Significance
Hydroxynitrile lyase (HNL) has been isolated from plants and bacteria and is a valuable tool in the chiral-specific synthesis of cyanohydrins, which are important building blocks of fine chemicals and pharmaceuticals. To discover more efficient and stable HNLs, we focused on the invasive cyanogenic millipede as a bioresource. The HNL identified from the millipede showed not only the highest specific activity toward benzaldehyde among known HNLs, including the almond HNL in industrial use, along with wide temperature and pH stabilities, but also high enantioselectivity in the synthesis of various cyanohydrins. These properties make it suitable as an industrial biocatalyst. Arthropods are likely to be valuable sources of potential biocatalysts for the next generation of industrial biotechnology.
Keywords: millipede hydroxynitrile lyase, bioresource exploration, biocatalysis, white biotechnology, arthropod
Abstract
Hydroxynitrile lyase (HNL) catalyzes the degradation of cyanohydrins and causes the release of hydrogen cyanide (cyanogenesis). HNL can enantioselectively produce cyanohydrins, which are valuable building blocks for the synthesis of fine chemicals and pharmaceuticals, and is used as an important biocatalyst in industrial biotechnology. Currently, HNLs are isolated from plants and bacteria. Because industrial biotechnology requires more efficient and stable enzymes for sustainable development, we must continuously explore other potential enzyme sources for the desired HNLs. Despite the abundance of cyanogenic millipedes in the world, there has been no precise study of the HNLs from these arthropods. Here we report the isolation of HNL from the cyanide-emitting invasive millipede Chamberlinius hualienensis, along with its molecular properties and application in biocatalysis. The purified enzyme displays a very high specific activity in the synthesis of mandelonitrile. It is a glycosylated homodimer protein and shows no apparent sequence identity or homology with proteins in the known databases. It shows biocatalytic activity for the condensation of various aromatic aldehydes with potassium cyanide to produce cyanohydrins and has high stability over a wide range of temperatures and pH values. It catalyzes the synthesis of (R)-mandelonitrile from benzaldehyde with a 99% enantiomeric excess, without using any organic solvents. Arthropod fauna comprise 80% of terrestrial animals. We propose that these animals can be valuable resources for exploring not only HNLs but also diverse, efficient, and stable biocatalysts in industrial biotechnology.
Enzymes are very specific and efficient catalysts. They are able to catalyze reactions without extreme conditions, such as high temperature and pressure, that are often required in chemical synthetic processes. Industrial biotechnologies using these biocatalysts have the advantages of higher reaction rates, efficiencies, and enantioselectivities. This technology also reduces energy consumption and decreases the production of hazardous chemical waste. Conventional industrial chemistry is gradually being replaced by industrial biotechnology. For example, nitrile hydratase is used for the synthesis of acrylamide from acrylonitrile. This strategy produces more than 600,000 tons of acrylamide worldwide every year (1).
Hydroxynitrile lyase (HNL) is found as a molecular component in plant defense systems and plays a role in the decomposition of stored cyanogenic glycosides into hydrogen cyanide and aldehydes (2). The enzyme’s reverse activity is used industrially in the enantioselective production of cyanohydrins. Cyanohydrins are valuable building blocks for the synthesis of fine chemicals, pharmaceuticals, and agrochemicals such as denopamine (β1-adrenergic receptor agonist) (3, 4), clopidogrel (platelet aggregation inhibitor) (5), pyrethroids (insecticides) (6), and (R)-pantolactone [starting compound for synthesis of (R)-pantothenic acid, (R)-panthenol, and (R)-pantetheine] (7). The enantioselective condensation of benzaldehyde with hydrogen cyanide in the asymmetric synthesis of mandelonitrile using emulsin from the almond, Prunus amygdalus, as an HNL source (PaHNL), has been demonstrated (8). Versatile HNLs, homologous to FAD-dependent oxidoreductase, α/β-hydrolase, carboxypeptidase, a Zn2+-dependent alcohol dehydrogenase, and a Mn2+-dependent cupin structure have all been identified from plants and bacteria (9–12). Because PaHNL shows the highest specific activity in the synthesis of mandelonitrile among known HNLs (13), along with a wide substrate specificity and high stability, it serves as a valuable catalyst in industrial biotechnology. Although many scientists have identified HNLs in the last two decades (14, 15), further screening for better enzyme(s) with high reaction rates and stabilities from unexplored bioresources is necessary to enrich the current tool box for sustainable industrial development.
The millipede Chamberlinius hualienensis (16) (Movie S1), originally from Hualien, Taiwan, invaded Okinawa Island, Japan in 1983 and has been expanding its habitat in Kyushu, Japan. Large swarms of the millipedes enter houses and sometimes cause train delays (17). The animal secretes mandelonitrile, benzaldehyde, and hazardous hydrogen cyanide as defense chemicals, as previously reported for polydesmid millipede species (18, 19). In the millipede species Apheloria corrugate, a predicted enzyme is thought to play a role in the decomposition of mandelonitrile and the release of hydrogen cyanide and benzaldehyde (20). If the enzyme derived from C. hualienensis is an HNL, the large population of invasive millipedes could be used as an important resource for HNL purification. Here we identify HNL from the invasive cyanogenic millipede, characterize its physicochemical properties and inhibitor susceptibility, and demonstrate its substrate specificity, kinetic parameters, and gene expression. We describe the potential of the millipede HNL to be widely used as a biocatalyst for the enantioselective synthesis of cyanohydrins, building blocks for fine chemicals and pharmaceuticals. Furthermore, we demonstrate the environmentally friendly synthesis of (R)-mandelonitrile from benzaldehyde and potassium cyanide using this HNL with 99.0% enantiomeric excess (ee) in 5 min without the use of organic solvents.
Results and Discussion
Identification and Purification of HNL from the Invasive Cyanogenic Millipede C. hualienensis.
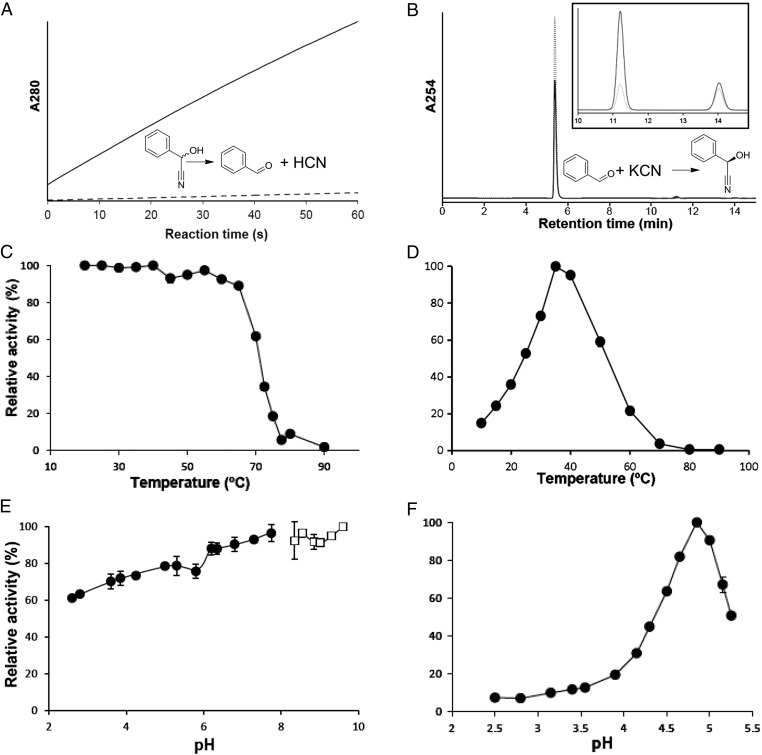
To confirm whether the millipede enzyme(s) can catalyze the decomposition of mandelonitrile into benzaldehyde and hydrogen cyanide, we measured the cleavage activity in millipede body extract. The extract decomposed racemic mandelonitrile into benzaldehyde in a time-dependent manner, with unambiguously higher reaction than the control (Fig. 1A). These results indicate that C. hualienensis has HNL in its body. On the other hand, the millipede extract enantioselectively catalyzed the synthesis of (R)-mandelonitrile from benzaldehyde and potassium cyanide (Fig. 1B). Thus, we designated this enzyme(s) from C. hualienensis as ChuaHNL and considered the invasive millipedes as a potential source for the purification of HNL.
Fig. 1.
Characterization of ChuaHNL. (A) Cleavage reaction of racemic mandelonitrile by ChuaHNL. Production of benzaldehyde in 100 mM citrate buffer, pH 5.5, at 22 °C was measured by monitoring the absorbance at 280 nm. Solid and dotted lines indicate absorbance with and without the enzyme, respectively. (B) Synthesis of (R)-mandelonitrile from 50 mM benzaldehyde in 400 mM citrate buffer, pH 4.2, at 22 °C. ChuaHNL specifically produces (R)-mandelonitrile, whereas the chemical reaction produces a scant amount of (R)- and (S)-mandelonitrile. Production of mandelonitrile was measured by monitoring the absorbance at 254 nm using an HPLC equipped with a chiral column. Retention times of (R)- and (S)-mandelonitrile were 11.4 min and 14.3 min, respectively. (Inset) Magnified view in the range of 10–15 min. Solid and dotted lines indicate the synthetic reaction of mandelonitrile from benzaldehyde and potassium cyanide with and without ChuaHNL, respectively. (C) Temperature stability. Enzyme samples were incubated at each temperature for 1 h and assayed for the remaining HNL activity (as shown in B). (D) Optimum temperature. Enzyme samples were assayed at each temperature (as shown in B). (E) pH stability. Enzyme samples were incubated at each temperature for 1 h and assayed for the remaining HNL activity (as shown in B). ●, enzymatic activity in a citrate-phosphate buffer; □, enzymatic activity in a glycine-sodium hydroxide buffer. (F) Optimum pH. Enzyme samples were assayed at each pH value (as shown in B). Values are the mean ± SD; n = 3.
