampling
We used repeated specimen-based subsampling (rarefaction) to standardize specimen sample sizes among the different time bins (16, 75). The specimen subsampling quota was set as high as possible without losing resolution in the 3.9–1.5 Ma interval (88 in the full bovid dataset, 245 in the Turkana dataset). Subsampling was repeated 100 times, with diversity metrics recorded for each trial, and means and SDs calculated from these.
Turnover Metrics
-
i)
Single-interval species (singletons) are the number of species only known from the time bin in question (i.e., species with a first and a last appearance datum in the same time bin). Singletons are excluded from origination and extinction measurements.
-
ii)
Originations are the number of species with a first appearance datum in the time bin, not counting singletons.
-
iii)
Extinctions are the number of species with a last appearance datum in the time bin, not counting singletons.
-
iv)
Through-ranging species are species that originate before the time bin and go extinct after the time bin [bin crossers (36)]. This includes species that may not necessarily be sampled in the time bin.
-
v)
Foote origination metric is the number of originations (ori) relative to the number of through-ranging species (thr) in a bin as an exponential decay coefficient, calculated as −ln(thr/(thr+ori)) (36). This metric does not count singletons among originations.
-
vi)
Foote extinction metric is the number of extinctions (ext) relative to the number of through-ranging species in a bin as an exponential decay coefficient, calculated as −ln(thr/(thr+ext)) (36). This metric does not count single-interval species among extinctions.
-
vii)
Thru-species diversity is the sum of originations, extinctions, and through-ranging species (but not single-interval species) in a bin.
-
viii)
To provide an independent measure of turnover that is not susceptible to edge effects and the pull of the Recent, we also calculated corrected two-timer origination and extinction rates (34, 75). Two-timers only look at short-term ranges and thus provide measures of origination and extinction that are less affected by edge effects because they do not take into account the appearance or disappearance of lineages outside the immediate vicinity of the bin in question. The two-timer extinction rate is given as ttext = ln (2ti/3ti), and the two-timer origination rate is ttori = ln (2ti+1/3ti), where 2t (two-timers) are those species that are sampled immediately before and within a bin, and 3t (three-timers) are two-timers that are also sampled in the following bin. The two-timer metric tends to overestimate rates due to failures of sampling, which do not represent biologically meaningful absences. However, in contrast to total range data, two-timer rates can be corrected by the three-timer sampling completeness metric: Ps,i = 3ti /(3ti + pti). Corrected extinction rates (µ) are calculated as µi = ln (2ti/3ti) + ln (Ps,i+1) and corrected origination rates as λi = ln (2ti+1/3ti) + ln (Ps,i-1).
-
ix)
Sampled in-bin diversity (SIB) is the total number of species actually sampled in a bin (regardless of their status as originations, extinctions, or singletons).
-
x)
Although classical subsampling methods such as rarefaction produce samples of the same size (specimens in our case), random culling favors the more common species and thus provides a measure of evenness. Alroy’s (76) SIB diversity through shareholder quorum seeks to mitigate this by subsampling to a predetermined coverage of the underlying abundance distribution in each bin. This proportion (the quorum value) is determined by first calculating the sampling coverage of each bin (u), which is
where n1 is the number of species represented by only one specimen in that bin and O is the total number of specimens in that bin. Bins with high numbers of species represented by only a single specimen (i.e., many very rare taxa) will have low values of u. The smallest value of u in the time series is called q. Each bin’s quorum value is then determined as q/u. The shareholder quorum analysis will randomly draw specimens from a bin until it has satisfied q/u proportion of the total in-bin richness in that bin. Bins that have fewer rare taxa (with u approaching 1) will have a smaller proportion of their in-bin richness sampled than bins that have many rare taxa (i.e., those with u approaching 0), with 100% sampling of the richness of the most coverage-poor bin.
