Abstract
The Phosphatase of Regenerating Liver (PRL) family, comprising PRL-1, PRL-2, and PRL-3, is a group of prenylated phosphatases, which are candidate cancer biomarkers and therapeutic targets. Although several studies have documented that altered expression of PRL-1 or PRL-3 can influence cell proliferation, migration and invasion, there is a dearth of knowledge about the biological functions of PRL-2. Thus, in the current study we have evaluated the role of PRL-2 in cell migration and invasion in human cancer cells. We found four human lung cancer cells, including A549 cells, over-express PRL-2 when compared with normal lung cells. PRL-2 knockdown by RNA interference markedly inhibited cell migration and invasion and this inhibition can be restored by over-expressing the siRNA resistant vector HA-PRL-2m. PRL-2 suppression by siRNA decreased p130Cas and vinculin expression, and decreased ERK phosphorylation, while increasing the phosphorylation of ezrin on tyrosine 146. We found no significant changes in total p53, Akt and c-Src expression levels or their phosphorylation status, suggesting PRL-2 knockdown could inhibit tumor cell migration and invasion through a Src-independent p130Cas signaling pathway. Ectopic expression of wild type PRL-2, a catalytic inactive C101S mutant and a C-terminal CAAX deletion revealed a requirement for both the PRL-2 catalytic functionality and prenylation site. Expression of wild type but not mutant forms of PRL-2 caused ERK phosphorylation and nuclear translocation. These results support a model in which PRL-2 promotes cell migration and invasion through an ERK-dependent signaling pathway.
Keywords: PRL-2, lung cancer, cell invasion, ERK
Introduction
The Phosphatase of Regenerating Liver (PRLs) family represents a novel subfamily of protein tyrosine phosphatases (PTPs), comprising three family members. PRL-1 was originally identified as an immediate early gene in regenerating liver (Diamond et al., 1994). Subsequently, PRL-2 and PRL-3 were discovered. The three PRLs share approximately 80% identity in total amino acid sequence and have identical signature PTP catalytic site sequences: CVAGLGR (Stephens et al., 2005). The PRLs are unique among the PTPs because they possess a conserved C-terminal CAAX domain for prenylation, where C is cysteine, A is an aliphatic amino acid, and X is any amino acid. The CAAX domain and its prenylation are thought to be important for targeting proteins to intracellular membranes, therefore, PRLs are typically localized to the plasma membrane and intracellular punctuate structures (Cates et al., 1996; Zeng et al., 2000).
Although the PRLs are highly homologous, their expression pattern differs among tissues, suggesting PRLs could function divergently. With regard to cancer, individual PRLs are over-expressed in a variety of cancer cell lines and tissues. For example, PRL-3 is dramatically up-regulated in metastatic colorectal carcinomas (Kato et al., 2004; Peng et al., 2004; Wang et al., 2007b), breast carcinoma (Radke et al., 2006, Wang et al., 2006), and gastric carcinomas (Li et al., 2007; Miskad et al., 2004). The elevated PRL-3 is associated with the progression of these types of human cancer (Kato et al., 2004; Li et al., 2007; Peng et al., 2004; Radke et al., 2006; Wang et al., 2006), making PRL-3 a promising biomarker. In contrast, the role of PRL-1 and PRL-2 in cancer is less well studied. Initial evidence indicates elevated expression of PRL-1 in melanoma, pancreatic cancer, lung cancer cell lines and esophageal squamous cell carcinoma (Achiwa and Lazo 2007; Han et al., 2002; Wang et al., 2002a). In addition, elevated levels of PRL-2 have been detected in pancreatic, prostate cancer cell lines, pediatric acute myeloid leukemia, and prostate and breast tumor tissues (Hardy et al., 2010; Stephens et al., 2008; Wang et al., 2002b; Yagi et al., 2003).
Functionally, PRL phosphatases, especially PRL-3 and PRL-1, have been implicated in tumor development and metastasis (Cates et al., 1996; Wu et al., 2004; Zeng et al., 2003), although the molecular processes they affect remain largely undefined. We know ectopic expression of PRL-1 or PRL-3 in non-tumorigenic cells can enhance the migratory and invasive properties of cells (Liang et al., 2007; Zeng et al., 2003). RNA interference-mediated knockdown of the endogenous PRL-1 or PRL-3 in cancer cells can abrogate cell motility and ability to metastasize in a mouse model (Achiwa and Lazo 2007; Kato et al., 2004). Moverover, we suspect the Rho family GTPases (Fiordalisi et al., 2006), Src (Achiwa and Lazo 2007; Liang et al., 2007; Luo et al., 2009), PI3K (Wang et al., 2007a), p53 (Min et al., 2009; Wang et al., 2007a) and ERK (Ming et al., 2009, Peng et al., 2009) could be important for PRL-1 and/or PRL-3 mediated changes on cell invasion and metastasis. Independent studies demonstrate the involvement of Src in PRL-1 or PRL-3 signaling pathways (Achiwa and Lazo 2007; Liang et al., 2007; Luo et al., 2009). PRL-3 down-regulates PTEN expression and has been reported to promote Epithelial-Mescenchymal Transition through PI3K signaling pathway (Wang et al., 2007a). PRL-1 and PRL-3 appears to be p53 targets (Basak et al., 2008; Min et al., 2009). PRL-3 promotes LoVo colon cancer cell invasion through PRL-3-intergrin β1-ERK1/2 signaling (Peng et al., 2009), while PRL-3 also facilitates angiogenesis and metastasis by increasing ERK phosphorylation in lung cancer (Ming et al., 2009).
Because PRL-2 is highly homologous to PRL-1 and PRL-3, we hypothesized that PRL-2, like its other family members, could also have a fundamental role in regulating tumor cell invasion. In the current study, we demonstrated the effects of PRL-2 over-expression in lung cancer cell lines. Using A549 lung cancer cells, we confirmed a role of PRL-2 in regulating tumor cell migration and invasion, and also identified the involvement of ERK1/2 phosphorylation in the PRL-2 signaling pathway. Together, these results provide seminal evidence that PRL-2 participates in lung cancer cell invasion and encourages greater attention on this PRL family member.