Using ∼29 kg of collected millipedes in preliminary experiments, we optimized each purification step. After fractionation by 50–70% saturated ammonium sulfate precipitation to remove some recalcitrant impurities, ChuaHNL was purified 1,230-fold from 1 kg of frozen millipedes by a combination of ion exchange chromatography (Toyopearl DEAE-650M, Q Sepharose Fast Flow, and Mono Q 5/50 GL), hydrophobic interaction chromatography (Toyopearl Butyl-650M), and gel filtration (Superdex 75 10/300 GL). The specific activity in the synthesis of (R)-mandelonitrile from benzaldehyde and potassium cyanide was 7,420 U⋅mg−1 (Table 1). This value is superior not only to the 31.5, 136, and 220 U⋅mg−1 specific activities of HNLs extracted, respectively, from loquats (Eriobotrya japonica) (21), passion fruit (Passiflora edulis) (22), and Japanese apricots (Prunus mume) (23) but also to the 1,450 U⋅mg−1 specific activity of HNL from almonds (Prunus amygdalus) (13), which is used in industry.
Table 1.
Purification of ChuaHNL from the millipede C. hualienensis
| Purification step | Activity, U* | Protein, mg | Specific activity, U⋅mg−1 | Yield,% | Purification fold |
| Body crude homogenate | 26,600 | 4,390 | 6.10 | 100 | 1.00 |
| Saturated ammonium sulfate fractionation (50–70%) | 19,600 | 1,000 | 19.6 | 73.7 | 3.20 |
| DEAE-Toyopearl | 11,600 | 180 | 64.5 | 43.8 | 10.9 |
| Butyl-Toyopearl | 5,160 | 14.0 | 369 | 19.4 | 61.5 |
| Q Sepharose Fast Flow | 1,900 | 1.40 | 1,360 | 7.14 | 226 |
| Mono Q | 1,270 | 0.46 | 2,760 | 4.77 | 450 |
| Superdex 75 | 890 | 0.12 | 7,420 | 3.35 | 1,230 |
Synthesis of (R)-mandelonitrile from benzaldehyde was monitored using an HPLC equipped with a chiral column.
Characterization of Enzymatic and Physicochemical Properties of ChuaHNL.
Next, we assessed the inhibitor susceptibility and physicochemical properties of ChuaHNL in the synthesis of mandelonitrile from benzaldehyde and potassium cyanide. Most of the enzyme inhibitors tested did not obviously affect the HNL activity, except sulfhydryl reagents, iodoacetic acid, and iodoacetamide (18% and 28% remaining activity, respectively) (Table S1). The inhibitor profile is similar to FAD-containing HNLs from E. japonica (loquats) (21), P. mume (Japanese apricot) (23), and P. amygdalus (almond) (24). These results suggest that cysteine and/or serine residue(s) are likely to be important for the enzymatic activity. ChuaHNL has a molecular mass of 47,300 Da, as determined by gel filtration, and 25,000 Da, as determined from SDS/PAGE (Fig. S1A), suggesting that it is a homodimer consisting of two identical subunits. Periodic acid-Schiff (PAS) staining identified the enzyme as a glycoprotein (Fig. S1B). ChuaHNL is stable up to 65 °C after 1 h of incubation and is inactive at higher temperatures (Fig. 1C); the enzyme can catalyze the synthesis of (R)-mandelonitrile in a wide temperature range of 15–60 °C, and the optimum temperature is 35 °C (Fig. 1D). It is stable in the pH range of 2.6–9.6 (Fig. 1E). The optimum pH is 4.9 (Fig. 1F). The temperature and pH stabilities are comparable to those of PaHNL (25). Based on these properties, this enzyme is a good candidate biocatalyst for industrial applications.
Table S1.
Effects of inhibitors on synthetic activity of ChuaHNL
| Candidate inhibitor | Remaining activity, % |
| Sulfhydryl reagent | |
| N-Ethylmaleimide | 100 |
| Dithionitrobenzoic acid | 97 |
| Iodoacetic acid (10 mM) | 18 |
| Iodoacetamide (10 mM) | 28 |
| p-Chloromercuribenzoic acid | 100 |
| His modifiers | |
| Diethylpyrocarbonate | 100 |
| Serine protease inhibitors | |
| Trypsin inhibitor (T-9003) | 88 |
| Trypsin inhibitor (T-9378) | 85 |
| Trypsin-chymotrypsin inhibitor (T-9777) | 91 |
| PMSF | 91 |
| Aspartic (Ser, Cys, Thr) protease inhibitors | |
| Pepstatin A (100 µg/mL) | 89 |
| Leupeptin (100 µg/mL) | 86 |
| Reducing agents | |
| 2-Mercaptoethanol (10 mM) | 95 |
| Hydrazine | 100 |
| DTT | 100 |
| Glutathione (reduced) (0.5 mM) | 93 |
| Glutathione (oxidized) (0.5 mM) | 95 |
| Biotin reagent | |
| Avidin (100 µg/mL) | 96 |
| Chelating reagents | |
| EDTA (10 mM) | 100 |
| Sodium barbital | 93 |
| Metals | |
| Ammonium thiocyanate (1 mM) | 43 |
| PbCl2 (0.1 mM) | 98 |
| NiCl2 | 93 |
| CoCl2 | 95 |
| CrCl3 | 88 |
| CuSO4 | 100 |
| FeCl3 | 100 |
| HgCl2 | 64 |
| ZnSO4 | 100 |
| CdCl2 | 99 |
| MnSO4 | 106 |
| AgNO3 | 68 |
| Others | |
| Phenylthiourea | 100 |
| Diphenyl hydantoin | 100 |
Enzyme (1–1.5 U) was used for the reaction. Concentration of applied inhibitor was 0.1 mM except where indicated. Each point represents the means of three independent experiments.
Fig. S1.
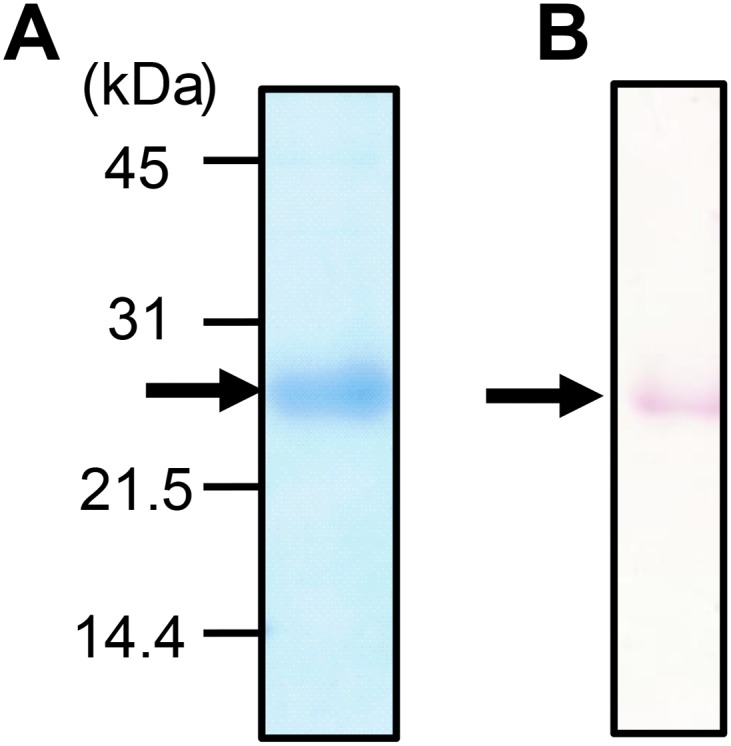
SDS/PAGE analysis and PAS staining of isolated native ChuaHNL. (A) 15% SDS/PAGE profile of isolated enzyme. ChuaHNL has a molecular mass of 25,000 Da. (B) PAS staining of ChuaHNL after separation with SDS/PAGE. Arrow indicates the migration of ChuaHNL.
Expression and Localization of the Gene Encoding ChuaHNL in the Body of the Millipede.
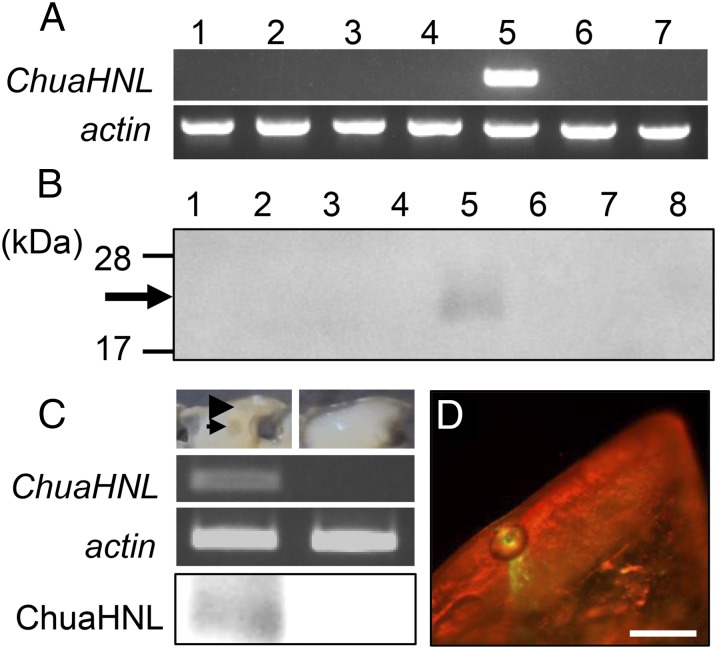
ChuaHNL transcript and ChuaHNL were accumulated in the paraterga of the millipede (Fig. 2 A and B). Because Western blotting did not detect this enzyme in the hemolymph (Fig. 2B), the enzyme is likely to be localized in defensive secretory gland(s) in the paraterga. There are two types of paraterga. One houses a reaction chamber and a storage chamber, whereas the other does not (arrowhead and arrow in Fig. 2C). The millipede specifically expressed ChuaHNL in the paraterga housing the reaction chamber and the storage chamber (Fig. 2C) and exuded the gene product at the ozopore through a duct of the reaction chamber (Fig. 2D). In A. corrugate, a “factor E” involving the decomposition of mandelonitrile to benzaldehyde and hydrogen cyanide is thought to be stored in a smaller compartment of a gland (20). The factor E in the millipede seems likely to be an HNL, much like the one characterized in this study.
