Abstract
Purpose
To explore the role of inducible focal adhesion (FA) protein Hic-5 in actin cytoskeletal reorganization, FA formation, fibrogenic activity, and expression of myocilin in trabecular meshwork (TM) cells.
Methods
Using primary cultures of human TM (HTM) cells, the effects of various external factors on Hic-5 protein levels, as well as the effects of recombinant Hic-5 and Hic-5 small interfering RNA (siRNA) on actin cytoskeleton, FAs, myocilin, α-smooth muscle actin (αSMA), and collagen-1 were determined by immunofluorescence and immunoblot analyses.
Results
Hic-5 distributes discretely to the FAs in HTM cells and throughout the TM and Schlemm's canal of the human aqueous humor (AH) outflow pathway. Transforming growth factor-β2 (TGF-β2), endothelin-1, lysophosphatidic acid, hydrogen peroxide, and RhoA significantly increased Hic-5 protein levels in HTM cells in association with reorganization of actin cytoskeleton and FAs. While recombinant Hic-5 induced actin stress fibers, FAs, αv integrin redistribution to the FAs, increased levels of αSMA, collagen-1, and myocilin, Hic-5 siRNA suppressed most of these responses in HTM cells. Hic-5 siRNA also suppressed TGF-β2-induced fibrogenic activity and dexamethasone-induced myocilin expression in HTM cells.
Conclusions
Taken together, these results reveal that Hic-5, whose levels were increased by various external factors implicated in elevated intraocular pressure, induces actin cytoskeletal reorganization, FAs, expression of fibrogenic markers, and myocilin in HTM cells. These characteristics of Hic-5 in TM cells indicate its importance in regulation of AH outflow through the TM in both normal and glaucomatous eyes.
Keywords: Hic-5, TGFB1I1, actin cytoskeleton, myocilin, TGF-β2, RhoA, dexamethasone
This study reveals that Hic-5, whose levels were increased by various external factors implicated in elevated IOP, induces actin cytoskeletal reorganization, focal adhesions, expression of collagen-1, and myocilin in the trabecular meshwork cells, suggesting its critical role in aqueous humor outflow.
Homeostasis of aqueous humor (AH) drainage through the trabecular meshwork (TM) is essential for maintenance of normal intraocular pressure.1 Importantly, blockage or increased resistance to AH outflow through the TM leads to elevated IOP, which is a major risk factor for primary open-angle glaucoma,2,3 a leading cause of blindness in the United States.4 The conventional AH outflow pathway of human eyes through which most AH is drained consists of the TM, juxtacanalicular (JCT) tissue, and Schlemm's canal (SC).5 The cells derived from these different tissues of the AH outflow pathway exhibit endothelial and mesenchymal characteristics.5,6 Various physiological and etiological mechanisms have been reported to account for homeostatic and deregulated AH outflow, respectively, through the conventional or trabecular pathway.2,3,5,7–10 Importantly, abundant experimental evidence also points to a role for the cellular properties of TM and SC, including contractile characteristics, actin cytoskeletal organization, cell adhesive interactions, mechanical and mechanosensing properties, and extracellular matrix (ECM) organization and production, in regulating AH outflow through the TM.2 To explore additional intracellular molecular mechanisms regulating the TM cell contractile, cell adhesive, and biomechanical properties for a thorough understanding of both homeostatic and deregulated AH outflow and IOP, we hypothesized that Hic-5, a multidomain adaptor protein, may play a crucial role in regulation of TM cell integrin–matrix interactions, actin cytoskeletal organization, and glucocorticoid-induced gene expression, eventually influencing AH outflow through the TM.
Hic-5 is an inducible focal adhesion (FA) protein that belongs to the family of paxillin proteins expressed abundantly in smooth muscle cells of various tissues.11–14 Hic-5 was originally isolated as a hydrogen peroxide (H2O2)- and TGF-β1-inducible (TGFB1I1) gene that encodes a 55-kDa protein.11,12,15 Interestingly, in separate studies, this protein has also been identified and characterized as an androgen or glucocorticoid receptor coactivator (ARA55) involved in transcriptional regulation and senescence.16–19 Hic-5 contains four LD (leucine-aspartate repeat) motifs at the amino terminus and four LIM domains at the carboxyl terminus and serves as a multiprotein interacting adaptor protein.11,12 The involvement of Hic-5 in both cytoskeletal organization and transcriptional regulation occurs primarily via the scaffolding properties of this protein.12,15,20,21 Hic-5 exhibits a significant homology to paxillin and binds to FA kinase, protein tyrosine kinase (PYK2), protein tyrosine phosphatase (PTP-PEST), and vinculin, and competes with paxillin to regulate integrin-mediated cell adhesion, actin cytoskeletal organization, contractile activity, and cell spreading.12,20,22–31 Moreover, Hic-5 has been considered as a therapeutic target since elevated levels of this protein have been reported in various diseases,32 and because Hic-5 induces cell plasticity, epithelial to mesenchymal transition (EMT) and fibrosis via Rho/Rho kinase, serum response factor/myocardin transcriptional regulators, and SMAD signaling.14,23
Considering the multiple characteristics of Hic-5 that appear to be relevant to TM cytoskeletal biology, TGF-β signaling, myocilin, Rho GTPase signaling, AH outflow, and steroid-induced glaucoma, we asked ourselves whether Hic-5 plays a critical role in regulation of AH outflow and IOP. To address this possibility, in this initial study we examined Hic-5 expression and distribution in the AH outflow pathway and its regulation and effects on actin cytoskeletal organization, FAs, integrins, myocilin, and fibrogenic activity in human TM (HTM) cells. To the best of our knowledge, this is the first study on Hic-5 demonstrating its regulation and effects in TM cells.
Materials and Methods
Reagents and Antibodies
Oleoyl-L-α-lysophosphatidic acid sodium salt (LPA), human recombinant transforming growth factor-β2 (TGF-β2), endothelin-1, dexamethasone, H2O2, TRITC-phalloidin, mouse monoclonal antibodies against vinculin, α-smooth muscle actin (αSMA), and β-actin were from Sigma-Aldrich Corp. (St. Louis, MO, USA). Mouse anti-Hic-5 monoclonal antibody (BD Transduction Laboratories, San Jose, CA, USA), rabbit anti Hic-5 antibody and Hic-5 blocking peptide (Abcam, Cambridge, MA, USA), rabbit anti-collagen type 1 antibody (Rockland Immunochemicals, Inc., Limerick, PA, USA), rabbit anti-SMAD2/3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), phospho-SMAD3 antibody (Cell Signaling Technology, Danvers, MA, USA), SIS3 (Calbiochem, Gibbstown, NJ, USA), Y-27632 and NSC23766 (Tocris Bioscience, Bristol, UK), rabbit anti-ponsin antibody (Upstate Biotechnology, Lake Placid, NY, USA), mouse IgG (Jackson Immuno Research, West Grove, PA, USA), Phalloidin-Alexa 647 and Hoechst 33258 (Invitrogen, Carlsbad, CA, USA), protease inhibitor cocktail tablets and PhosSTOP (Roche, Basel, Switzerland), Alexa Fluor 568 goat-anti-rabbit (Invitrogen), and mAb AP-5 against β3 integrin (The Blood Center of Wisconsin, Milwaukee, WI, USA) were obtained from the indicated commercial sources. Rabbit anti–green fluorescent protein (GFP) and rabbit anti-myocilin antibodies were provided by the laboratory of Daniel Stamer, Department of Ophthalmology, and Duke University.
Cell Culture
Human primary TM cells were cultured from TM tissue isolated from corneal rings used for corneal transplantation at the Duke Ophthalmology clinical service as we described earlier.33 Donor age ranged from 18 to 64 years, and all HTM cell culture experiments conducted in this study used cells derived from passages between 4 and 6. Cells were cultured at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS (fetal bovine serum) and penicillin-streptomycin-glutamine.33 All experiments were performed using confluent cell cultures serum starved for at least 24 hours unless noted otherwise. For experiments involving activation of β3 integrin, glass coverslips were coated with 8 μg/mL β3 integrin-activating mAb (AP5) resuspended in 2% gelatin. Mouse IgG (8 μg/mL 2% gelatin)-coated coverslips without AP5 were used as controls. Porcine TM cells were isolated and cultured as we described earlier.34
Plasmid Transfection
Plasmids expressing GFP-Hic-5 or GFP in the pLEGFP-C1 backbone (gift from Christopher Turner, Department of Cell and Developmental Biology, SUNY Upstate Medical University) were amplified and purified using a Qiagen Plasmid Plus Maxi Kit (Qiagen, San Jose, CA, USA) according to user guidelines. Human TM cells were transfected with respective plasmids using an endothelial Nucleofector Kit (Lonza, Basel, Switzerland) per manufacturer's instructions. Transfected cells were plated either on gelatin-coated glass coverslips or in plastic petri plates. Visualization based on GFP was used to determine the transfection efficiency, and cells transfected at >80% efficiency were used. Changes in cellular morphology were recorded, after which the cells were fixed and immunostained or lysed for immunoblot analysis for proteins of interest.