Results
PRL-2 silencing by siRNA and shRNA
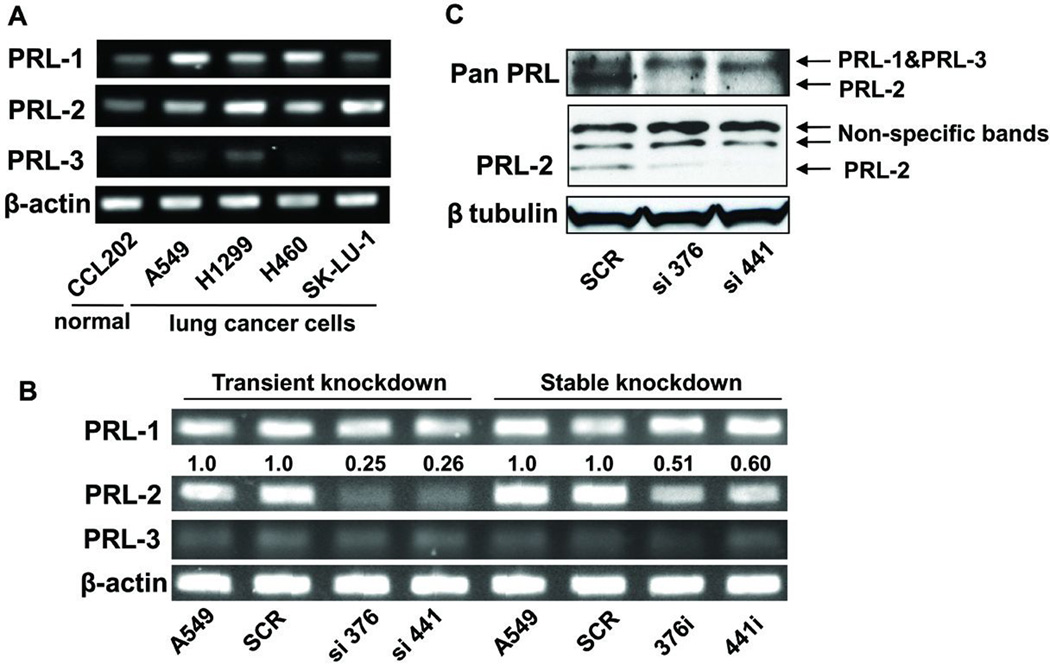
Because our previous study (Achiwa and Lazo 2007) indicated that at least one lung cancer cell line, A549, expressed PRL-2, we extended our examination to three other lung cancer cell lines. As indicated in Figure 1A, we found significant amounts of PRL-2 mRNA in H1299, H460, SK-LU-1 and A549 cells. All four human lung cancer cell lines had higher PRL-2 mRNA levels relative to those found in the normal human lung fibroblast cell line CCL202. A549, H1299 and H460 but not SK-LU-1 cells had detectable PRL-1 mRNA and none of the cells had detectable PRL-3 mRNA. SK-LU-1 cells were initially considered as an attractive cell type to study but its poor growth, migration and invasive characteristics disqualified it. Therefore, A549 lung cancer cells were selected for further study because they migrate and invade well in vitro and because our previously interrogation of PRL-1 with this cell line provided a valuable isogenic comparison (Achiwa and Lazo 2007). To examine the biological functions of endogenous PRL-2, we established A549 cells that stably expressed shRNA for PRL-2. Four different 21-nucleotide sequences were designed for shRNA and the two that provided the best knockdown were selected and named according to the starting nucleotide number of the targeting sequence: sh376 and sh441. Clones made with each of the two targeting sequences that persistently exhibited suppressed PRL-2 expression were selected and renamed 376i and 441i; one of the clones obtained with scrambled shRNA (SCR) was also used as a control. The sh376 and sh441 cells chronically expressing PRL-2 shRNA had 50% and 40% less PRL-2 mRNA, respectively (Figure 1B). PRL-1 and PRL-3 mRNA levels were unaffected in the PRL-2 knockdown cells (Figure 1B).
Figure 1. PRL-2 mRNA and protein levels were significantly suppressed in the PRL-2 knockdown cells.
The cell clone numbers 376 and 441 indicate the starting nucleotide number of siRNA- or shRNA-targeting sequences of PRL-2 mRNA. RT-PCR and Western blotting images were processed and quantified with Fuji Multi Gauge software (Fujifilm). A) RT-PCR demonstrated that PRL-2 was over-expressed in several lung cancer cells including A549 cells. β-actin was used as internal control. B) PRL-2 was selectively down-regulated at the mRNA level in cells with transient and stable PRL-2 knockdown. The siRNA transiently transfected cells displayed ~80% decrease in PRL-2 mRNA level, while there was a ~50% decrease in the shRNA transfected stable knockdown cells. β-actin was used as the internal control to determine expression levels. The average fold changes, as shown above the blot, were calculated from three independent experiments and normalized to relative PRL-2 levels in the parental A549 cells. C) PRL-2 protein level was selectively down-regulated. The upper blot was obtained with an antibody from R & D Systems (MAB32191) that recognizes all three PRL proteins and the middle blot was produced with a PRL-2 specific polyclonal antibody from Bethyl (BL1205). β tubulin was used as an internal control.
To improve mRNA suppression, we also transiently transfected A549 cells with siRNA targeting the identical shRNA sequences. PRL-2 levels were selectively decreased 75% by two siRNA (Figure 1B). We elected to use the transient knockdown cells for some experiments because they gave superior PRL-2 silencing and amplified the effects on the signaling pathways. We also confirmed that PRL-2 was selectively down-regulated at the protein level by Western blotting using two different antibodies (Figure 1C). As shown in Figure 1C, PRL-2 protein was selectively down-regulated in cells transiently transfected with siRNA directed against PRL-2. The specificity of PRL antibodies were confirmed by Western blotting with recombinant GST-PRL protein (Supplementary Figure 1).
PRL-2 knockdown inhibited cell migration and invasion
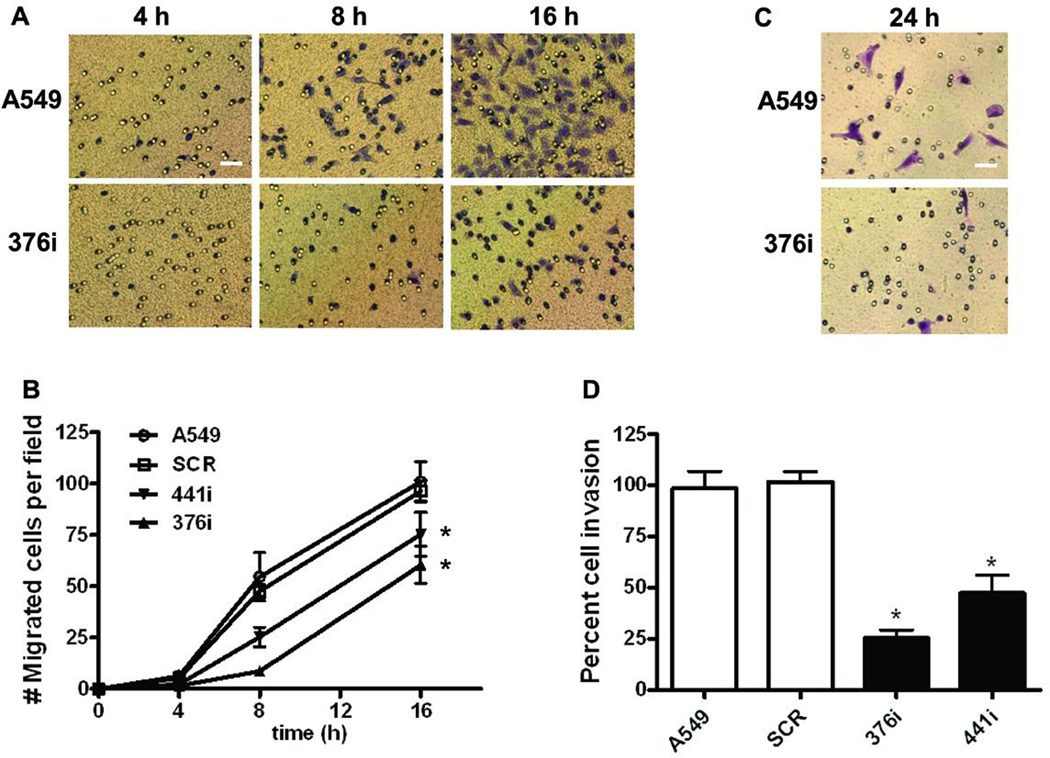
To investigate the role of endogenous PRL-2 in cancer cell metastatic processes, we interrogated cell migration and invasion assays using Transwell migration and Matrigel invasion chambers, respectively. PRL-2-silenced cells migrated slower than the control cells in the cell migration assay (Figure 2A and 2B). After 24 h when cell migration was saturated, control cells achieved the same number of migrated cells per field as did the PRL-2-silenced clones (data not shown). Cell invasion was measured at 24 h when the migration control wells were saturated. There was significant invasion with the PRL-2-silenced clones compared to the parental or scramble cells (p<0.001) for both (Figure 2C and 2D).
