Significance
Automated chemical processes, such as DNA sequencing and nucleic acid and peptide synthesis, have transformed the fields of genetics and biotechnology. There is no analogous automated or semiautomated process, however, to provide unimolecular, sequence-defined synthetic polymers to those interested in studying them. The combination of multistep continuous flow chemistry and polymer synthesis by iterative exponential growth (Flow-IEG) enables the semiautomated synthesis of perfect polymers reported herein. The user-friendly nature, scalability, and modularity of Flow-IEG provides a general strategy for the automated synthesis of sequence and architecturally defined, uniform macromolecules. We envision this polymer synthesis machine will serve as an enabling tool for both fundamental explorations and advanced applications in biotechnology, medicinal chemistry, and materials science.
Keywords: polymers, automation, continuous flow chemistry, unimolecular macromolecules, sequence-controlled polymers
Abstract
We report a semiautomated synthesis of sequence and architecturally defined, unimolecular macromolecules through a marriage of multistep flow synthesis and iterative exponential growth (Flow-IEG). The Flow-IEG system performs three reactions and an in-line purification in a total residence time of under 10 min, effectively doubling the molecular weight of an oligomeric species in an uninterrupted reaction sequence. Further iterations using the Flow-IEG system enable an exponential increase in molecular weight. Incorporating a variety of monomer structures and branching units provides control over polymer sequence and architecture. The synthesis of a uniform macromolecule with a molecular weight of 4,023 g/mol is demonstrated. The user-friendly nature, scalability, and modularity of Flow-IEG provide a general strategy for the automated synthesis of sequence-defined, unimolecular macromolecules. Flow-IEG is thus an enabling tool for theory validation, structure–property studies, and advanced applications in biotechnology and materials science.
The automation of chemical synthesis provides nonexperts with access to enabling technologies that revolutionize entire fields of scientific inquiry. For example, automation of DNA sequencing (1) provided a user-friendly technology that helped unlock how hereditable information is passed between generations, eventually enabling the Human Genome Project (2). Likewise, automating processes for solid-phase synthesis (SPS) provided routine access to biological polymers such as peptides (3), nucleic acids (4), and polysaccharides (5) for a variety of fundamental and applied explorations in biotechnology, medicinal chemistry, and immunology.
Sequence-defined synthetic polymers hold immense potential for applications in a wide range of fields, including biomedicine, nanotechnology, and high-density information storage (6). The automated synthesis of perfect polymers would provide these materials to those best equipped to exploit their potential. Despite their promise, the efficient and scalable syntheses of unimolecular, sequence-defined, and nonbioresemblant polymers remains a major challenge for chemical synthesis (7). Herein, we report a general strategy for the scalable, semiautomated synthesis of macromolecules with uniform mass and precisely defined primary sequence by integrating iterative exponential growth (IEG) (8), an underused methodology in synthetic polymer chemistry, with modern multistep continuous flow synthesis (9).
Chemical methods for the synthesis of unimolecular, sequence-defined synthetic polymers typically rely on either co-opting biological machinery—for example, in PCR (10) or in vitro protein expression (11, 12)—or using SPS (13). Although these powerful methods have enabled transformative advances in biotechnology and have recently been revisited for the synthesis of sequence-defined synthetic polymers (14–16), alternative strategies are needed that provide a general solution for access to sequence-defined synthetic polymers in a scalable and sustainable manner. In contrast, step- or chain-growth polymerization methods that use differences in monomer reactivity, living polymerizations, monomer design, and/or templating strategies to impart sequence regulation have been recently reported (17–23). These scalable methods, however, still rely on stochastic monomer addition and thus do not generate unimolecular macromolecules.
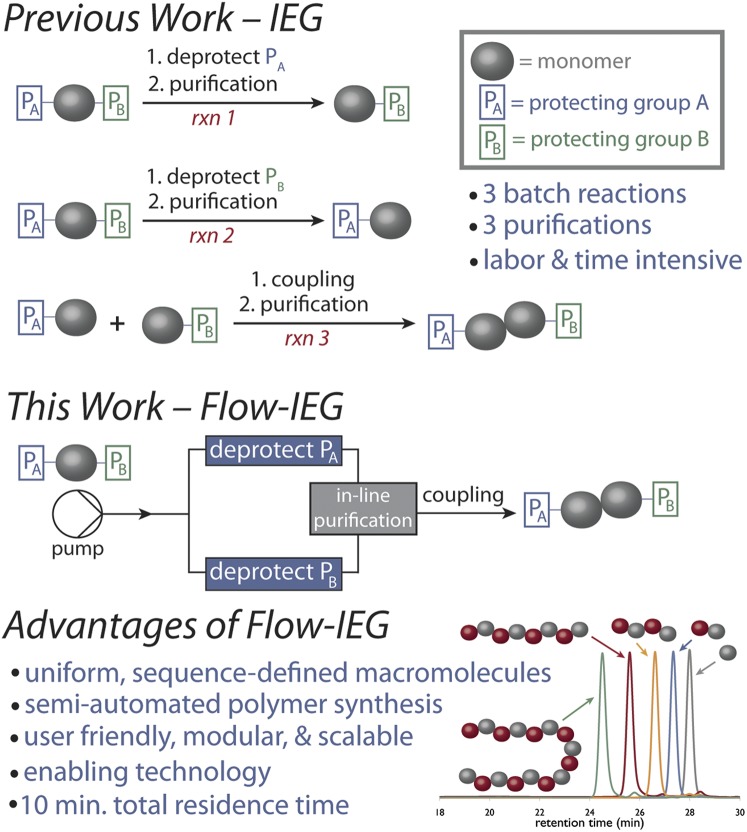
IEG, pioneering by Whiting and coworkers for the synthesis of unimolecular samples of polyethylene (24, 25), is a method where a common starting material is partitioned in half, with each half undergoing two separate and complementary deprotections, followed by coupling of the two halves to give a new molecule with approximately twice the original molecular weight (Fig. 1) (8). Because each doubling of molecular weight requires three different reactions, this method is exceedingly laborious when done under standard batch conditions and thus has not been broadly adopted for routine polymer synthesis.
Fig. 1.
Flow-IEG improves on previous work to enable the synthesis of unimolecular polymers by conducting multiple reactions and purifications in a continuously flowing system.
To overcome the challenges associated with IEG, we envisioned a union of IEG with continuous flow chemistry (Flow-IEG). Continuous flow methods can have considerable advantages in terms of mass and heat transfer, reproducibility, throughput, and telescoping reactions (9, 26, 27). We sought to leverage these advantages by merging the multistep flow methods being developed for organic and medicinal chemistry (28) with the capabilities of polymer synthesis in-flow (29, 30). The automated and exacting control over mixing, thermal conditions, in-line purification, and residence time make continuous flow methods ideal for IEG, as optimized conditions can be repeatedly applied to the iterative coupling of polymer building blocks in a continuous system.
The Flow-IEG system reported herein performs three reaction steps and an in-line purification in a total residence time of under 10 min. The optimized Flow-IEG system generates unimolecular polymers of molecular weight >4,000 g/mol in a scalable fashion. Further, because the Flow-IEG system is designed to allow “plug-and-play” monomer selection (31), the semiautomated synthesis of sequence and architecturally defined, unimolecular polymers is demonstrated, and their structure–property relationships are compared.
Design and Optimization of the Flow-IEG System
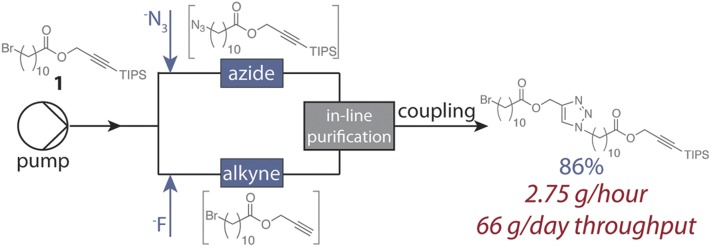
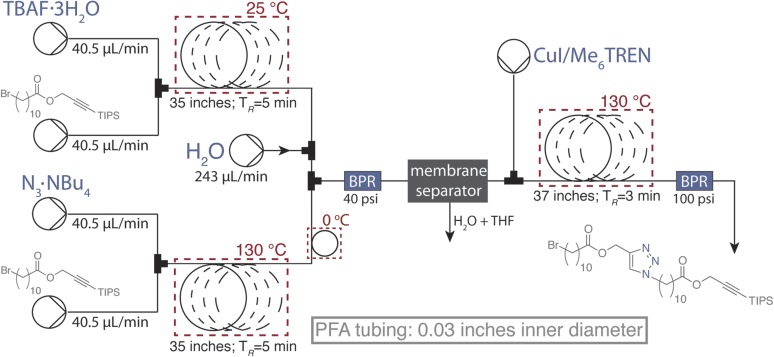
The copper catalyzed azide–alkyne cycloaddition (CuAAC) is an ideal candidate for Flow-IEG systems because of its efficiency, chemoselectivity, and simple preparation of coupling partners, as demonstrated by Drockenmuller and coworkers (32). Monomer 1 was thus designed to contain the requisite masked functional groups for IEG: a triisopropylsilyl (TIPS) protected alkyne and an alkyl bromide (Fig. 2). Initial investigations demonstrated that the tetrabutylammonium salts of the both azide (TBAA) and fluoride (TBAF) were excellent reagents to unveil the azide and alkyne derivatives of 1, respectively. Optimization (SI Materials and Methods) identified conditions that provided chemoselective conversion of 1 into the requisite azide and alkyne coupling partners over a range of substrate concentrations. Specifically, azide substitution reached full conversion in flow at 130 °C with a residence time (tR) of 5 min, and the silyl deprotection reached full conversion with a tR of 5 min at room temperature. To quench and remove excess or unreacted fluoride and azide reagents immediately before the CuAAC, we incorporated an aqueous workup into the continuous system by taking advantage of an in-line, membrane-based liquid–liquid separator developed by Jensen and coworkers (33). Its straightforward implementation, small footprint, and integrated pressure control allows the use of excess fluoride and azide reagents to ensure complete conversion without deleterious cross reactivity in subsequent steps. [The aqueous workup also serves to remove tetrabutylammonium salts and tetrahydrofuran (THF), thus both purifying the flow stream and concentrating the coupling partners into toluene before the CuAAC reaction.]
Fig. 2.
Ester monomer 1 was optimized and implemented into the Flow-IEG system, where three reactions and an in-line purification are performed in a continuous system and iterative coupling provides exponential increases in molecular weight. The system has been scaled to provide 2.75 grams of coupled product per hour.