Small Interfering RNA Transfection
Small interfering RNA (siRNA) directed against the human Hic-5 sequence (C. No. sc-37685) and a corresponding, scrambled control siRNA (C. No. sc-37007) were purchased from Santa Cruz Biotechnology. Human TM cells were transfected using 25 pmol Hic-5 siRNA or control siRNA per well of a six-well plate, using a Lipofectamine RNAiMax reagent (Invitrogen) per manufacturer's instructions. Following transfection, cells were plated either on glass coverslips or in plastic petri plates. After 48 to 72 hours following transfection, the cells were fixed and immunostained or immunoblotted for proteins of interest.
Construction of Replication-Deficient Recombinant Adenovirus Expressing Hic-5
Generation of recombinant adenovirus expressing Hic-5 (AdHic-5) and DsRed (AdDsRed) was performed using the ViraPower Adenoviral Expression System (K4930-00; Invitrogen). Hic-5 (1386 nt) and DsRed (678 nt) DNA fragments were amplified by high-fidelity PCR (Advantage-HF 2 PCR kit, 639123; Clontech, Mountain View, CA, USA) from pLEGFP-Hic-5-C1 and pIRES2-DsRed-Express2 (Clontech) plasmids, respectively. The PCR products were purified, sequenced, and cloned into the pENTR/D-TOPO cloning vector (K243520; Invitrogen). The pENTR-Hic-5 and pENTR-DsRed constructs generated were then recombined with the pAd/CMV/V5-DEST vector (V493-20; Invitrogen), using Gateway technology and the LR Clonase II enzyme mix (11791020; Invitrogen) to create the pAdCMV-Hic-5 and pAdCMV-DsRed. The pAdCMV-Hic-5 and pAdCMV-DsRed were then linearized by PacI (RO547S; New England Biolabs, Ipswich, MA, USA) digestion and transfected into 293A cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) to produce recombinant adenoviruses expressing Hic-5 and DsRed, which were subsequently amplified in 293A cells yielding crude lysates containing adenovirus particles.
Adenovirus-Mediated Gene Transduction
Replication-defective recombinant adenoviral vectors encoding either DsRed or Hic-5, GFP alone, or constitutively active RhoA (RhoAV14) and GFP, provided by Patrick Casey, Department of Pharmacology and Cancer Biology, Duke University School of Medicine, were amplified and purified as we described earlier.35 Human TM cells grown either on gelatin-coated glass coverslips or in plastic petri dishes were infected with adenovirus for the various experiments at 50 MOI (multiplicity of infection). When cells showed adequate transduction (>80%, as assessed based on GFP or DsRed fluorescence), usually after 24 to 36 hours, cells were serum starved for 48 hours prior to use in experiments.
Immunohistochemistry
Tissue sections from formalin-fixed, paraffin-embedded human donor eye whole globes were immunostained as previously described.36 Briefly, 5-μm-thick tissue sections were deparaffinized and rehydrated using xylene, absolute ethyl alcohol, and water. To unmask the antigen epitopes, heat-induced antigen retrieval was performed using 0.1 M citrate buffer pH 6.0 (Vector Laboratories, Burlingame, CA, USA) for 20 minutes at 100°C. The slides were then blocked for nonspecific interaction with Biocare Medical's (Concord, CA, USA) Sniper Background Reducer (C. No. BS966). The tissue sections were then incubated overnight at 4°C in a humidified chamber either with rabbit polyclonal primary antibody raised against Hic-5, or with antibody preincubated with Hic-5 blocking peptide as a control, at a final dilution of 1:50. Primary antibody dilutions were made in 1% bovine albumin in Tris-buffered saline (TBS). After incubation with primary antibody, the slides were washed with TBS and incubated with Alexa Fluor-594 goat anti-rabbit secondary antibodies for 2 hours at room temperature. Immunostaining analysis was carried out in triplicate, and two negative controls (primary antibody preincubated with blocking peptide or secondary antibody alone) were run simultaneously. After staining and washing, tissue sections were coverslipped using Aqua mount (Lerner Laboratories, Pittsburg, PA, USA). The slides were then viewed and imaged using a Nikon Eclipse 90i confocal laser-scanning microscope (Nikon Instruments, Melville, NY, USA) with ×20 and ×60 objectives.
Immunofluorescence Staining
Human TM cells were grown on gelatin (2%)-coated glass coverslips until they attained confluency. After appropriate treatment, cells were washed in PBS twice and then fixed, permeabilized, and immunostained as we described previously.37 The slides were viewed and imaged using a Nikon Eclipse 90i confocal laser-scanning microscope.
Immunoblotting
Total protein cell lysates were prepared from serum-starved confluent cultures of HTM cells derived from the various experiments described and from porcine TM tissue. Briefly, TM cells and tissue were homogenized in hypotonic Tris buffer (10 mM Tris, 0.2 mM MgCl2), pH 7.4, containing phosphatase and protease inhibitor cocktail using a probe sonicator and centrifuged at 800g at 4°C for 15 minutes. The resultant supernatant was used for the immunoblot analysis. Protein assay reagent (Bio-Rad, Hercules, CA, USA) was used to determine protein concentration of lysates. Samples containing equal amounts of protein were mixed with Laemmli buffer and separated by SDS-PAGE (10% and 5% acrylamide), followed by transfer of resolved proteins to nitrocellulose membranes. Membranes were blocked for 2 hours at room temperature in Tris-buffered saline containing 0.1% Tween 20 and 5% (wt/vol) nonfat dry milk and subsequently probed with primary antibodies (anti-αSMA, anti-SMAD2/3, anti-pSMAD3, anti-Col-1, and anti-GFP) in conjunction with horseradish peroxidase-conjugated secondary antibodies. In the case of collagen-1 detection, the protein samples along with the Laemmli buffer were boiled for 30 minutes and then loaded onto the gel. Detection of immunoreactivity was performed by enhanced chemiluminescence. Densitometric analysis of immunoblots was performed using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Data were normalized relative to the specified loading controls.
Statistical Analyses
All data represent the average of a minimum of four to six independent observations. Quantitative data were analyzed by the Student's t-test, and a P < 0.05 was used to define statistically significant differences between test and control samples.
Results
Expression and Distribution of Hic-5 in HTM Cells and the AH Outflow Pathway
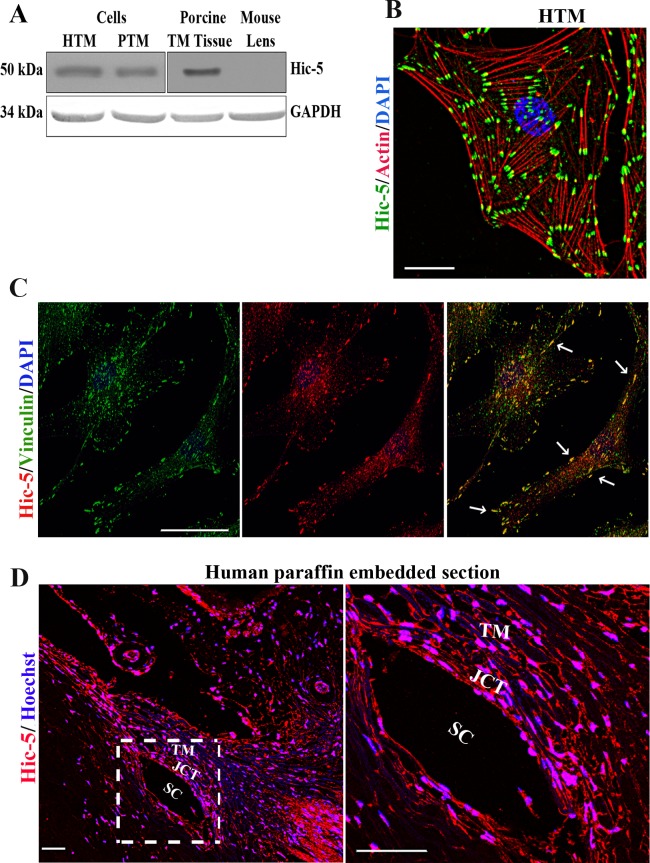
To determine the expression of Hic-5, cell lysates (800g supernatant) derived from human and porcine primary TM cell cultures and from porcine TM tissue were immunoblotted using Hic-5 monoclonal antibody. Trabecular meshwork cell and tissue lysates of human and porcine origin showed a single immunopositive band corresponding to the molecular mass of Hic-5 at ∼50 kDa (Fig. 1A). In contrast, no Hic-5 immunopositive band was noted in the mouse lens lysates (Fig. 1A). Following confirmation of expression in TM cells, we evaluated Hic-5 distribution by immunofluorescence analysis and confocal imaging. In HTM cells, Hic-5 exhibits an intense and clustered distribution (in green) localizing discretely to the leading tips of actin stress fibers labeled with rhodamine-phalloidin (in red) (Fig. 1B). To confirm Hic-5 distribution to FAs, HTM cells were examined by immunofluorescence analysis for codistribution of Hic-5 (red) with the well-characterized FA proteins vinculin (green) and ponsin (Supplementary Fig. S1).12,38 As can be seen in Figure 1C (far right image), Hic-5 exhibits codistribution (in yellow, indicated with arrows) with vinculin, confirming the localization of Hic-5 to FAs in TM cells. Similarly, Hic-5 (using Hic-5 monoclonal antibody) also exhibited colocalization with ponsin (see arrows in Supplementary Fig. S1). The polyclonal rabbit Hic-5 antibody used for colocalization analyses did not yield as discrete a staining pattern relative to that seen with the Hic-5 monoclonal antibody (Fig. 1B) shown in Figure 1C.
