Significance
Dental microwear is among the most common proxies paleontologists use for diet reconstruction. Recent models have suggested that while quartz grit adherent to food produces wear of tooth enamel, softer particles, such as silica phytoliths found in many plants, do not. Some have therefore suggested that microwear patterns better reflect habitat than diet. This is important to paleobiologists because reconstructions of species from the earliest vertebrates to human ancestors have relied on dental microwear as a proxy for diet. Here we present an in vitro study demonstrating that softer particles produce microwear under conditions mimicking chewing. Enamel wear occurs not because an abrasive is hard but because it exceeds the binding force of proteins that hold together hydroxyapatite crystallites.
Keywords: dental microwear, diet reconstruction, tooth wear
Abstract
Paleoanthropologists and vertebrate paleontologists have for decades debated the etiology of tooth wear and its implications for understanding the diets of human ancestors and other extinct mammals. The debate has recently taken a twist, calling into question the efficacy of dental microwear to reveal diet. Some argue that endogenous abrasives in plants (opal phytoliths) are too soft to abrade enamel, and that tooth wear is caused principally by exogenous quartz grit on food. If so, variation in microwear among fossil species may relate more to habitat than diet. This has important implications for paleobiologists because microwear is a common proxy for diets of fossil species. Here we reexamine the notion that particles softer than enamel (e.g., silica phytoliths) do not wear teeth. We scored human enamel using a microfabrication instrument fitted with soft particles (aluminum and brass spheres) and an atomic force microscope (AFM) fitted with silica particles under fixed normal loads, sliding speeds, and spans. Resulting damage was measured by AFM, and morphology and composition of debris were determined by scanning electron microscopy with energy-dispersive X-ray spectroscopy. Enamel chips removed from the surface demonstrate that softer particles produce wear under conditions mimicking chewing. Previous models posited that such particles rub enamel and create ridges alongside indentations without tissue removal. We propose that although these models hold for deformable metal surfaces, enamel works differently. Hydroxyapatite crystallites are “glued” together by proteins, and tissue removal requires only that contact pressure be sufficient to break the bonds holding enamel together.
Dental microwear, the study of microscopic use-wear on tooth surfaces, is among the most common proxies used today for the reconstruction of diet in fossil vertebrates. The first analysis to focus on the etiology of microwear was Baker et al.’s (1) work on sheep teeth. They suggested that both exogenous grit from the soil and phytoliths found in the grasses sheep eat wear their teeth. Phytoliths are formed as silicic acid from the soil is taken up by a plant and deposited as hydrated amorphous silica within the lumen of its epidermal cells. Support for their hypothesis came from the observation of both angular quartz (soil) fragments and fractured phytoliths in sheep feces, along with hardness tests suggesting to them that phytoliths were harder than (and therefore capable of wearing) dental enamel. Walker et al. (2) made a similar argument for hyraxes. They found microscopic wear striations on the teeth of grass-eating hyraxes with fractured phytoliths in their fecal pellets, but none on the teeth of sympatric bush-eating individuals without phytoliths in their feces.
Studies since have focused on matching patterns of microscopic tooth wear with diet, especially for mammals. Analyses of teeth ranging from those of antelopes to zebras, bats to moles, pigs to sheep, and marsupials to large cats, and especially primates, have since found consistent associations between dental microwear patterning and diet (3). Species reported or observed to consume harder items (like nuts or bone) tend to have a higher ratio of pits to scratches on their cheek teeth than do closely related ones that prefer tougher foods (like grass blades or meat). The focus of microwear from the outset has been on chewing movements (4). The inference is that although abrasives in or on foods cause the wear, fracture properties of items eaten produce pattern variation; hard ones are crushed, causing pits, and tough ones are sheared, causing scratches (5). Nevertheless, details about what causes tooth wear still elude consensus. All researchers have acknowledged that quartz grit can cause tooth wear, but the same cannot be said for phytoliths.