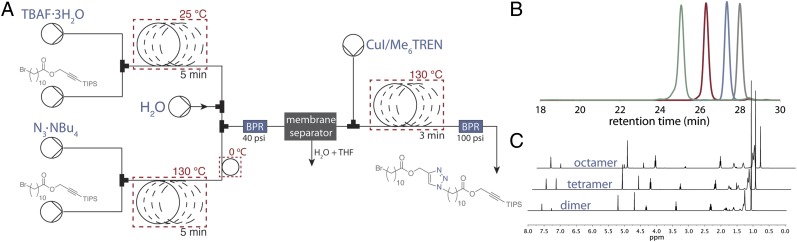
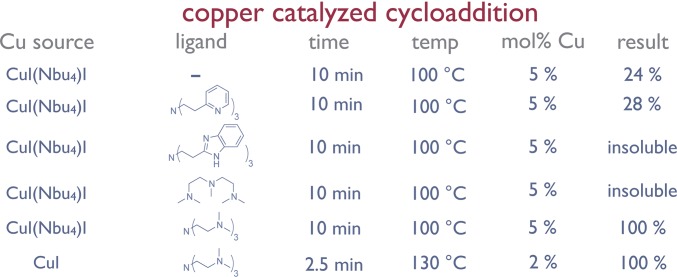
Having optimized the preparation and in-line purification of the azide and alkyne coupling partners, we turned our attention to joining them via CuAAC with a copper–ligand combination that was both highly active and completely soluble under the reaction conditions (34). After evaluating several conditions, we discovered that a 0.1 M solution of CuI with 1.1 equivalents of the ligand tris[2-(dimethylamino)ethyl]amine (Me6TREN) provided a CuAAC that reached full conversion in 3 min at 130 °C with only 3 mol% copper loading. These conditions provided excellent chemoselective reactivity at a variety of substrate concentrations. Optimizing other system design elements, such as cooling the flow stream containing the azide and quenching the silyl deprotection before mixing further, prevented deleterious cross-reactivity. The optimized end-to-end Flow-IEG system (Fig. 3A) affects three reactions and an in-line purification in a total average tR of only 10 min. This system design generally provided coupled product in high conversion with no high-molecular-weight impurities present. Collection of the material, solvent removal, and chromatography provides the pure coupled product of 1 in 86% isolated yield over the three reaction steps.
Fig. 3.
A machine for automated IEG. (A) Schematic of the Flow-IEG system detailing reaction times and flow sequence. (B) SEC traces of unimolecular macromolecules derived from 1. (C) 1H-NMR spectra of the unimolecular macromolecules derived from 1.
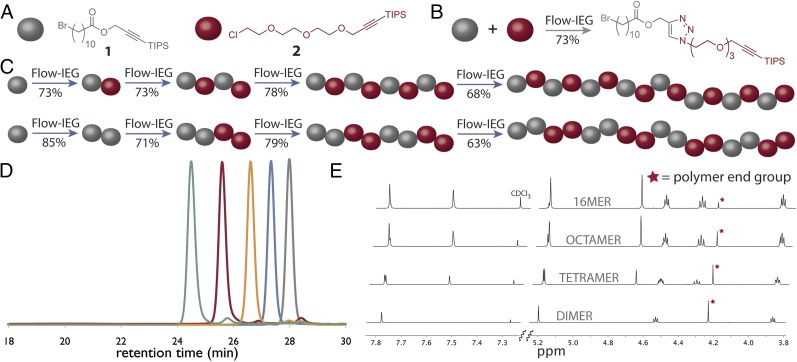
Critical to the success of Flow-IEG is its ability to produce high-molecular-weight species. Therefore, the purified dimer of monomer 1 was reintroduced in the Flow-IEG system. (For reactions generating larger oligomeric species, chlorobenzene was found to provide superior solubility while having no observable effect on reactivity.) The dimer was converted to the tetrameric polyester via Flow-IEG in 87% isolated yield; subsequently, Flow-IEG transformed the tetramer into the octamer in 78% isolated yield. Because these long-chain aliphatic esters are prone to crystallization, solubility became a significant challenge in this system when attempting to make structures larger than the octamer. This rapid construction of large unimolecular species required only three runs through the Flow-IEG system; including solution preparation, running the Flow-IEG system, purification, and isolation, the entire semiautomated process can be easily completed in a routine 8-h work day. Therefore, octamer synthesis by a skilled practitioner would require only 3 workdays. In contrast, nine reaction and purification steps would typically be required using traditional batch chemistry. The polyester octamer with a molecular weight of 2,317 g/mol was synthesized by Flow-IEG in an isolated yield of 58% from 1.
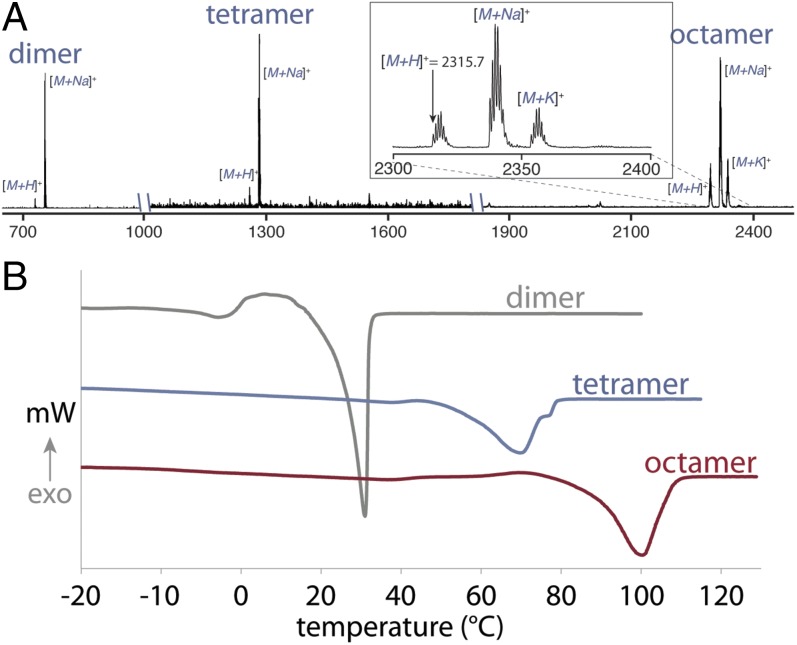
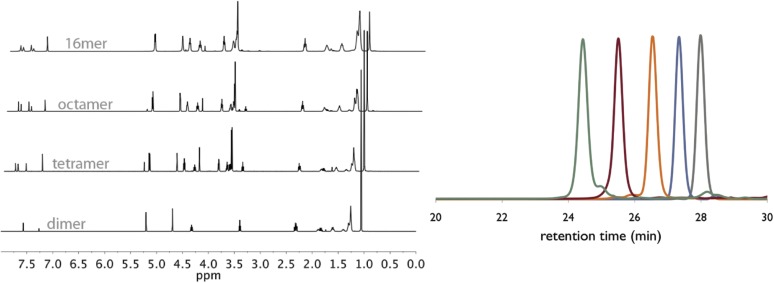
Full characterization of these oligomeric species was accomplished by 1H-NMR, 13C-NMR, size exclusion chromatography (SEC), matrix-assisted laser desorption ionization (MALDI) MS, thermal gravemetric analysis (TGA), and differential scanning calorimetry (DSC). SEC clearly demonstrates both the growth and purity of the oligomeric species (Fig. 3B). As the substrates grow exponentially in size, the peaks in the SEC shift to shorter retention times while maintaining their narrow and monomodal peak shape. The dispersity (Đ) of each species is below 1.01 compared with polystyrene standards, thus corroborating the unimolecular nature of the polymers. 1H-NMR proved diagnostic for tracking the growth of Flow-IEG derived polymers. The unique resonances of the polymer end groups were clearly visible by 1H-NMR (at 4.25 and 3.21 ppm), and their integration relative to the polymer backbone and triazole signals after each growth step confirmed the iterative coupling (Fig. 3C). For example, if the integration of the propargylic proton at 4.25 ppm is set to 2.0 protons, the integration of the triazole peak at 7.58 ppm increases from 1.0 to 3.0 to 7.0 protons for the dimer, tetramer, and octamer oligomers, respectively. MS, particularly MALDI, provided valuable information on the unimolecular nature of the polymers. As shown in Fig. 4A, the molecular ion for the dimer, tetramer, and octamer is observed as the single species in each spectrum. A closer look at the octamer (Fig. 4A, Inset) shows not only the correct mass of the molecular ion ([M+H]+) at 2,315.7 Da, but also displays the characteristic isotopic fine structure associated with a species of the correct chemical formula (C121H204BrN21O16Si).
Fig. 4.
Structure–property relationships of Flow-IEG derived polymers. (A) MALDI MS spectra of the unimolecular copolymers derived from 1. (Inset) Zoom of the octamer spectra demonstrating the mass of the molecular ion [M+H]+ along with its isotopic fine structure. (B) DSC traces demonstrating the increasing melting transitions of the unimolecular oligomers derived from 1. The third heating cycle of the DSC is shown from samples heated at 5 °C/min.
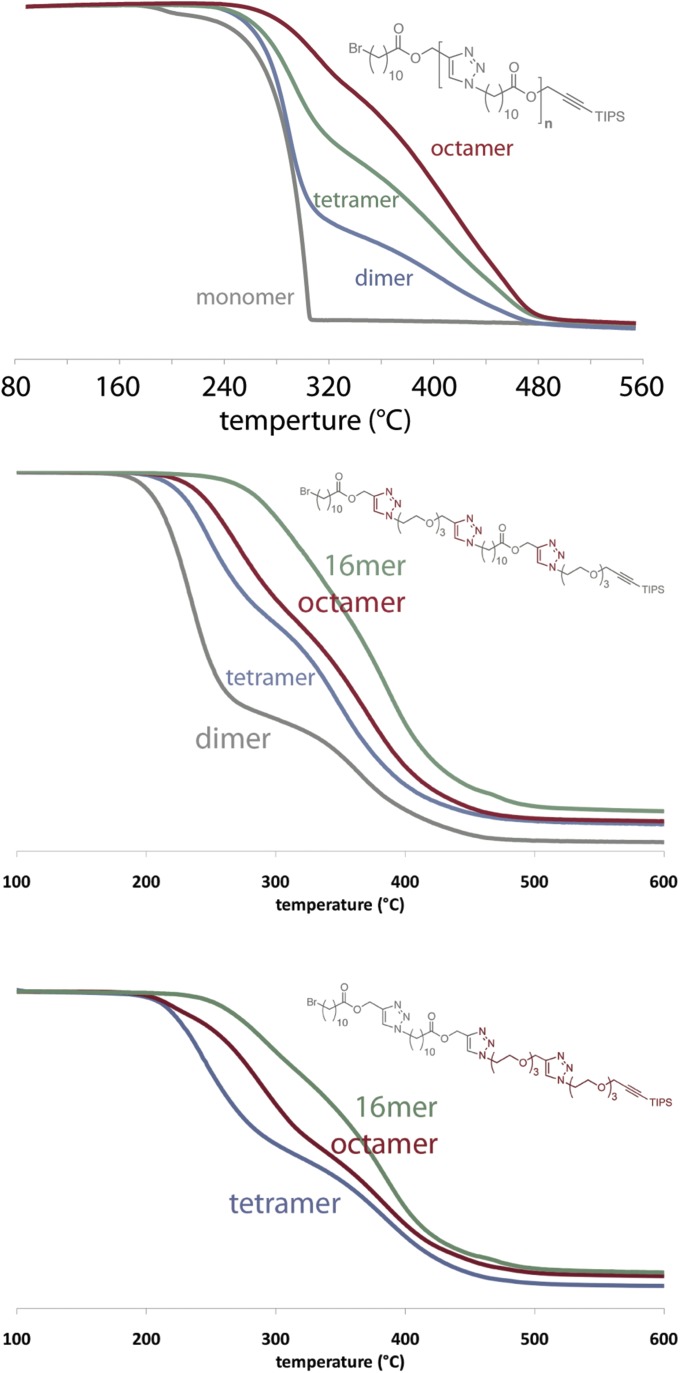
The IEG approach has previously proven valuable in gaining insight into the structure–property relationships of industrially important polymers, such as nylon (35), poly(ethylene terephthalate) (36), and a variety of polyesters (37–39). Accordingly, we used the oligomers made by Flow-IEG to study the evolution of the thermal properties of these unimolecular species. As observed in previous IEG studies, the decomposition temperature of these oligomers increases with increasing molecular weight (TGA in SI Materials and Methods). Most importantly, DSC clearly demonstrates how polymer molecular weight influences both the glass transition temperature (Tg) and the crystallization behavior of these oligomers. The Tg increases significantly with increasing molecular weight, going from −53.1 °C for the dimer to −23.9 °C for the tetramer to −16.5 °C for the octamer, commensurate with Flory–Fox theory (40). Further, as seen in Fig. 4B, the melting temperature (Tm) of these polymers also evolves with increasing molecular weight. Although the dimer is a sticky solid and melts just above room temperature (31.0 °C), increasing the molecular weight exponentially increases the Tm to 70.0 °C for the tetramer and 100.4 °C for the octamer. This dramatic increase in Tm even at low molecular weights demonstrates not only chain growth, but also the strong propensity of these polymers to crystallize and quickly approach the Tm of the parent polymer, which has been reported to be 112 °C (41). We envision Flow-IEG will serve as an enabling tool for theory validation and future structure–property studies like these on a wide range of materials.