Figure 1.
Expression and distribution of Hic-5 in human TM cells and the AH outflow pathway. (A) Immunoblotting analysis reveals Hic-5 expression in lysates of human TM cells, porcine TM cells, and porcine TM tissue. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) Distribution of Hic-5 (green) and actin stress fibers (red) in HTM cells based on immunofluorescence (using Hic-5 monoclonal antibody) and rhodamine-phalloidin fluorescence, respectively. Cell nuclei are stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (C) Colocalization of Hic-5 (red) with vinculin (green) in HTM cells based on immunofluorescence analysis using polyclonal Hic-5 and monoclonal vinculin antibodies. (D) Immunofluorescence analysis of Hic-5 distribution (red) in tissues of the aqueous humor outflow pathway (including TM, JCT, and SC) of human donor specimens, using a polyclonal Hic-5 antibody. Nuclei are stained with Hoechst (blue). On the right is a magnified image of the area marked on the left. Scale bars: 50 μm.
Hic-5 distribution in the human AH outflow pathway (donor age, 80 years) was then assessed using paraffin-embedded tissue sections and a polyclonal anti-Hic-5 antibody. The results indicate that Hic-5 is distributed throughout the human AH outflow pathway, including the TM, JCT, and SC tissues (Fig. 1D). In Figure 1D, the image on the right shows a higher magnification of the area indicated at the left with a white square box. The distribution of Hic-5 is evident in both the cytoplasmic and nuclear regions. Nuclei in the AH outflow pathway were stained with Hoechst (blue stain). To confirm specificity of the Hic-5 antibody used in immunofluorescence analysis, antibody preincubated with the specific cognate peptide antigen (1: 5 ratio) was used as a control, along with secondary antibody control. Both controls did not yield any positive signals (Supplementary Fig. S2), confirming the specificity of Hic-5 antibody used in this study.
TGF-β2, LPA, Endothelin-1, H2O2, and RhoA Stimulation Leads to Increased Hic-5 Protein Levels and Actin Cytoskeletal Reorganization in HTM Cells
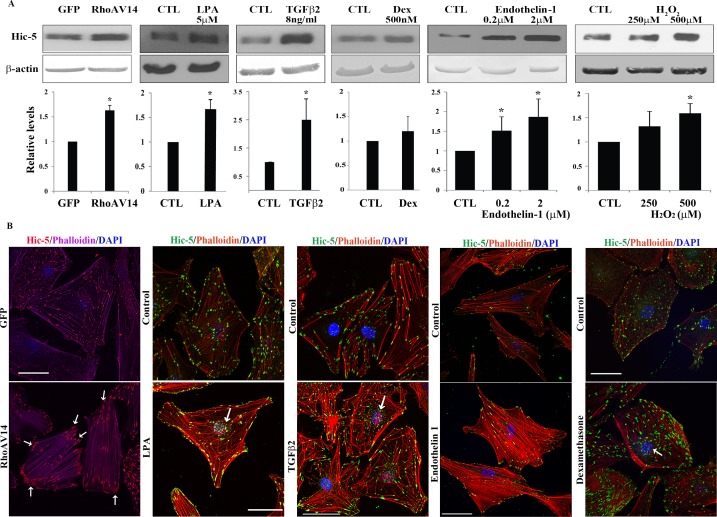
Since Hic-5 has been identified as an inducible protein,12 we explored whether TGF-β2, endothelin-1, LPA, H2O2, and dexamethasone, each of which has been implicated in pathobiology of elevated IOP and glaucoma,2 regulate the expression of Hic-5 in HTM cells. Toward this objective, serum-starved (for 24 hours) confluent cultures of HTM cells were treated with the above-described agents followed by immunoblotting analysis for changes in Hic-5 protein levels. To evaluate the effects of RhoA on Hic-5, HTM cells expressing adenovirus-encoded constitutively active RhoAV1435 were similarly monitored by immunoblotting analysis. Adenovirus-treated HTM cells were maintained in 10% serum for the first 24 hours following infection, followed by a 24-hour period of serum starvation prior to analysis of Hic-5 protein levels. Under the conditions described above, TGF-β2 (8 ng/mL for 14 hours), endothelin-1 (0.2 and 2 μM for 24 hours), H2O2 (0.5 mM for 3 hours), LPA (5 μM for 24 hours), and RhoAV14 expression (for 48 hours) were found to stimulate significant increases (P < 0.05) in the level of Hic-5 protein in HTM cells compared to the respective controls, based on four independent analyses. While a lower concentration of H2O2 (0.250 mM for 3 hours) and dexamethasone (0.5 μM for 4 days) both supported an increasing trend in Hic-5 protein levels, the observed effect was not significant relative to untreated controls (Fig. 2A).
Figure 2.
Upregulation of expression and redistribution of Hic-5 in human TM cells by various physiological factors and Rho GTPase. (A) Confluent serum-starved HTM cells expressing either adenovirus-encoded RhoAV14 or GFP, or treated with LPA (5 μM for 24 hours), TGF-β2 (8 ng/mL for 14 hours), dexamethasone (500 nM for 4 days), endothelin-1 (0.2 and 2 μM for 24 hours), or H2O2 (250 and 500 μM for 3 hours), exhibit a significant increase in Hic-5 protein levels except with dexamethasone relative to untreated control cells, as assessed by immunoblotting and densitometric quantification. β-actin was immunoblotted as a loading control (n = 6 for dexamethasone; for all other analyses n = 4, mean ± SD). *P < 0.05. (B) HTM cells expressing RhoAV14/GFP or GFP and wild-type cells were grown to semiconfluence on gelatin-coated glass coverslips. The wild-type cells were treated with LPA, TGF-β2, endothelin-1 (2 μM), or dexamethasone (500 nM) as described above. All cells were serum starved prior to being immunostained for Hic-5 and labeled for F-actin (using rhodamine-phalloidin) followed by confocal microscopy imaging to evaluate changes in Hic-5 distribution and actin cytoskeletal reorganization. Expression of RhoAV14/GFP or treatment of HTM cells with LPA, TGF-β2, and endothelin-1 led to appearance of a contractile cell morphology with increased actin stress fibers (in cells either expressing RhoAV14 or treated with endothelin-1) and tangled or cross-linked actin (cells treated with LPA, TGF-β2, or dexamethasone). Hic-5 redistributed to the leading ends of actin stress fibers (in cells either expressing RhoAV14 or treated with endothelin-1) and tangled actin (cells treated with LPA, TGF-β2, or dexamethasone) relative to GFP-expressing controls or untreated controls. Also seen is increased localization of Hic-5 to the nucleus or nuclear matrix (arrows) in cells treated with LPA, TGF-β2, and dexamethasone. Scale bars: 50 μm.
In the experiments discussed above, we also recorded changes in actin cytoskeletal organization and redistribution of Hic-5 in HTM cells using rhodamine-phalloidin fluorescence and immunofluorescence analysis, respectively (Fig. 2B). The RhoAV14-expressing HTM cells showed a robust increase in actin stress fibers distributing straight from one end (posterior) to the other end (anterior) of cells, together with Hic-5 redistributing discretely and intensely to the leading tips of actin stress fibers (see arrows), compared to GFP-expressing control cells, in which actin stress fibers and Hic-5 were found to be distributed throughout the cell body. Cells treated with LPA exhibited not only an increase in actin stress fibers but also an increase in actin filament cross-linking or tangling with Hic-5 distributing to the leading edges of HTM cells. We also noted increased Hic-5 around the nucleus under LPA treatment compared to untreated controls (see arrows in Fig. 2B). Interestingly, TGF-β2 also induced actin cytoskeletal reorganization with increased cross-linked actin-like structures in HTM cells, together with increased Hic-5 clustering at the nucleus (see arrow) and at the leading edges of cells compared to the controls. Similar to the changes observed upon RhoAV14 expression, treatment of HTM cells with endothelin-1 also stimulated a robust increase in actin stress fibers in HTM cells, with Hic-5 redistributing to the leading tips of the actin stress fibers and organizing at the leading edges of the cells compared to the respective untreated controls. While dexamethasone treatment led to increased actin stress fiber formation in HTM cells, the actin stress fibers were shorter and exhibited tangling or cross-linked actin filament structures. Under dexamethasone treatment, there seemed to be a dramatic reorganization of Hic-5, with large clusters localizing to the tips of actin stress fibers. Additionally, there appeared to be an increased clustering of Hic-5 to the nuclear region (see arrows) compared to the controls. The observations described above are consistent with a definitive association between Hic-5 and actin cytoskeletal reorganization in HTM cells.