Fig. 2.
Expression and localization of ChuaHNL. (A) ChuaHNL expression. RT-PCR detected ChuaHNL transcript in the paraterga; actin expression was used as an internal control. (B) Localization of ChuaHNL. Western blotting detected an immunoreactive material with a molecular mass of 25,000 Da in the paraterga. 1, antenna; 2, leg; 3, head; 4, integument; 5, paraterga; 6, fat body; 7, gut; 8, hemolymph. (C) ChuaHNL transcript and ChuaHNL were specifically accumulated in the paraterga. The arrowhead and arrow indicate the storage chamber and the reaction chamber, respectively. (D) Immunohistochemical localization. ChuaHNL was transferred through a duct of the reaction chamber and released from the ozopore. (Scale bar, 200 μm.)
Substrate Specificity and Kinetic Studies in the Synthesis of Cyanohydrins Using ChuaHNL.
Because millipedes exude defensive secretions of aromatic compounds, such as mandelonitrile and benzaldehyde (19), millipede HNLs seem likely to have substrate specificity toward aromatic compounds. Thus, we sought to synthesize cyanohydrins using ChuaHNL in a citrate buffer, using the aromatic aldehydes in our chemical library (26, 27) as starting materials, and evaluated the chiral configuration and ee, which are important indices for fine chemical syntheses. ChuaHNL catalyzed the synthesis of cyanohydrins from monosubstituted, disubstituted, biphenyl, heteroaromatic, and bicyclic aldehydes with an almost exclusive R configuration and an ee range of 7.5–90% (Table S2). Overall, ChuaHNL displayed wide substrate specificity in cyanohydrin synthesis in an aqueous system at a mild temperature (22 °C) and was shown to be a potent biocatalyst. Although other HNLs from almond or Japanese apricot showed a wide substrate spectrum, they required biphasic systems containing organic solvents (13, 26). Catalyzing reactions in aqueous medium (citrate buffer in this research) is closer to a green chemistry concept (28). ChuaHNL catalyzes the synthesis of many cyanohydrins including mandelonitrile, an intermediate in the synthesis of several chemicals such as (S)-amphetamines (nervous system drug) (29) and (R)-mandelic acid (anti-bacterial agent) (30). para-Methoxybenzaldehyde cyanohydrin has applications as a synthetic intermediate (e.g., in natural hydroxyl amides with insecticidal and adrenaline activity) and it is present in the structure of (−)-tempamide, an antiemetic, and (−)-denopamine, a cardiac drug used in the treatment of congestive heart failure and angina (4). ChuaHNL synthesizes 4-bromomandelonitrile (and some other cyanohydrins of benzaldehyde) as precursors of 2,3-disubstituted transaziridines (naturally occurring mitosanes). The aziridine ring is essential for antitumor activity (31). Based on the preliminary screening (Table S2), we selected the substrates with high ee and determined steady-state kinetic parameters for the synthesis of cyanohydrins. The Michaelis–Menten constants (Km) for benzaldehyde, 4-bromobenzaldehyde, 3-methylbenzaldehyde, 4-methylbenzaldehyde, 3-methoxybenzaldehyde, 4-methoxybenzaldehyde, 2,4-dimethylbenzaldehyde, 2-thiophenecarboxaldehyde, and 4-biphenylcarboxaldehyde are in the range of 3.0–18 mM. In contrast, the catalytic efficiency (kcat/Km) of these compounds is in the wider range of 4.7–1,100 mM−1⋅s−1 (Table 2). The highest catalytic efficiency belongs to mandelonitrile synthesis at 22 °C. The enzyme not only accepts benzaldehyde and its monosubstitutes but also exhibits remarkable activity toward bulkier substrates such as 2-thiophene carboxaldehyde. ChuaHNL exhibited a decreased kcat/Km toward bulkier substrates possessing an additional ring, such as 4-biphenylcarboxaldehyde, which has a flexible, nonplanar structure and exhibits a low kcat. The enzyme showed as high a catalytic efficiency in the synthesis of the sterically demanding 4-methylbenzaldehyde cyanohydrin as in mandelonitrile synthesis. More structure–function relationships will emerge upon solving the crystal structure of this glycoprotein.
Table S2.
Screening for substrate specificity of ChuaHNL toward aromatic aldehydes
| HPLC methods | ||||||
| Substrate | Configuration | ee, % | Solvent n-hexane:2-propanol | Retention time for aldehyde,min | Retention time for (R)-cyanohydrin,min | Retention time for (S)-cyanohydrin,min |
| Benzaldehyde | R | 77.7 | 85:15 | 5.4 | 11.4 | 14.3 |
| 2-Chlorobenzaldehyde | R | 11.0 | 97.5:2.5 | 6 | 36.4 | 38.7 |
| 4-Bromobenzaldehyde | R | 90.0 | 95:5 | 7.9 | 30.5 | 31.8 |
| 2-Methylbenzaldehyde | R | 44.9 | 85:15 | 5 | 8.4 | 9.8 |
| 3-Methylbenzaldehyde | R | 89.6 | 85:15 | 5 | 9.7 | 11.2 |
| 4-Methylbenzaldehyde | R | 86.9 | 85:15 | 5.3 | 11 | 14.9 |
| 2-Methoxybenzaldehyde | S | 49.6 | 90:10–98:2 (gradient) | 7.3 | 17.1 | 17.6 |
| 3-Methoxybenzaldehyde | R | 72.8 | 85:15 | 6.6 | 14 | 16.8 |
| 4-Methoxybenzaldehyde | R | 73.2 | 85:15 | 8.3 | 22.7 | 26.3 |
| 3-(Trifluoromethyl)benzaldehyde | R | 36 | 85:15 | 4.9 | 6.11 | 6.5 |
| 4-(Trifluoromethyl)benzaldehyde | R | 45 | 95:5 | 6.2 | 17 | 18.8 |
| 3-Nitrobenzaldehyde | R | 39.6 | 85:15 | 16.2 | 19.6 | 22.2 |
| 4-Nitrobenzaldehyde | R | 12.9 | 85:15 | 15.7 | 22 | 26.6 |
| 2,3-Dichlorobenzaldehyde | R | 7.5 | 95:5 | 5.4 | 7.6 | 7.9 |
| 2,4-Dichlorobenzaldehyde | S | 19.0 | 99:1 | 8.4 | 60.4 | 70.7 |
| 2,5-Dichlorobenzaldehyde | S | 18.9 | 99:1 | 6 | 32.9 | 35.4 |
| 2,6-Dichlorobenzaldehyde | R | 14.9 | 95:5 | 8 | 26.6 | 28.4 |
| 3,4-Dichlorobenzaldehyde | R | 64.6 | 97.5:2.5 | 8.3 | 42.9 | 45.1 |
| 2,4-Dimethylbenzaldehyde | R | 58.8 | 85:15 | 5 | 8.1 | 10.2 |
| 3,5-Dimethoxybenzaldehyde | R | 18.7 | 85:15 | 10.5 | 51 | 55.8 |
| Piperonal | R | 70.6 | 85:15 | 10.8 | 22.1 | 24.3 |
| Terephthalaldehyde | R | 8.6 | 85:15 | 13.6 | 20.6 | 25.1 |
| 4-Biphenylcarboxaldehyde | R | 83.3 | 85:15 | 12.2 | 27.8 | 30.1 |
| 2-Furancarboxaldehyde | S | 40.1 | 85:15 | 7.6 | 13.3 | 10.4 |
| 2-Thiophenecarboxaldehyde | S | 89.2 | 85:15 | 7.7 | 17.1 | 12.4 |
| 3-Pyridinecarboxaldehyde | R | 23.7 | 85:15 | 9.2 | 10.3 | 11.5 |
| 2-Quinolinecarboxaldehyde | R | 15.3 | 85:15 | 21.2 | 47 | 50.1 |
| 1-Naphthalenecarboxaldehyde | R | 48.4 | 85:15 | 7.3 | 15.6 | 18.4 |
| 2-Naphthalenecarboxaldehyde | R | 29.9 | 85:15 | 9.5 | 19 | 23.7 |
Six units of enzyme was used in citrate buffer, pH 4.2, at room temperature (22 °C), without using organic solvent in the screening step. The (S) configuration was assigned according to Cahn–Ingold–Prelog rules (46).
Table 2.