We further envision Flow-IEG can provide a practical means to generate unimolecular polymers in multigram to kilogram quantities, thus differentiating it from SPS. With an eye toward these applications, the Flow-IEG system was scaled to approximately four times the flow rate of the screening reactions, and the reactors were lengthened to maintain the appropriate reaction times. Monomer 1 was coupled at an initial concentration of 0.50 M in toluene to provide the ester dimer. Collection of the product over 1 h of steady-state operation provided 2.75 g of material in 84% isolated yield. Extrapolation of this production rate corresponds to Flow-IEG producing 66.0 g/d (24 kg/y) of coupled product. Further, scaling using known engineering principles will provide significant throughput improvements in future systems (42).
SI Materials and Methods
General Methods.
All commercially obtained solvents and reagents were used without further purification. Anhydrous THF was purified before use via a SG Water USA solvent column system. Flash column chromatography was performed using either Silicycle silica gel (230–400 mesh) or a Biotage Isolera flash purification system on SNAP KP-SIL columns. Analytical TLC was performed on 0.2-mm coated silica gel plates (EMD 60-F254) and visualized using a UV lamp (254 nm) and KMnO4 stain.
Characterization.
1H NMR and 13C NMR spectra were recorded on a Varian Inova 500 NMR spectrometer. Chemical shifts are reported in δ, parts per million (ppm) relative to the residual solvent peak of CDCl3. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, dt = doublet of triplets, ddt = doublet of doublet of triplets, dtd = doublet of triplet of doublets, m = multiplet), coupling constant (J) in Hertz (Hz), and integration. Gel permeation chromatography (GPC) was performed in THF on an HP/Agilent series 1100 GPC system and analyzed using a HP/Agilent refractive index detector. TGA was performed on a TA Instruments Discovery TGA. Samples were run in platinum TGA pans at a ramp rate of 10 °C/min from 50 °C to 600 °C. DSC was performed on a TA Instruments Discovery DSC. Samples were run in Tzero aluminum pans with hermetic lids. Data for the determination of glass transition temperature were taken from the third heating cycle of a run where the sample was cycled at a rate of 10 °C/min from −50 °C to 150 °C. Data for the determination of crystallization temperature were taken from the third heating cycle of a run where the sample was cycled at a rate of 5 °C/min from −50 °C to 150 °C. MALDI MS was conducted on a Bruker model Microflex MALDI-TOF instrument using an α-cyano-4-hydroxycinnamic acid matrix.
Flow equipment.
Syrris Asia model pumps with the yellow 50-μL/100-μL syringes were used for pumping all reagent or solvent solutions unless otherwise noted. A Harvard Apparatus syringe pump, model PHD2000, with an 8-mL stainless steel syringe was used to flow the copper solution. All PFA tubing and connectors, mixers, back pressure regulators, etc. were purchased from IDEX Health & Science. PFA tubing with a 0.03-in inner diameter was used unless otherwise noted. T-mixers with an internal diameter of 0.02 in were used for mixing all of the reagents. Heating the reactors was accomplished by immersion in an oil bath (Fig. S2) with temperature regulation provided by an IKA hot plate. The membrane liquid–liquid separator was purchased from Zaiput Flow Technologies and used as is.
Fig. S2.
The optimization results of running the CuAAC in flow. Me6TREN turned out to be a superior combination of both solubility and activity for this reaction.
Synthetic Methods.
(3-Bromoprop-1-yn-1-yl)triisopropylsilane (45), the oligoethylene glycol monomer (2) (32), and the oligoethylene glycol dimer (32) were prepared according to previously published procedures.
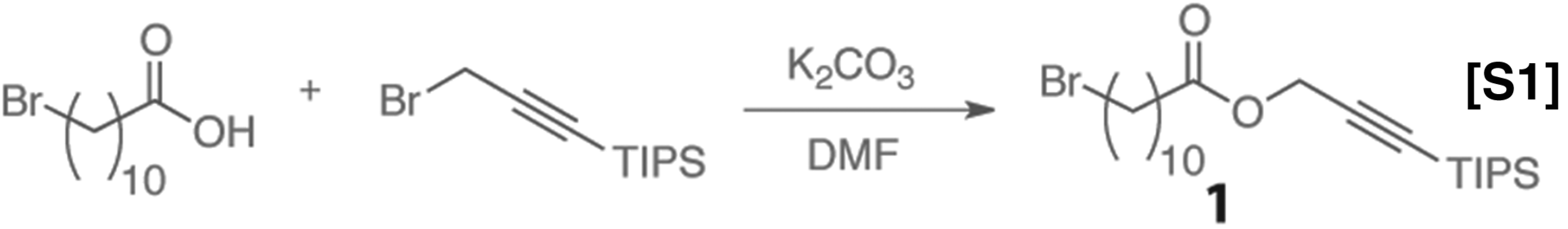
The synthesis of 3-(Triisopropylsilyl)prop-2-yn-1-yl 11-bromoundecanoate is shown in Eq. S1. To a flame-dried 100-mL round bottom flask equipped with a stir bar was added 11-bromoundecanoic acid (2.77 g, 10.44 mmol), 15 mL dry DMF, (3-bromoprop-1-yn-1-yl)triisopropylsilane (4.00 g, 14.6 mmols), and anhydrous K2CO3 (2.76 g, 20 mmol). The reaction mixture was capped with a rubber septa and stirred overnight. The solution was quenched with saturated solution of ammonium chloride (75 mL) and extracted three times with Et2O (120 mL total). The combined organic phases were washed with brine and dried over MgSO4. After solvent removal, chromatography (2.5% EtOAc:Hex) afforded the product as a colorless oil (3.77 g, 79% yield); 1H NMR (500 MHz, CDCl3) δ 4.70 (s, 2H), 3.40 (t, J = 6.9 Hz, 2H), 2.34 (t, J = 7.5 Hz, 2H), 1.85 (q, J = 8.6 Hz, 2H), 1.63 (q, J = 7.4 Hz, 2H), 1.44–1.24 (m, 12H), 1.06 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 173.00, 101.31, 88.13, 52.71, 34.19, 34.09, 32.98, 29.53, 29.46, 29.37, 29.17, 28.90, 28.31, 25.04, 18.67, 11.23.; HR-MS (TOF-ESI) calc for C23H43BrO2Si: [M+H]+ 459.2288. Found: [M+H]+ 459.2280.
Reaction Optimization.
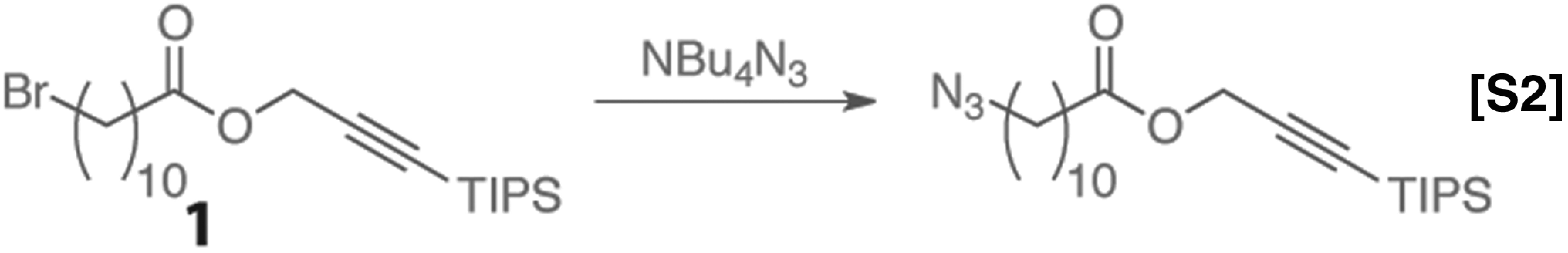
Azide displacement.
Ester 1 was dissolved in toluene at a concentration of 0.50 M, and a separate solution of TBAA was prepared at a concentration of 0.55 M. The solutions were loaded into separate vials and mixed in-flow using a T-mixer and allowed to react at the temperatures and times specified in Fig. S1. The system was allowed to run for three residence times to reach steady state before the product was collected for analysis. The product distribution was measured by 1H-NMR.
Fig. S1.

The results of reaction optimization for the azide displacement (Left) and TBAF deprotection (Right) of substrate 1.
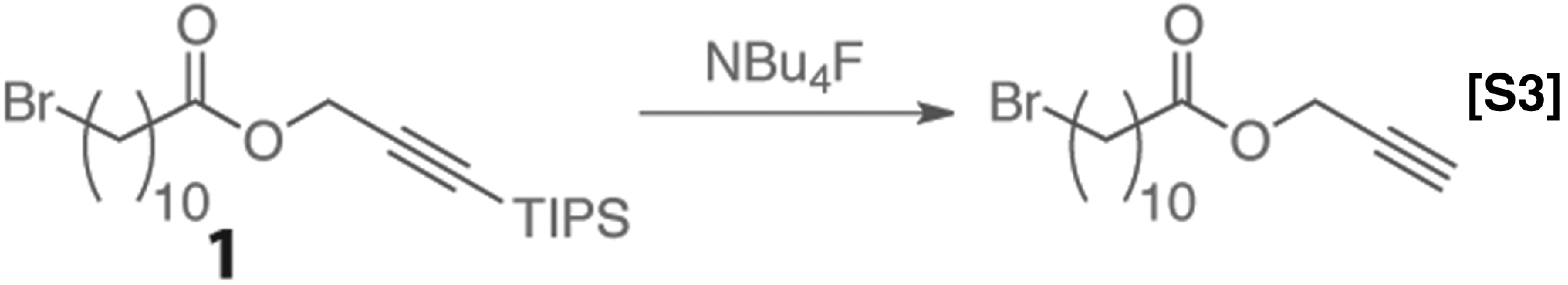
TBAF deprotection.