TGF-β2-Induced Expression of Hic-5 in TM Cells Is Mediated via SMAD Signaling
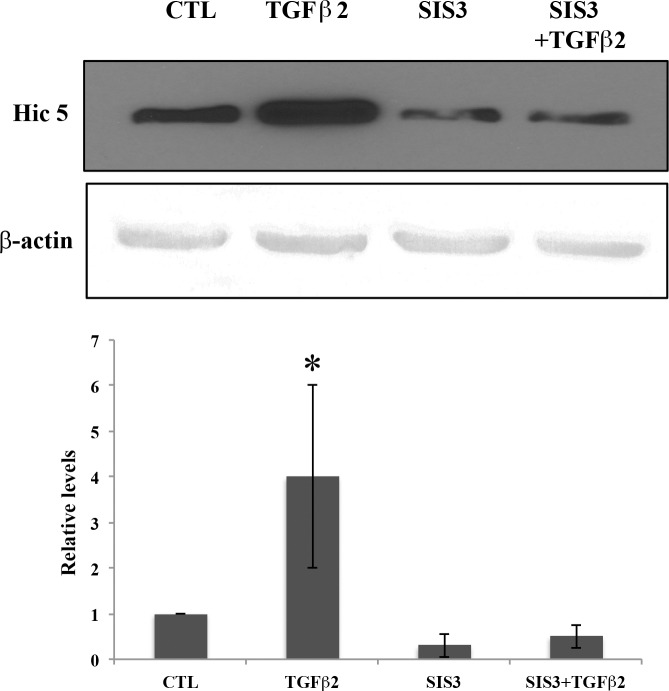
To delineate the molecular mechanism mediating induction of Hic-5 by TGF-β2 in TM cells, we looked into the possible involvement of canonical TGF-β2 signaling via SMAD. For this, serum-starved HTM cells were stimulated with TGF-β2 in the presence or absence of SIS3, a specific inhibitor of SMAD2/3, and probed for changes in Hic-5 protein levels by immunoblotting analysis. Transforming growth factor-β2 by itself (8 ng/mL for 14 hours) significantly increased the levels of Hic-5 protein as described earlier (Fig. 3; n = 4, P < 0.05). In contrast, treatment with SIS3 (10 μM for 12 hours) caused a significant inhibition of the TGF-β2-mediated increase and basal level of Hic-5 expression in these cells. Interestingly, neither the Rho kinase inhibitor Y27632 (10 μM for 12 hours) nor Rac1 inhibitor NSC23766 (20 μM for 12 hours) had any effect on Hic-5 protein levels in HTM cells (data not shown).
Figure 3.
Involvement of SMAD3 signaling in TGF-β2-induced expression of Hic-5 in HTM cells. Serum-starved HTM cells were treated with TGF-β2 (8 ng/mL for 14 hours) alone or in the presence of SMAD3 inhibitor-SIS3 (10 μM for 12 hours) and examined for changes in levels of Hic-5 by immunoblotting analysis. β-actin was immunoblotted as a loading control. While stimulation with TGF-β2 resulted in an increase in Hic-5 protein levels, treatment with SIS3 either alone or in the presence of TGF-β2 led to significant reduction in the levels of Hic-5 to below those of untreated controls (n = 4, mean ± SD). *P < 0.05.
Recombinant Hic-5 Induces Actin Stress Fiber and FAs Formation in HTM Cells
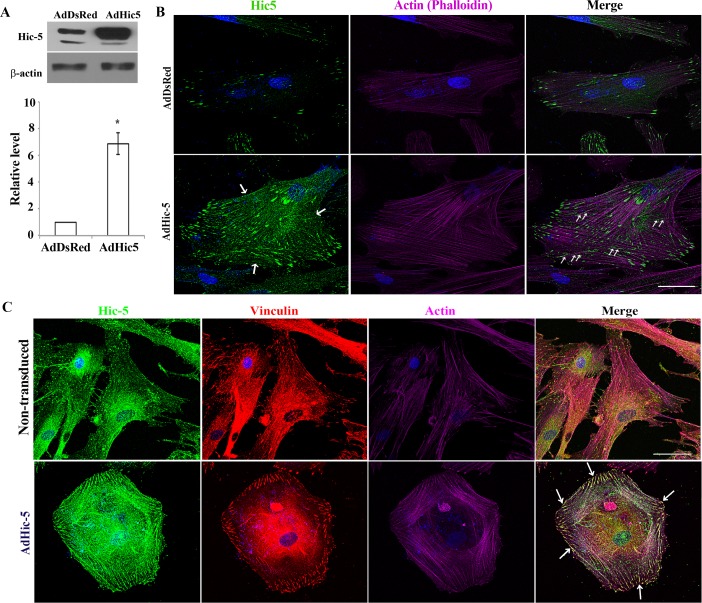
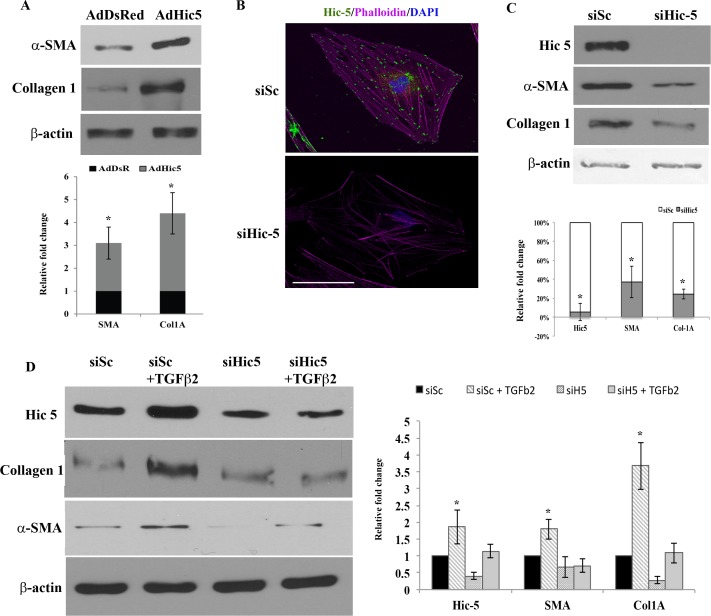
To determine the direct influence of Hic-5 on actin cytoskeletal organization and FAs, replication-deficient adenovirus was used to express recombinant Hic-5 in HTM cells. For controls, we used adenovirus with the same backbone expressing the DsRed reporter gene. Following virus infection, cells were serum starved for 48 hours, and cell lysates (800g supernatant) were immunoblotted for Hic-5. Hic-5 protein levels in the Ad-Hic-5-infected HTM cells were significantly higher (6- to 8-fold) compared to Ad-DsRed-infected cells (Fig. 4A). Ad-Hic-5- and Ad-DsRed-infected HTM cells grown on glass coverslips were serum starved for 48 hours, fixed and stained for actin stress fibers, Hic-5, or vinculin, and examined for changes in actin cytoskeletal organization and redistribution of Hic-5 and vinculin based on rhodamine-phalloidin fluorescence and immunofluorescence, respectively. Ad-Hic-5-infected cells showed a robust increase in actin stress fibers with contractile cell morphology compared with the Ad-DsRed-treated cells (Fig. 4B). Moreover, in Hic-5–overexpressing cells, Hic-5 not only distributed to the leading tips of actin stress fibers but also along the actin stress fibers (Fig. 4B, green stain at bottom right, see arrows). Additionally, overexpression of Hic-5 induced FA formation, redistribution of vinculin (in red) to the FAs, and vinculin/Hic-5 colocalization at FAs in HTM cells compared to the DsRed-expressing controls (Fig. 4C, see arrows).
Figure 4.
Hic-5 induces formation of actin stress fibers and FAs in HTM cells. (A) Serum-starved HTM cells infected with adenovirus expressing either Hic-5 or DsRed and immunoblotted using an anti-Hic-5 antibody exhibit a significant increase in Hic-5 protein levels compared to DsRed controls (n = 4, mean ± SD). *P < 0.05. β-actin was immunoblotted as a loading control. (B, C) Expression of recombinant Hic-5 induces formation of actin stress fibers and a contractile morphology in HTM cells, with Hic-5 distributing to the leading ends of actin stress fibers and along the actin stress fibers (arrows). Additionally, Hic-5 colocalizes with vinculin to the FAs (arrows) in HTM cells overexpressing Hic-5 compared with DsRed-expressing cells. Scale bar: 50 μm.