Steady-state kinetic parameters of ChuaHNL for aldehydes in the synthesis reaction of cyanohydrins
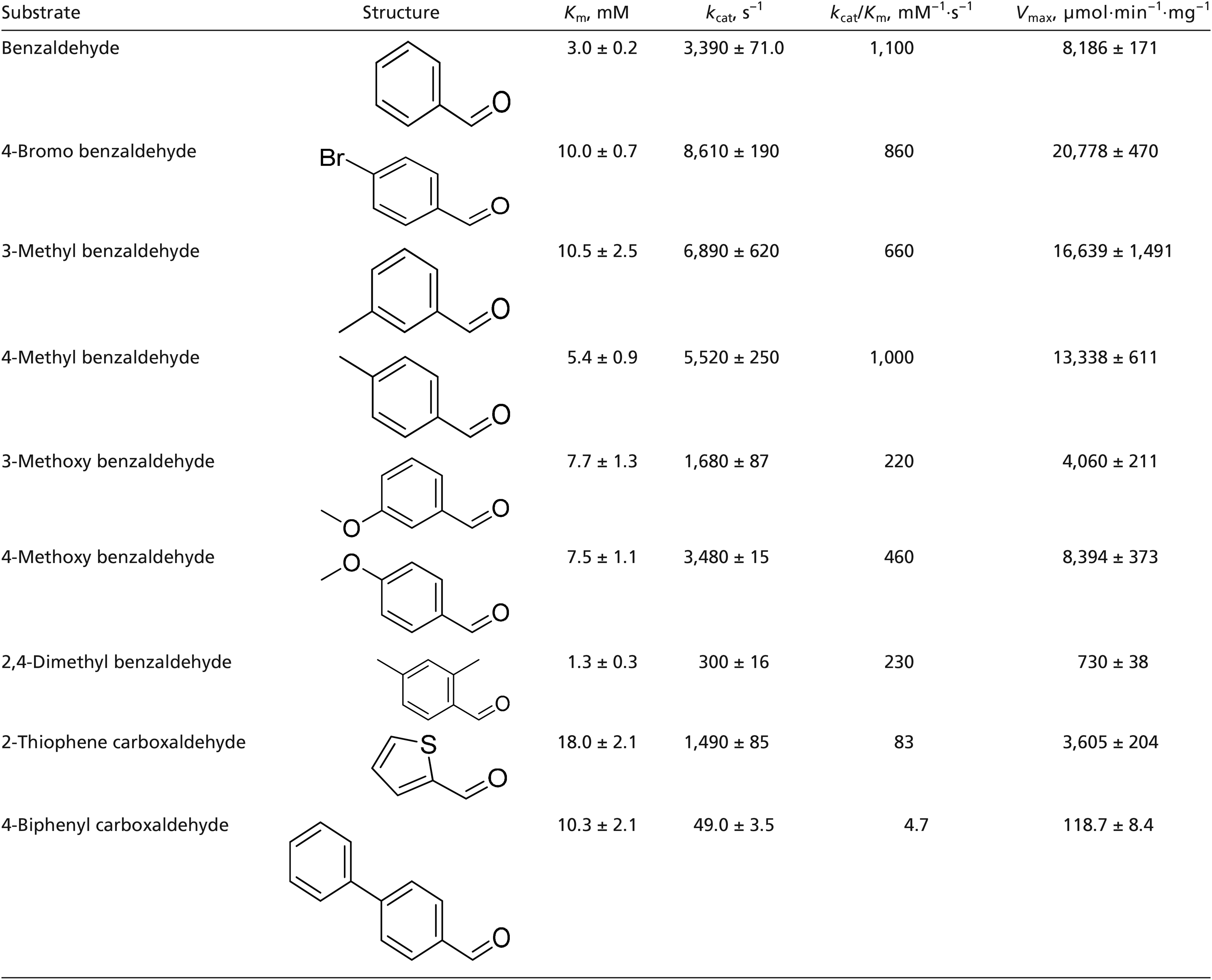 |
ChuaHNL (2.5 U) was used for the reactions. Values are the mean ± SD; n = 3.
To summarize, ChuaHNL can broadly catalyze the condensation of various aromatic aldehydes with potassium cyanide in the synthesis of cyanohydrins.
Unique Amino Acid Sequence of ChuaHNL.
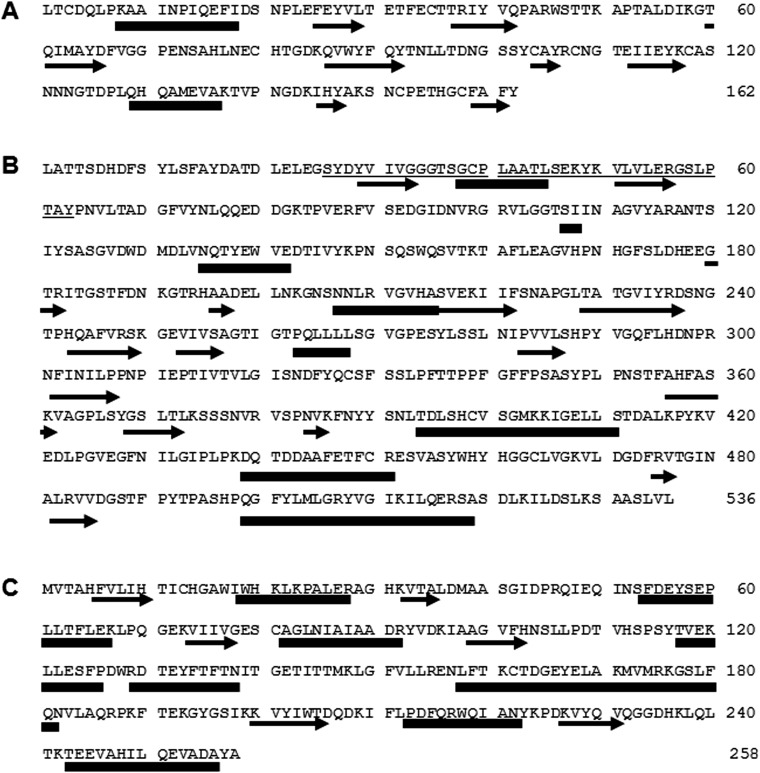
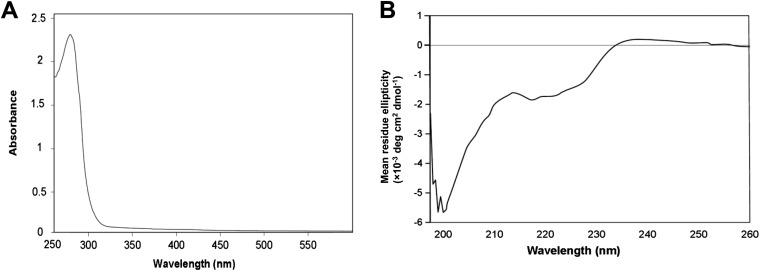
Information regarding the cDNA sequence is indispensable to obtain an unlimited recombinant protein for industrial uses. Based on the N-terminal and internal amino acid sequences determined from the purified ChuaHNL, we designed primers and applied RACE. The cDNA sequence of 783 bp encoded 183 aa residues, including a 21-aa-long signal peptide. The deduced protein included the three previously determined amino acid sequences, indicating that the cloned cDNA encodes ChuaHNL. The calculated molecular mass and pI were 18,225 Da and 4.97, respectively. The protein had one predicted O-glycosylation site at position 2 and three predicted N-glycosylation sites at positions 99, 109, and 123 (Fig. S2). This prediction is in agreement with the results of the PAS staining (Fig. S1B), suggesting that ChuaHNL most likely consists of 27% oligosaccharides. It is noteworthy that ChuaHNL shares no amino acid sequence identity with any of the HNLs previously reported in the blastp search. The deduced protein comprised two α-helices and eight β-structures. The ChuaHNL sequence contains no FAD-binding domain (Fig. S3A). This information is in agreement with the UV-visible (UV-Vis) spectrum of the purified millipede HNL at the range of 200–600 nm (Fig. S4A), distinguishing ChuaHNL from the HNLs of P. amygdalus (almond) (FAD-dependent oxidoreductase type) and Manihot esculenta (α/β-hydrolase type) (refs. 32 and 33 and Fig. S3 B and C). The CD spectra of ChuaHNL (Fig. S4B) suggests that the enzyme contains little α-helix (222 nm) and more random structures, based on a decrease in mean residue ellipticity at 200–210 nm (34). CD spectral analysis using K2D2 (35) also suggests 10.3% and 30.7% for α-helices and β-strands, respectively. DichroWeb (36) predicted 9.1% α-helices, 26% β-strands, 7% turns, and 58% unordered structures.
Fig. S2.
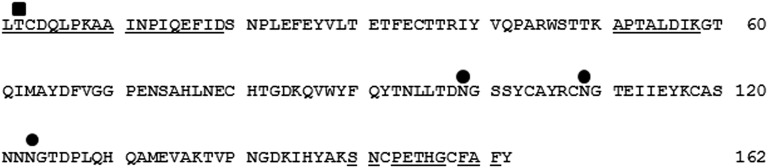
Deduced amino acid sequence of ChuaHNL. Underlined amino acid residues were determined by protein sequencing. ■ and ● are potential O- and N-glycosylation sites, respectively.
Fig. S3.
Predicted secondary structure of ChuaHNL and typical HNLs. (A) ChuaHNL. (B) PaHNL (FAD-dependent oxidoreductase type). (C) MeHNL (α/β-hydrolase type). Thick bars and arrows indicate predicted α-helix and β-structure, respectively. Underline in B indicates predicted FAD binding domain. ChuaHNL showed no homology to PaHNL and MeHNL at the amino acid level and no similarity at the secondary structure level. The deduced amino acid sequences of PaHNL (ref. 32, accession no. AF412329) and MeHNL (ref. 33, accession no. Z29091) were analyzed using the SingalP 4.1 server and Jpred 4.
Fig. S4.
Secondary structure of native ChuaHNL. (A) UV-Vis spectrum of a purified fraction of the native ChuaHNL (1.5 mg⋅mL−1 in 20 mM potassium phosphate buffer, pH 7.0). (B) The far-UV CD spectra of a 0.15 mg⋅mL−1 enzyme solution suggest the enzyme contains few α-helices (222 nm) and a decrease at 210–200 might be related to amount of random structures. The CD data analysis tools, K2D2 and DichroWeb, estimation of the secondary structure of this millipede HNL are in agreement with this prediction.
An Excellent EE of the ChuaHNL in the Synthesis of Mandelonitrile in Aqueous Medium.