Ester 1 was dissolved in toluene at a concentration of 0.50 M, and a separate solution of TBAF was prepared at a concentration of 0.55 M. The solutions were loaded into separate vials and mixed in-flow using a T-mixer and allowed to react at the temperatures and times specified in Fig. S1. The system was allowed to run for three residence times to reach steady state before the product was collected for analysis. The product distribution was measured by 1H-NMR.
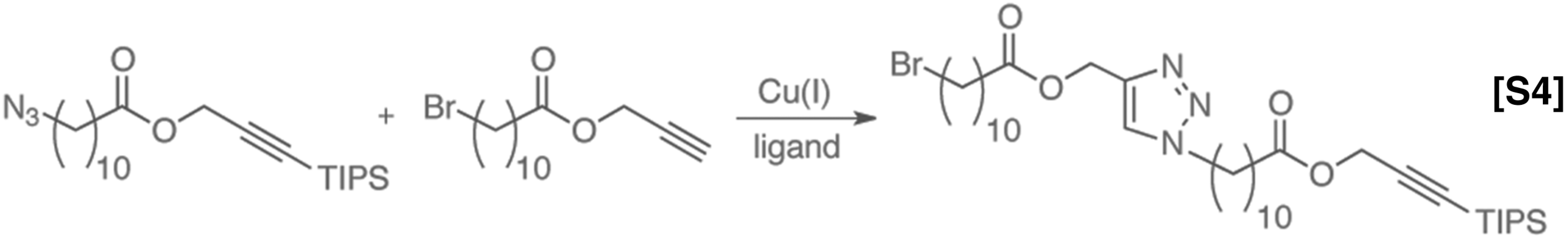
Copper-catalyzed azide/alkyne cycloaddition.
The coupling partners were prepared and mixed in solution at a total concentration of 0.50 M in toluene. A 1:1 mixture of copper source and ligand was prepared in THF at a concentration of 0.10 M. All solutions were degassed by sparging with argon for 20 min before use. The flow rates were calibrated to lead to the desired catalyst loading in each reaction. The system was allowed to run for three residence times to reach steady state before the product was collected for analysis. Solubility limited most of the Cu/ligand concentrations, as any insolubility in flow chemistry leads to reactor clogging. The results of the reaction trails are shown in Fig. S2.
General procedure for running the Flow-IEG system.
All Flow-IEG reactions were performed in the system shown in Fig. S3. The substrates of interest were dissolved in toluene or chlorobenzene to generate a solution of ∼10 wt%. The TBAA and TBAF were dissolved in THF to generate a solution that contained 1.1 molar equivalents of TBAA and TBAF to substrate compared with substrate. As shown in Fig. S3, equal flow rates of the TBAA, TBAF, and the substrate solutions were used. All solvent and reagent solutions were degassed by sparging with argon for 20 min before use. Syrris Asia pumps equipped with 50/100-μL syringes are used for all solvents and reagents except the Me6TREN/CuI solution, which used a Harvard PHD2000 pump with an 8-mL stainless steel syringe. The system was allowed to run for 2.5 residence times to reach steady state before collecting and analyzing product composition. Heating the reactors was accomplished by immersing the PFA tubing into an oil bath. A photograph of the setup with parts labeled can be seen in Fig. S4.
Fig. S3.
Flow diagram for the Flow-IEG system used to build the polymers described in this manuscript.
Fig. S4.
Picture of the Flow-IEG system while it is running.
Fig. S5.
1H-NMR spectra (Left) and SEC chromatograms (Right) for the doubly alternating copolymers.
Fig. S6.
1H-NMR spectra for the branched copolymers (SEC in main text).
Fig. S7.
TGA of the oligomers studied in this manuscript.
Example procedure for running the Flow-IEG system.
Two 0.50-M solutions of 1 were prepared by dissolving 690 mg of 1 in toluene at a total volume of 3 mL. A 0.55-M solution of TBAA was prepared by dissolving 469 mg TBAA in THF at a total volume of 3 mL. A 0.55 M solution of TBAF was prepared by dissolving 521 mg TBAF•3 H2O in THF at a total volume of 3 mL. A 0.10-M solution of CuI/Me6TREN was prepared by dissolving 92 mg Me6TREN into 4 mL degassed THF and transferring that solution into a 6-mL vial containing 76 mg CuI. All reagent and solvent solutions were degassed before use. The pumps were turned on, and the system was allowed to run 25 min to reach steady state. The product was collected for 49 min. The solvent was evaporated, and the product was loaded directly on a column and run at 40% (vol/vol) EtOAc/Hex to yield the product as a white solid (618 mg, 86% yield from 1).
Comment regarding yields.
Although all our individual control reactions show full conversion, the yields in the full system are not quantitative. The reviewers of this manuscript raised this question, and we have also been asked it in other venues; thus, we wanted to provide a complete answer to this query. We believe the less-than-quantitative yields for the Flow-IEG system are a result of a combination of both engineering and chemistry factors. First, the Syrris Asia pumps we are using rely on a piston action to draw material from a reservoir and pump it through our reactors. Each time the pistons transition, there is a small “hiccup” that temporarily throws our system out of steady state. These repeated fluctuations subtly alter stoichiometry over time and affect yield. Second, although the membrane separator is a key enabling tool for our system, there are random occurrences of slugs of organic phase not crossing the membrane and thus being shuttled to waste. Pressure fluctuations caused by the pump’s piston action most likely exaggerate the separator failures and affect our overall yields. Further, the high temperatures we use for our cycloaddition reaction does seem to lead to a small amount (1–3%) of Glaser coupling of the terminal alkynes. Last, we believe the consistently lower yields seen with oligomers containing the oligoethylene glycol monomer are a result of a small amount of those polymers partitioning to the aqueous phase. The combination of these three factors leads to less than quantitative yields of the course of the Flow-IEG system. Overall, however, the yields we observe are synthetically useful, especially considering the ultimate ease of running Flow-IEG compared with its batch counterpart.
Characterization.
Polyesters: 1H-NMR spectra and SEC chromatograms in main text.
Ester dimer (n = 1).
1H NMR (500 MHz, CDCl3) δ 7.57 (s, 1H), 5.20 (s, 2H), 4.69 (s, 2H), 4.32 (t, J = 7.3 Hz, 2H), 3.38 (t, J = 6.8 Hz, 2H), 2.32 (dt, J = 9.9, 7.6 Hz, 4H),1.94–1,79 (m, 4H), 1.65–1.56 (m, 4H), 1.43–1.22 (m, 22H), 1.06 (s, 18H);); 13C-NMR (150 MHz, CDCl3) δ 174.05, 173.23, 143.19, 123.81, 101.43, 88.39, 57.82, 52.91, 50.70, 34.44, 34.36, 34.36, 33.11, 30.58, 29.65, 29.62, 29.60, 29.58, 29.49, 29.48, 29.36, 29.31, 29.27, 29.03, 28.45, 26.78, 25.18, 25.12, 18.84, 11.39. GPC: Tret= 28.0 min, Đ = 1.009.
Ester tetramer (n = 3).
1H NMR (500 MHz, CDCl3) δ 7.58 (s, 3H), 5.21 (d, J = 1.0 Hz, 6H), 4.70 (s, 2H), 4.33 (t, J = 7.3 Hz, 6H), 3.31 (t, J = 6.8 Hz, 2H), 2.37–2.28 (m, 8H), 1.96–1.80 (m, 8H), 1.66–1,55 (m, 8H), 1.46–1.22 (m, 44H), 1.06 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 174.05, 174.02, 173.23, 143.16, 123.82, 101.43, 88.40, 57.82, 52.91, 50.70, 50.70, 34.43, 34.42, 34.38, 34.35, 33.11, 30.58, 30.57, 29.64, 29.62, 29.60, 29.58, 29.56, 29.49, 29.47, 29.45, 29.36, 29.34, 29.31, 29.27, 29.24, 29.02, 28.44, 26.78, 26.76, 25.17, 25.12, 25.10, 18.83, 11.38. GPC: Tret= 27.3 min, Đ = 1.004.
Ester octamer (n = 7).
1H NMR (500 MHz, CDCl3) δ 7.57 (s, 7H), 5.19 (s, 14H), 4.69 (s, 2H), 4.32 (t, J = 7.3 Hz, 14H), 3.39 (t, J = 6.9 Hz, 2H), 2.30 (t, J = 7.6 Hz, 16H), 1.96–1.80 (m, 16H), 1.66–1,55 (m, 16H), 1.46–1.22 (m, 92H), 1.06 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 174.00, 173.22, 143.14, 123.82, 123.81, 101.42, 88.39, 57.81, 52.90, 50.69, 34.43, 34.41, 34.39, 34.35, 33.10, 30.56, 29.64, 29.61, 29.57, 29.55, 29.48, 29.46, 29.44, 29.35, 29.33, 29.30, 29.26, 29.23, 29.01, 28.44, 26.75, 25.09, 18.83, 11.38. GPC: Tret= 25.0 min, Đ = 1.012.
Alternating copolymers: 1H-NMR spectra and SEC chromatograms in main text.
Alternating copolymer (dimer).
1H NMR (500 MHz, CDCl3) δ 7.78 (s, 1H), 5.20 (s, 2H), 4.53 (t, J = 5.5 Hz, 2H), 4.23 (s, 2H), 3.86 (t, J = 5.5 Hz, 2H), 3.71–3.58 (m, 8H), 3.38 (t, J = 7.0 Hz, 2H), 2.31 (t, J = 7.6 Hz, 2H), 1.80 (q, J = 7.6 Hz, 2H), 1.64–1.54 (m, 2H), 1.42–1.23 (m, 12H), 1.05 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 173.90, 143.05, 125.15, 103.39, 88.04, 70.83, 70.77, 70.73, 69.70, 68.95, 59.44, 57.77, 53.77, 50.58, 34.42, 33.83, 30.77, 29.63, 29.61, 29.48, 29.38, 28.80, 25.12, 18.87, 11.42, 7.69. GPC: GPC: Tret= 27.3 min, Đ = 1.006.
Alternating copolymer (tetramer, n = 1).
1H NMR (500 MHz, CDCl3) δ 7.78 (s, 1H), 7.77 (s, 1H), 7.52 (s, 1H), 5.19 (s, 2H), 5.18 (s, 2H), 4.66 (s, 2H), 4.52 (m, 4H), 4.31 (t, J = 7.3 Hz, 2H), 4.22 (s, 2H), 3.85 (t, J = 7.4 Hz, 2H), 3.84 (t, J = 6.2 Hz, 2H), 3.72–3.58 (m, 16H), 3.31 (t J = 7.0 Hz, 2H), 2.30 (t, J = 7.6, 4H), 1.93–1.71 (m, 4H), 1.64–1.54 (m, 4H), 1.44–1.17 (m, 24H), 1.05 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 173.58, 144.93, 142.76, 124.97, 124.94, 122.53, 103.25, 87.74, 70.59, 70.57, 70.55, 70.52, 70.48, 69.69, 69.45, 68.73, 64.67, 59.19, 57.54, 53.67, 50.39, 50.34, 34.19, 34.17, 34.16, 32.87, 30.38, 29.41, 29.36, 29.34, 29.25, 29.23, 29.13, 29.11, 29.03, 28.78, 28.20, 26.55, 24.89, 24.87, 18.66, 11.19. GPC: Tret= 26.6 min, Đ = 1.007.