Phytoliths are often considered a defense mechanism developed by plants to deter predation (6, 7). Not only do mammalian grazers avoid phytolith-rich foods when they can (8), but grasses, for example, generate more phytoliths when their blades are eaten by grazers (9). The assumption is that mammals (and other animals) avoid these foods to prevent excessive wear of the feeding apparatus. Evidence for the role of phytoliths in tooth wear comes from the discovery of siliceous plant opals embedded in tooth enamel at the ends of microwear scratches (10), as well as experimental study by Gügel et al. (11) demonstrating that cereals with differing phytolith loads leave different microwear patterns in enamel after simulated chewing. It makes sense, then, that primate species known to consume more phytolith-rich foods tend to have thicker tooth enamel (12).
On the other hand, some have questioned the role of phytoliths in tooth wear. They argue instead that phytoliths deter predation by reducing nutrient uptake during feeding (13) or by mimicking grit without actually wearing teeth (14). Indeed, Sanson et al. (15) challenged Baker et al.’s (1) original assertion that phytoliths are harder than tooth enamel and therefore capable of wearing it. More recent studies by Erickson (16) and Lucas et al. (14) also found phytoliths to be softer than enamel. Lucas et al. (14) argued that phytoliths are more likely to rearrange enamel on the nanoscale, creating ridges alongside an indentation, than to separate tissue from the surface.
Here we address apparent contradictions and discrepancies in the literature concerning the role of phytoliths in tooth wear. We assess the potential for amorphous silicon dioxide (SiO2) and other particles softer than enamel (aluminum and brass spheres) to wear teeth under controlled conditions and develop a model to explain our results. The recent suggestion that soil-derived quartz grit but not siliceous phytoliths remove enamel tissue and wear teeth may call into question the efficacy of dental microwear as a diet proxy. If microwear was caused by environmental grit alone, variation among samples could well reflect habitat differences rather than diet per se. This is a fundamental issue for paleobiologists today because microwear analysis is commonly used to reconstruct diets of fossil species ranging from Paleozoic conodonts (17) to Pleistocene hominins (18).