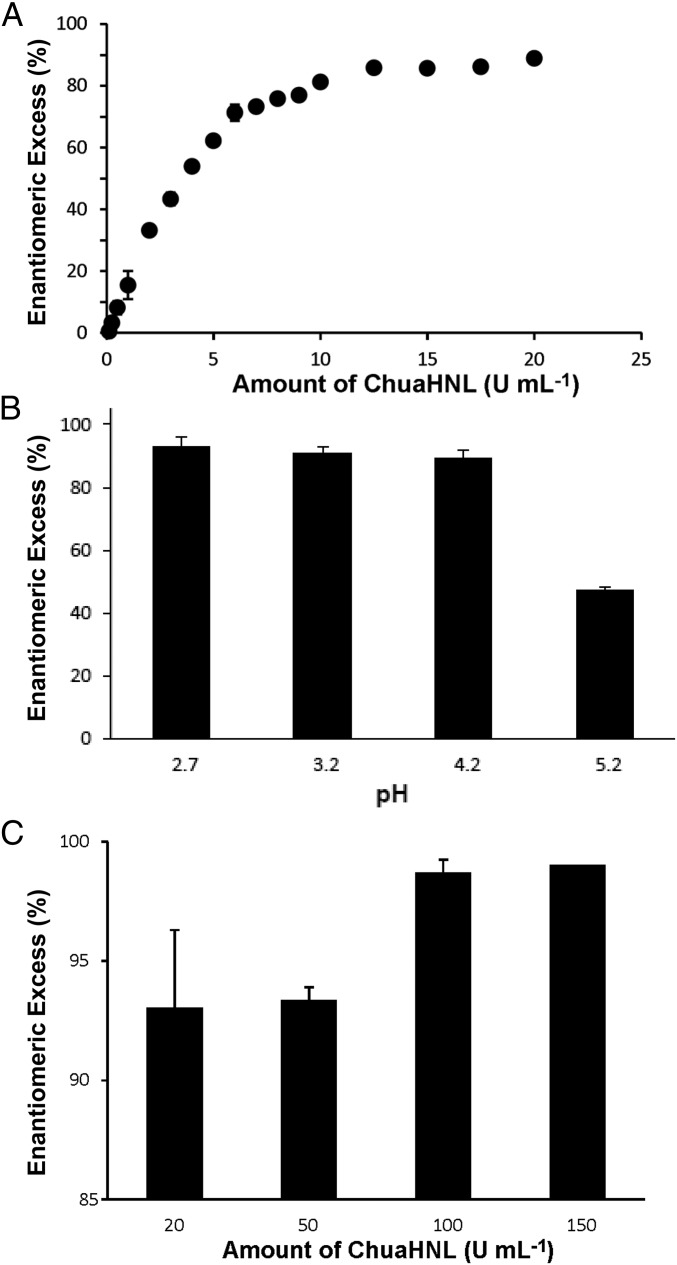
Industrial chemistry requires chirally pure chemicals not only to allow simple synthetic steps without additional separation but also to decrease the amount of hazardous waste. Therefore, we examined the (R)-mandelonitrile synthesis from benzaldehyde and cyanide as a “model reaction” using purified recombinant ChuaHNL from Pichia pastoris and evaluated the ee values under various experimental conditions without organic solvents. ChuaHNL (20 U⋅mL−1) catalyzed the synthesis of (R)-mandelonitrile with a maximum of 88.9% ee (Fig. 3A). It was reported that a low pH suppresses the spontaneous chemical reaction producing racemic mandelonitrile (9). Thus, we exposed 20 U⋅mL−1of ChuaHNL to benzaldehyde in various pH conditions. ChuaHNL still had enzymatic activity at a pH of 2.7, as presented in Fig. 1 E and F, and gave a maximum ee of 93.0% (Fig. 3B). Furthermore, we measured the amount of (R)-mandelonitrile produced by the enzyme (20–150 U⋅mL−1) in the same pH condition as in Fig. 3B. ChuaHNL (150 U⋅mL−1) gave a maximum ee of 99.0% in 5 min (Fig. 3C), which is sufficient for industrial use. We have shown that the enzyme catalyzes cyanohydrin synthesis with high efficiency and enantiopurity at room temperature (22 °C) and in a citrate buffer system, unlike other HNLs, which normally catalyze these reactions in biphasic systems containing organic solvents.
Fig. 3.
(R)-Mandelonitrile synthesis with an excellent ee value using ChuaHNL in aqueous solution. (A) Relationship between the amount of ChuaHNL (0–20 U⋅mL−1) and ee (%). The reaction was performed in 400 mM citrate buffer, pH 4.2, at 22 °C for 5 min. (B) Relationship between pH and ee. ChuaHNL (20 U⋅mL−1) was reacted with the 50 mM substrate benzaldehyde at 22 °C and at various pH values. (C) (R)-mandelonitrile synthesis under optimized conditions. ChuaHNL (20–150 U⋅mL−1) was reacted with substrate in 400 mM citrate buffer, pH 2.7.
Conclusion
ChuaHNL is derived from an arthropod. Because the global arthropod species richness is at least 5 million species (37), the animal kingdom is likely to be the next bioresource for desired enzymes. This millipede HNL, which shows no homology with reported HNLs, exhibits high temperature and pH stability and wide substrate specificity toward aromatic carbonyl aldehydes, and it enables the production of enantioselectively pure mandelonitrile. In addition, in the synthesis of (R)-mandelonitrile from benzaldehyde and potassium cyanide, the enzyme shows a fivefold higher specific activity compared with PaHNL (the already industrialized and highly active almond enzyme), effectively saving materials and energy previously needed for the reaction. In HNL research, cycles of enzyme identification, physicochemical property characterization, and rational protein engineering have been repeated for the sustainable development of industrial biotechnology for a century (38). In this respect, the discovery of millipede HNL can serve as a template for the isolation of promising and efficient enzymes and the design of tailor-made enzymes by rational protein engineering, which are needed to spur the industrial synthesis of fine chemicals and pharmaceuticals in the next generation.
Materials and Methods
Materials and methods are described completely in SI Materials and Methods.
Nucleotide Sequence Accession Number.
The nucleotide sequence data of ChuaHNL cDNA have been deposited in the DNA Data Bank of Japan (accession no. LC004755).
Description of SI Materials and Methods.
The materials used in this study and the methods of millipede collection, enzyme assays, enzyme purification, protein sequencing, detection of sugar molecules in the HNL, UV-Vis, and CD spectra of the purified millipede HNL, cDNA cloning, recombinant gene expression, tissue collection, RT-PCR, Western-blot analysis, immunohistochemistry, and NMR data for the synthesized cyanohydrins are described in SI Materials and Methods.
SI Materials and Methods
Millipede Collection.
The invasive millipedes Chamberlinius hualienensis were manually collected using a brush and a plastic box (18 × 12 × 5 cm) in the Japanese cedar forest located in Kyushu, Japan from 2010 to 2013. We collected a total of 30 kg of millipede during the 4 y. Empirically, the invasive millipedes could not be collected from the same site during the next season because of our mass collection. For protein purification, the collected animals were frozen with dry ice, transferred to our laboratory, and stored at −80 °C until used. For other analyses, these animals were fed on slices of sweet potato and water and were kept in plastic boxes (26 × 19 × 9 cm) at 17 °C and 70–90% relative humidity until used.
Enzyme Assays.
Synthesis reaction (including characterization, substrate screening, and kinetics).
All chemicals were purchased from various commercial suppliers. The synthesis of cyanohydrins from aromatic aldehydes was monitored using an HPLC equipped with a chiral column (26). The enzyme sample was added to 1 mL of 400 mM citrate buffer, pH 4.2, containing 50 mM aromatic aldehydes and 100 mM potassium cyanide, mixed, and incubated at 22 °C for 5 min. One hundred microliters of reactant was transferred to 900 µL of n-hexane:2-propanol (see the ratios in Table S2), mixed vigorously, and centrifuged at 16,000 × g for 3 min. Five microliters of the organic phase was analyzed using an HPLC (UFLC Prominence Liquid Chromatograph LC-20AD connected to a Prominence UV-Vis Detector SPD-20A; Shimadzu) equipped with a CHIRALCEL OJ-H column (particle size: 5 μm; 4.6 mm i.d. × 250 mm; Daicel) under the following conditions: mobile phase, n-hexane:2-propanol (see ratio in Table S2); flow rate 1 mL⋅min−1 and absorbance 254 nm.
All authentic and enzymatic-synthesized cyanohydrins (Table 2) were determined using HPLC and proton NMR. The cyanohydrins used for the calculation of HNL enzymatic activity including kinetic studies (Table 2) were prepared by chemical synthesis, because these chemicals are not commercially available (26), with the exception of racemic mandelonitrile, which was purchased from Sigma-Aldrich. The amount of product was calculated using a standard curve prepared with a known amount of each authentic chemical. As a control, the synthetic rate without enzyme was simultaneously determined for each assay and subtracted from the total rate. Each point represents the mean value of three independent experiments. One unit of activity is defined as the amount of the enzyme that catalyzes the production of 1 μmol of optically active cyanohydrin from the corresponding substrate per 1 min (12).
Various concentrations of substrates (0.1–100 mM) were used to plot the substrate saturation curve for kinetic study of the enzyme (ca. ∼2.5 U toward benzaldehyde) in the synthesis of cyanohydrins (Table 2) from benzaldehyde, 4-bromobenzaldehyde, 3-methylbenzaldehyde, 4-methylbenzaldehyde, 3-methoxybenzaldehyde, 4-methoxybenzaldehyde, 2,4-dimethyl benzaldehyde, 2-thiophene carboxaldehyde, and 4-biphenyl carboxaldehyde. Steady-state kinetic parameters were calculated using KaleidaGraph version 4.1 (Synergy Software), fitting the data to the Michaelis–Menten equation.
The optimum temperature was evaluated by monitoring the mandelonitrile synthetic activity for 5 min at various temperature ranges from 10 °C to 90 °C. The effect of temperature was determined by measuring the activity after 1 h of incubation at various temperatures ranging from 0 °C to 90 °C. The optimum pH was evaluated by monitoring the synthetic activity for 5 min in 1 mL of 400 mM citrate buffer in various pH ranges (2.5–6.5). The effect of pH was determined by measuring the remaining activity after incubation for 1 h in 400 mM citrate-phosphate or glycine-sodium hydroxide buffer at various pH ranges (2.5–9.6).
The effect of potential inhibitors on the synthetic activity of ChuaHNL was monitored as follows. The inhibitors (0.1–10 mM) listed in Table S1 were incubated with 1.0–1.5 U ChuaHNL in 1 mL of 20 mM potassium phosphate buffer at 22 °C for 1 h. The remaining enzymatic activity was detected as described above.
The ee was evaluated as follows. Recombinant ChuaHNL (1–150 U) was added to 1 mL of 400 mM citrate buffer, pH 2.7–5.2, containing 50 mM benzaldehyde, and the mixture was incubated at 22 °C for 5 min. The amount of (R)- and (S)-mandelonitrile produced was measured by the method described above. The ee was determined as described in a previous study (27).
Cleavage reaction.