Alternating copolymer (octamer, n = 3).
1H NMR (500 MHz, CDCl3) δ 7.77 (s, 3H), 7.76 (s, 1H), 7.52 (s, 3H), 5.18 (s, 2H), 5.17 (s, 6H), 4.64 (s, 6H), 4.50 (t, 8H, J = 5 Hz), 4.30 (t, 6H, J = 10 Hz), 4.21 (s, 2H), 3.84 (t, 8H, J = 5 Hz), 3.72–3.48 (m, 32H), 3.34 (t, 2H, J = 10 Hz), 2.28 (td, 8H, J = 5 & 10 Hz), 2.00–1.65 (m, 10H), 1.65–1.45 (m, 8H), 1.40–1.10 (m, 48H), 1.03 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 173.86, 145.15, 142.99, 142.97, 125.14, 125.11, 122.66, 103.39, 88.01, 70.81, 70.76, 70.74, 70.70, 69.90, 69.67, 68.93, 64.89, 59.41, 57.74, 50.61, 50.55, 50.54, 45.48, 34.40, 34.38, 33.80, 32.89, 30.74, 30.59, 29.64, 29.60, 29.57, 29.55, 29.45, 29.43, 29.35, 29.33, 29.24, 29.11, 28.76, 27.12, 26.76, 25.10, 25.08, 18.85, 18.84, 11.40, 7.70. GPC: Tret= 25.6 min, Đ = 1.009.
Alternating copolymer (hexadecamer, n = 7).
1H NMR (500 MHz, CDCl3) δ 7.78 (s, 8H), 7.53 (s, 7H), 5.19 (s, 16H), 4.66 (s, 14H), 4.52 (t, J = 5.1 Hz, 16H), 4.32 (t, J = 7.3 Hz, 14H), 4.23 (s, 2H), 3.86 (t, J = 5.0 Hz, 16H), 3.73–3.56 (m, 64H), 3.31 (t, J = 7.0 Hz, 2H), 2.30 (t, J = 7.6 Hz, 16H), 1.93–1.71 (m, 20H), 1.64–1.54 (m, 16H), 1.43–1.21 (m, 104H), 1.05 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 173.90, 145.18, 143.00, 125.17, 122.70, 103.41, 88.05, 70.83, 70.79, 70.78, 70.73, 69.93, 69.70, 68.96, 64.92, 59.44, 57.77, 50.65, 50.58, 45.51, 34.43, 34.42, 32.92, 30.61, 30.00, 29.67, 29.60, 29.58, 29.48, 29.46, 29.38, 29.35, 29.27, 29.14, 27.15, 26.79, 25.13, 25.11, 18.88, 11.43. GPC: Tret= 24.5 min, Đ = 1.032.
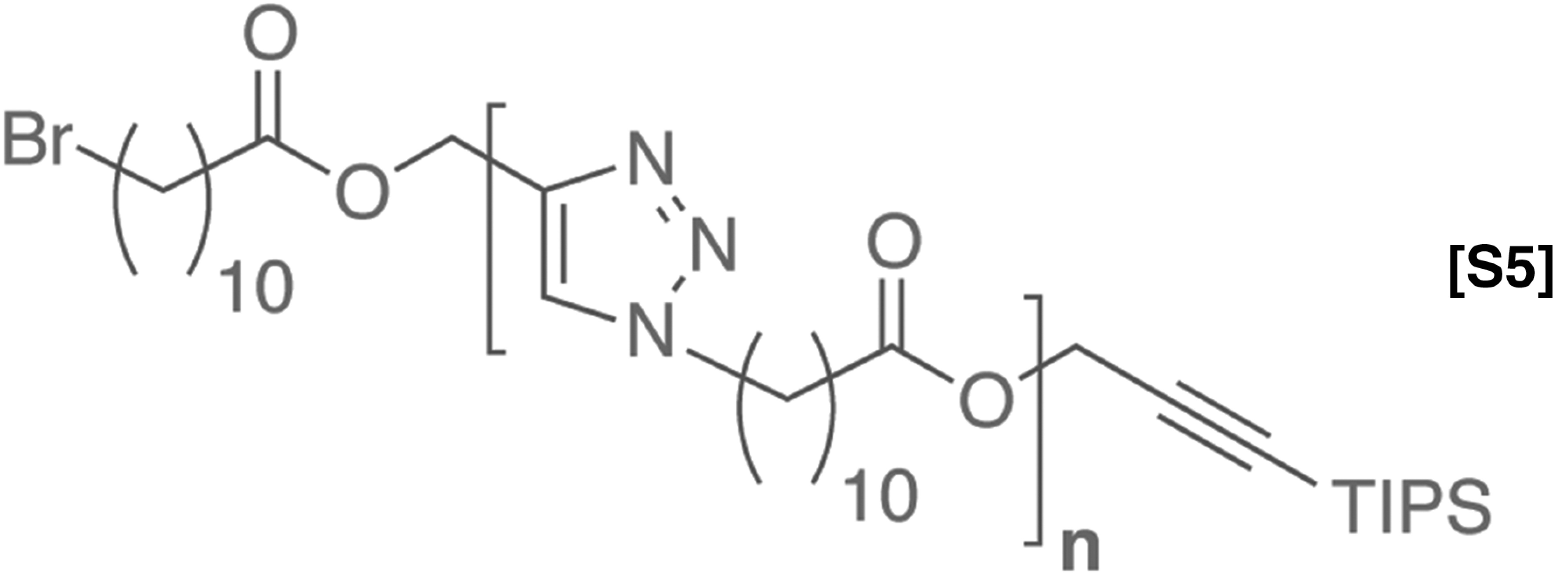
Doubly alternating copolymer (tetramer).
1H NMR (500 MHz, CDCl3) δ 7.78 (s, 1H), 7.73 (s, 1H), 7.57 (s, 1H), 5.20 (s, 2H), 5.19 (s, 2H), 4.66 (s, 2H), 4.53 (t, J = 5.1 Hz, 4H), 4.33 (t, J = 7.3 Hz, 2H), 4.23 (s, 2H), 3.86 (t, J = 7.4 Hz, 4H), 3.72–3.56 (m, 16H), 3.40 (t, J = 6.8 Hz, 2H), 2.31 (td, J = 7.6, 3.2 Hz, 4H), 1.93–1.78 (m, 4H), 1.64–1.54 (m, 4H), 1.44–1.19 (m, 24H), 1.05 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ 174.41, 174.26, 145.41, 143.54, 143.37, 125.55, 124.53, 124.21, 103.81, 88.43, 72.03, 71.21, 71.17, 71.12, 71.08, 70.25, 70.14, 70.08, 69.33, 65.27, 65.18, 59.81, 58.20, 58.14, 51.07, 50.95, 50.91, 50.90, 43.45, 34.80, 34.79, 34.77, 33.48, 31.15, 30.95, 30.38, 30.02, 29.97, 29.96, 29.94, 29.84, 29.83, 29.73, 29.62, 29.40, 29.17, 28.82, 27.13, 25.49, 25.48, 19.26, 11.80. GPC: Tret= 26.5 min, Đ = 1.005.
Doubly alternating copolymer (octamer).
1H NMR (500 MHz, CDCl3) δ 7.79 (s, 2H), 7.73 (s, 2H), 7.58 (s, 2H), 7.53 (s, 1H), 5.20–5.18 (m, 8H), 4.67 (s, 4H), 4.66 (s, 2H), 4.56–4.50 (m, 8H), 4.36–4.30 (m, 6H), 4.23 (s, 2H), 3.86 (t, 8H, J = 5 Hz), 3.72–3.57 (m, 32H), 3.40 (t, 2H, J = 6.9 Hz), 2.36–2.28 (m, 8H), 1.94–1.80 (m, 10H), 1.65–1.54 (m, 8H), 1.44–1.20 (m, 48H), 1.04 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ174.43, 174.40, 174.28, 145.57, 145.42, 143.52, 143.38, 125.55, 124.53, 124.21, 123.09, 103.81, 88.45, 71.23, 71.21, 71.19, 71.18, 71.16, 71.13, 71.10, 70.27, 70.27, 70.15, 70.09, 69.34, 65.32, 65.19, 59.83, 58.21, 58.21, 58.16, 51.09, 51.03, 50.96, 50.91, 50.90, 45.90, 34.82, 34.80, 33.31, 31.01, 30.96, 30.05, 29.99, 29.98, 29.96, 29.86, 29.85, 29.75, 29.73, 29.66, 29.64, 29.53, 27.54, 27.18, 27.15, 25.51, 25.50, 25.49, 19.27, 11.81. GPC: Tret= 25.5 min, Đ = 1.005.
Doubly alternating copolymer (hexadecamer).
1H NMR (500 MHz, CDCl3) δ 7.77 (s, 4H), 7.72 (s, 4H), 7.57 (s, 4H), 7.53 (s, 3H), 5.20–5.18 (m, 16H), 4.65 (bs, 14H), 4.56–4.50 (m, 16H), 4.38–4.27 (m, 14H), 4.22 (s, 2H), 3.85 (t, 16H, J = 5.1 Hz), 3.72–3.52 (m, 64H), 3.38 (t, 2H, J = 7.0 Hz), 2.30 (t, 16H, J = 7.5 Hz), 1.94–1.80 (m, 20H), 1.65–1.54 (m, 18H), 1.44–1.20 (m, 96H), 1.05 (s, 18H); 13C-NMR (150 MHz, CDCl3) δ174.41, 174.29, 145.58, 145.45, 143.57, 143.52, 143.39, 125.57, 124.58, 124.55, 124.23, 123.13, 123.10, 103.82, 88.46, 71.22, 71.20, 71.17, 71.14, 71.11, 70.33, 70.29, 70.15, 70.09, 69.35, 65.32, 65.20, 59.84, 58.21, 58.16, 54.16, 51.10, 51.06, 50.98, 50.93, 45.91, 34.83, 34.81, 33.32, 31.01, 30.97, 29.99, 29.99, 29.97, 29.86, 29.75, 29.74, 29.66, 29.64, 27.55, 27.16, 25.50, 25.49, 19.28, 11.82. GPC: Tret= 24.4 min, Đ = 1.021.