Figure 2. Cell migration and invasion were significantly inhibited after PRL-2 knockdown.
A) Representative images from the transwell migration assay showing Wright Giemsa stained control and PRL-2 knockdown cells that reached the lower side of the membrane 4, 8 and 16 h after plating; bar, 10 εm. B) Quantification of the cell migration time course of wildtype A549 cells or A549 cells stably expressing scrambled or PRL-2 shRNA. After the indicated times, cells that migrated to the lower chamber were fixed, stained, and counted with a microscope. The mean values of three independent experiments measured in triplicate are indicated; bars equal the SEM. All mean values at 16 h time point were compared to the parental A549 cells using a two-tailed Student’s t-test. *, p<0.01. C) Representative images of Wright Geimsa stained control and PRL-2 knockdown cells on the lower side of a membrane from the Matrigel-coated transwell invasion assay; bar, 10 εm. D) Quantification of cell invasion after PRL-2 knockdown. Invasive activity was determined as the percent invasion of the wildtype A549 cells through the Matrigel matrix and membrane relative to the migration through the control membrane. The mean values of three independent experiments measured in triplicate are indicated and the bars equal the SEM. All mean values were compared to the parental A549 cells using a two-tailed Student’s t-test. *, p<0.001.
PRL-2 knockdown decreased p130Cas and vinculin expression and ERK phosphorylation independent of Src, Akt and p53
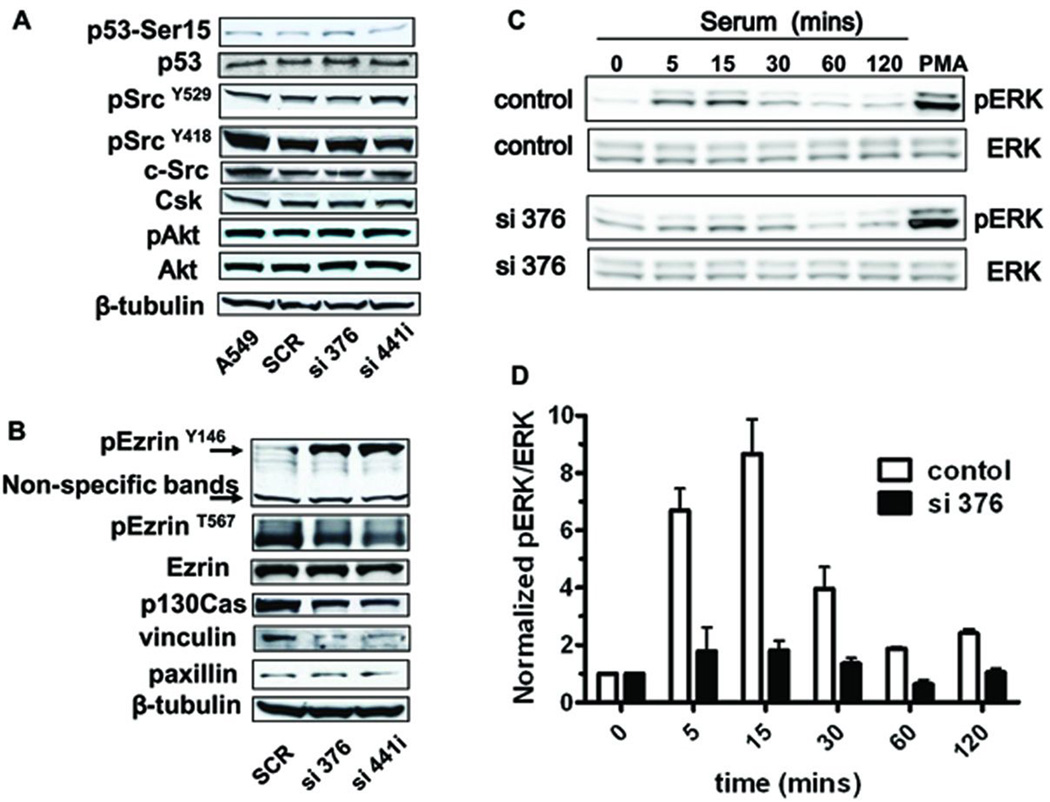
To study the potential mechanisms of PRL-2 mediated phenotypic changes on cell migration and invasion, we first examined the putative participants involved in PRL-1 or PRL-3 signaling pathways, such as p53 (Min et al., 2009), Akt (Wang et al., 2007a), and Src (Achiwa and Lazo 2007; Liang et al., 2007). As shown in the Figure 3A, total Src expression was not altered after PRL-2 knockdown. Moreover, there was no detectable change in c-Src stimulation as measured by phosphorylation on Tyr418, Tyr529, and the expression level of Csk, a negative Src regulator. In addition, total p53 or Akt expression levels, or their phosphorylation status were not altered. Silencing of PRL-2 was confirmed in these samples by RT-PCR (Supplemental Figure S2).
Figure 3. Effect of PRL-2 knockdown on ezrin, p130Cas, Src, Akt, vinculin, and ERK activity.
A549 cells were transiently transfected with scramble or PRL-2 siRNA. After 2 d, cells were harvested for Western blotting to permit an analysis of the changes in ezrin status, p130Cas, Src, vinculin, and ERK phosphorylation. A) Western blotting showed hyper-phosphorylation on phosphorylated ezrin Y146 and no significant change on phosphorylated ezrin T567 after PRL-2 knockdown. The p130Cas and vinculin levels were decreased after transient PRL-2 knockdown. B) PRL-2 silencing did not alter total p53, Src, Csk and Akt expression and p53, Src, and Akt phosphorylation status after transient PRL-2 knockdown. C) Total ERK or pERK were examined in lysates from cells with normal or silencing PRL-2. The cells were pre-treated with serum free medium for 6 h, and then serum was added for time course. Phorbol ester (PMA) was used as a positive control for ERK phosphorylation. D) Quantification of ERK phosphorylation status. N=3; bars = SEM.