Results
All of the soft particles (aluminum and brass spheres) and silicon dioxide drawn across enamel surfaces cause striations in the direction of drag. Our analyses demonstrate that these grooves result from tissue loss rather than plastic deformation.
Microscale Experiments.
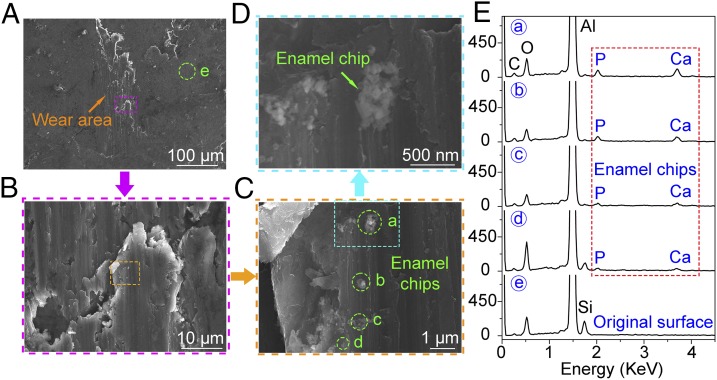
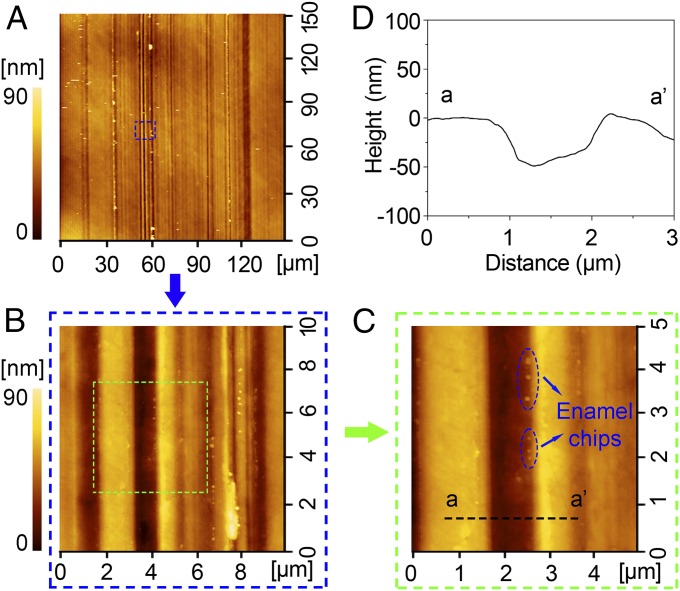
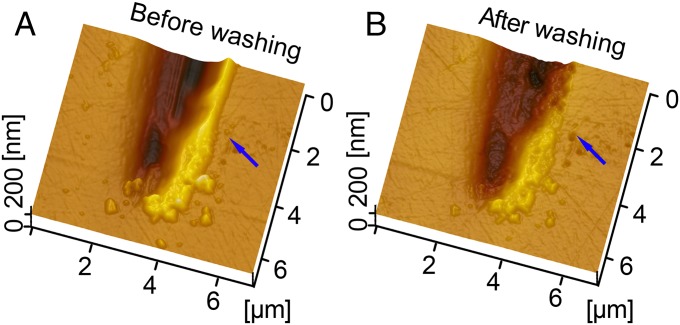
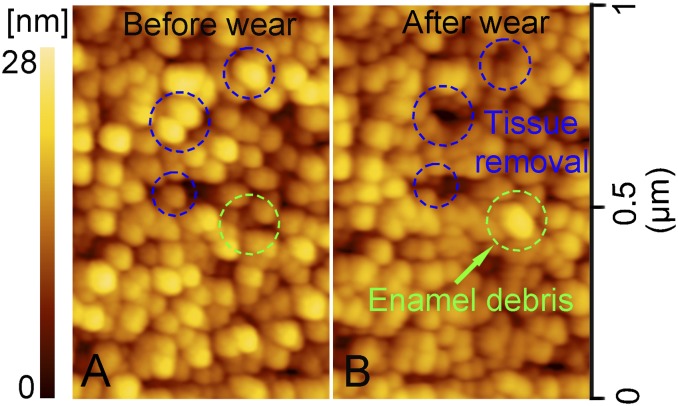
Even though the aluminum sphere (with hardness of 1.29 GPa; Table 1) is much softer than tooth enamel (4.62 GPa), it removed enamel chips ranging between 50 nm and 500 nm in diameter. Indeed, enamel chips were found embedded in the aluminum sphere after only one cycle of movement under the maximum contact pressure of 0.98 GPa (Fig. 1). Because individual hydroxyapatite crystals average ∼70 nm in diameter, this debris varied from single crystallites to large clumps of them. In addition, both aluminum and enamel debris were found in the scratch grooves themselves, with individual enamel particles ranging between 60 nm and 80 nm across—again, the diameter of individual hydroxyapatite crystals (Fig. 2). Enamel debris also built up on the edges of the individual striations, but this was clearly not the result of plastic deformation, or “pileup” (19) because the debris was easily washed away by water flow for 30 s (Fig. 3). More wear cycles resulted in more debris. After 60 cycles, substantively more enamel chips were observed embedded in the aluminum sphere (see Fig. S1). Tooth wear by soft particles was also effected by brass spheres (with hardness of 2.60 GPa; see Fig. S2).
Table 1.
Means and standard deviations of nanoindentation hardness values obtained for soft particles and the enamel surface
| Property | Enamel (n = 25) | Al (n = 17) | Brass (n = 20) |
| Hardness, GPa | 4.62 (0.14) | 1.29 (0.078) | 2.60 (0.15) |
| Data range | 4.33–4.99 | 1.20–1.40 | 2.26–2.91 |
Here, n denotes number of measurements.