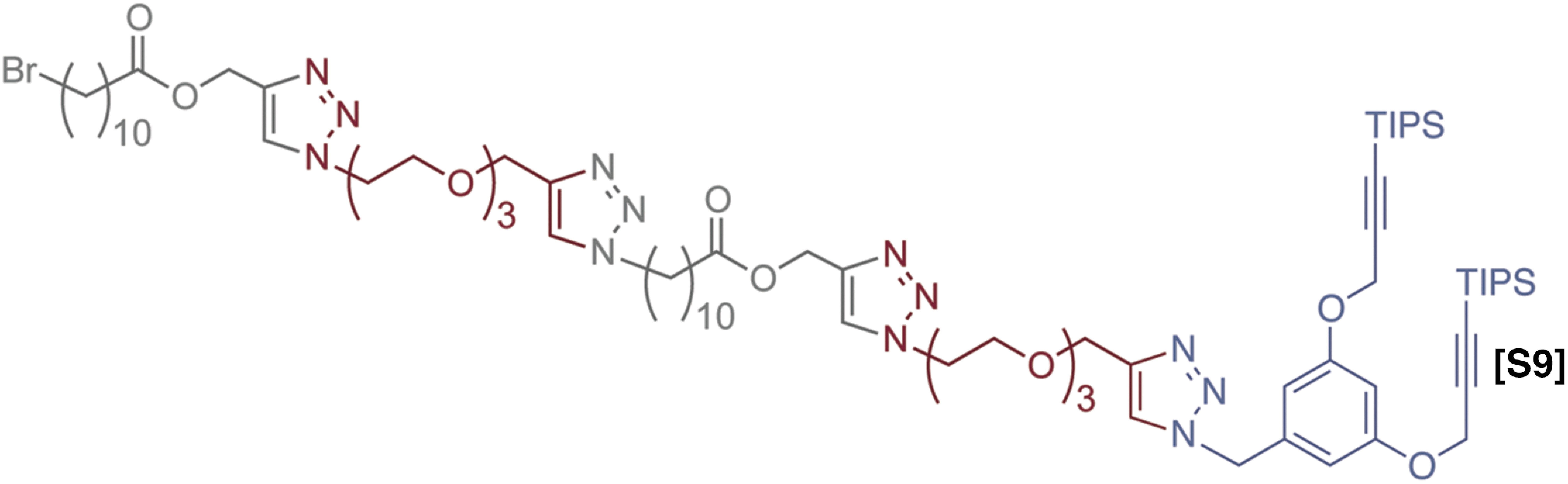
Alternating copolymer with branching unit.
1H NMR (500 MHz, CDCl3) δ 7.77 (s, 1H), 7.75 (s, 1H), 7.52 (s, 1H), 7.43 (s, 3H), 6.63 (t, J = 2.3 Hz, 1H), 6.49 (d, J = 2.2 Hz, 2H), 5.38 (s, 2H), 5.17 (s, 2H), 5.16 (s, 2H), 4.65 (s, 4H), 4.62 (s, 2H), 4.40–4.45 (m, 4H), 4.30 (t, J= 7.3 Hz, 2H), 3.87–3.80 (m, 4H), 3.69–3.54 (m, 16H), 3.37 (t, J= 7.0 Hz, 2H), 2.34–2.22 (m, 4H), 1.90–1.64 (m, 6H), 1.62–1.53 (m, 4H), 1.48–1.18 (m, 26H), 1.01 (s, 36H); 13C-NMR (150 MHz, CDCl3) δ 174.30, 174.26, 159.90, 146.03, 145.57, 143.40, 143.38, 137.01, 125.56, 125.55, 123.19, 123.10, 108.68, 103.45, 102.05, 100.49, 90.29, 71.22, 71.20, 71.18, 71.16, 70.36, 70.31, 70.08, 70.07, 65.30, 65.27, 59.93, 58.16, 58.14, 57.57, 54.76, 54.18, 51.03, 50.96, 45.90, 34.81, 34.79, 34.21, 33.31, 31.15, 31.00, 30.05, 29.99, 29.96, 29.87, 29.85, 29.76, 29.74, 29.66, 29.52, 29.18, 27.54, 27.18, 25.51, 25.49, 25.00, 20.50, 19.20, 14.45, 11.74. GPC: Tret= 26.2 min, Đ = 1.004.
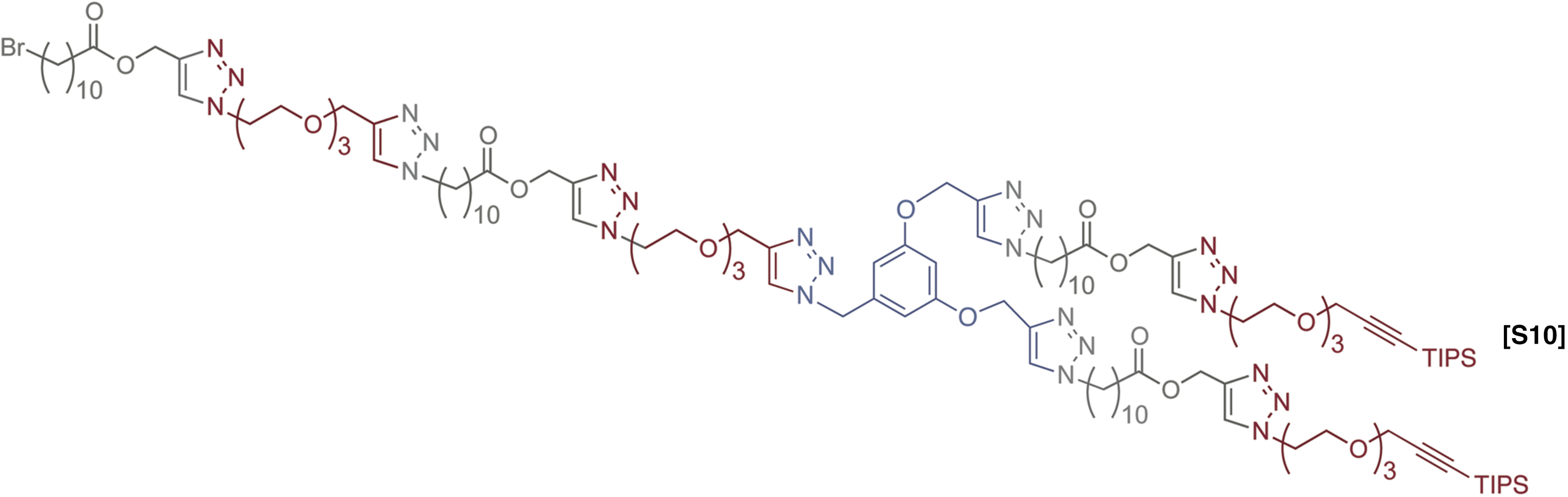
Fully branched alternating copolymer.
1H NMR (500 MHz, CDCl3) δ 7.77–7.75 (m, 4H), 7.62 (s, 2H), 7.52 (s, 1H), 7.51 (s, 1H), 6.61 (t, J = 2.2 Hz, 1H), 6.50 (d, J = 2.2 Hz, 2H), 5.42 (s, 2H), 5.19 (s, 4H), 5.18 (s, 2H), 5.16 (s, 2H), 5.12 (bs, 4H), 4.66 (s, 2H), 4.64 (s, 2H), 4.54–4.45 (m, 8H), 4.37–4.28 (m, 6H), 4.22 (s, 4H), 3.88–3.80 (m, 8H), 3.73–3.54 (m, 32H), 3.40 (t, J = 7.0 Hz, 2H), 2.33–2.26 (m, 8H), 1.95–1.70 (m, 10H), 1.62–1.54 (m, 8H), 1.44–1.16 (m, 52H), 1.02 (s, 36H) 13C-NMR (150 MHz, CDCl3) δ 174.32, 174.28, 174.27, 160.59, 146.14, 145.56, 144.04, 143.41, 143.37, 137.66, 125.56, 125.54, 123.45, 123.40, 123.09, 108.11, 103.80, 102.44, 88.44, 71.22, 71.20, 71.18, 71.17, 71.16, 71.12, 70.42, 70.31, 70.09, 70.07, 69.35, 65.31, 62.75, 59.83, 58.16, 58.15, 54.71, 51.17, 51.03, 50.97, 50.96, 50.95, 45.91, 34.82, 34.80, 33.31, 31.01, 30.99, 30.06, 29.99, 29.97, 29.88, 29.86, 29.77, 29.75, 29.66, 29.53, 27.54, 27.18, 25.50, 19.27, 11.82. GPC: Tret= 25.4 min, Đ = 1.062.
Making Sequence and Architecturally Defined Macromolecules
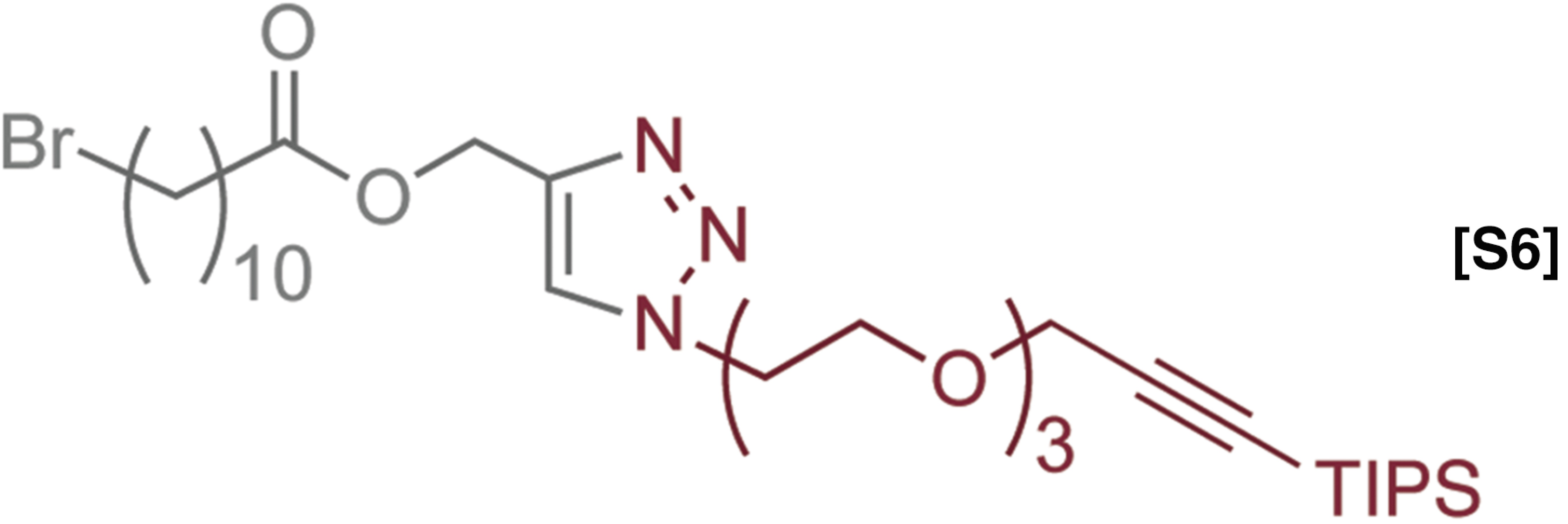
The Flow-IEG system in Fig. 3A can be adapted to different monomers while maintaining its efficiency and user-friendly nature. To tackle such a challenge, we chose to incorporate monomer 2 into the Flow-IEG system because of its previous utility in IEG and hydrophilic nature (Fig. 5) (32). During the processes of testing each of the three reactions individually with 2, we discovered that azide displacement of the alkyl chloride with TBAA required 7.5 min at 130 °C; thus, the reactor for the azide displacement was lengthened accordingly. To demonstrate the potential of Flow-IEG for sequence-defined polymer synthesis, a perfectly alternating (ABAB)n polymer and its structural isomer, a polymer with the repeat unit (AABB)n, were targeted. These materials demonstrate the power and flexibility of Flow-IEG, allowing the user to combine either two different monomers in the case of the (ABAB)n polymer or two different dimers in the case of the (AABB)n polymer. Further, synthesis of these two structural isomers will probe how sequence influences polymer properties.
Fig. 5.