Our previous study (Achiwa and Lazo 2007) suggested that in A549 cells the close family member PRL-1 could associate with focal adhesion complex (FAC) and regulate adhesion turnover, which are involved in regulating cell migration. Thus, we examined the effect of PRL-2 knockdown on FAC and the downstream pathways. Vinculin, which normally is recruited during FAC activation, was decreased while paxillin, another FAC recruit, remain unchanged after PRL-2 knockdown (Figure 3B). Furthermore, PRL-2 silencing significantly decreased the total expression level of p130Cas (Figure 3B), an adaptor protein for FAC. In addition, ezrin, a linker between cytoskeleton and plasma membrane, was phosphorylated on tyrosine 146 with no apparent effect on the threonine 567 site (Figure 3B). We observed no increase in ezrin tyrosine phosphorylation after silencing PRL-1 in A549 cells (Supplementary Figure 3).
To examine the possible role of PRL-2 in modifying ERK activity, which is downstream of FAC and participates in cell migration and invasion, we first measured ERK1/2 phosphorylation by Western blotting. The cells were pre-treated in serum free medium for 6 h, and then serum was added as a stimulus for ERK1/2 phosphorylation. The Western blots (Figure 3C) and the quantitative data (Figure 3D) clearly showed a dramatic decrease in serum time course of ERK1/2 phosphorylation.
Collectively, the above results indicated that suppression of PRL-2 protein levels decreased p130Cas and vinculin expression, and decreased ERK1/2 phosphorylation, and presumably inhibited migration and invasion through effects on the FAC and associated signaling pathways.
Ectopically expressed PRL-2 promoted cell migration and invasion, and induced ERK activation and translocation into the nucleus
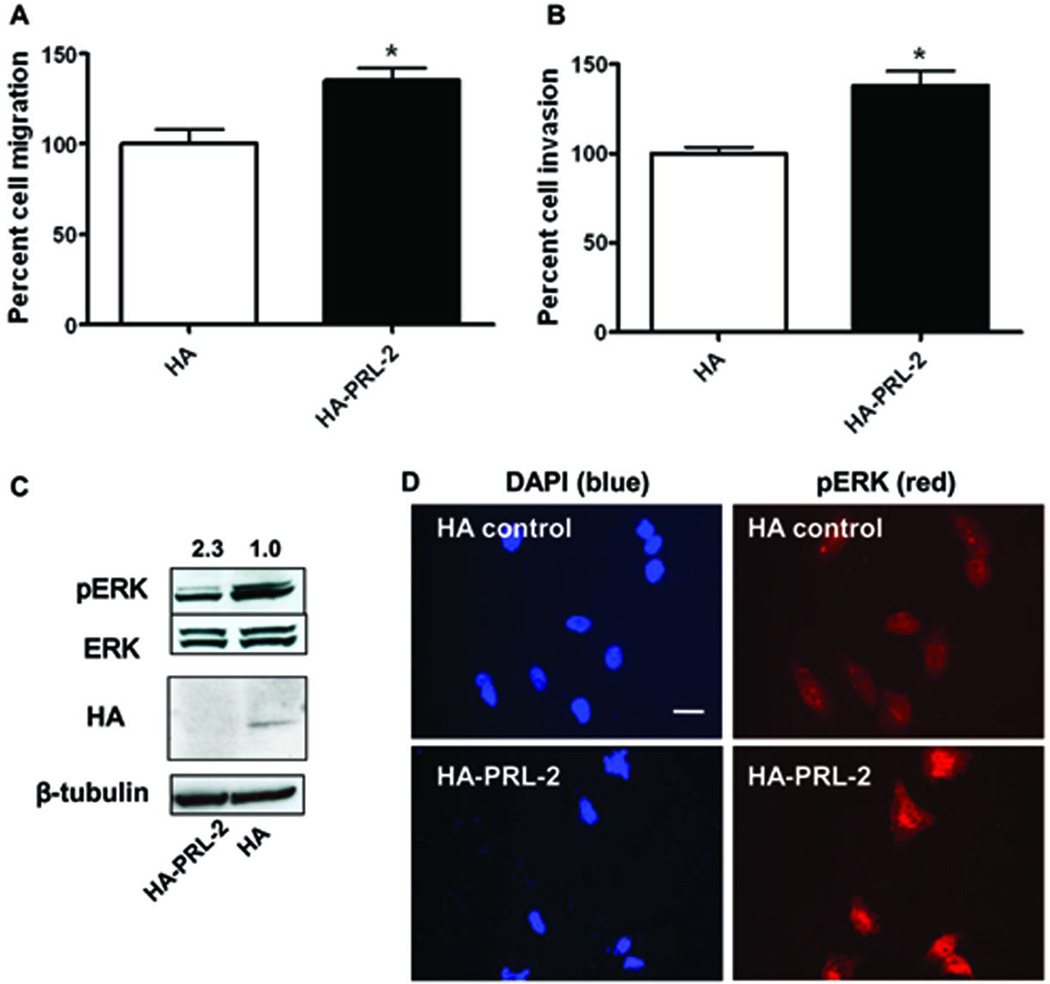
We next examined the role of ectopic PRL-2 on A549 cell migration and invasion using the Transwell migration and Matrigel invasion chambers, respectively. Cells transfected with the HA-tagged PRL-2 construct migrated 35% faster than control cells transfected with the HA containing plasmid (Figure 4A). We observed a 40% increase in cellular invasion in cells transfected with the HA-PRL-2 plasmid compared to the control cells (Figure 4B). The expression of HA-PRL-2 was confirmed by Western blotting with HA antibody (Figure 4C). The increase in total PRL-2 expression, however, was estimated to be only 20–30% based on Western blotting, which might be responsible for the modest but reproducible increase in cell migration and invasion.
Figure 4. Ectopically expressed PRL-2 promoted cell migration and invasion, and induced ERK activation and translocation into the nucleus.
A549 cells were transfected with empty HA vector control or HA-PRL-2 vector. After 24 h, cells were harvested for Western blotting, Transwell migration or invasion assay. A) After 16 h, cells that migrate to the lower chamber were fixed, stained, and counted with a microscope. Percent cell migration was determined by normalizing the number of migrated cells in the HA-PRL-2 wells to HA vector controls. The mean values of three independent experiments measured in triplicate are indicated; bars equal SEM. The mean values were compared using a two-tailed Student’s t-test. *, p<0.01. B) Cells were plated and after 24 h cells that invade to the lower chamber were fixed, stained, and counted with a microscope. Percent cell invasion indicated on the ordinate was determined as invaded cells in HA-PRL-2 wells normalized to HA vector control wells. The means of three independent experiments measured in triplicate are indicated; bars are SEM and significance determined as mentioned above. *, p<0.001. C) Western blotting showed increased ERK phosphorylation after over-expressing PRL-2. The mean values of three independent Western blots for relative pERK and ERK are shown. The over-expression of HA-PRL-2 was confirmed by Western blotting using an anti-HA antibody. D) Immunofluorescent staining of cell transfected with HA-PRL-2. A549 cells were transfected with HA control or HA-PRL-2. After 24 h, cells were fixed, permealized, and stained with DAPI (blue) and pERK (red). In the cells with over-expressed HA-PRL-2, ERK was activated; pERK signal was greater and intense in the nucleus; bar, 10 µm.