-
xi)
Sampling completeness is the proportion of through-ranging species that have actually been sampled in a time bin. A completeness value of one has all of the through-taxa that should be in the bin actually sampled in that bin. A time bin with a value of 0.5 has only half the through-taxa that should be in the bin actually represented there. Range endpoints are not included in the calculations because these are always sampled and would inflate the sampling completeness estimates. This is the same as the CIbda metric of Maas et al. (105) or Ri of Foote (36).
-
xii)
Evenness is a metric reflecting the rank abundance distribution of the species sampled in that bin, using Pielou’s evenness measure J: J = H/Hmax, where H is the Shannon−Wiener index and Hmax is the logarithm of the number of species in that bin. High values indicate assemblages with more-even species abundances (1 would mean that all species have exactly the same abundance), whereas low-value assemblages are those dominated by just one or a few species.
Bovid AA Frequencies
We use the raw relative abundance of specimens of AA among bovids, a metric Vrba (60) pioneered as a relative measure of aridity or open habitats at African fossil sites. Even though fossil species differed from their living relatives in many ways, early fossils and phylogenetic divergence dates support similar tribal-level ecological affinities going back to at least the early Pliocene. From all specimens securely identified to tribal level (without cf., aff., etc.) in the bovid dataset, the relative proportion of AA was calculated on the raw data (subsampling produces identical proportions) by site area (Afar, Omo, East Turkana, West Turkana, and southern Kenya and northern Tanzania) for all time bins (250 ka) with more than 40 bovid specimens identified to tribe.
Species Durations
Species durations were calculated as the difference between the first and last appearance datum for all extinct species in the Bovidae and Turkana datasets (raw data, not subsampled). Species with durations of <250 ka were excluded, as shorter-lived species are disproportionately known from younger time intervals where chronological control is more refined. Naturally, some of the calculated first and last appearance data represent migration events rather than speciation and extinction, especially in the Turkana Basin data. For clade comparisons, only bovid tribes and Turkana large mammal families with four or more species were included. The results are graphed as beanplots (106).
Species Occupancy Curves
These largely follow Foote (54). Only extinct species occurring in three or more time bins and more than one site were analyzed. Sites were East Turkana, West Turkana, Kanapoi, Lothagam, Middle Awash, Hadar, Laetoli, Olduvai, Rusinga, Mfangano, Woranso-Mille, Mpesida, Lukeino, Manonga, Nkondo, Kazinga, Kaiso, Hohwa, Kyeoro, Nyabusosi, Konso, Lainyamok, Buia, Melka Kunture, Gona, Asbole, Omo, Lee Adoyta, Mursi, and Kibish. A total of 29 bovid species and 52 Turkana large mammals fulfilled these criteria. For each time bin, the number of sites occupied by each species per time bin was first divided by the number of mammal sites in that bin (to correct for the variable number of sampled sites over time) and then by that species' maximum occupancy in a bin. Species durations were scaled from 0 (origination) to 1 (extinction) to make them comparable, as were occupancies. Gaps in the record were filled with “NA” and are not considered in the trajectories. Mean and SE of relative abundances per unit duration (40 units used) were calculated using bootstrap resampling with 1,000 iterations.
Bovid Body Size Estimates
Estimates were derived using the regressions of Damuth (77) for selenodonts and Janis (78) for bovids using anteroposterior tooth lengths (upper and lower molar rows and second molars) or cranial occipital height. Dental or cranial metrics of fossil species were taken from measurements by F.B. or from the literature. We used a single mass estimate per species, sometimes averaged from multiple specimen estimates. When possible, extant species masses were also estimated from the dental regressions (rather than from direct weight data), to be consistent with the fossil data.
Supplementary Material
Acknowledgments
We thank the compilers of the Turkana Public Database and the Comprehensive Olduvai Database Initiative for making specimen data available publicly; J.-R. Boisserie, T. White, K. Reed, D. Su, D. Geraads, and Y. Haile-Selassie for making specimen data available to us; J.-R. Boisserie, T. White, and E. Vrba for critically reading (but not necessarily endorsing) drafts of this manuscript; and the VolkswagenStiftung (to W.K.) and Leibniz-DAAD (Deutscher Akademischer Austauschdienst) fellowship program (to F.B.) for support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504538112/-/DCSupplemental.