The degradation of mandelonitrile to benzaldehyde was monitored using a UV-Vis spectrophotometer (27, 39). The enzyme sample was added to 1 mL of 100 mM citrate buffer, pH 5.5, containing 2 mM of racemic mandelonitrile. After mixing gently, the amount of benzaldehyde produced was immediately measured by monitoring the absorbance at 280 nm and 22 °C for 2 min using a UV-2600 UV-Vis spectrophotometer (Shimadzu). Because the racemic mandelonitrile decomposes slowly under our experimental conditions, as a control, the cleavage rate without enzyme was simultaneously determined for each assay and subtracted from the total rate. One unit of activity in the cleavage of mandelonitrile to benzaldehyde is defined as the amount of enzyme that catalyzes the production of 1 μmol of benzaldehyde per 1 min.
Enzyme Purification.
The enzymatic activity of the (R)-mandelonitrile synthesis from benzaldehyde in each purification step was measured using the method described above. The protein concentration was determined with a protein assay kit (Bio-Rad Laboratories) using BSA as the standard protein. The protein purity was assessed by 15% SDS/PAGE.
Frozen millipedes were ground in liquid nitrogen using a mortar and pestle in a chemical fume hood. The fine millipede powder was suspended in 20 mM of ice-cold potassium phosphate buffer, pH 7.0, and stirred for 3 h to extract the protein. All subsequent steps were performed at 4 °C. The solution was carefully passed through four layers of cheesecloth to remove unground tissues. After the addition of 0.05% protamine sulfate (Nacalai Tesque) and incubation for 30 min, the solution was centrifuged at 28,500 × g for 30 min at 4 °C to remove debris.
After precipitation with 50–70% saturated ammonium sulfate, the active fraction was dialyzed against 20 mM potassium phosphate buffer, pH 8.2, and loaded onto a column containing Toyopearl DEAE-650M (Tosoh) (i.d. 50 mm; column volume 200 mL). The protein was eluted at a flow rate of 0.5 mL⋅min−1. Enzymatic activity was detected in the flow-through fraction. After adding ammonium sulfate at 30% saturation, the DEAE-unbound fraction was loaded onto another column containing Toyopearl Butyl-650M (Tosoh) (i.d. 25 mm; column volume 25 mL). The protein was eluted with a linear gradient of ammonium sulfate from 30 to 0% in the same buffer. The active fraction was dialyzed against 20 mM potassium phosphate buffer, pH 8.2, loaded onto a Tricorn 10/300 column (GE Healthcare) packed with Q Sepharose Fast Flow (GE Healthcare), and eluted with a linear gradient of sodium chloride from 0 mM to 300 mM at a flow rate of 2 mL⋅min−1. The active fractions were then dialyzed against 20 mM Tris⋅HCl, pH 9, loaded onto a MonoQ 5/50 GL anion exchange column (GE Healthcare), and eluted with a linear gradient of sodium chloride from 0 mM to 400 mM in the same buffer at a flow rate of 1 mL⋅min−1. The active fractions were pooled and concentrated using a centrifugal filtration device (Amicon Ultra-0.5 Centrifugal Filter Unit with Ultracel-10 membrane; EMD Millipore). The protein was loaded onto a Superdex 75 10/300 GL gel filtration column (GE Healthcare) and eluted with 20 mM potassium phosphate buffer, pH 7.0, containing 150 mM of sodium chloride at a flow rate of 0.5 mL⋅min−1.
Protein Sequencing.
The purified enzyme was separated using 15% SDS/PAGE and electrophoretically transferred to a PVDF membrane (Clear Blot Membrane-p, 85 × 90 mm; ATTO) using a blotting system (WSE-4020 HorizeBLOT 2M-R; ATTO). The PVDF membrane was stained with freshly prepared Coomassie Brilliant Blue R-250 for 10 min and then destained in a solution containing 10% (vol/vol) acetic acid and 40% (vol/vol) methanol. N-terminal and internal amino acid sequences were determined by Edman degradation or by in-gel digestion (Apro Life Science Institute).
Detection of Sugar Molecules in the HNL.
The purified native HNL was separated using 15% SDS/PAGE. Sugar molecules in the separated HNL were detected by the PAS method using a Pierce Glycoprotein Staining Kit (Thermo Fisher Scientific). The staining procedure was performed according to the manufacturer’s instructions.
UV-Vis and CD Spectra of the Purified Millipede HNL.
To detect a possible prosthetic group, the purified protein (1.5 mg⋅mL−1 in 20 mM potassium phosphate buffer, pH 7.0) was scanned at the range of 200–600 nm in a 1-cm cell path length Ultra-Micro Cell (Hellma Analytics) using a UV-2600 spectrophotometer (Shimadzu). To estimate the secondary structure, the enzyme solution (0.025–0.3 mg⋅mL−1) was scanned at 180–300 nm in a 1-cm quartz cuvette (Tosoh) using a Jasco 720 circular dichroism spectrophotometer (Shimadzu) under the following conditions: temperature, 20 °C; cell length, 0.02 cm; resolution, 0.2 nm; bandwidth, 2.0 nm; sensitivity, 100 mdeg; response, 1 s; speed, 100 nm⋅min−1; and accumulation, 10–20. To analyze the CD spectra, we used web-based software, K2D2 (35) and DichroWeb (36), in the range of 190–240 nm.
cDNA Cloning.
The dissected paraterga was immediately homogenized in TRIzol reagent (Thermo Fisher Scientific) using a BioMasher II (Nippi). RNA extraction was performed according to the manufacturer’s instructions. The cDNAs for 5′- and 3′-RACE were synthesized using a SMART RACE cDNA Amplification Kit and SMARTScribe Reverse Transcriptase (Clontech Laboratories) and subsequently treated with RNase H (Takara Bio).
Degenerate primers (Table S3) were designed based on the N-terminal and internal amino acid sequences determined by protein sequencing as described in SI Materials and Methods. The following PCR program was performed using the degenerate primers and Dream Taq DNA polymerase (Thermo Fisher Scientific) or Advantage GC2 Polymerase Mix (Clontech Laboratories): 94 °C for 3 min, 70 cycles of 94 °C for 1 min, 40 °C for 1 min, and 72 °C for 1 min. The PCR products were gel-purified using a Wizard SV PCR and Gel Clean-Up System (Promega) and ligated into the EcoRV recognition site of pBluescript II SK (+) (Agilent Technologies). The DNA sequence was determined using a 3500 Genetic Analyzer (Applied Biosystems). The sequences obtained were assembled and analyzed by ATGC and Genetyx Ver. 12 (Genetyx), respectively. Based on the DNA sequence, gene-specific primers were designed (Table S3). The following PCR program was performed using KOD plus neo (Toyobo): 94 °C for 2 min, 35 cycles of 98 °C for 10 s, and 68 °C for 1 min. Other procedures were performed as described above. The full-length cDNA sequence was determined using 18 independent clones to avoid PCR-derived sequence errors.
Table S3.
Primers for cDNA cloning, RT-PCR, and vector construction
| Primer | Sequence |
| PKAAINPIQEf1 | 5′-CC(A/C/G/T)AA(A/G)GC(A/C/G/T)GC(A/C/G/T)AT(A/T/C)AA(C/T)CC(A/C/G/T)AT(A/T/C)CA(A/G)GA-3′ |
| APTALDIKf1 | 5′-GC(A/C/G/T)CC(A/C/G/T)AC(A/C/G/T)GC(A/C/G/T)TT(A/G)GA(C/T)AT(A/C/T)AA-3′ |
| APTALDIKf2 | 5′-GC(A/C/G/T)CC(A/C/G/T)AC(A/C/G/T)GC(A/C/G/T)CT(A/C/G/T)GA(C/T)AT(A/C/T)AA-3′ |
| APTALDIKr1 | 5′-TT(A/G/T)AT(A/G)TC(C/T)AA(A/C/G/T)GC(A/C/G/T)GT(A/C/G/T)GG(A/C/G/T)GC-3′ |
| APTALDIKr2 | 5′-TT(A/G/T)AT(A/G)TC(A/C/G/T)AG(A/C/G/T)GC(A/C/G/T)GT(A/C/G/T)GG(A/C/G/T)GC-3′ |
| AAINPIQEf | 5′-GC(A/C/G/T)GC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| ATINPIQEf | 5′-GC(A/C/G/T)AC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| LAINPIQEf1 | 5′-TT(A/G)GC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| LAINPIQEf2 | 5′-CT(A/C/G/T)GC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| LTINPIQEf1 | 5′-TT(A/G)AC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| LTINPIQEf2 | 5′-CT(A/C/G/T)AC(A/C/G/T)AT(A/C/T)AA(C/T)CC(A/C/G/T)AT(A/C/T)CA(A/G)GA-3′ |
| AAINPIQEr | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GC(A/C/G/T)GC-3′ |
| ATINPIQEr | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GT(A/C/G/T)GC-3′ |
| LAINPIQEr1 | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GC(C/T)AA-3′ |
| LAINPIQEr2 | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GC(A/C/G/T)AG-3′ |
| LTINPIQEr1 | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GT(C/T)AA-3′ |
| LTINPIQEr2 | 5′-TC(C/T)TG(A/G/T)AT(A/C/G/T)GG(A/G)TT(A/G/T)AT(A/C/G/T)GT(A/C/G/T)AG-3′ |
| NCPETHGCFAFf | 5′-AA(C/T)TG(C/T)CC(A/C/G/T)GA(A/G)AC(A/C/G/T)CA(C/T)GG(A/C/G/T)TG(C/T)TT(C/T)GC(A/C/G/T)TT-3′ |
| NCPETHGCFAFr | 5′-AA(A/C/G/T)GC(A/G)AA(A/G)CA(A/C/G/T)CC(A/G)TG(A/C/G/T)GT(C/T)TC(A/C/G/T)GG(A/G)CA(A/G)TT-3′ |
| ChuaHNL-1 | 5′-CTGACTGAAACCTTCGAATGCACCACTCG-3′ |
| ChuaHNL-2 | 5′-GGCATAATGAATCTTGTCGCCGTTTGGAAC-3′ |
| ChuaHNL-3 | 5′-TTTGGTAGTGGACCAGCGAGCAGGTTGCAC-3′ |
| ChuaHNL-4 | 5′-ATAATCCCTTTAAAGTTCAGGTGCAATTAG-3′ |
| ChuaHNL-5 | 5′-ATACCAACACATCAAACTTACCAAGCTTAG-3′ |
| ChuaHNL-6 | 5′-ATTATGGCTTACGATTTCGTCGGTGGTCC-3′ |
| XhoIkex2-mChuaHNL | 5′-GCGCTCGAGAAAAGACTGACTTGTGATCAACTTCCC-3′ |
| ChuaHNLstop-XbaI | 5′-CGCTCTAGATTAGTAAAAAGCAAAGCAACCGTGGGTTTC-3′ |
Bioinformatics analysis was performed using the BLAST program (blast.ncbi.nlm.nih.gov/Blast.cgi) (40), PeptideMass, NetNGlyc 1.0 Server (www.cbs.dtu.dk/services/), NetOGlyc 4 Server (41), and Jpred 3 (42). The algorithm parameters of blastp were set as follows: Max target sequences, 100; Expect threshold, 10; Max matches in a query range; Matrix, BLOSUM62; Gap Costs, Existence:11 Extension:1; Compositional adjustments, Conditional compositional score matrix adjustment.