ERK1/2 phosphorylation has been reported to be involved in PRL-3 signaling in colon and lung cancer (Ming et al., 2009; Peng et al., 2009). ERK was also recently observed to be hyper-phosphorylated after over-expression of PRL-2 in breast cancer cells (Hardy et al., 2010). To determine the role of ERK in PRL-2 signaling in lung cancer cells, we first examined the total ERK and its phosphorylation status by Western blotting with ectopic HA-PRL-2 expression and observed a 2.3 fold increase in ERK1/2 phosphorylation with no change in total ERK1/2 protein levels (Figure 4C). ERK activation was further confirmed by microscopic observation in A549 cells transfected transiently with HA-PRL-2 (Figure 4D). Endogenous pERK (red) in control cells was weak and throughout the entire cells (Figure 4D upper panels), whereas pERK in PRL-2-expressing cells was more prominent as well as being more intense in nucleus (Figure 4D bottom panels).
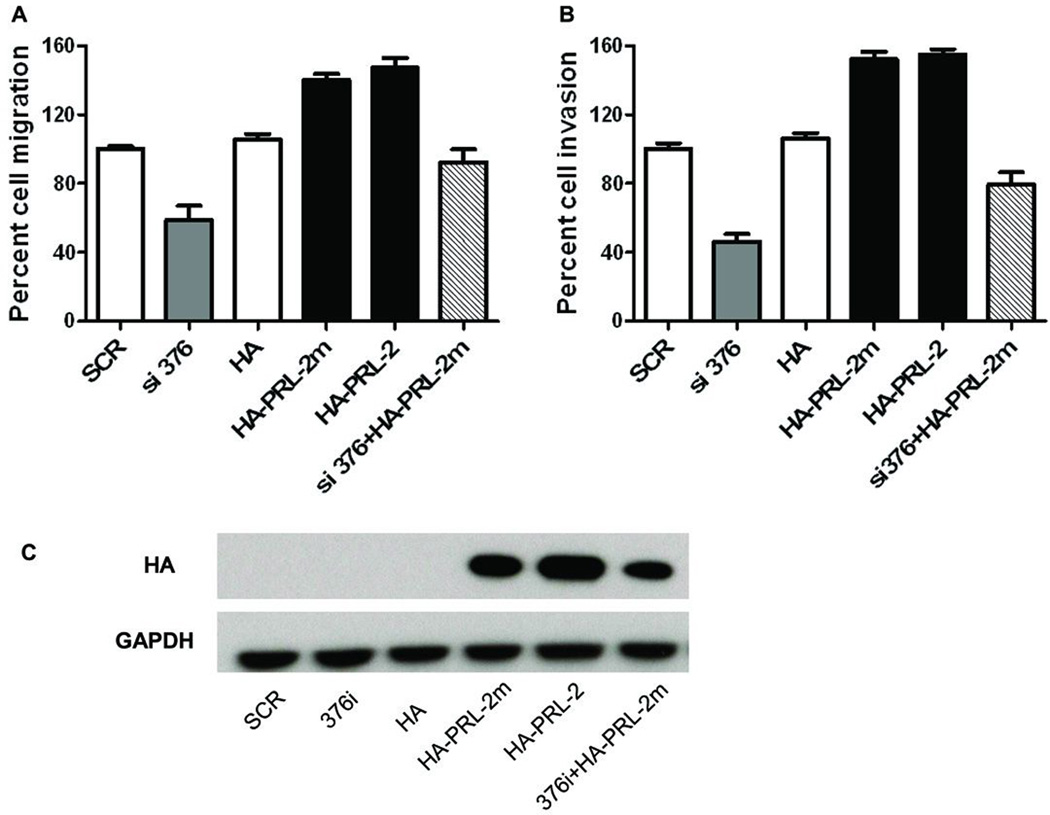
The siRNA resistant mutant HA-PRL-2m increased cell migration and invasion in PRL-2 silenced cells
To further investigate the effects of PRL-2 on A549 cell migration and invasion, we constructed a siRNA resistant mutant HA-PRL-2m by replacing two nucleotides in the si376 targeted region without changing the encoded amino acids. The A549 cells were transfected with empty HA vector control, siRNA alone, wildtype HA-PRL-2 vector or HA-PRL-2m mutant alone, or co-transfected with si376 and HA-PRL-2m. Interestingly, the siRNA resistant PRL-2 mutant restored cell migration (Figure 5A) and invasion (Figure 5B) in PRL-2 silenced cells. The expression levels of ectopic HA-PRL-2 or its mutant were confirmed by Western blotting using HA antibody (Figure 5C). These results strengthened the notion that PRL-2 has an fundamental role in regulating cell migration and invasion.
Figure 5. The siRNA resistant mutant HA-PRL-2m increased cell migration and invasion in PRL-2 silenced cells.
A549 cells were transfected with empty HA vector, siRNA alone, wildtype HA-PRL-2 vector or HA-PRL-2m mutant alone, or co-transfected with si376 and HA-PRL-2m. 24 h later, cells were harvested for Western blotting, Transwell migration or invasion assay. A) After 16 h, cells that migrate to the lower chamber were fixed, stained, and counted with a microscope. Percent cell migration was determined by normalizing the number of migrated cells in the each well to scramble siRNA control cells. The mean values of three independent experiments measured in triplicate are indicated; bars equal SEM. B) Cells were plated and after 24 h cells that invade to the lower chamber were fixed, stained, and counted with a microscope. Percent cell invasion indicated on the ordinate was determined as invaded cells in each wells normalized to scramble siRNA control wells. The means of three independent experiments measured in triplicate are indicated. C) The over-expression of HA-PRL-2 or its mutant was confirmed by Western blotting using an anti-HA antibody.