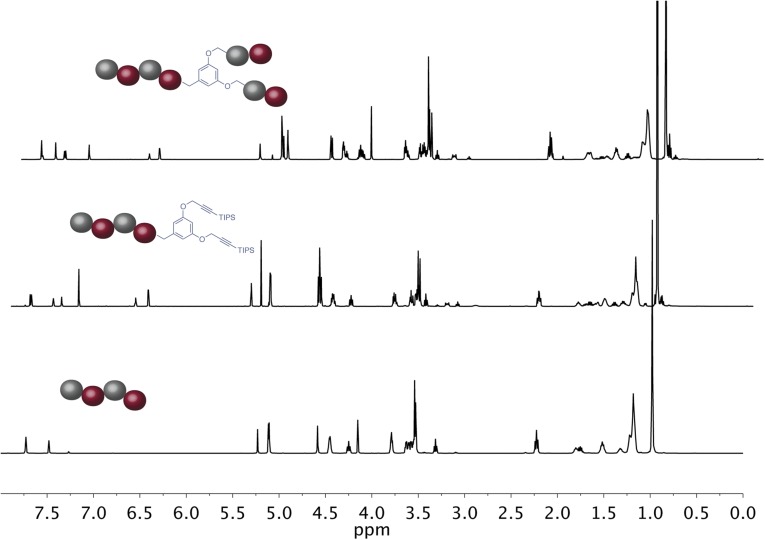
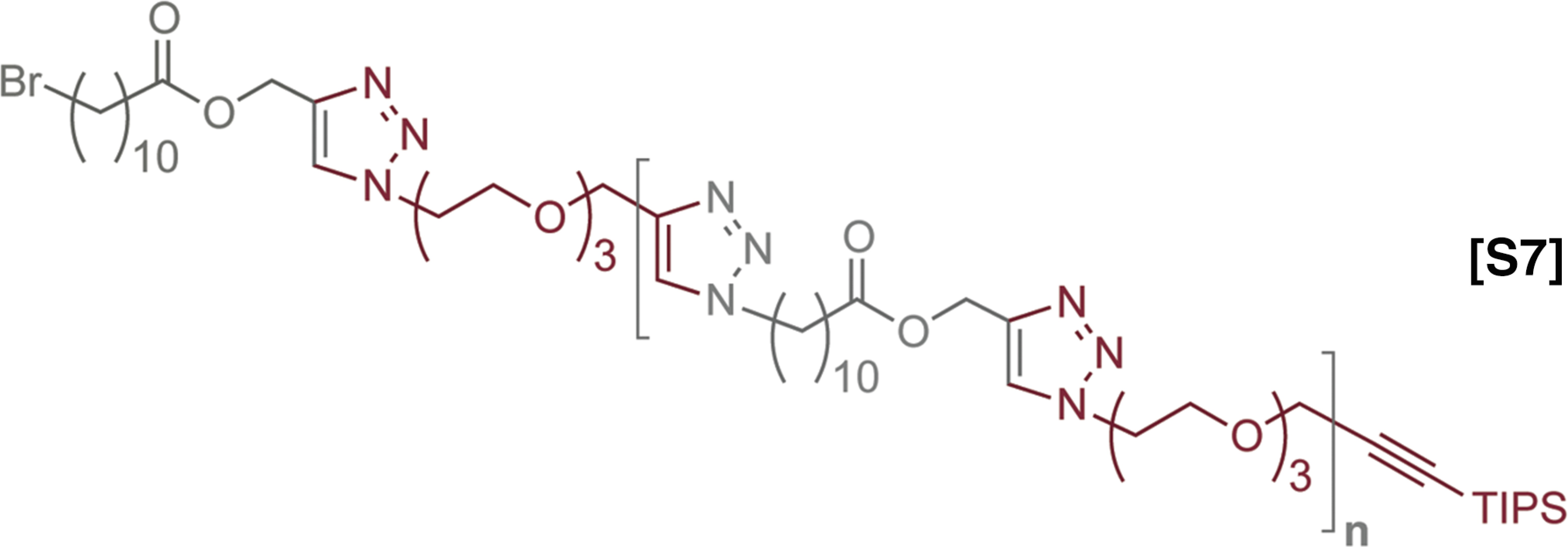
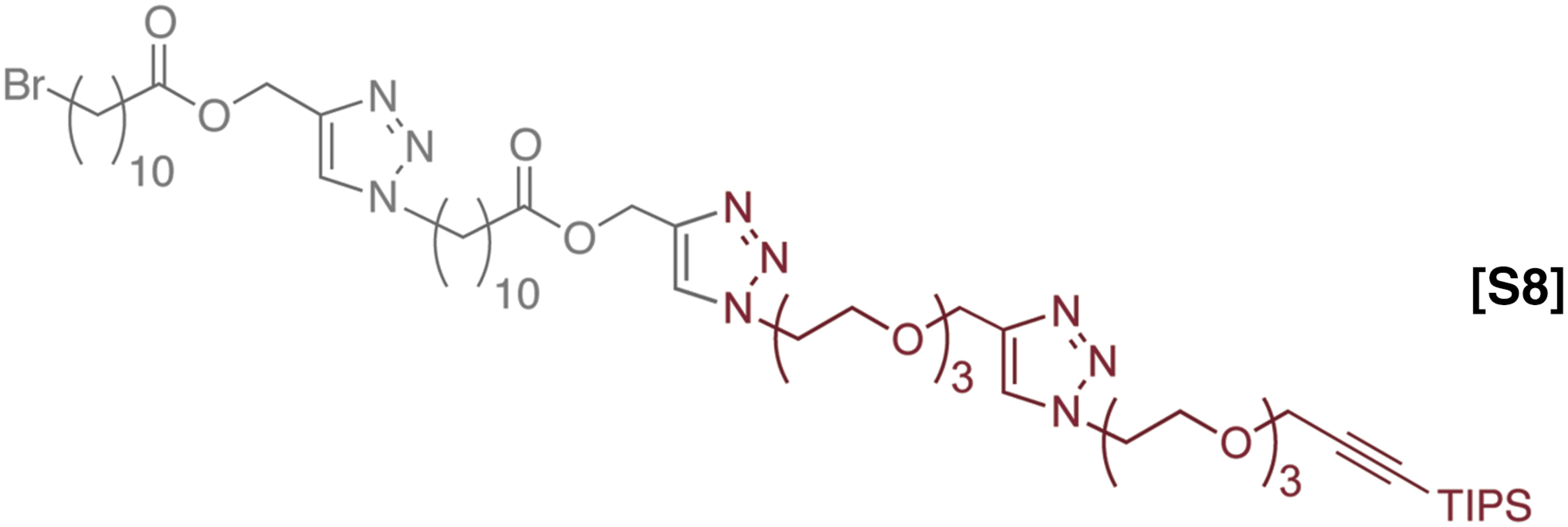
The synthesis and characterization of sequence defined unimolecular polymers by Flow-IEG. (A) Legend for the two monomers used. (B) Example of one round of Flow-IEG incorporating 2. (C) The yields of each Flow-IEG iteration are shown, along with the (D) SECs and the (E) 1H-NMR spectra of the perfectly alternating (ABAB)n unimolecular copolymer.
The synthetic sequences and overall yields of Flow-IEG for these materials are summarized in Fig. 5C. Yields remain high over the three-step Flow-IEG sequence, with variations possibly due to fluctuations in the membrane separator operation with the more hydrophilic materials. As a result of the improved solubility of these sequence-defined polymers, hexadecamers could be synthesized from octamers by Flow-IEG, providing access to unimolecular, sequence-defined polymers with molecular weights reaching 4,023 g/mol in >95% purity. The SECs and 1H-NMRs of the perfectly alternating copolymer (ABAB)n are shown in Fig. 5 D and E, respectively, highlighting both the unimolecular nature and efficient growth of these sequence-defined macromolecules. A closer examination of the 1H-NMRs provides key structural insights (Fig. 5E). The appearance of a second triazole proton (7.51 ppm) is clearly visible in the aromatic region when two AB-dimers are coupled to make an ABAB tetramer. Further, at 7.78 ppm, two distinct singlets are observed signifying the two chemically inequivalent triazole “end-group” protons in the tetramer product. As the oligomers are coupled to the octamer and subsequently 16mer, the singlets coalesce into a single peak as a result of the triazole protons within the polymer chain having a nearly identical chemical shift and those beginning to dominate the spectrum in longer polymer chains. This same phenomenon can be observed with the peak of the protons between the ester and triazole at 5.20 ppm. Further, the peak assigned to the propargylic protons of the polymer end-group at 4.24 ppm gets smaller compared with the peaks of protons from the polymer backbone as the polymer grows in size, demonstrating the increasing molecular weight of the Flow-IEG products.
Comparing the structure–property relationships of these isomeric copolymers illustrates the important role that sequence plays in influencing polymer properties. The Tds of the two polymers show similar trends and increase in the progression from tetramer to octamer to hexadecamer (TGA in SI Materials and Methods). Interestingly, the melting behavior is significantly different between the structural isomers. For instance, although the tetramers of the two samples show similar melting behavior, the (AABB)n octamer has a melting transition at 41 °C, whereas the alternating octamer (ABAB)n shows no Tm under the same conditions. Comparing the two 16-mers, the (AABB)n copolymer has a Tm at 44 °C and the (ABAB)n has two melting transitions at 29 °C and 64 °C. All samples that do have crystalline properties exhibit cold crystallization, where crystallization occurs between the Tg and Tm during heating (43). The structure–property relationships of these sequence-defined, unimolecular structural isomers demonstrates the important role that sequence plays in determining properties and how Flow-IEG can be used to evaluate and even tune these physical properties.
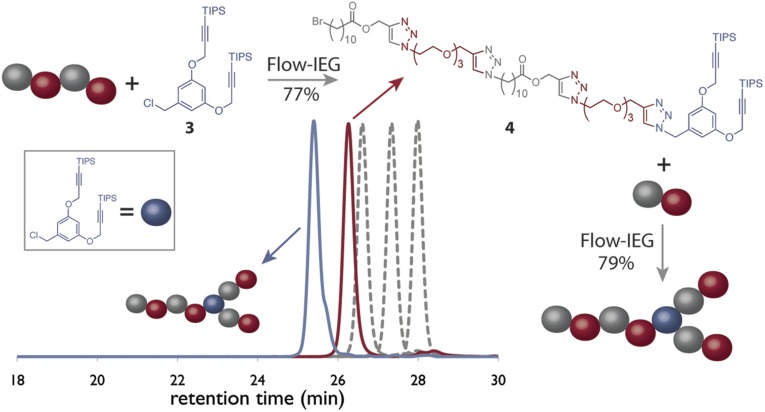
Flow-IEG is not only useful for the synthesis of sequence-defined macromolecules but it can also be modified to generate architecturally defined materials. The concept of IEG has many similarities to dendrimer synthesis, and we drew inspiration from the synthesis of triazole-based dendrimers to design monomer 3 (44). The two protected alkynes in 3 provide a branching point within a polymer and, by judiciously choosing when to introduce 3, the branching point can be located precisely within a sequence-defined, unimolecular macromolecule (Fig. 6). This degree of control is necessary for fundamental studies on chain architecture and multivalency.
Fig. 6.
Control of polymer architecture is accomplished through the use of monomer 3. The synthesis by Flow-IEG and SEC traces of the copolymers are shown.