Construction of the Pichia Expression Vector and Expression of Recombinant ChuaHNL.
The inserted DNA was amplified by PCR using an XhoIkex2-mChuaHNL primer, a ChuaHNLstop-XbaI primer (Table S3), PfuUltra II fusion HS DNA polymerase (Agilent Technologies), and template cDNA prepared from the paraterga. The PCR was performed as follows: 95 °C for 2 min, 30 cycles of 95 °C for 20 s, 40 °C for 20 s, and 72 °C for 3 min. The PCR amplicon was treated with DpnI (New England Biolabs) at 37 °C for 1 h. After heat inactivation, the PCR product was digested with XhoI and XbaI (New England Biolabs), gel-purified, and ligated into the corresponding recognition sites of the restriction enzymes of pPICZαA (Thermo Fisher Scientific).
The expression vector was linearized with SacI (Takara Bio). Pichia competent cells were prepared from the GS115 strain by the method reported previously (43). Transformation and screening for transformants were performed according to the manufacturer’s instructions for an Easy Select Pichia Expression Kit (Thermo Fisher Scientific). The selected transformants were inoculated in 5 mL of yeast extract peptone dextrose broth and agitated at 300 rpm and 30 °C for 1 d. After harvesting by centrifugation, the cells were transferred to buffered minimal glycerol supplemented by histidine medium and agitated at 175 rpm at 30 °C for 2 d. The cells were then transferred to buffered minimal methanol supplemented by histidine medium and agitated at 225 rpm at 30 °C for 3 d. Protein expression was induced by adding 0.5% methanol daily during this period. The cultured medium was centrifuged to remove cells and used for purification.
Tissue Collection.
Antenna and leg samples were collected using fine forceps under a microscope, after anesthetizing the millipede on ice. Hemolymph was collected from wounds on the antennal sclerite or the leg by centrifugation at 8,000 × g for 2 min at 4 °C. Then, the body was immobilized with insect pins on a piece of rubber sheet attached to a Petri dish and immersed in ice-cold PBS. Paraterga, integument, head, fat body, and gut were collected from the body.
RT-PCR.
The cDNA was synthesized using a SMART RACE cDNA Amplification Kit (Clontech Laboratories) and with SuperScript II (Thermo Fisher Scientific) as a reverse transcriptase. The following PCR program was performed using the primers ChuaHNL-1 and ChuaHNL-2 (Table S3) and KOD plus neo (Toyobo) as a Taq DNA polymerase: 94 °C for 2 min, 25 cycles of 98 °C for 10 s, and 68 °C for 1 min. The expression of actin, as an internal control, was detected as previously reported (44). The PCR product was separated on a 1.5% (wt/vol) agarose gel, visualized under UV using a gel documentation system (AE-6933FXES-U Printgraph; ATTO), and cropped using Adobe Photoshop CS6 Extended software (Adobe).
Western Blot Analysis.
Rabbit anti-ChuaHNL serum was raised against a synthetic peptide, GGPENSAHLNE, in ChuaHNL (Operon Biotechnology). Each experimental tissue was collected after anesthetizing on ice as described above. The tissue was immediately homogenized in SDS sample buffer using BioMasher II (Nippi), boiled for 5 min, and centrifuged at 16,000 × g for 10 min to remove debris. For the antenna, leg, head, paraterga, and gut, 0.3 animal equivalents were analyzed using SDS/PAGE. However, for the integument and hemolymph, two body segments-equivalents and 1 µL were analyzed, respectively. The protein was separated using 15% SDS/PAGE (e-PAGEL; ATTO) and transferred to a PVDF membrane (ATTO) using an electroblotter, as described above. The transferred membrane was washed with TBS-T (10 mM Tris⋅HCl, pH 7.5, 100 mM sodium chloride, and 0.1% Tween-20) and blocked with Blocking One (Nacalai Tesque) for 1 h. The membrane was washed and reacted with a 1:1,000 dilution of the primary antibody, rabbit anti-ChuaHNL serum in Canget Signal Immunoreaction Enhancer Solution 1 (Toyobo), for 1 h. After washing, the membrane was reacted with a 1:30,000 dilution of the secondary antibody, HRP-conjugated anti-rabbit IgG (Anti-Rabbit IgG, HRP-Linked Whole Ab Donkey; GE Healthcare) in Canget Signal Immunoreaction Enhancer Solution 2 (Toyobo) for 1 h. The immunoreactive material was visualized using an ECL Prime Western Blotting Detection Reagent (GE Healthcare) according to the manufacturer’s instruction. Chemiluminescence was detected using a luminescent image analyzer (LAS-1000 mini; GE Healthcare).
Immunohistochemistry.
Immunohistochemistry was performed using a slightly modified method (45). In brief, the paraterga of a millipede was dissected in PBS containing 4% (wt/vol) paraformaldehyde. The tissue was fixed for an additional 2 h using the same fixative. After washing twice in PBS containing 0.1% Triton X-100 (PBST) at room temperature for 15 min, the specimen was treated with an ethanol series. After rehydration, the specimen was washed twice in PBST for 15 min and blocked with PBST containing 10% (vol/vol) normal goat serum (Wako Pure Chemical Industries) at 4 °C overnight. The specimen was reacted with a 1:1,000 dilution of the rabbit anti-ChuaHNL antiserum at 4 °C overnight. After washing three times in PBST for 30 min, the specimen was blocked and reacted with a 1:1,000 dilution of Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific) at 4 °C overnight. After washing in PBST, the specimen was embedded in Vectashield Mounting Medium (Vector Laboratories) and observed under a fluorescence microscope (M165 FC-Fluorescence; Leica Microsystems). The image was captured using imaging software (LAS V4.3; Leica Microsystems) and cropped using Adobe Photoshop CS6 Extended (Adobe).
NMR Data for Synthesized Cyanohydrins.
1H NMR spectra were obtained in CDCl3 using a Bruker Biospin Avance II 400 spectrometer. The remaining proton signal for CHCl3 (7.26 ppm) was used as the internal standard.
2-Hydroxy-2-phenylacetonitrile: 1H NMR: δ 5.54 (1H, s), 7.43–7.45 (3H, m), 7.53–7.55 (2H, m).
2-(4-Bromophenyl)-2-hydroxyacetonitrile: 1H NMR: δ 5.52 (1H, s), 7.41–7.43 (2H, m), 7.57–7.60 (2H, m).
2-Hydroxy-2-(m-tolyl) acetonitrile: 1H NMR: δ 2.39 (3H, s), 5.49 (1H, s), 7.23 (1H, m), 7.31–7.35 (3H, m).
2-Hydroxy-2-(p-tolyl) acetonitrile: 1H NMR: δ 2.40 (3H, s), 5.51 (1H, s), 7.26 (2H, m), 7.43 (2H, m).
2-Hydroxy-2-(3-methoxyphenyl) acetonitrile: 1H NMR: δ 3.84 (3H, s), 5.52 (1H, s), 6.96 (1H, brdd, J = 2.2 and 8.0 Hz), 7.07 (1H, m), 7.11 (1H, brd, J = 8.0 Hz), 7.36 (1H, t, J = 8.0 Hz).
2-Hydroxy-2-(4-methoxyphenyl) acetonitrile: 1H NMR: δ 3.82 (3H, s), 5.50 (1H, s), 6.93 (2H, m), 7.46 (2H, m).
2-(2, 4-Dimethylphenyl)-2-hydroxyacetonitrile: 1H NMR: δ 2.34 (3H, s), 2.41 (3H, s), 5.62 (1H, s), 7.06–7.09 (2H, m), 7.48 (1H, d, J = 7.7 Hz).
2-Hydroxy-2-(thiophen-2-yl) acetonitrile: 1H NMR: δ 5.74 (1H, brs), 7.05 (1H, dd, J = 3.5 and 5.1 Hz), 7.31 (1H, m), 7.43 (1H, dd, J = 1.3 and 5.1 H).
2-([1, 1′-Biphenyl]-4-yl)-2-hydroxyacetonitrile: 1H NMR: δ 5.61 (3H, s), 7.39 (1H, m), 7.46 (2H, m), 7.59–7.63 (4H, m), 7.68 (2H, m).
Supplementary Material
Acknowledgments
We thank T. Tanabe, M. Kamakura, T. Sakaki, M. Kameya, H. Komeda, Y. Takakura, and K. Isobe for supporting our experiments and valuable discussions. This work was supported by the Exploratory Research for Advanced Technology program of the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The cDNA sequence reported in this paper has been deposited in the DNA Data Bank of Japan (accession no. LC004755).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508311112/-/DCSupplemental.
References
- 1.Asano Y. 2015. Hydrolysis of nitriles to amides. Science of Synthesis: Biocatalysis in Organic Synthesis, eds Faber K, Fessner WD, Turner N (Georg Thieme, Stuttgart), Vol 1, pp 255–276.