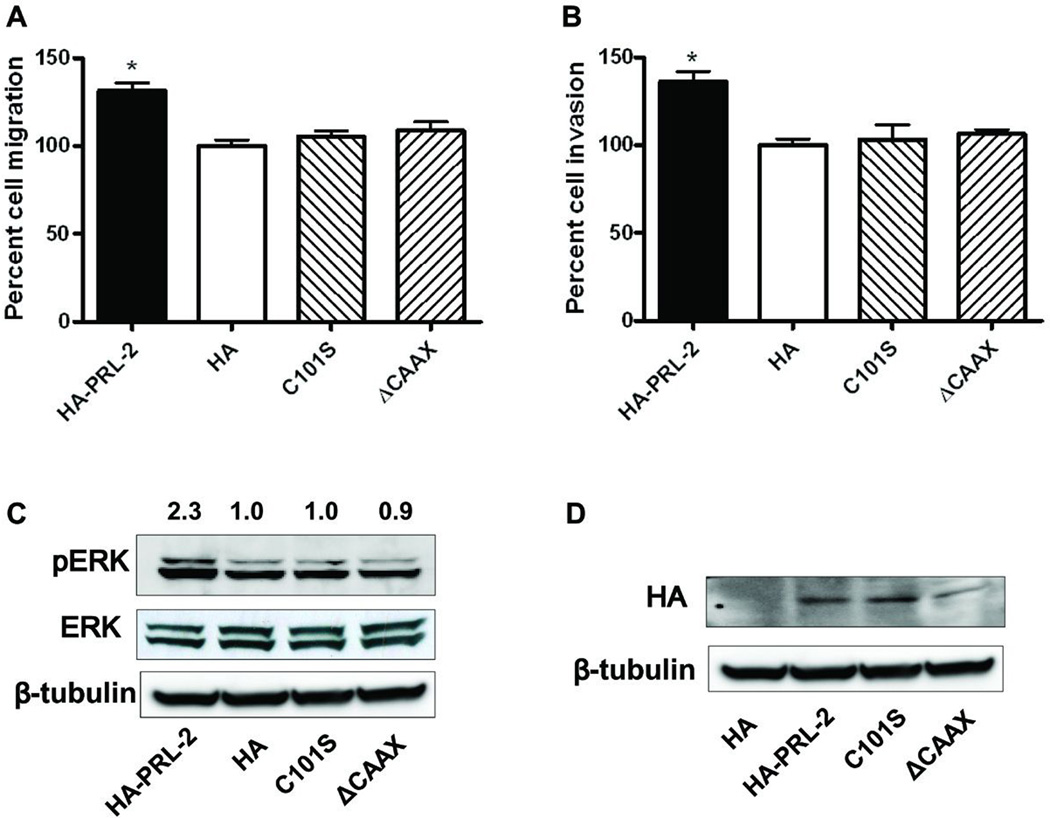
Both catalytic activity and C-terminal CAAX domain were critical for PRL-2 function
To study the biochemical importance of the PRL-2 phosphatase activity and membrane localization, we constructed a phosphatase inactive mutant HA-PRL-2 by replacing the catalytic cysteine with a serine (C101S), and a C-terminal CAAX domain deleted mutant (ΔCAAX). The cells were transfected with empty HA vector control, wildtype HA-PRL-2 vector, or the mutants C101S or ΔCAAX. Interestingly, only wildtype PRL-2 over-expression significantly promoted cell migration (Figure 5A) and invasion (Figure 5B), but not the catalytic inactive mutant or the CAAX deleted mutant. In addition, ERK hyper-phosphorylation was seen only with wild type PRL-2 and not with the mutants (Figure 6C). The expression levels of ectopic HA-PRL-2 or its mutants were equivalent, which was confirmed by Western blotting using HA antibody (Figure 6D). Thus, it is unlikely that the wild type HA-PRL-2 promoted cell migration and invasion were due to a protein dosage effect. These results suggested that both catalytic activity and C-terminal CAAX domain for prenylation are important for the biological function of PRL-2.
Figure 6. Both catalytic activity and C-terminal CAAX domain were critical for PRL-2 function.
A) A549 cells were transfected with HA control, HA-PRL-2, the catalytic dead mutant C101S or ΔCAAX deletion mutant. After 16 h, cells that migrate to the lower chamber were fixed, stained, and counted with a microscope. Percent cell migration was determined by normalizing the number of migrated cells in the HA-PRL-2 wells to HA vector controls. The mean values of three independent experiments measured in triplicate are show; bars equal SEM. *, p<0.01. B) Cells were plated on Matrigel matrix and after 24 h, cells that invade to the lower chamber were fixed, stained, and counted as above. N=3; bars = SEM. *, p<0.01. C) Increased pERK after expression of wild type HA-PRL-2 but not the mutants. A549 cells were transfected with HA control, HA-PRL-2, the catalytic dead mutant C101S or ΔCAAX deletion mutant. Cells were harvested 24 h later for Western blotting. Average fold changes from three independent experiments normalized to HA vector control cells were shown above the Western blots. D) Western blotting confirmed the over-expression of HA-PRL-2 and its mutants.
Discussion
Although attention has been directed toward understanding the potential roles of PRL-1 and PRL-3 in tumor development and metastasis, there has been a noticeable absence of information about PRL-2. Previous data concerning this enigmatic phosphatase indicate it is expressed in pancreatic, prostate, and breast cancer cells (Hardy et al., 2010; Stephens et al., 2008; Wang et al., 2002b; Yagi et al., 2003). The current study suggests for the first time that PRL-2 could function as an important participant in lung cancer cell migration and invasion. Reduction of endogenous PRL-2 by RNA interference dramatically inhibited cell migration and invasion in A549 lung cancer cells (Figures 2 and 5), while ectopic expression of PRL-2 significantly promoted tumor cell migration and invasion (Figures 4 and 5). Moreover, introduction of a siRNA-resistant PRL-2 in PRL-2 silenced cells restored cell migration and invasion (Figure 5). Placed in the context of previous work, our results suggest PRL-2 shares a similar functionality with the other two PRL family members. For example, both PRL-1 and PRL-3 have been found to be over-expressed in a variety of different cancer cell lines and tumor tissues, and the elevated PRLs are associated with enhanced tumor progression (Cates et al., 1996; Wu et al., 2004; Zeng et al., 2003). Ectopic PRL-1 or PRL-3 expression in different cancer cell types or mouse models are correlated with the induction of metastatic phenotypes, such as enhanced cell motility and invasiveness, or experimental metastasis in mice (Liang et al., 2007; Zeng et al., 2003)(Guo et al., 2004; Zeng et al., 2003). Likewise, ablation of endogenous PRL-1 or PRL-3 by interfering RNA has an opposite effect (Achiwa and Lazo 2007; Kato et al., 2004; Luo et al., 2009).
The biochemical signaling pathways exploited by PRLs are not firmly established, although several have been proposed including p53, PI3K/Akt, Src and Rho family GPTases. PRL-3 was reported to be a p53 target involved in cell-cycle regulation (Basak et al., 2008). It has been hypothesized that PRL-3 promotes HEK293 cell invasion by down-regulation of Csk leading to Src activation (Liang et al., 2007), while PRL-1 also appears to promote cell invasion through Src and Rho pathway (Achiwa and Lazo 2007; Luo et al., 2009; Fiordalisi et al., 2006). Nonetheless, when we suppressed PRL-2 in A549 cells, we observed decreased p130Cas and vinculin expression, and decreased ERK phosphorylation, but did not detect any difference on the total level or phosphorylation status of p53, Akt or Src (Figure 3). This suggests that PRL-2 may signal through a pathway different than that employed by PRL-1 or PRL-3. This notion is reinforced when one compares our current PRL-2 data with our previously published PRL-1 data in which using the same lung cancer cell line we found PRL-1 silencing regulates c-Src level and cell invasion (Achiwa and Lazo 2007). While both PRL-1 and PRL-2 depleted cells lost their ability to invade, PRL-2 did not alter Src expression or phosphorylation; and PRL-2 knockdown cells did not exhibit a preference for fibronectin nor did they appear to regulate adhesion turnover as PRL-1 silenced cells did (Achiwa and Lazo 2007). This leads us to speculate that while PRL-2 may be functionally similar to PRL-1 and PRL-3, it could signal through somewhat different pathways.