Fig. 1.
Enamel chips embedded in the surface of the aluminum sphere after one cycle of drag under a contact pressure of 0.98 GPa. (A) SEM image of wear topography on the aluminum sphere. (B−D) The detail of the wear area in A, B, and C, respectively. (E) Enamel chips (a−d) in C were confirmed by the Ca and P peaks in the energy-dispersive X-ray spectroscopy (EDX) spectrum. The EDX detection on the original surface (e) in A is presented for comparison. Because the EDX instrument detection depth and area were about 1.5 μm and 1.5 μm2, respectively, and the diameter of the enamel chips was only 50–500 nm, Al made up a large percentage of the composition. However, Ca and P peaks were still discernable [hydroxyapatite is Ca10(PO4)6(OH)2], which confirms that the chips were enamel.
Fig. 2.
Enamel debris on the enamel surface after a single cycle of wear by an aluminum sphere under the contact pressure of 0.98 GPa. Height scales for all images are 90 nm. (A) AFM topography of the wear area; (B and C) detail of the wear area in A and B, respectively; and (D) cross-sectional profile of the wear area in C.
Fig. 3.
AFM images of a scratch on the enamel surface produced by an aluminum sphere using contact pressure of 0.98 GPa and 10 wear cycles. (A) Before washing. (B) After washing by water flow for 30 s. The blue arrows refer to the pileup in A and the washed area in B.
Fig. S1.
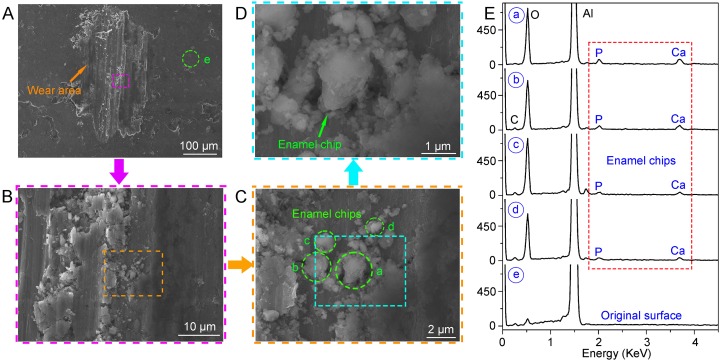
Enamel chips embedded in the surface of the aluminum sphere after 60 cycles. (A) SEM image of wear topography on the aluminum sphere. (B−D) Detail of the wear area in A, B, and C, respectively. (E) Enamel chips (a−d) in C were confirmed by the Ca and P peaks in the EDX spectrum. The EDX detection on the original area (e) in A was conducted for comparison (confirming the Aluminum substrate).
Fig. S2.
An AFM image and cross-section profiles of a wear area on an enamel surface after 60 simulated chew cycles using a brass sphere.
Nanoscale Experiments.
The silicon dioxide sphere (1 μm diameter) also produced clear grooves in enamel. In this case, the pattern of wear depends on contact pressure. When that pressure reaches 0.74 GPa, individual or clumps of crystallites separate from the surface as the bonds linking them are broken (Fig. 4). When the contact pressure is raised to 1.84 GPa, tissue is again removed, but there is also grain refinement, wherein individual crystallites decrease in diameter from ∼70 nm to 20 nm (20). Further increase in contact pressure results in larger-scale tissue loss and surface debris accumulation at the edges of individual striations. This is clearly not plastic deformation, as observed for metals (21), because the pileups of enamel result from the accumulation of debris, rather than aggregation from within the scratch, and can be removed by washing.
Fig. 4.
Crystallite removal and individual debris on the enamel surface after one cycle of wear by a SiO2 microsphere under the contact pressure of 0.74 GPa. Particles with diameters of ∼60 nm to ∼80 nm are hydroxyapatite crystallites on the enamel surface. (A) AFM image of the original surface before wear. (B) In situ AFM image of the worn surface.