- 2.Osbourn AE. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8(10):1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effenberger F, Ziegler T, Förster S. Enzyme-catalyzed cyanohydrin synthesis in organic solvents. Angew Chem Int Ed Engl. 1987;26(5):458–460. [Google Scholar]
- 4.Brown RFC, Donohue AC, Jackson WR, McCarthy TD. Synthetic applications of optically active cyanohydrins. Enantioselective syntheses of the hydroxyamides tembamide and aegeline, the cardiac drug denopamine, and some analogues of the bronchodilator salbutamol. Tetrahedron. 1994;50(48):13739–13752. [Google Scholar]
- 5.Effenberger F, Förster S, Kobler C. State of the art and applications in stereoselective synthesis of chiral cyanohydrins. In: Patel RN, editor. Biocatalysis in the Pharmaceutical and Biotechnology Industries. CRC; New York: 2007. pp. 677–698. [Google Scholar]
- 6.Holt J, Hanefeld U. Enantioselective enzyme-catalysed synthesis of cyanohydrins. Curr Org Synth. 2009;6(1):15–37. [Google Scholar]
- 7.Effenberger F, Eichhorn J, Roos J. Enzyme catalyzed addition of hydrocyanic acid to substituted pivalaldehydes-A novel synthesis of (R)-pantolactone. Tetrahedron Asymmetry. 1995;6(1):271–282. [Google Scholar]
- 8.Rosenthaler L. Durch enzyme bewirkte asymmetrische synthesen. Biochem Z. 1908;14:238–253. [Google Scholar]
- 9.Brussee J, van der Gen A. 2000. Biocatalysis in the enantioselective formation of chiral cyanohydrins, valuable building blocks in organic synthesis. Stereoselective Biocatalysis, ed Patel RN (CRC, Boca Raton, FL), pp 289–319.
- 10.Fechter MH, Griengl H. Hydroxynitrile lyases: Biological sources and application as biocatalysts. Food Technol Biotechnol. 2004;42(4):287–294. [Google Scholar]
- 11.Wiedner R, Gruber-Khadjawi M, Schwab H, Steiner K. Discovery of a novel (R)-selective bacterial hydroxynitrile lyase from Acidobacterium capsulatum. Comput Struct Biotechnol J. 2014;10(16):58–62. doi: 10.1016/j.csbj.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asano Y, et al. Screening for new hydroxynitrilases from plants. Biosci Biotechnol Biochem. 2005;69(12):2349–2357. doi: 10.1271/bbb.69.2349. [DOI] [PubMed] [Google Scholar]
- 13.Glieder A, et al. Comprehensive step-by-step engineering of an (R)-hydroxynitrile lyase for large-scale asymmetric synthesis. Angew Chem Int Ed Engl. 2003;42(39):4815–4818. doi: 10.1002/anie.200352141. [DOI] [PubMed] [Google Scholar]
- 14.Dadashipour M, Asano Y. Hydroxynitrile lyases: Insights into biochemistry, discovery, and engineering. ACS Catal. 2011;1(9):1121–1149. [Google Scholar]
- 15.Hanefeld U. Immobilisation of hydroxynitrile lyases. Chem Soc Rev. 2013;42(15):6308–6321. doi: 10.1039/c3cs35491a. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-C, Golovatch SI, Chang H-W, Chen S-H. Revision of the Taiwanese millipede genus Chamberlinius Wang, 1956, with descriptions of two new species and a reclassification of the tribe Chamberlinini (Diplopoda, Polydesmida, Paradoxosomatidae, Paradoxosomatinae) ZooKeys. 2011;98(98):1–27. doi: 10.3897/zookeys.98.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niijima K, Arimura T. Obstruction of trains by the outbreaks of a millipede Chamberlinius hualienensis WANG (Diplopoda: Polydesmida) Edaphologia. 2002;69:47–49. [Google Scholar]
- 18.Noguchi S, Mori N, Higa Y, Kuwahara Y. Identification of mandelonitrile as a major secretory compound from Chamberlinius hualienensis Wang (Polydesmida: Paradoxosomatidae) Jpn J Environ Entomol Zool. 1997;8(4):208–214. [Google Scholar]
- 19.Blum MS. Chemical Defenses of Arthropods. Academic; New York: 1981. [Google Scholar]
- 20.Eisner T, Meinwald J. Defensive secretions of arthropods. Science. 1966;153(3742):1341–1350. doi: 10.1126/science.153.3742.1341. [DOI] [PubMed] [Google Scholar]
- 21.Ueatrongchit T, Kayo A, Komeda H, Asano Y, H-Kittikun A. Purification and characterization of a novel (R)-hydroxynitrile lyase from Eriobotrya japonica (Loquat) Biosci Biotechnol Biochem. 2008;72(6):1513–1522. doi: 10.1271/bbb.80023. [DOI] [PubMed] [Google Scholar]
- 22.Ueatrongchit T, Tamura K, Ohmiya T, H-Kittikun A, Asano Y. Hydroxynitrile lyase from Passiflora edulis: Purification, characteristics and application in asymmetric synthesis of (R)-mandelonitrile. Enzyme Microb Technol. 2010;46(6):456–465. doi: 10.1016/j.enzmictec.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Fukuta Y, et al. Characterization of a new (R)-hydroxynitrile lyase from the Japanese apricot Prunus mume and cDNA cloning and secretory expression of one of the isozymes in Pichia pastoris. Biosci Biotechnol Biochem. 2011;75(2):214–220. doi: 10.1271/bbb.100187. [DOI] [PubMed] [Google Scholar]
- 24.Wajant H, Förster S, Selmar D, Effenberger F, Pfizenmaier K. Purification and characterization of a novel (R)-mandelonitrile lyase from the fern Phlebodium aureum. Plant Physiol. 1995;109(4):1231–1238. doi: 10.1104/pp.109.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen I, Woker R, Kula MR. Purification and protein characterization of hydroxynitrile lyases from Sorghum and Almond. Biotechnol Appl Biochem. 1992;15(1):90–99. [Google Scholar]
- 26.Nanda S, Kato Y, Asano Y. A new (R)-hydroxynitrile lyase from Prunus mume: Asymmetric synthesis of cyanohydrins. Tetrahedron. 2005;61(46):10908–10916. [Google Scholar]
- 27.Dadashipour M, et al. S-selective hydroxynitrile lyase from a plant Baliospermum montanum: molecular characterization of recombinant enzyme. J Biotechnol. 2011;153(3-4):100–110. doi: 10.1016/j.jbiotec.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Anastas PT, Warner JC. Green Chemistry: Theory and Practice. Oxford Univ Press; New York: 1998. p. 30. [Google Scholar]
- 29.Effenberger F, Jӓger J. Stereoselective synthesis of (S)-3,4-methylenedioxyamphetamines from (R)-cyanohydrins. Chemistry. 1997;3(8):1370–1374. [Google Scholar]
- 30.Yamamoto K, Oishi K, Fujimatsu I, Komatsu K. Production of R-(-)-mandelic acid from mandelonitrile by Alcaligenes faecalis ATCC 8750. Appl Environ Microbiol. 1991;57(10):3028–3032. doi: 10.1128/aem.57.10.3028-3032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritzen B, van Oers MCM, van Delft FL, Rutjes FPJT. Enantioselective chemoenzymatic synthesis of trans-aziridines. J Org Chem. 2009;74(19):7548–7551. doi: 10.1021/jo901548t. [DOI] [PubMed] [Google Scholar]
- 32.Dreveny I, Gruber K, Glieder A, Thompson A, Kratky C. The hydroxynitrile lyase from almond: A lyase that looks like an oxidoreductase. Structure. 2001;9(9):803–815. doi: 10.1016/s0969-2126(01)00639-6. [DOI] [PubMed] [Google Scholar]
- 33.Hughes J, Carvalho FJ, Hughes MA. Purification, characterization, and cloning of alpha-hydroxynitrile lyase from cassava (Manihot esculenta Crantz) Arch Biochem Biophys. 1994;311(2):496–502. doi: 10.1006/abbi.1994.1267. [DOI] [PubMed] [Google Scholar]
- 34.Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell. 2010;143(7):1121–1135. doi: 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Iratxeta C, Andrade-Navarro MA. 2008. K2D2: Estimation of protein secondary structure from circular dichroism spectra. BMC Struct Biol 8:25.
- 36.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668-73. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odegaard F. How many species of arthropods? Erwin’s estimate revised. Biol J Linn Soc Lond. 2000;71(4):583–597. [Google Scholar]
- 38.Purkarthofer T, Skranc W, Schuster C, Griengl H. Potential and capabilities of hydroxynitrile lyases as biocatalysts in the chemical industry. Appl Microbiol Biotechnol. 2007;76(2):309–320. doi: 10.1007/s00253-007-1025-6. [DOI] [PubMed] [Google Scholar]
- 39.Hanefeld U, Straathof AJJ, Heijnen JJ. Study of the (S)-hydroxynitrile lyase from Hevea brasiliensis: Mechanistic implications. Biochim Biophys Acta. 1999;1432(2):185–193. doi: 10.1016/s0167-4838(99)00108-9. [DOI] [PubMed] [Google Scholar]
- 40.Johnson M, et al. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008;36(Web Server issue):W5-9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steentoft C, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36(Web Server issue):W197-201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin-Cereghino J, et al. Condensed protocol for competent cell preparation and transformation of the methylotrophic yeast Pichia pastoris. Biotechniques. 2005;38(1):44–48, 46, 48. doi: 10.2144/05381BM04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishida Y, Leal WS. Cloning of putative odorant-degrading enzyme and integumental esterase cDNAs from the wild silkmoth, Antheraea polyphemus. Insect Biochem Mol Biol. 2002;32(12):1775–1780. doi: 10.1016/s0965-1748(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 45.Ishida Y, et al. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc Natl Acad Sci USA. 2014;111(10):3847–3852. doi: 10.1073/pnas.1323928111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eliel EL, Wilen SH, Mander LN. Stereochemistry of Organic Compounds. Wiley; New York: 1994. pp. 101–152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.