References
- 1.Barnosky AD. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J Vertebr Paleontol. 2001;21(1):172–185. [Google Scholar]
- 2.Jablonski D. Biotic interactions and macroevolution: Extensions and mismatches across scales and levels. Evolution. 2008;62(4):715–739. doi: 10.1111/j.1558-5646.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 3.Benton MJ. The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science. 2009;323(5915):728–732. doi: 10.1126/science.1157719. [DOI] [PubMed] [Google Scholar]
- 4.Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. Climate change and the past, present, and future of biotic interactions. Science. 2013;341(6145):499–504. doi: 10.1126/science.1237184. [DOI] [PubMed] [Google Scholar]
- 5.Fortelius M, et al. Evolution of Neogene mammals in Eurasia: Environmental forcing and biotic interactions. Annu Rev Earth Planet Sci. 2014;42:579–604. [Google Scholar]
- 6.Mayr E. Animal Species and Evolution. Harvard Univ Press; Cambridge, MA: 1963. [Google Scholar]
- 7.Eldredge N, Gould SJ. Punctuated equilibria: An alternative to phyletic gradualism. In: Schopf TJM, editor. Models in Paleobiology. Freeman Cooper; San Francisco: 1972. pp. 82–115. [Google Scholar]
- 8.Vrba ES. Environment and evolution: Alternative causes of the temporal distribution of evolutionary events. S Afr J Sci. 1985;81(5):229–236. [Google Scholar]
- 9.Vrba ES. Mammals as a key to evolutionary theory. J Mammal. 1992;73(1):1–28. [Google Scholar]
- 10.Vrba ES. Turnover-pulses, the Red Queen, and related topics. Am J Sci. 1993;293A:418–452. [Google Scholar]
- 11.Vrba ES. On the connections between paleoclimate and evolution. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. Yale Univ Press; New Haven, CT: 1995. pp. 24–45. [Google Scholar]
- 12.Azanza B, Alberdi MT, Prado JL. Large mammal turnover pulses correlated with latest Neogene glacial trends in the northwestern Mediterranean region. Geol Soc Lond Spec Publ. 2000;181(1):161–170. [Google Scholar]
- 13.Foote M. Pulsed origination and extinction in the marine realm. Paleobiology. 2005;31(1):6–20. [Google Scholar]
- 14.van Dam JA, et al. Long-period astronomical forcing of mammal turnover. Nature. 2006;443(7112):687–691. doi: 10.1038/nature05163. [DOI] [PubMed] [Google Scholar]
- 15.White TD. African omnivores: Global climatic change and Plio-Pleistocene hominids and suids. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. Yale Univ Press; New Haven, CT: 1995. pp. 369–384. [Google Scholar]
- 16.Alroy J. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr Palaeoclimatol Palaeoecol. 1996;127(1):285–311. [Google Scholar]
- 17.Behrensmeyer AK, Todd NE, Potts R, McBrinn GE. Late pliocene faunal turnover in the Turkana Basin, Kenya and Ethiopia. Science. 1997;278(5343):1589–1594. doi: 10.1126/science.278.5343.1589. [DOI] [PubMed] [Google Scholar]
- 18.Alroy J, Koch PL, Zachos JC. Global climate change and North American mammalian evolution. Paleobiology. 2000;26(sp4):259–288. [Google Scholar]
- 19.Werdelin L, Lewis ME. Plio-Pleistocene Carnivora of eastern Africa: Species richness and turnover patterns. Zool J Linn Soc. 2005;144(2):121–144. [Google Scholar]