Functional Interaction Between Hic-5 and Integrin αvβ3 in HTM Cells
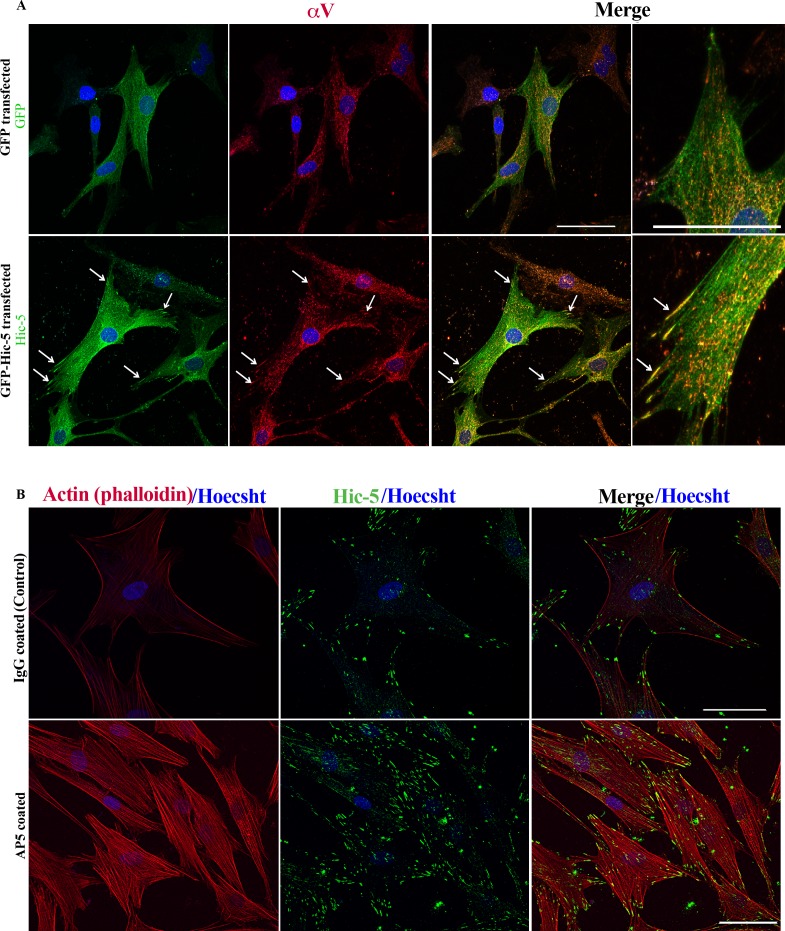
Integrins, a family of transmembrane cell surface proteins, act as tension sensors and form the link between actin cytoskeleton, FAs, and ECM, playing a major role in mechanosensing and inside-out and outside-in signaling.39 In TM cells, integrin αvβ3 has been demonstrated to regulate actin cytoskeletal reorganization and FAs.40,41 To explore potential and mutual interactions between Hic-5 and αvβ3 in HTM cells with respect to actin cytoskeletal organization and FA formation, we examined for the effects of Hic-5 on redistribution of αv integrin in HTM cells expressing the recombinant Hic-5-GFP. Three days after transfection, HTM cells expressing Hic-5-GFP were serum starved for 24 hours prior to being monitored for colocalization of integrin αv with Hic-5-GFP by immunofluorescence using anti-αv and anti-GFP antibodies. In Hic-5-GFP-expressing cells, αv integrin (in red) exhibited a redistribution to the FAs (see arrows) and colocalized with Hic-5 (see arrows at right) compared to control cells expressing GFP (upper) (Fig. 5A). To seek further insights into potential functional interaction between Hic-5 and αvβ3 integrin in regulating actin cytoskeletal organization in TM cells, we tested the effects of β3 integrin on Hic-5 distribution and actin cytoskeletal organization using antibody raised against activated epitope of β3 integrin (AP5). Human TM cells grown on glass coverslips coated with AP5 antibody (activated β3 integrin) exhibited increased Hic-5-specific immunofluorescence (in green) compared to the cells grown on IgG-coated glass coverslips. Additionally, HTM cells grown on AP5-coated glass coverslips showed increased actin stress fibers (in red) and Hic-5 localizing to the tips of actin stress fibers compared to the cells grown on mouse IgG-coated glass coverslips, revealing the influence of β3 integrin on Hic-5 expression, distribution, and actin cytoskeletal organization in HTM cells (Fig. 5B).
Figure 5.
Hic-5 induces redistribution of αv integrin to FAs, and activation of β3 integrin stimulates Hic-5 expression in HTM cells. (A) Expression of recombinant Hic-5 using pLEGFP-Hic-5 plasmid in HTM cells induces αv redistribution to the Hic-5-containing FAs (arrows) relative to GFP-expressing controls, as determined by immunofluorescence analysis. (B) Culturing HTM cells on glass coverslips coated with AP5 antibody (activated β3 integrin) appears to induce Hic-5 (green) and formation of actin stress fibers (red) relative to control cells (IgG coated), based on immunofluorescence analysis of Hic-5 and rhodamine-phalloidin fluorescence, respectively. In (A), far right shows magnified images shown in column 3 from left. Scale bar: 50 μm.
Hic-5-Induced Fibrogenic Activity in HTM Cells
Since contractile force generation associated with increased actomyosin assembly and FA formation has been found to influence plasticity and fibrogenic activity in the TM33 and other cell types,23 we asked whether the FA protein Hic-5 plays a role in fibrogenic activity in TM cells. Immunoblotting analysis was used to evaluate the effects of recombinant Hic-5 on levels of αSMA and collagen-1, which are both recognized as fibrogenic markers.33,42 Forty-eight hours following infection with adenovirus-expressing Hic-5 or DsRed (control), HTM cells were serum starved (for 24 hours) before preparation of total protein lysates (800g supernatant) that were subjected to immunoblotting analysis of α-SMA and collagen-1 levels. Expression of recombinant Hic-5 in HTM cells significantly increased (P < 0.05; n = 4) the levels of α-SMA and collagen-1 in comparison to DsRed-expressing control HTM cells (Fig. 6A). To further substantiate the role of Hic-5 in modulating αSMA and collagen-1 expression in HTM cells, we created Hic-5 deficiency using a specific siRNA (siHic-5). Human TM cells transfected with siHic-5 (for 24 hours) exhibited changes in cell morphology, exhibiting a somewhat elongated profile compared to cells transfected with scrambled siRNA control (data not shown). Additionally, siHic-5-transfected HTM cells presented a decrease in Hic-5 immunostaining (Fig. 6B), and a consistent and significant decrease (>90%; P < 0.05, n = 4) in Hic-5 protein levels compared to cells transfected with a scrambled siRNA. Cells transfected with siHic-5 showed significantly decreased (P < 0.05, n = 4) levels of both αSMA and collagen-1 compared to cells transfected with scrambled siRNA, revealing a direct involvement of Hic-5 in the expression of αSMA and collagen-1 (Fig. 6C).
Figure 6.
Hic-5 induces fibrogenic activity in HTM cells. (A) Immunoblotting of cell lysates (72 hours post infection) derived from serum-starved HTM cells expressing adenovirus-encoded recombinant Hic-5 showed a significant increase in α-SMA and collagen-1 compared to control cells infected with adenovirus encoding DsRed (n = 4, mean ± SD). *P < 0.05. (B) HTM cells transfected with Hic-5 siRNA (72 hours post transfection) exhibit a decrease in Hic-5 expression (green immunofluorescence staining) and actin stress fibers (rhodamine-phalloidin labeling in red) compared to cells transfected with scrambled siRNA (siSc). Scale bar: 50 μm. (C) HTM cells transfected with Hic-5 siRNA show a significant decrease in Hic-5 (>80%), α-SMA, and collagen-1 compared to cells treated with siSc based on immunoblot analyses. β-actin was probed as a loading control. The histograms show a densitometry-based representation of the decrease in the levels of Hic-5, α-SMA, and collagen-1 proteins (values are mean ± SD, n = 4, *P < 0.05). (D) Knockdown of Hic-5 expression in HTM cells suppresses TGF-β2-induced expression of αSMA and collagen-1. Serum-starved HTM cells treated with TGF-β2 (8 ng/mL for 24 hours) exhibited significant increase in expression of Hic-5, αSMA, and collagen-1 by immunoblotting analysis. These effects of TGF-β2 were suppressed in cells transfected with Hic-5 siRNA. Histograms represent the densitometry-based values from an average (mean ± SD) of four independent experiments. *P < 0.05. β-actin was immunoblotted as loading control.
We then evaluated the effects of TGF-β2 on αSMA and collagen-1 levels in the presence and absence of siHic-5 since TGF-β2 induces Hic-5 expression (Fig. 2) in HTM cells. Human TM cells transfected with either scrambled siRNA (siSc) or siHic-5 were treated with TGF-β2 (8 ng/mL) for 24 hours, prior to being lysed in preparation for assessment of α-SMA and collagen-1 levels by immunoblot analysis. The siSc-transfected (control) cells treated with TGF-β2 showed a significant increase in Hic-5, α-SMA, and collagen-1 compared to those cells treated with siSc alone (P < 0.05, n = 4). On the other hand, HTM cells transfected with siHic-5 alone exhibited significant decreases in the levels of both α-SMA and collagen-1. Additionally, siHic-5-transfected cells treated with TGF-β2 failed to show an increase in levels of either α-SMA or collagen-1, indicating the requirement for Hic-5 in the TGF-β2-induced fibrogenic process (Fig. 6D).