Focal adhesions are dynamic structures through which the cytoskeleton of a cell connects to the extracellular matrix via interactions with integrin receptors. Ligand binding by integrins results in recruitment of adaptor, kinases and other components to the FAC. Besides Src family kinases and focal adhesion kinase, the adaptor protein p130Cas is critical downstream component in this integrin signaling, and its involvement in cell motility as a component of the integrin signaling machinery is well established (Defilippi et al., 2006). Vinculin and paxillin are also important components that usually recruited to FAC (Zamir and Geiger 2001). In the context of PRL-3 studies, p130Cas phosphorylation was increased as was the interaction between p130Cas and vinculin in PRL-3 expressing HEK-293 cells (Liang et al., 2007). In the current study, we observed decreased p130Cas and vinculin expression while paxillin unchanged after PRL-2 knockdown in A549 cells (Figure 3B). This is consistent with our previous observation in PRL-1 silencing A549 cells (Achiwa and Lazo 2007), in which p130Cas was also decreased while paxillin unchanged (vinculin levels unknown). The fact that paxillin level remained unchanged is most likely because the association between p130Cas and vinculin is more important in A549 cells as the paxillin expression is hardly detected in this cell line. In addition, we observed a p130Cas cleavage after PRL-2 knockdown (Supplemental Figure S4). Etoposide was used as a control to study p130Cas cleavage, because it is a known p130Cas cleavage inducer and generates a 31 KD product leading to apoptosis (Kim et al., 2004). We do not know the biological function of the 80 KD cleavage product that was generated after PRL-2 knockdown.
The ERK phosphorylation cascade is distal to integrin signaling, which functions in cellular proliferation, migration, differentiation, and survival. Its inappropriate activation is a common occurrence in human cancers (Reddy et al., 2003). It was reported (Peng et al., 2009) that PRL-3 associates with integrin β1 and its expression has been positively correlated with ERK1/2 phosphorylation in colon cancer tissues. Depletion of integrin by siRNA abrogates the activation of ERK1/2 and also abolishes PRL-3-induced LoVo cell invasion (Peng et al., 2009). Recently, Hardy and coworkers found that enhanced tumor growth correlates with increased ERK1/2 phosphorylation when PRL-2 is over-expressing in a mouse tumor model (Hardy et al., 2010). Consistent with this, we found that PRL-2 silenced cells had significantly less ERK1/2 phosphorylation than control cells in a serum stimulated time course (Figure 3C and 3D). In contrast, PRL-2 over-expression promoted tumor cell invasion, which correlated with increased ERK1/2 phosphorylation (Figure 4C). Importantly, we found the activated ERK1/2 translocated into the nucleus (Figure 4D), where it regulated the transcription of various target genes, presumably controlling cell migration and invasion. We also observed PRL-2 expression caused some minor changes in nuclear morphology (Figure 4D), which merits further investigation. It is noteworthy that PRL-2 depletion did not significantly alter basal levels of pERK (Figure 4C). This could be a gene dosage effect or a reflection of the differences in the regulation of pERK in stimulated and unstimulated cells. Clearly, identifying the PRL-2 regulated genes will be important for uncovering the roles of PRL-2 in tumor progression.
The phosphatase activity of PRL-3 appears to be important for functionality. For example, a catalytically inactive PRL-3 mutant has significantly reduced migration-promoting activity (Zeng et al., 2003). EGF-PRL-3 expressing CHO cells rapidly induced metastatic tumor formation in lung while the catalytic inactive mutant expressing ones lose this metastatic activity (Guo et al., 2004). As PRL-2 has conserved catalytic domain with PRL-3, we hypothesized that the catalytic activity is also required for PRL-2 function. PRL-2 functionality required both its phosphatase activity and membrane binding properties (Figure 6). Although PRLs have a clear phosphatase domain, their substrates are not well defined. Only ezrin, a linker between plasma membrane and cytoskeleton, has been reported to be a direct target of protein PRL-3 (Forte et al., 2008). In vitro dephosphorylation assays suggest that ezrin-Thr567 is a substrate of PRL-3, which challenges the current believe that PRLs belongs to PTP family. Interestingly, ezrin was hyper-phosphorylated on Tyr 146 in our PRL-2 silencing cells while no change on Thr 567 (Figure 3B). Unfortunately, we have insufficient evidence to document it as a direct substrate for PRL-2. Suppressing PRL-1 by siRNA in the same cell type, however, did not alter the phosphorylation state of Tyr 146, suggesting potential differential functionality of PRL-1 and PRL-2 in A549 cells.
Collectively, our results provide support for the involvement of PRL-2 in promoting tumor cell invasion via ERK signaling pathway. To date, most studies have focused on the role of PRL-1 and PRL-3 in tumor progression. Here we reported for the first time that PRL-2 regulates cell migration and invasion in non-small cell lung cancer. Notably we showed that the PRL-2 stimulated cell invasion was associated with ERK1/2 phosphorylation, and activated ERK in the nucleus might participate in PRL-2 mediated tumor cell invasion.
Materials and Methods
Cell line, antibodies, and reagents
Cell lines were obtained from the American Type Culture Collection (Manassas, VA) and maintained in a humidified atmosphere of 5% CO2 at 37°C. A549 cells were authenticated by RADIL (Columbia, MO) and maintained in BME (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Gemini). Antibodies and reagents were obtained from the following sources: rabbit anti-PRL-2 polyclonal antibody (Bethyl, Montgomery, TX); Pan-PRL antibody (R&D Systems, Minneapolis, MN); recombinant GST-tagged PRLs (BIOMOL International, Plymouth Meeting, PA); anti-p130Cas, anti-paxillin, and anti-Csk antibodies (BD Transduction Laboratories, San Diego, CA); anti-ezrin antibody (Sigma-Aldrich, St. Louis, MO) anti-c-Src, and anti-phospho Tyr146 ezrin (Santa Cruz, CA); anti-GAPDH, anti-ERK1/2 (p44/42 MAP kinase) and phospho-Erk (Thr202/Tyr204), Thr567 ezrin, Akt, phospho-Akt, and Tyr418 Src and Tyr529 Src (Biosource International, Camarillo, CA); and anti-GST (Upstate Biotechnology, Lake Placid, NY).