- 20.Frost SR. African Pliocene and Pleistocene cercopithecid evolution and global climatic change. In: Bobe R, Alemseged Z, Behrensmeyer AK, editors. Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence. Springer, Dordrecht; The Netherlands: 2007. pp. 51–76. [Google Scholar]
- 21.Van Valen L. A new evolutionary law. Evol Theory. 1973;1:1–30. [Google Scholar]
- 22.Liow LH, Van Valen L, Stenseth NC. Red Queen: From populations to taxa and communities. Trends Ecol Evol. 2011;26(7):349–358. doi: 10.1016/j.tree.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Alroy J. Equilibrial diversity dynamics in North American mammals. In: McKinney ML, Drake JA, editors. Biodiversity Dynamics: Turnover of Populations, Taxa and Communities. Columbia Univ Press; New York: 1998. pp. 232–288. [Google Scholar]
- 24.Jernvall J, Fortelius M. Maintenance of trophic structure in fossil mammal communities: Site occupancy and taxon resilience. Am Nat. 2004;164(5):614–624. doi: 10.1086/424967. [DOI] [PubMed] [Google Scholar]
- 25.Foote M, et al. Rise and fall of species occupancy in Cenozoic fossil mollusks. Science. 2007;318(5853):1131–1134. doi: 10.1126/science.1146303. [DOI] [PubMed] [Google Scholar]
- 26.Quental TB, Marshall CR. How the Red Queen drives terrestrial mammals to extinction. Science. 2013;341(6143):290–292. doi: 10.1126/science.1239431. [DOI] [PubMed] [Google Scholar]
- 27.White F. The Vegetation of Africa: A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa. United Nations; Paris: 1983. [Google Scholar]
- 28.Vrba ES. Late Pliocene climatic events and hominid evolution. In: Grine FE, editor. The Evolutionary History of the Robust Australopithecines. Aldine de Gruyter; New York: 1988. pp. 405–426. [Google Scholar]
- 29.Vrba ES. The fossil record of African antelopes (Mammalia, Bovidae) in relation to human evolution and paleoclimate. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. Yale Univ Press; New Haven, CT: 1995. pp. 385–424. [Google Scholar]
- 30.deMenocal PB. Anthropology. Climate and human evolution. Science. 2011;331(6017):540–542. doi: 10.1126/science.1190683. [DOI] [PubMed] [Google Scholar]
- 31.Ravelo AC, Andreasen DH, Lyle M, Olivarez Lyle A, Wara MW. Regional climate shifts caused by gradual global cooling in the Pliocene epoch. Nature. 2004;429(6989):263–267. doi: 10.1038/nature02567. [DOI] [PubMed] [Google Scholar]
- 32.deMenocal PB. African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet Sci Lett. 2004;220(1-2):3–24. [Google Scholar]
- 33.Vrba ES. Climate, heterochrony, and human evolution. J Anthropol Res. 1996;52(1):1–28. [Google Scholar]
- 34.Alroy J. Colloquium paper: Dynamics of origination and extinction in the marine fossil record. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11536–11542. doi: 10.1073/pnas.0802597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrba ES. Mass turnover and heterochrony events in response to physical change. Paleobiology. 2005;31(2):157–174. [Google Scholar]
- 36.Foote M. Origination and extinction components of taxonomic diversity: General problems. Paleobiology. 2000;26(4):74–102. [Google Scholar]
- 37.Bobe R, Behrensmeyer AK. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;207(3-4):399–420. [Google Scholar]