Discussion
Recent challenges to the efficacy of microwear as a tool for reconstructing diet center on the notion that “the geometry of microwear features depends critically on the hardness (i.e., resistance to indentation) of microscopic particles in relation to that of enamel,” and that phytoliths are not “hard enough to create steep, sharp angled pits or scratches on enamel surfaces” (22). These arguments are based on the innovative nanowear experiments of Lucas et al. (19).
Lucas et al. (19) suggested that phytoliths can alter surface patterns without actual wear. This seems to be based in part on Atkins et al.’s (21) model wherein softer particles can rub against surfaces and create prows in ductile metals. In metals, atoms are linked by metallic bonds to become malleable. As such, metal object surfaces and subsurfaces can deform back and forth before releasing debris diverse in size and shape from fatigue, rubbing, sliding, abrasion, and corrosion (23). However, enamel works differently owing to its different bonding mechanism. Individual hydroxyapatite crystallites are glued together by a thin protein layer (no more than 2 nm thick) that holds the crystallites on the surface with its elastic polymeric backbone (24, 25). Crystallites can only change position by breaking the protein layer; the crystallites do not link back up unless new proteins are formed, which is possible but may require that teeth be soaked in water or saliva for hours (26–28), during which Brownian motion cannot allow the nanometer-scale debris to sit still nearby. Herbivorous mammals can chew thousands or even tens of thousands of cycles per day, which should lead to debris removal and act against reattachment of crystallites and regrowth of enamel. In any event, given the fundamental differences in bonding mechanics between metal objects and teeth, models based on one should not be used to understand nanoscale wear in the other (see Supporting Information).
Concerning Lucas et al.’s prowing phenomenon, pileups on the edges of scratches in our in vitro studies are clearly enamel debris rather than plastic deformation of the surface. Were this prowing, it could not be washed away easily with water—yet it is (Fig. 3). Although the image of a diamond scratching glass is a compelling one, it has been known for some time that softer materials can and do wear harder ones (29). As Lucas et al. (19) acknowledge, there is more (e.g., toughness) to tooth wear than hardness alone. There is little doubt, for example, that amorphous silica can and does remove dental enamel under the conditions of mastication.
Walker et al. (2) made a compelling case that phytoliths cause microwear based on scratch patterns evinced by sympatric hyrax species, as did Gügel et al. (11) based on their experimental study abrading teeth with phytoliths. “Smoking gun” phytoliths found embedded in the ends of wear scratches by Fox et al. (10) are equally persuasive. In other words, phytoliths can and do contribute to microwear patterning. Dental microwear remains a valuable tool for reconstructing diets of fossils, as has been confirmed by the many studies showing patterns separating closely related extant species in ways that make sense given reported differences in their diets (30).
Methods
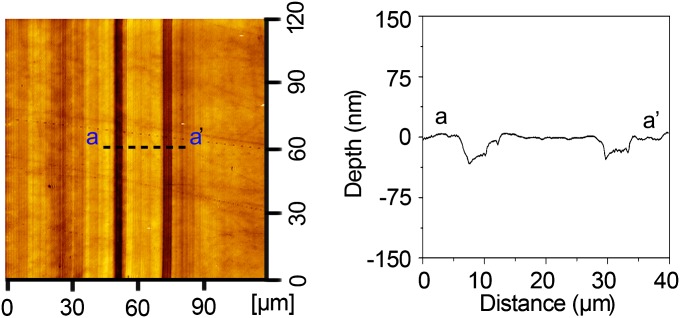
Specimens were prepared from intact permanent human molars stored in deionoized water at 4 °C before use. Teeth were embedded in epoxy resin, and a buccolingual section of each crown was made. To obtain a flat surface for wear tests, specimens were ground with abrasive papers of 1,200 and 1,500 grit and then polished with a 0.5-μm diamond paste, all using a rotary polishing machine and constant water irrigation. Specimens were then cleaned with deionized water before testing. The topographic relief of the enamel surface following polishing and cleaning was determined by atomic force microscope (AFM, SPI 3800N; Seiko) to be about 1.5 nm (RMS roughness) in each 40 × 40 μm2 area. All of the wear tests were conducted on the occlusal surface of the enamel, where prism orientation was virtually perpendicular to the plane chosen for tests.