Hic-5 Regulates Dexamethasone-Induced Myocilin Expression in HTM Cells
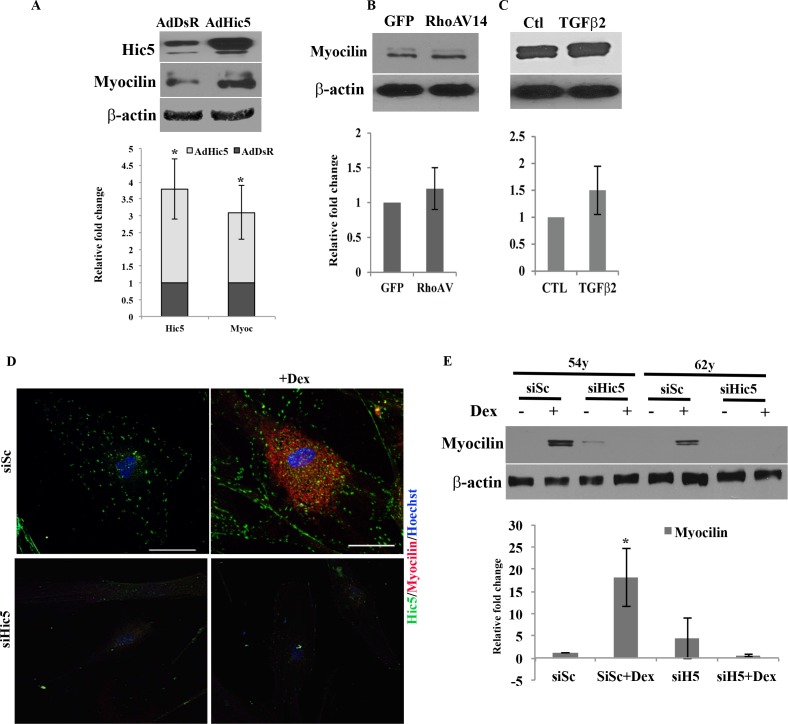
Hic-5, in addition to its role in FA formation and actin cytoskeletal organization, has been identified as a steroid receptor coactivator for several classes of steroids, including the glucocorticoids.17,43 Dexamethasone is known to elevate intraocular pressure by increasing resistance to AH outflow through the TM in association with altered actin cytoskeletal organization.44,45 We therefore asked whether Hic-5 plays a role in mediating the effects of dexamethasone via affecting the expression of myocilin in light of the fact that a mutation in the myocilin (MYOC) gene is linked to development of juvenile primary open-angle glaucoma46 Most importantly, MYOC is a well-characterized dexamethasone-inducible gene.46,47 To gain insight into a potential role for Hic-5 in regulation of expression of myocilin, we examined the effects of expressing adenovirus-encoded recombinant Hic-5 in HTM cells, which were infected with Ad-Hic-5 or DsRed control for 96 hours, serum starved (for 24 hours) prior to cell lysate preparation (800g supernatant) for immunoblot analysis. As expected, Ad-Hic-5 infection led to a robust increase in Hic-5 protein levels compared to the DsRed-expressing controls (Fig. 7). Evaluation of lysates from these cells showed a significant increase (P < 0.05; n = 4) in myocilin levels compared to the DsRed-expressing controls (Fig. 7A). We then assessed the effects of dexamethasone on myocilin and Hic-5 levels in HTM cells by immunofluorescence and immunoblot analyses, respectively, in the presence and absence of Hic-5-specific siRNA (siHic-5) as described earlier. As reported in earlier studies,47 treatment of HTM cells with dexamethasone induced myocilin expression based on immunofluorescence analysis (red stain, Fig. 7D), with the same cells also exhibiting increased levels of Hic-5 (green stain, Fig. 7D). In contrast, transfection of HTM cells with siHic-5 prior to dexamethasone treatment suppressed the expression of both myocilin and Hic-5 based on immunofluorescence analysis. Immunoblotting analysis of myocilin levels was also performed for HTM cells treated with dexamethasone in the presence and absence of siHic-5. Quantitative immunoblot analysis demonstrated that the presence of Hic-5 siRNA consistently and significantly suppresses dexamethasone induced myocilin expression (P < 0.05; n = 4) in HTM cells, substantiating the importance of Hic-5 in mediating the effects of dexamethasone on myocilin expression (Fig. 7E). The effects of TGF-β2 and RhoAV14 on the expression of myocilin were also evaluated since these proteins induce the expression of Hic-5 in HTM cells. Human TM cells expressing RhoAV14 or treated with TGF-β2, however, did not show a significant change in myocilin levels compared to their respective controls (Figs. 7B, 7C).
Figure 7.
Hic-5 regulates myocilin expression in HTM cells. (A) Immunoblotting of protein extracts derived from serum-starved HTM cells infected with adenovirus-expressing recombinant Hic-5 (72 hours post infection) showed a significant increase in Hic-5 and myocilin compared to control cells expressing DsRed (n = 4, mean ± SD). *P < 0.05. In contrast, RhoAV14 (B) expression or treatment with TGF-β2 ([C] 8 ng/mL for 48 hours) did not influence myocilin expression in HTM cells relative to control cells as determined by immunblotting analysis (n = 4, mean ± SD). (D) Dexamethsone-induced myocilin expression in HTM cells was suppressed by Hic-5 siRNA. Immunofluorescence staining revealed an increase in myocilin levels in dexamethasone (500 nM for 4 days)-treated HTM cells that were also transfected with siSc, with the response being attenuated in cells transfected with siHic-5. Scale bar: 50 μm. (E) Immunoblotting for myocilin in HTM cells (two independent cell strains) transfected with siSc and treated with dexamethasone as described above led to a significant increase in myocilin levels, while transfection with siHic-5 prior to dexamethasone treatment prevented the increase in myocilin levels in HTM cells. Values are shown as mean ± SD, n = 4, *P < 0.05.
Discussion
Although it is now well recognized that TM cell contractile activity, mechanical force, cell–matrix interactions, and ECM organization play an important role in regulation of AH outflow through the trabecular pathway and IOP in normal and glaucoma patients,2,8,10,48 we still do not have a thorough understanding regarding different molecular mechanisms controlling these characteristics of TM and other cell types of the AH outflow pathway. Toward this, for the first time, we report the expression and distribution of Hic-5 in the AH outflow pathway of humans, a well-characterized FA protein with its demonstrated role(s) in actin cytoskeletal organization and transcriptional regulation.21,23,43 Hic-5 reveals tissue specificity of expression, and was detected in both HTM cells and tissue but not in lens tissue or cultured human retinal pigment epithelial cells (data not shown), consistent with the abundant expression of this protein in smooth muscle cells.13,14 Importantly, Hic-5 expression in HTM cells is significantly upregulated by TGF-β2, RhoA, LPA, endothelin-1, and H2O2 that also influence the redistribution of Hic-5 to the FAs and nuclear matrix in association with actin cytoskeletal reorganization. Upregulation and redistribution of Hic-5 in TM cells appear to be associated with an increase in actin stress fiber formation and their tangling or cross-linking, indicating that Hic-5 likely participates in cytoskeletal reorganization and formation of FAs in response to TGF-β2, RhoA, LPA, endothelin-1, and H2O2. Since each of these physiological agents has been demonstrated to influence AH outflow through the TM,8,34–36,49–52 it is reasonable to speculate on a potential role for Hic-5 in regulation of AH outflow acting downstream of these important external cues. In addition to the increased levels of Hic-5 observed in the current study, it is likely that tyrosine phosphorylation-mediated activation of Hic-553 might also contribute to the effects of the physiological agents tested in our experiments with HTM cells.
Expression of recombinant Hic-5 in TM cells induced a dramatic increase in actin stress fibers, FAs, contractile cell morphology, and redistribution of vinculin and αv integrin to the FAs, supporting the contention that Hic-5 directly influences actin cytoskeletal organization, contractility, and cell–ECM adhesive interactions in TM cells. We also noted that Hic-5 relocalizes along actin stress fibers in addition to FAs, indicating that this protein likely impacts actin stress fiber formation and bundling. Additionally, this study also provides a novel molecular insight into αvβ3 integrin-mediated changes in actin cytoskeletal organization and cell adhesive properties in TM cells.40,41 Our results showed not only that Hic-5 has direct effects on redistribution of αV integrin to the FAs, but also that activated β3 integrin, which is known to induce cross-linked actin networks (CLANs) in TM cells, appears to stimulate Hic-5 expression and causes Hic-5 to redistribute to FAs in association with increased actin stress fiber formation. Taken together, these data indicate the existence of a plausible functional interaction between αVβ3 and Hic-5 and suggest that these proteins mutually regulate TM cell contractile and cell adhesive properties. Interestingly, syndecan-4, which has been shown to influence formation of CLANs in coordination with β3 integrin in TM cells,40 has been reported to interact with Hic-5 through syndesmos.26
The contractile properties of smooth muscle cells are regulated directly by actin stress fiber formation, cell–matrix interactions, and mechanotransduction via FAs, which are then in turn known to influence cell plasticity, migration, and ECM production and reorganization of different cell types.23,33,54–56 Our studies revealed that expression of Hic-5 in TM cells leads to robust increases in the levels of collagen-1 and αSMA in TM cells, which are well-recognized markers of fibrogenic activity, demonstrating that Hic-5 plays a role in control of TM cell fibrogenic activity and ECM synthesis. Consistent with these observations, our previous studies showed that RhoA GTPase-induced fibrogenic activity in TM cells is associated with increased actin stress fiber formation, supporting the significance of cell contractile activity and mechanical force in influencing cell plasticity and fibrogenic activity.33,37 Moreover, our results also demonstrate a critical role for Hic-5 in TGF-β2-induced expression of collagen-1 and αSMA in TM cells, as evidenced by the blunted response to TGF-β2 in HTM cells deficient for Hic-5. The current study also provided evidence for participation of SMAD signaling in TGF-β2-induced Hic-5 expression, based on the inhibitory effect of SIS3 on Hic-5 levels in the presence of TGF-β2. Collectively, these observations present experimental evidence for the participation of Hic-5 in TGF-β-mediated SMAD signaling and fibrogenic activity in TM cells. Consistent with our observations in HTM cells, Hic-5 has been shown to participate in TGF-β-induced fibrosis in different tissues.23,32,57,58 Although Rho/Rho kinase has been reported to participate in Hic-5-induced EMT and fibrosis,14,23 neither Rho kinase inhibitor nor Rac1 inhibitor had a significant effect on Hic-5 expression in TM cells.