- 38.Cooke HBS. Plio-Pleistocene Suidae in relation to African hominid deposits. In: Beden M, editor. L’Environnement des Hominidés au Plio-Pléistocène. Masson; Paris: 1985. pp. 101–117. [Google Scholar]
- 39.Turner A. The evolution of the guild of larger terrestrial carnivores during the Plio-Pleistocene in Africa. Geobios. 1990;23(3):349–368. [Google Scholar]
- 40.Bonnefille R. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global Planet Change. 2010;72(4):390–411. [Google Scholar]
- 41.Bibi F, Souron A, Bocherens H, Uno K, Boisserie J-R. Ecological change in the lower Omo Valley around 2.8 Ma. Biol Lett. 2013;9(1):20120890. doi: 10.1098/rsbl.2012.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobe R, Behrensmeyer AK, Chapman RE. Faunal change, environmental variability and late Pliocene hominin evolution. J Hum Evol. 2002;42(4):475–497. doi: 10.1006/jhev.2001.0535. [DOI] [PubMed] [Google Scholar]
- 43.Geraads D. Evolution of bovid diversity in the Plio-Pleistocene of Africa. Hist Biol. 1994;7(3):221–237. [Google Scholar]
- 44.Bobe R, Behrensmeyer AK, Eck GG, Harris JM. Patterns of abundance and diversity in late Cenozoic bovids from the Turkana and Hadar Basins, Kenya and Ethiopia. In: Bobe R, Alemseged Z, Behrensmeyer AK, editors. Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence. Springer; Dordrecht, The Netherlands: 2007. pp. 129–157. [Google Scholar]
- 45.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;307(1-4):75–89. [Google Scholar]
- 46.Feakins SJ, deMenocal P, Eglinton TI. Biomarker records of late Neogene changes in northeast African vegetation. Geology. 2005;33(12):977–980. [Google Scholar]
- 47.Reed KE. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol. 1997;32(2-3):289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- 48.Brierley CM, Fedorov AV. Relative importance of meridional and zonal sea surface temperature gradients for the onset of the ice ages and Pliocene-Pleistocene climate evolution. Paleoceanography. 2010;25(2):PA2214. [Google Scholar]
- 49.Vrba ES. Evolutionary pattern and process in the sister-group Alcelaphini-Aepycerotini (Mammalia: Bovidae) In: Eldredge N, Stanley SM, editors. Living Fossils. Springer; New York: 1984. pp. 62–79. [Google Scholar]
- 50.Vrba ES. Ecology in relation to speciation rates: Some case histories of Miocene-Recent mammal clades. Evol Ecol. 1987;1(4):283–300. [Google Scholar]
- 51.Price SA, Hopkins SSB, Smith KK, Roth VL. Tempo of trophic evolution and its impact on mammalian diversification. Proc Natl Acad Sci USA. 2012;109(18):7008–7012. doi: 10.1073/pnas.1117133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vrba ES. Major features of Neogene mammalian evolution in Africa. In: Partridge TC, Maud RR, editors. The Cenozoic of Southern Africa. Oxford Univ Press; Oxford: 2000. pp. 277–304. [Google Scholar]
- 53.White T. Paleoanthropology. Early hominids—Diversity or distortion? Science. 2003;299(5615):1994–1997. doi: 10.1126/science.1078294. [DOI] [PubMed] [Google Scholar]
- 54.Foote M. Symmetric waxing and waning of marine invertebrate genera. Paleobiology. 2007;33(4):517–529. [Google Scholar]
- 55.Liow LH, Stenseth NC. 2007. The rise and fall of species: Implications for macroevolutionary and macroecological studies. Proc R Soc B 274(1626):2745−2752.