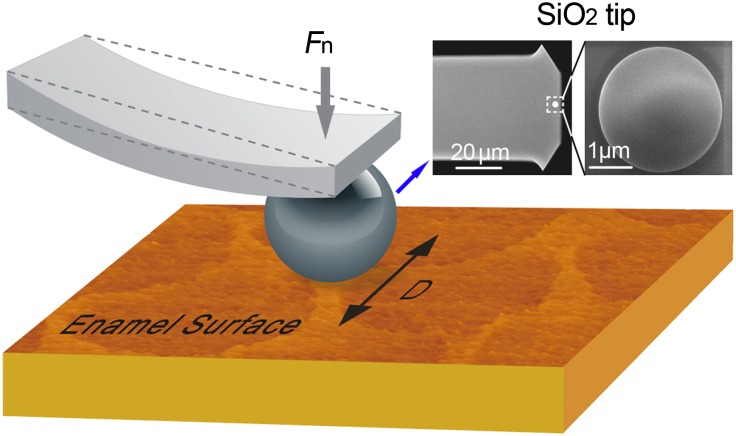
Chewing was simulated by reciprocating sliding wear tests between soft particles and the enamel surface. Separate experiments were conducted on the microscale using an aluminum (Al) sphere with a radius of 1.5 mm and a brass sphere (Cu-62 wt% Zn) with a radius of 0.9 mm, and on the nanoscale using a silicon dioxide (SiO2) sphere with a radius of 1 μm. The Al sphere and brass sphere were mounted on a tip cantilever attached to a self-developed pin-on-flat microtribometer and dragged across enamel surfaces with a fixed normal load (Fn = 7 N; the corresponding maximum Herzian contact pressure Pc is estimated to be 0.98 GPa for the Al sphere and 1.61 GPa for the brass sphere), sliding speed (v = 0.1 mm/s), and sliding span (D = 1 mm) for 1 cycle and 60 cycles. The SiO2 tip was attached to an AFM and also dragged across the enamel surface with a fixed normal load (Fn = 1 μN and Pc = 0.74 GPa), sliding speed (v = 0.8 μm/s), and sliding span (D = 1 μm) for one cycle (Fig. 5). Contact pressures associated with human mastication have been reported to vary between ∼0 GPa and ∼2.5 GPa (25). As such, tests here have been conducted using the maximum Hertzian contact pressures of ∼0.74 GPa to ∼1.61 GPa.
Fig. 5.
Illustration showing the nanoscale wear test on the enamel surface. The SiO2 microsphere with a radius of 1 μm moved horizontally on the enamel surface over a distance D under an applied load Fn. The upper right Insets show SEM images of the SiO2 tip.
The topography of scratch-induced damage on the enamel was documented using the AFM with a standard Si3N4 tip (MLCT; Veeco) with nominal radius of 10 nm, and the morphology and composition of the debris generated were studied by means of a scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX, JSM-7001F, JEOL, Japan). During the EDX tests, the accelerating voltage was set to 15 kV, and the probe current was 5 nA.
Mean indentation hardnesses of the Al sphere, brass sphere, and enamel were obtained by a nanoindentation tester (T750, Hysitron, Inc.) using a Berkovich diamond tip (Table 1). The SiO2 tip used in the nanowear tests was amorphous, with hardness and elastic modulus values estimated from the literature (31) as 5.7 GPa and 81.5 GPa, respectively.