Conclusions
Flow-IEG is an enabling tool for the semiautomated synthesis of sequence-defined unimolecular macromolecules. The simplicity of Flow-IEG compared with the alternative batch procedures, its capacity for scale-up, and the semiautomated nature of this methodology make it attractive for both exploratory and large-scale applications in polymer chemistry. Specifically, Flow-IEG is one of the only methodologies available for the modular synthesis of unimolecular polymers that has the ability to control both sequence and architecture independently. This advance has important consequences for potential structure–activity screening of synthetic polymers in biomedical applications, as Flow-IEG can provide synthetic polymer libraries where the influences of functional group density, sequence, and chain architecture can by assayed for a desired activity.
Flow-IEG represents a technology for the semiautomated synthesis of sequence and architecturally defined synthetic polymers. Flow-IEG telescopes three reactions and an in-line purification in an uninterrupted system with a total residence time of under 10 min, and a modest scale-up provides the capacity to produce significant quantities (≥60 g/d, as shown herein). Flow-IEG is also an enabling tool for polymer theory validation and structure–property studies on a wide range of materials. Overall, Flow-IEG is a flexible and modular tool empowered by the convergence of multistep continuous flow chemistry and polymer synthesis.
Materials and Methods
All Flow-IEG reactions were performed in the system shown in Fig. 2A. The substrates of interest were dissolved in toluene or chlorobenzene to generate a solution of ∼10 wt%. The TBAA and TBAF were dissolved in THF to generate a solution that contained 1.1 molar equivalents of TBAA and TBAF compared with substrate. Equal flow rates of the TBAA, TBAF, and the substrate solutions were used. All solvent and reagent solutions were degassed by sparging with argon for 20 min before use. Syrris Asia pumps equipped with 50/100-μL syringes are used for all solvents and reagents except the Me6TREN/CuI solution, which used a Harvard PHD2000 pump with an 8-mL stainless steel syringe. The system was allowed to run for 2.5 residence times to reach steady state before collecting and analyzing product composition. Heating the reactors was accomplished by immersing the PFA tubing into an oil bath.
Acknowledgments
F.A.L. is supported by a National Science Foundation Science, Engineering and Education for Sustainability (SEES) postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508599112/-/DCSupplemental.
References
- 1.Prober JM, et al. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- 2.Schmutz J, et al. Quality assessment of the human genome sequence. Nature. 2004;429(6990):365–368. doi: 10.1038/nature02390. [DOI] [PubMed] [Google Scholar]
- 3.Merrifield RB, Stewart JM, Jernberg N. Instrument for automated synthesis of peptides. Anal Chem. 1966;38(13):1905–1914. doi: 10.1021/ac50155a057. [DOI] [PubMed] [Google Scholar]
- 4.Caruthers MH. Gene synthesis machines: DNA chemistry and its uses. Science. 1985;230(4723):281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- 5.Seeberger PH. Automated oligosaccharide synthesis. Chem Soc Rev. 2008;37(1):19–28. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 6.Ouchi M, Badi N, Lutz J-F, Sawamoto M. Single-chain technology using discrete synthetic macromolecules. Nat Chem. 2011;3(12):917–924. doi: 10.1038/nchem.1175. [DOI] [PubMed] [Google Scholar]
- 7.Lutz JF, Ouchi M, Liu DR, Sawamoto M. Sequence-controlled polymers. Science. 2013;341(6146):1238149–1238149. doi: 10.1126/science.1238149. [DOI] [PubMed] [Google Scholar]
- 8.Binauld S, Damiron D, Connal LA, Hawker CJ, Drockenmuller E. Precise synthesis of molecularly defined oligomers and polymers by orthogonal iterative divergent/convergent approaches. Macromol Rapid Commun. 2011;32(2):147–168. doi: 10.1002/marc.201000548. [DOI] [PubMed] [Google Scholar]
- 9.Ingham RJ, et al. Organic synthesis: March of the machines. Angew Chem Int Ed. 2015;54(11):144–148. doi: 10.1002/anie.201410744. [DOI] [PubMed] [Google Scholar]
- 10.Saiki RK, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Schultz PG. Expanding the genetic code. Angew Chem Int Ed Engl. 2004;44(1):34–66. doi: 10.1002/anie.200460627. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Residue-specific incorporation of non-canonical amino acids into proteins: Recent developments and applications. Curr Opin Chem Biol. 2010;14(6):774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merrifield RB. Solid phase peptide synthesis: The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85(14):2149–2154. [Google Scholar]
- 14.Roy RK, et al. Design and synthesis of digitally encoded polymers that can be decoded and erased. Nat Commun. 2015;6:7237. doi: 10.1038/ncomms8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porel M, Alabi CA. Sequence-defined polymers via orthogonal allyl acrylamide building blocks. J Am Chem Soc. 2014;136(38):13162–13165. doi: 10.1021/ja507262t. [DOI] [PubMed] [Google Scholar]
- 16.Solleder SC, Meier MAR. Sequence control in polymer chemistry through the Passerini three-component reaction. Angew Chem Int Ed Engl. 2014;53(3):711–714. doi: 10.1002/anie.201308960. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer S, Lutz J-F. A facile procedure for controlling monomer sequence distribution in radical chain polymerizations. J Am Chem Soc. 2007;129(31):9542–9543. doi: 10.1021/ja0717616. [DOI] [PubMed] [Google Scholar]
- 18.Moatsou D, Hansell CF, O’Reilly RK. Precision polymers: A kinetic approach for functional poly(norbornenes) Chem Sci (Camb) 2014;5(6):2246–2250. [Google Scholar]
- 19.Gody G, Maschmeyer T, Zetterlund PB, Perrier S. Rapid and quantitative one-pot synthesis of sequence-controlled polymers by radical polymerization. Nat Commun. 2013;4:2505. doi: 10.1038/ncomms3505. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Matta ME, Hillmyer MA. Synthesis of sequence-specific vinyl copolymers by regioselective ROMP of multiply substituted cyclooctenes. ACS Macro Lett. 2012;1(12):1383–1387. doi: 10.1021/mz300535r. [DOI] [PubMed] [Google Scholar]
- 21.Lo PK, Sleiman HF. Nucleobase-templated polymerization: Copying the chain length and polydispersity of living polymers into conjugated polymers. J Am Chem Soc. 2009;131(12):4182–4183. doi: 10.1021/ja809613n. [DOI] [PubMed] [Google Scholar]
- 22.Niu J, Hili R, Liu DR. Enzyme-free translation of DNA into sequence-defined synthetic polymers structurally unrelated to nucleic acids. Nat Chem. 2013;5(4):282–292. doi: 10.1038/nchem.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutekunst WR, Hawker CJ. J Am Chem Soc. 2015;137(25):8038–8041. doi: 10.1021/jacs.5b04940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paynter OI, Simmonds DJ, Whiting MC. The synthesis of long-chain unbranched aliphatic compounds by molecular doubling. J Chem Soc Chem Commun. 1982:1165–1166. [Google Scholar]
- 25.Bidd I, Whiting MC. The synthesis of pure n-paraffins with chain-lengths between one and four hundred. J Chem Soc Chem Commun. 1985:543–544. [Google Scholar]
- 26.Longstreet AR, McQuade DT. Organic reaction systems: Using microcapsules and microreactors to perform chemical synthesis. Acc Chem Res. 2013;46(2):327–338. doi: 10.1021/ar300144x. [DOI] [PubMed] [Google Scholar]
- 27.Lévesque F, Seeberger PH. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew Chem Int Ed Engl. 2012;51(7):1706–1709. doi: 10.1002/anie.201107446. [DOI] [PubMed] [Google Scholar]
- 28.Webb D, Jamison TF. Continuous flow multi-step organic synthesis. Chem Sci (Camb) 2010;1(6):675–680. [Google Scholar]
- 29.Tonhauser C, Natalello A, Löwe H, Frey H. Microflow technology in polymer synthesis. Macromolecules. 2012;45(24):9551–9570. [Google Scholar]
- 30.Myers RM, Fitzpatrick DE, Turner RM, Ley SV. Flow chemistry meets advanced functional materials. Chemistry. 2014;20(39):12348–12366. doi: 10.1002/chem.201402801. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Pesak DJ, Ludwick JL, Moore JS. Geometrically-controlled and site specifically-functionalized phenylacetylene macrocycles. J Am Chem Soc. 1994;116(10):4227–4239. [Google Scholar]
- 32.Binauld S, Hawker CJ, Fleury E, Drockenmuller E. A modular approach to functionalized and expanded crown ether based macrocycles using click chemistry. Angew Chem Int Ed Engl. 2009;48(36):6654–6658. doi: 10.1002/anie.200903156. [DOI] [PubMed] [Google Scholar]
- 33.Adamo A, Heider PL, Weeranoppanant N, Jensen KF. Membrane-based, liquid–liquid separator with integrated pressure control. Ind Eng Chem Res. 2013;52(31):10802–10808. [Google Scholar]
- 34.Presolski SI, Hong V, Cho S-H, Finn MG. Tailored ligand acceleration of the Cu-catalyzed azide-alkyne cycloaddition reaction: Practical and mechanistic implications. J Am Chem Soc. 2010;132(41):14570–14576. doi: 10.1021/ja105743g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy SS, Dong X, Murgasova R, Gusev AI, Hercules DM. Synthesis and secondary-ion mass spectrometry of linear single oligomers of nylon–6. Macromolecules. 1999;32(5):1367–1374. [Google Scholar]
- 36.Brooke GM, Cameron NR, MacBride JAH, Whiting MC. The synthesis of oligomers related to poly(ethylene terephthalate) Polymer (Guildf) 2002;43(4):1139–1154. [Google Scholar]
- 37.Williams JB, Chapman TM, Hercules DM. Synthesis of discrete mass poly(butylene gluterate) oligomers. Macromolecules. 2003;36(11):3898–3908. doi: 10.1021/ac030061q. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa K, Tang C, Hawker CJ. Molecularly defined caprolactone oligomers and polymers: Synthesis and characterization. J Am Chem Soc. 2008;130(5):1718–1726. doi: 10.1021/ja077149w. [DOI] [PubMed] [Google Scholar]
- 39.Takizawa K, Nulwala H, Hu J, Yoshinaga K, Hawker CJ. Molecularly defined (L)-lactic acid oligomers and polymers: Synthesis and characterization. J Polym Sci A Polym Chem. 2008;46(18):5977–5990. [Google Scholar]
- 40.Fox TG, Flory PJ. Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys. 1950;21(6):581–591. [Google Scholar]
- 41.Schwartz E, Breitenkamp K, Fokin VV. Synthesis and postpolymerization functionalization of poly(5-iodo-1,2,3-triazole)s. Macromolecules. 2011;44(12):4735–4741. [Google Scholar]
- 42.McMullen JP, Jensen KF. Rapid determination of reaction kinetics with an automated microfluidic system. Org Process Res Dev. 2011;15(2):398–407. [Google Scholar]
- 43.Wunderlich B. Single Component Materials. Thermal Analysis of Polymeric Materials. Springer; Berlin: 2005. pp. 591–704. [Google Scholar]
- 44.Wu P, et al. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(i)-catalyzed ligation of azides and alkynes. Angew Chem Int Ed Engl. 2004;43(30):3928–3932. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]
- 45.Hoogboom J, Swager TM. Increased alignment of electronic polymers in liquid crystals via hydrogen bonding extension. J Am Chem Soc. 2006;128(47):15058–15059. doi: 10.1021/ja065662o. [DOI] [PubMed] [Google Scholar]