- 56.Vrba ES, DeGusta D. Do species populations really start small? New perspectives from the Late Neogene fossil record of African mammals. Philos Trans R Soc Lond B Biol Sci. 2004;359(1442):285–292, discussion 292–293. doi: 10.1098/rstb.2003.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roopnarine PD. Extinction cascades and catastrophe in ancient food webs. Paleobiology. 2009;32(1):1–19. [Google Scholar]
- 58.Vrba ES. The significance of bovid remains as indicators of environment and predation patterns. In: Behrensmeyer AK, Hill A, editors. Fossils in the Making: Vertebrate Taphonomy and Paleoecology. Univ Chicago Press; Chicago: 1980. pp. 247–271. [Google Scholar]
- 59.Greenacre MJ, Vrba ES. Graphical display and interpretation of antelope census-data in African wildlife areas, using correspondence-analysis. Ecology. 1984;65(3):984–997. [Google Scholar]
- 60.Vrba ES. Some evidence of chronology and palaeoecology of Sterkfontein, Swartkrans and Kromdraai from the fossil Bovidae. Nature. 1975;254(5498):301–304. [Google Scholar]
- 61.Vrba ES. Chronological and ecological implications of fossil Bovidae at Sterkfontein australopithecine site. Nature. 1974;250(5461):19–23. [Google Scholar]
- 62.Bobe R, Eck GG. Responses of African bovids to Pliocene climatic change. Paleobiology. 2001;27(2):1–47. [Google Scholar]
- 63.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 64.Sepulchre P, et al. Tectonic uplift and Eastern Africa aridification. Science. 2006;313(5792):1419–1423. doi: 10.1126/science.1129158. [DOI] [PubMed] [Google Scholar]
- 65.Everett MA. 2010. The paleoecology of the Pleistocene Upper Busidima Formation, Gona, Afar Depression, Ethiopia. PhD thesis (Indiana University, Bloomington)
- 66.Bailey GN, Reynolds SC, King GCP. Landscapes of human evolution: Models and methods of tectonic geomorphology and the reconstruction of hominin landscapes. J Hum Evol. 2011;60(3):257–280. doi: 10.1016/j.jhevol.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Ditchfield P, Harrison T. Sedimentology, lithostratigraphy and depositional history of the Laetoli area. In: Harrison T, editor. Paleontology and Geology of Laetoli: Human Evolution in Context. Springer; Dordrecht, The Netherlands: 2011. pp. 47–76. [Google Scholar]
- 68.Gentry AW. Fossil Bovidae (Mammalia) from Langebaanweg, South Africa. Ann S Afr Mus. 1980;79(8):213–337. [Google Scholar]
- 69.Shipman P, Harris JM. Evolutionary History of the “Robust” Australopithecines. Aldine de Gruyter; New York: 1988. Habitat preference and paleoecology of Australopithecus boisei in Eastern Africa; pp. 343–381. [Google Scholar]
- 70.Vrba ES. An hypothesis of heterochrony in response to climatic cooling and its relevance to early hominid evolution. In: Corruccini R, Ciochon R, editors. Integrative Paths to the Past: Paleoanthropological Advances in Honour of F. Clark Howell. Prentice Hall; Englewood Cliffs, NJ: 1994. pp. 345–376. [Google Scholar]
- 71.Vrba ES. Ecology, evolution, and development: Perspectives from the fossil record. In: Hall BK, Pearson RD, Muller GB, editors. Environment, Development, and Evolution. MIT Press; Cambridge, MA: 2004. pp. 85–105. [Google Scholar]
- 72.Bell RHV. The use of the herb layer by grazing ungulates in the Serengeti. In: Watson A, editor. Animal Populations in Relation to their Food Resources. Blackwell Sci; Oxford: 1969. pp. 111–124. [Google Scholar]
- 73.Alroy J. Cope’s rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280(5364):731–734. doi: 10.1126/science.280.5364.731. [DOI] [PubMed] [Google Scholar]
- 74.Bobe R, Behrensmeyer AK, Leakey MG, Mbua E. The Turkana Database: An archive of vertebrate evolution in East Africa. Evol Anthropol. 2011;20(6):256. [Google Scholar]
- 75.Alroy J. Speciation and extinction in the fossil record of North American mammals. In: Butlin RK, Bridle JR, Schluter D, editors. Speciation and Patterns of Diversity. Cambridge Univ Press; Cambridge, UK: 2009. pp. 301–323. [Google Scholar]
- 76.Alroy J. 2010. Fair sampling of taxonomic richness and unbiased estimation of origination and extinction rates. Quantitative Methods in Paleobiology, eds Alroy J, Hunt G, Paleontological Society Papers (Paleontol Soc, Boulder, CO), Vol 16, pp 55–80.