Abrasion of Enamel Surface by Scratching an Aluminum Sphere for 60 Cycles
Figs. 1 and 2 show a microwear striation on the enamel surface formed after a single cycle of drag using an aluminum sphere. Further tests involving 60 drag cycles (each with maximum Hertzian contact pressure of 0.98 GPa) were conducted to simulate chewing. As shown in Fig. S1, more enamel chips were found embedded in the surface of the aluminum sphere after 60 cycles, indicating that the amount of enamel wear depends on number of chew cycles.
Microwear on Enamel Surface by Brass Sphere
To further confirm tooth wear by soft particles, chewing simulations were repeated for 60 cycles using a brass sphere (0.9 mm in radius) with a maximum Hertzian contact pressure of 1.61 GPa. As shown in Fig. S2, resulting scratches on the dental surfaces indicate that enamel is also abraded by brass particles.
Unique Microwear Mechanism of Tooth Enamel
Tooth enamel differs fundamentally in the way it wears compared with normal metal materials. According to Hertzian contact theory, the critical contact pressure Py corresponding to the initial yield of material can be determined by (32)
| [S1] |
Here, is the yield stress of the material. For most materials, the hardness H is about 2.8 times yield stress (19, 33),
| [S2] |
Combining Eqs. S1 and S2, we obtain
| [S3] |
Given the hardness of enamel H = 4.62 GPa, the Py for the initial yield of enamel was estimated as 2.63 GPa based on Eq. S3. However, based on our experimental results (see Fig. 4), the Py was estimated as 0.74 GPa, only 0.16 times its hardness. Such low critical contact pressure Py may be attributed to the unique microstructure of tooth enamel. Hydroxyapatite crystallites are glued together by proteins, and tissue removal requires only that contact pressure be sufficient to break the bonds holding the enamel together.
Acknowledgments
This project was funded by the National Science Foundation of China (91323302, 51222511, and 51375409).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509491112/-/DCSupplemental.
References
- 1.Baker G, Jones LHP, Wardrop ID. Cause of wear in sheeps’ teeth. Nature. 1959;184(Suppl 20):1583–1584. doi: 10.1038/1841583b0. [DOI] [PubMed] [Google Scholar]
- 2.Walker A, Hoeck HN, Perez L. Mecrowear of mammalian teeth as an indicator of diet. Science. 1978;201(4359):908–910. doi: 10.1126/science.684415. [DOI] [PubMed] [Google Scholar]
- 3.Ungar PS. Mammalian dental function and wear: A review. Biosurf Biotribol. 2015;1(1):25–41. [Google Scholar]
- 4.Simpson GG. Mesozoic Mammalia, IV; the multituberculates as living animals. Am J Sci. 1926;11(63):228–250. [Google Scholar]
- 5.Teaford MF, Walker A. Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. Am J Phys Anthropol. 1984;64(2):191–200. doi: 10.1002/ajpa.1330640213. [DOI] [PubMed] [Google Scholar]
- 6.Piperno DR. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. Altamira Press; Lanham, MD: 2006. [Google Scholar]
- 7.Rabenold D, Pearson OM. Scratching the surface: A critique of Lucas et al. (2013)'s conclusion that phytoliths do not abrade enamel. J Hum Evol. 2014;74:130–133. doi: 10.1016/j.jhevol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Vicari M, Bazely DR. Do grasses fight back? The case for antiherbivore defences. Trends Ecol Evol. 1993;8(4):137–141. doi: 10.1016/0169-5347(93)90026-L. [DOI] [PubMed] [Google Scholar]
- 9.Massey FP, Ennos AR, Hartley SE. Herbivore specific induction of silica-based plant defences. Oecologia. 2007;152(4):677–683. doi: 10.1007/s00442-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 10.Fox CL, Pérez-Pérez A, Juan J. Dietary information through the examination of plant phytoliths on the enamel surface of human dentition. Science. 1994;21(1):29–34. [Google Scholar]