In addition to the cellular effects of Hic-5 discussed above, one of the most intriguing characteristics of Hic-5 is its ability to serve as a coactivator of steroid nuclear receptors and regulate transcriptional activity of steroids including the glucocorticoids.16,17,21,43,59 Hic-5 has been demonstrated to shuttle from cytosol to nucleus, regulate both cell adhesive properties and transcriptional activity of steroids, and transduce mechanical signals from the FAs to the nucleus.12,43,60 This aspect of Hic-5 is particularly interesting as it relates to the effects of steroids on TM cells. It is well known that glucocorticoids including dexamethasone induce IOP in human patients and increase resistance to AH outflow through the TM.9 Moreover, dexamethasone has been reported to induce changes in actin cytoskeletal organization, cell adhesive properties, and ECM synthesis and reorganization in TM cells.41,45,61,62 Importantly, dexamethasone induces expression of myocilin, whose mutation results in hereditary juvenile glaucoma with elevated IOP.46 Additionally, overexpression of myocilin has been reported to increase resistance to AH outflow and induce changes in cytoskeletal organization and cell adhesive properties.41,46,63–68 However, an understanding of the molecular basis for dexamethasone- or myocilin-induced actin cytoskeletal changes, ECM synthesis, and AH outflow, as well as the induction of myocilin expression by dexamethasone, has remained elusive. Toward this, our results in this study imply that Hic-5 induces myocilin expression and that Hic-5 deficiency decreases dexamethasone-induced myocilin expression in TM cells along with significant effects on actin cytoskeletal reorganization and ECM synthesis in TM cells. These observations collectively illuminate significant molecular insight into the role of Hic-5 in dexamethasone- and myocilin-induced actin cytoskeletal changes and cell adhesive properties, as well as dexamethasone-induced myocilin expression. In contrast to the effect of dexamethasone, which induces expression of both Hic-5 and myocilin in HTM cells, TGF-β2 and RhoAV14 stimulate the expression of Hic-5 but have no effect on myocilin levels. These observations indicate that either both dexamethasone and Hic-5 or high levels of Hic-5 are required to induce transcriptional activation of myocilin expression through the glucocorticoid receptor pathway. Further studies are necessary for a thorough understanding of the role of Hic-5 in dexamethasone-induced expression of myocilin.
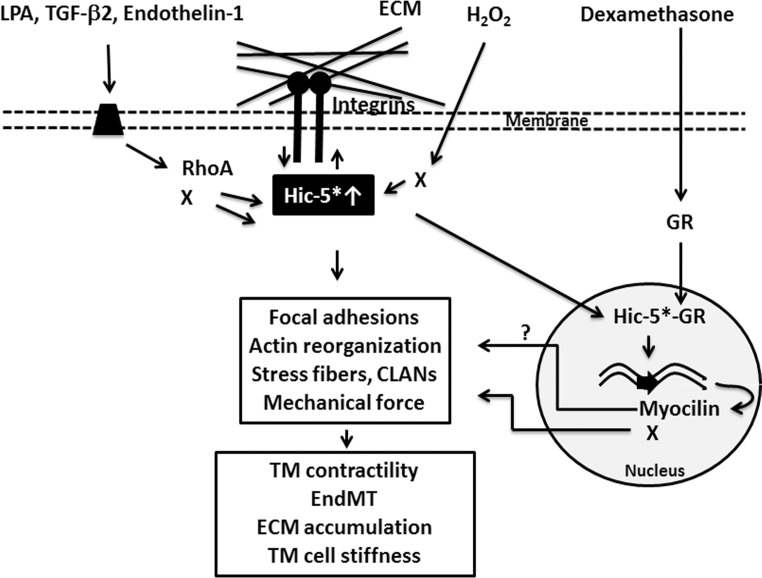
In conclusion, this initial study offers a novel insight into the molecular link between Hic-5, actin cytoskeleton, FAs, fibrogenic activity, glucocorticoid receptors, dexamethasone, and myocilin in TM cells and their collective role in AH outflow. Figure 8 provides a schematic representation of the role of Hic-5 in regulating cellular events in HTM cells, in the context of AH outflow and IOP. It is possible that deregulated Hic-5 expression and activation in the TM might pose a double-edged sword response (via steroid-induced gene expression and fibrogenic activity) by increasing resistance to AH outflow through the trabecular pathway in the presence of dexamethasone and increased levels of TGF-β2, endothelin-1, and LPA.
Figure 8.
Schematic illustration of the role and regulatory effects of Hic-5 in TM cells. Increased levels of Hic-5 induced by external factors (e.g., LPA, H2O2, TGF-β2, endothelin-1, and dexamethasone) and a possible activation of Hic-5 via tyrosine phosphorylation (indicated with asterisk) lead to activation of intracellular signaling pathways including the RhoA, SMADs, and others (X) in TM cells. These signaling pathways regulate FA formation, integrin–extracellular matrix (ECM) interaction, actin cytoskeletal reorganization, contractility, actin cytoskeletal tangling/cross-linking, cell mechanical properties, endothelial to mesenchymal transition (EndMT), and ultimately accumulation of ECM components. Additionally, elevated levels of Hic-5 might conceivably enhance the transcriptional activity of the glucocorticoid receptor (GR) in the presence of dexamethasone via nuclear translocation and manifestation of steroid receptor coactivator function, resulting in upregulation of myocilin and other (X) target genes. Dexamethasone-induced expression of myocilin and other gene products may possibly in turn influence cell adhesive interactions and actin cytoskeletal reorganization in TM cells.
Supplementary Material
Acknowledgments
The authors thank Christopher Turner and Patrick Casey for their generous help in providing the pLEGFP-C1 Hic-5 plasmid and adenovirus expressing RhoAV14, respectively. We also thank Daniel Stamer for anti-GFP and anti-myocilin antibody, Goldis Malek for providing the human RPE cells, and Paloma Liton for providing entry clone of pENTR-D-TOPO and DsRed plasmids.
Supported by National Institutes of Health Grant R01EY018590.
Disclosure: P.P. Pattabiraman, None; P.V. Rao, None
References
- 1. Acott TS,, Kelley MJ,, Keller KE,, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014; 30: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stamer WD,, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012; 23: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamm ER,, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007; 52 (suppl 2): S101–S104. [DOI] [PubMed] [Google Scholar]
- 4. Quigley HA,, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999; 18: 91–119. [DOI] [PubMed] [Google Scholar]
- 6. Alvarado JA,, Betanzos A,, Franse-Carman L,, Chen J,, Gonzalez-Mariscal L. Endothelia of Schlemm's canal and trabecular meshwork: distinct molecular functional, and anatomic features. Am J Physiol Cell Physiol. 2004; 286: C621–C634. [DOI] [PubMed] [Google Scholar]
- 7. Keller KE,, Aga M,, Bradley JM,, Kelley MJ,, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiederholt M,, Thieme H,, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000; 19: 271–295. [DOI] [PubMed] [Google Scholar]
- 9. Clark AF,, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009; 88: 752–759. [DOI] [PubMed] [Google Scholar]
- 10. Rao VP,, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007; 21: 167–177. [DOI] [PubMed] [Google Scholar]
- 11. Shibanuma M,, Mashimo J,, Kuroki T,, Nose K. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem. 1994; 269: 26767–26774. [PubMed] [Google Scholar]
- 12. Thomas SM,, Hagel M,, Turner CE. Characterization of a focal adhesion protein Hic-5, that shares extensive homology with paxillin. J Cell Sci. 1999; 112 (pt 2): 181–190. [DOI] [PubMed] [Google Scholar]
- 13. Kim-Kaneyama JR,, Suzuki W,, Ichikawa K,, et al. Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells in vivo. J Cell Sci. 2005; 118: 937–949. [DOI] [PubMed] [Google Scholar]
- 14. Wang X,, Hu G,, Betts C,, et al. Transforming growth factor-beta1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J Biol Chem. 2011; 286: 41589–41599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shibanuma M,, Mochizuki E,, Maniwa R,, et al. Induction of senescence-like phenotypes by forced expression of hic-5, which encodes a novel LIM motif protein, in immortalized human fibroblasts. Mol Cell Biol. 1997; 17: 1224–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujimoto N,, Yeh S,, Kang HY,, et al. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J Biol Chem. 1999; 274: 8316–8321. [DOI] [PubMed] [Google Scholar]
- 17. Gao Z,, Schwartz LM. Identification and analysis of Hic-5/ARA55 isoforms: implications for integrin signaling and steroid hormone action. FEBS Lett. 2005; 579: 5651–5657. [DOI] [PubMed] [Google Scholar]
- 18. Rahman MM,, Miyamoto H,, Lardy H,, Chang C. Inactivation of androgen receptor coregulator ARA55 inhibits androgen receptor activity and agonist effect of antiandrogens in prostate cancer cells. Proc Natl Acad Sci U S A. 2003; 100: 5124–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang L,, Guerrero J,, Hong H,, DeFranco DB,, Stallcup MR. Interaction of the tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol Biol Cell. 2000; 11: 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohanian J,, Pieri M,, Ohanian V. Non-receptor tyrosine kinases and the actin cytoskeleton in contractile vascular smooth muscle [published online ahead of print November 28, 2014] J Physiol. doi:http://dx.doi.org/10.1113/jphysiol.2014.284174. [DOI] [PMC free article] [PubMed]