- 77.Damuth J. Problems in estimating body masses of archaic ungulates using dental measurements. In: Damuth J, MacFadden BJ, editors. Body Size in Mammalian Paleobiology. Cambridge Univ Press; Cambridge, UK: 1990. pp. 229–255. [Google Scholar]
- 78.Janis CM. Correlation of cranial and dental variables with body size in ungulates and macropodoids. In: Damuth J, MacFadden BJ, editors. Body Size in Mammalian Paleobiology. Cambridge Univ Press; Cambridge, UK: 1990. pp. 255–300. [Google Scholar]
- 79.Gentry AW. The Bovidae of the Omo Group deposits, Ethiopia (French and American collections) In: Coppens Y, Howell FC, editors. Les faunes Plio-Pléistocènes de la basse Vallée de l’Omo (Ethiopie); I: Perissodactyles-Artiodactyles (Bovidae), Cahiers de Paléontologie. Vol 1985. CNRS; Paris: 1985. pp. 119–191. [Google Scholar]
- 80.Drapeau MSM, et al. The Omo Mursi Formation: A window into the East African Pliocene. J Hum Evol. 2014;75:64–79. doi: 10.1016/j.jhevol.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Rowan J, Faith JT, Gebru Y, Fleagle JG. Taxonomy and paleoecology of fossil Bovidae (Mammalia, Artiodactyla) from the Kibish Formation, southern Ethiopia: Implications for dietary change, biogeography, and the structure of the living bovid faunas of East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2015;420:210–222. [Google Scholar]
- 82.Vrba ES. New fossils of Alcelaphini and Caprinae (Bovidae; Mammalia) from Awash, Ethiopia, and phylogenetic analysis of Alcelaphini. Palaeontol Afr. 1997;34:127–198. [Google Scholar]
- 83.Gilbert WH. Bovidae. In: Gilbert WH, Asfaw B, editors. Homo Erectus: Pleistocene Evidence from the Middle Awash, Ethiopia. Univ California Press; Berkeley: 2008. pp. 45–94. [Google Scholar]
- 84.Haile-Selassie Y, Vrba ES, Bibi F. Bovidae. In: Haile-Selassie Y, WoldeGabriel G, editors. Ardipithecus kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia. Univ California Press; Berkeley: 2009. pp. 277–330. [Google Scholar]
- 85.Geraads D, Bobe R, Reed K. Pliocene Bovidae (Mammalia) from the Hadar Formation of Hadar and Ledi-Geraru, lower Awash, Ethiopia. J Vertebr Paleontol. 2012;32(1):180–197. [Google Scholar]
- 86.Geraads D, Melillo S, Haile-Selassie Y. Middle Pliocene Bovidae from hominid-bearing sites in the Woranso-Mille area, Afar region, Ethiopia. Palaeontol Afr. 2009;44:59–70. [Google Scholar]
- 87.Geraads D, Eisenmann V, Petter G. The large mammal fauna of the Oldowayan sites of Melka Kunture, Ethiopia. In: Chavaillon J, Piperno M, editors. Studies on the Early Palaeolithic Site of Melka Kunture, Ethiopia. Inst Italiano di Preistoria e Protostoria; Florence, Italy: 2004. pp. 169–192. [Google Scholar]
- 88.Geraads D, Alemseged Z, Reed D, Wynn J, Roman DC. The Pleistocene fauna (other than Primates) from Asbole, lower Awash Valley, Ethiopia, and its environmental and biochronological implications. Geobios. 2004;37(6):697–718. [Google Scholar]
- 89.Martínez-Navarro B, et al. The large fossil mammals from Buia (Eritrea): Systematics, biochronology and paleoenvironments. Riv Ital Paleontol Stratigr. 2004;110(suppl.):61–88. [Google Scholar]
- 90.Schwartz E. 1937. Die fossilen Antilopen von Oldoway. Wiss Ergeb Oldoway Exped 4:8−104.