- 11.Gügel IL, Grupe G, Kunzelmann KH. Simulation of dental microwear: Characteristic traces by opal phytoliths give clues to ancient human dietary behavior. Am J Phys Anthropol. 2001;114(2):124–138. doi: 10.1002/1096-8644(200102)114:2<124::AID-AJPA1012>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Rabenold D, Pearson OM. Abrasive, silica phytoliths and the evolution of thick molar enamel in primates, with implications for the diet of Paranthropus boisei. PLoS One. 2011;6(12):e28379. doi: 10.1371/journal.pone.0028379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massey FP, Ennos AR, Hartley SE. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J Anim Ecol. 2006;75(2):595–603. doi: 10.1111/j.1365-2656.2006.01082.x. [DOI] [PubMed] [Google Scholar]
- 14.Lucas PW, et al. The role of dust, grit and phytoliths in tooth wear. Ann Zool Fenn. 2014;51(1-2):143–152. [Google Scholar]
- 15.Sanson GD, Kerr SA, Gross KA. Do silica phytoliths really wear mammalian teeth? J Archaeol Sci. 2007;34(4):526–531. [Google Scholar]
- 16.Erickson KL. Prairie grass phytolith hardness and the evolution of ungulate hypsodonty. Hist Biol. 2014;26(6):737–744. [Google Scholar]
- 17.Purnell MA. Microwear on conodont elementsand macrophagy in the first vertebrates. Nature. 1994;374(27):798–800. [Google Scholar]
- 18.El Zaatari S, Grine FE, Ungar PS, Hublin JJ. Ecogeographic variation in Neandertal dietary habits: Evidence from occlusal molar microwear texture analysis. J Hum Evol. 2011;61(4):411–424. doi: 10.1016/j.jhevol.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Lucas PW, et al. Mechanisms and causes of wear in tooth enamel: Implications for hominin diets. J R Soc Interface. 2013;10(80):20120923. doi: 10.1098/rsif.2012.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng SY, et al. Investigation on the microtribological behavior of human tooth enamel by nanoscratch. Wear. 2011;271(9):2290–2296. [Google Scholar]
- 21.Atkins AG, Liu JH. Toughness and the transition between cutting and rubbing in abrasive contacts. Wear. 2007;262(1):146–159. [Google Scholar]
- 22.Strait DS, et al. Viewpoints: Diet and dietary adaptations in early hominins: The hard food perspective. Am J Phys Anthropol. 2013;151(3):339–355. doi: 10.1002/ajpa.22285. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DP. Wear Debris Atlas. Noria Corp; Tulsa, OK: 2012. [Google Scholar]
- 24.He LH, Swain MV. Contact induced deformation of enamel. Appl Phys Lett. 2007;90(17):171916. [Google Scholar]
- 25.He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater. 2008;1(1):18–29. doi: 10.1016/j.jmbbm.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Lippert F, Parker DM, Jandt KD. In situ remineralisation of surface softened human enamel studied with AFM nanoindentation. Surf Sci. 2004;553(1):105–114. [Google Scholar]
- 27.Gao SS, Huang SB, Qian LM, Yu HY, Zhou ZR. Wear behavior of early carious enamel before and after remineralization. Wear. 2009;267(5):726–733. [Google Scholar]
- 28.Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent Mater. 2009;25(7):884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson RCD. The wear of metals by relatively soft abrasives. Wear. 1968;11(4):245–275. [Google Scholar]
- 30.Ungar PS. Dental allometry in mammals: A retrospective. Ann Zool Fenn. 2014;51(1-2):177–187. [Google Scholar]
- 31.Thurn J, Cook RF. Stress hysteresis during thermal cycling of plasma-enhanced chemical vapor deposited silicon oxide films. J Appl Phys. 2002;91(4):1988–1992. [Google Scholar]
- 32.Bhushan B. Introduction to Tribology. 2nd Ed Wiley; New York: 2013. [Google Scholar]
- 33.Tabor D. The Hardness of Metals. Clarendon Press; Oxford: 1951. [Google Scholar]