- 21. Chodankar R,, Wu DY,, Schiller BJ,, Yamamoto KR,, Stallcup MR. Hic-5 is a transcription coregulator that acts before and/or after glucocorticoid receptor genome occupancy in a gene-selective manner. Proc Natl Acad Sci U S A. 2014; 111: 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deakin NO,, Ballestrem C,, Turner CE. Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA. PLoS One. 2012; 7: e37990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tumbarello DA,, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a RhoA/ROCK-dependent pathway. J Cell Physiol. 2007; 211: 736–747. [DOI] [PubMed] [Google Scholar]
- 24. Guignandon A,, Boutahar N,, Rattner A,, Vico L,, Lafage-Proust MH. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun. 2006; 343: 407–414. [DOI] [PubMed] [Google Scholar]
- 25. Shibanuma M,, Mori K,, Kim-Kaneyama JR,, Nose K. Involvement of FAK and PTP-PEST in the regulation of redox-sensitive nuclear-cytoplasmic shuttling of a LIM protein, Hic-5. Antioxid Redox Signal. 2005; 7: 335–347. [DOI] [PubMed] [Google Scholar]
- 26. Denhez F,, Wilcox-Adelman SA,, Baciu PC,, et al. Syndesmos, a syndecan-4 cytoplasmic domain interactor, binds to the focal adhesion adaptor proteins paxillin and Hic-5. J Cell Sci. 2002; 277: 12270–12274. [DOI] [PubMed] [Google Scholar]
- 27. Nishiya N,, Tachibana K,, Shibanuma M,, Mashimo JI,, Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and Hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001; 21: 5332–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Osada M,, Ohmori T,, Yatomi Y,, Satoh K,, Hosogaya S,, Ozaki Y. Involvement of Hic-5 in platelet activation: integrin alphaIIbbeta3-dependent tyrosine phosphorylation and association with proline-rich tyrosine kinase 2. Biochem J. 2001; 355: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishiya N,, Iwabuchi Y,, Shibanuma M,, Cote JF,, Tremblay ML,, Nose K. Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3 domain. J Biol Chem. 1999; 274: 9847–9853. [DOI] [PubMed] [Google Scholar]
- 30. Fujita H,, Kamiguchi K,, Cho D,, Shibanuma M,, Morimoto C,, Tachibana K. Interaction of Hic-5, a senescence-related protein, with focal adhesion kinase. J Cell Sci. 1998; 273: 26516–26521. [DOI] [PubMed] [Google Scholar]
- 31. Matsuya M,, Sasaki H,, Aoto H,, et al. Cell adhesion kinase beta forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J Biol Chem. 1998; 273: 1003–1014. [DOI] [PubMed] [Google Scholar]
- 32. Kim-Kaneyama JR,, Lei XF,, Arita S,, Miyauchi A,, Miyazaki T,, Miyazaki A. Hydrogen peroxide-inducible clone 5 (Hic-5) as a potential therapeutic target for vascular and other disorders. J Atheroscler Thromb. 2012; 19: 601–607. [DOI] [PubMed] [Google Scholar]
- 33. Pattabiraman PP,, Maddala R,, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014; 229: 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mettu PS,, Deng PF,, Misra UK,, Gawdi G,, Epstein DL,, Rao PV. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2004; 45: 2263–2271. [DOI] [PubMed] [Google Scholar]
- 35. Zhang M,, Maddala R,, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008; 295: C1057–C1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pattabiraman PP,, Rinkoski T,, Poeschla E,, Proia A,, Challa P,, Rao PV. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. Am J Pathol. 2015; 185: 496–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pattabiraman PP,, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010; 298: C749–C763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rao PV,, Maddala R. Abundant expression of ponsin, a focal adhesion protein, in lens and downregulation of its expression by impaired cytoskeletal signaling. Invest Ophthalmol Vis Sci. 2009; 50: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010; 2: a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filla MS,, Woods A,, Kaufman PL,, Peters DM. Beta1 and beta3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47: 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Filla MS,, Schwinn MK,, Nosie AK,, Clark RW,, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Invest Ophthalmol Vis Sci. 2011; 52: 2952–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalluri R,, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003; 112: 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heitzer MD,, DeFranco DB. Hic-5, an adaptor-like nuclear receptor coactivator. Nucl Recept Signal. 2006; 4: e019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clark AF,, Wilson K,, de Kater AW,, Allingham RR,, McCartney MD. Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest Ophthalmol Vis Sci. 1995; 36: 478–489. [PubMed] [Google Scholar]
- 45. Clark AF,, Wilson K,, McCartney MD,, Miggans ST,, Kunkle M,, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994; 35: 281–294. [PubMed] [Google Scholar]
- 46. Stone EM,, Fingert JH,, Alward WL,, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997; 275: 668–670. [DOI] [PubMed] [Google Scholar]
- 47. Nguyen TD,, Chen P,, Huang WD,, Chen H,, Johnson D,, Polansky JR. Gene structure and properties of TIGR an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Cell Sci. 1998; 273: 6341–6350. [DOI] [PubMed] [Google Scholar]
- 48. Tian B,, Geiger B,, Epstein DL,, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000; 41: 619–623. [PubMed] [Google Scholar]
- 49. Gottanka J,, Chan D,, Eichhorn M,, Lutjen-Drecoll E,, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 153–158. [DOI] [PubMed] [Google Scholar]
- 50. Fleenor DL,, Shepard AR,, Hellberg PE,, Jacobson N,, Pang IH,, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006; 47: 226–234. [DOI] [PubMed] [Google Scholar]
- 51. Yan DB,, Trope GE,, Ethier CR,, Menon IA,, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991; 32: 2515–2520. [PubMed] [Google Scholar]
- 52. Kahn MG,, Giblin FJ,, Epstein DL. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 1983; 24: 1283–1287. [PubMed] [Google Scholar]
- 53. Hetey SE,, Lalonde DP,, Turner CE. Tyrosine-phosphorylated Hic-5 inhibits epidermal growth factor-induced lamellipodia formation. Exp Eye Res. 2005; 311: 147–156. [DOI] [PubMed] [Google Scholar]
- 54. Piera-Velazquez S,, Li Z,, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011; 179: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J,, Zohar R,, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp Eye Res. 2006; 312: 205–214. [DOI] [PubMed] [Google Scholar]
- 56. Desmouliere A,, Chaponnier C,, Gabbiani G. Tissue repair contraction, and the myofibroblast. Wound Repair Regen. 2005; 13: 7–12. [DOI] [PubMed] [Google Scholar]
- 57. Inui S,, Shono F,, Noguchi F,, Nakajima T,, Hosokawa K,, Itami S. In vitro and in vivo evidence of pathogenic roles of Hic-5/ARA55 in keloids through Smad pathway and profibrotic transcription. J Dermatol Sci. 2010; 58: 152–154. [DOI] [PubMed] [Google Scholar]
- 58. Dabiri G,, Tumbarello DA,, Turner CE,, Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol. 2008; 128: 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heitzer MD,, DeFranco DB. Mechanism of action of Hic-5/androgen receptor activator 55, a LIM domain-containing nuclear receptor coactivator. Mol Endocrinol. 2006; 20: 56–64. [DOI] [PubMed] [Google Scholar]
- 60. Deakin NO,, Pignatelli J,, Turner CE. Diverse roles for the paxillin family of proteins in cancer. Genes Cancer. 2012; 3: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tane N,, Dhar S,, Roy S,, Pinheiro A,, Ohira A,, Roy S. Effect of excess synthesis of extracellular matrix components by trabecular meshwork cells: possible consequence on aqueous outflow. Exp Eye Res. 2007; 84: 832–842. [DOI] [PubMed] [Google Scholar]
- 62. Overby DR,, Bertrand J,, Tektas OY,, et al. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014; 55: 4922–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fautsch MP,, Bahler CK,, Vrabel AM,, et al. Perfusion of his-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest Ophthalmol Vis Sci. 2006; 47: 213–221. [DOI] [PubMed] [Google Scholar]
- 64. Kwon HS,, Tomarev SI. Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway. J Cell Physiol. 2011; 226: 3392–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwon HS,, Lee HS,, Ji Y,, Rubin JS,, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol. 2009; 29: 2139–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yuan Y,, Call MK,, Yuan Y,, et al. Dexamethasone induces cross-linked actin networks in trabecular meshwork cells through noncanonical wnt signaling. Invest Ophthalmol Vis Sci. 2013; 54: 6502–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ueda J,, Wentz-Hunter K,, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002; 43: 1068–1076. [PubMed] [Google Scholar]
- 68. Peters DM,, Herbert K,, Biddick B,, Peterson JA. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp Eye Res. 2005; 303: 218–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.