- 91.Gentry AW, Gentry A. 1978. Fossil Bovidae (Mammalia) of Olduvai Gorge, Tanzania; Part I. Bull Br Mus (Nat Hist) Geol 29(4):289−446.
- 92.Gentry AW. Bovidae. In: Harrison T, editor. Paleontology and Geology of Laetoli: Human Evolution in Context. Vol 2. Springer; New York: 2011. pp. 363–465. [Google Scholar]
- 93.Gentry AW, Su DF. Appendix. In: Harrison T, editor. Paleontology and Geology of Laetoli: Human Evolution in Context. Vol 2. Springer; New York: 2011. pp. 413–463. [Google Scholar]
- 94.Gentry AW. Fossil ruminants (Mammalia) from the Manonga Valley, Tanzania. In: Harrison T, editor. Neogene Paleontology of the Manonga Valley, Tanzania. Plenum; New York: 1997. pp. 107–135. [Google Scholar]
- 95.Faith JT, Choiniere JN, Tryon CA, Peppe DJ, Fox DL. Taxonomic status and paleoecology of Rusingoryx atopocranion (Mammalia, Artiodactyla), an extinct Pleistocene bovid from Rusinga Island, Kenya. Quat Res. 2011;75(3):697–707. [Google Scholar]
- 96.Tryon CA, et al. Late Pleistocene artefacts and fauna from Rusinga and Mfangano islands, Lake Victoria, Kenya. Azania. 2012;47(1):14–38. [Google Scholar]
- 97.Faith JT, Tryon CA, Peppe DJ, Beverly EJ, Blegen N. Biogeographic and evolutionary implications of an extinct Late Pleistocene impala from the Lake Victoria Basin, Kenya. J Mamm Evol. 2013;21(2):213–222. [Google Scholar]
- 98.Potts R, Deino A. Mid-Pleistocene change in large mammal faunas of East Africa. Quat Res. 1995;43(1):106–113. [Google Scholar]
- 99.Suwa G, et al. Plio-Pleistocene terrestrial mammal assemblage from Konso, southern Ethiopia. J Vertebr Paleontol. 2003;23(4):901–916. [Google Scholar]
- 100.Thomas H. 1980. Les bovidés du Miocène supérieur des couches de Mpesida et de la formation de Lukeino (district de Baringo, Kenya). Proceedings of the 8th Panafrican Congress of Prehistory and Quaternary Studies (Nairobi 1977), eds Leakey REF, Ogot BA Int Louis Leakey Meml Inst African Prehistory, Nairobi), pp 82−91.
- 101.Geraads D, Thomas H. 1994. Bovidés du Plio-Pléistocène d’Ouganda. Geology and Palaeobiology of the Albertine Rift Valley, Uganda-Zaire: Volume II, Palaeobiology, eds Senut B, Pickford M (CIFEG, Paris), Vol 29, pp 383-407. [Google Scholar]
- 102.Werdelin L, Lewis ME. New species of Crocuta from the early Pliocene of Kenya, with an overview of early Pliocene hyenas of eastern Africa. J Vertebr Paleontol. 2008;28(4):1162–1170. [Google Scholar]
- 103.Werdelin L, Lewis ME. 2013. The Carnivora, Koobi Fora Research Project (California Acad Sci, San Francisco), Vol 7.
- 104.Werdelin L. Pachycrocuta (hyaenids) from the Pliocene of east Africa. Paläontol Z. 1999;73(1-2):157–165. [Google Scholar]
- 105.Maas MC, Anthony MRL, Gingerich PD, Gunnell GF, Krause DW. Mammalian generic diversity and turnover in the Late Paleocene and Early Eocene of the Bighorn and Crazy Mountains Basins, Wyoming and Montana (USA) Palaeogeogr Palaeoclimatol Palaeoecol. 1995;115(1-4):181–207. [Google Scholar]
- 106.Kampstra P. Beanplot: A boxplot alternative for visual comparison of distributions. J Stat Softw. 2008;28(1):1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.