shRNAs and siRNAs
PRL-1 depletion was conducted as previously described (Achiwa and Lazo, 2007). To deplete endogenous PRL-2, we selected two different 21-nucleotide sequences according to the manufacturer's instructions (Ambion, Austin, TX): TGCAGTTCAGTTTATAAGACA (PRL-2 silencing site 376), AAATACCGACCTAAGATGCGA (PRL-2 silencing site 441). The numbers 376 and 441 indicate the starting nucleotide number of shRNA-targeting sequences on the coding PRL-2 mRNA based on the published sequence data from Genbank (accession no. NM_080391). The specificity of each sequence was verified by a BLAST search of the public databases. pSilencer 4.1-CMV puro expression vectors (Ambion) that produce shRNAs targeted against PRL-2 were also prepared according to the manufacturer's instructions. In brief, two sets of oligonucleotides were chemically synthesized: PRL2-376 sense, 5'-GATCC CAGTTCAGTTTATAAGACACTCAAGAGATGTCTTATAAACTGAACTGCAA-3'; PRL2-376 antisense, 5'-AGCTTTGCAGTTCAGTTTATAAGACATCTCTTGAG TGTCTTATAAACTGAACTGG-3'; PRL2-441 sense, 5'-GATCC ATACCGACCTAAGATGCGACTCAAGAGA TCGCATCTTAGGTCGGTATTTA-3'; PRL2-441 antisense, 5'-AGCTTAAATACCGACCTAAGATGCGATCTCTTGAG TCGCATCTTAGGTCGGTATG-3' (the underlined sequences contribute to forming shRNAs). The annealed oligonucleotides encoding shRNAs were then subcloned into the BamHI-HindIII site of the pSilencer 4.1-CMV puro vector. For transfection, 1 × 105 cells were plated in six-well plates 24 h before transfection in normal growth medium with four µg of plasmid DNA and 10 µL Lipofectamine 2000 (Invitrogen, Carlsbad, CA) were used. Twenty-four h after transfection, the medium was replaced with Basal Medium Eagle with 2 µg/mL puromycin and 10% fetal bovine serum. After 2 weeks, stable round colonies were harvested and cloned by limiting dilution method, named 376i and 441i. The two siRNAs, which were designed to target the identical PRL-2 sites as the two shRNAs, were synthesized by Invitrogen, and named si376 and si441. For siRNA transfection, 100 pmol of siRNA and 5 µL Lipofectamine 2000 were used according to the manufacture’s instructions (Invitrogen).
PRL-2 constructs and mutations
PRL-2 cDNA was subcloned into pCMV-HA vector with an HA-tag on the N-terminus. We constructed a phosphatase inactive mutant HA-PRL-2 by replacing the catalytic cysteine with a serine (C101S), and a C-terminal CAAX domain deleted mutant (ΔCAAX). We constructed a siRNA resistant mutant HA-PRL-2m (also named AA390CC) by replacing the two nucleotides AA at site 390 to CC without changing the coded amino acids. The mutation primers were design using Primer X, and PRL-2 mutants were generated using QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacture’s instructions. All mutations were confirmed by DNA sequencing.
RNA isolation and reverse transcription-PCR
Total RNA was extracted with the RNeasy Mini kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Reverse transcription-PCR (RT-PCR) for PRL-1, PRL-2, PRL-3, and actin as an internal control was carried out in a volume of 50 µL by SuperScript III One-step RT-PCR System (Invitrogen) as per manufacturer's instruction. Following primer pairs were used for each reaction: PRL-1, 5'-ACCTGGTTGTTGTATTGCTGTT-3' (forward) and 5'-GTTGTTTCTATGACCGTTGGAA-3' (reverse); PRL-2, 5'-AGCCAGGTTGCTGTGTTGCAG-3' (forward) and 5'-CACAGCAATGCCCATTGGTA-3' (reverse); PRL-3, 5'-AAGGTAGTGGAAGACTGGCT-3' (forward) and 5'-GGTGAGCTGCTTGCTGTTGAT-3' (reverse); actin, 5'-AAGAGAGGCATCCTCACCCT-3' (forward) and 5'-TACATGGCTGGGGTGTTGAA-3' (reverse).
Protein extraction and Western blotting
Cells were lysed in modified radioimmunoprecipitation assay buffer containing 0.1% SDS, 1% Triton X-100, protease inhibitor cocktail (Roche, South San Francisco, CA) and phosphatase inhibitors (2 mmol/L Na3VO4, 12 mmol/L β-glycerol phosphate, and 10 mmol/L NaF). The lysates were incubated on ice for 30 min with frequent vortexing, and were cleared by centrifugation at 13,000 × g for 15 min. Protein content was determined by the Bradford assay. Total cell lysates were resolved by SDS-PAGE using Tris-glycine gels, and incubated with primary antibodies at 4°C overnight and the secondary antibody at room temperature for 1 h. Proteins were visualized using Pierce enhanced chemiluminescence Western blotting substrate (Pierce Biotechnology). For quantification of protein expression levels, luminescence band intensities were measured on Multi Gauge V3.1 (Fujifilm).
Cell migration and invasion assays
The cell motility assay was performed using Transwell inserts (6.5-mm diameter, 8-µm pore size polycarbonate membrane) obtained from Corning (Cambridge, MA). Cells (1 × 105) in 0.5 mL serum-free medium were placed in the upper chamber, and the lower chamber was loaded with 0.8 mL medium containing 10% fetal bovine serum. Cells that migrated to the lower surface of filters were stained with Wright Giemsa solution (Sigma-Aldrich), and five fields of each well were counted after 4 to 24 h of incubation at 37°C with 5% CO2. Three wells were examined for each condition and cell type, and the experiments were repeated three times. The cell invasion assay was conducted using BD Biocoat Matrigel 24-well invasion chambers with filters coated with extracellular matrix on the upper surface (BD Biosciences, Bedford, MA). Control inserts were used for migration control. The experiments were done according to the manufacturer's protocol. We added 1 × 105 cells in 0.5 mL of serum-free culture medium to the upper chamber, and after incubation at 37°C for 24 h, we stained cells and determined total cell invasion and migration as described above. Invasiveness was expressed as the percent invasion for each cell type through the Matrigel matrix and membrane relative to the migration through the control membrane.
Immunofluorescence
Cells were seeded on glass slides and fixed at the indicated time points with 4% paraformaldehyde at room temperature for 15 min. After three washes with PBS, cells were permeabilized for 15 min with 0.1% Triton X-100. After blocking with 2% bovine serum albumin for 45 min, cells were incubated with the anti-HA antibody or anti-pERK antibody for 2 h followed by incubation with Alexa Fluor 588-conjugated anti-mouse IgG for HA or Alexa Fluor 594-conjugated anti-rabbit IgG for pERK and DAPI for nucleus. The fluorescence images of cells were captured and analyzed using an Olympus XI.
Statistical analysis
Results were expressed as means ± SE of at least three independent experiments. ANOVA and Student’s t tests were performed using Graphpad Prism 5 software. Differences were considered statistically different if p < 0.05. Western blots and autoradiograms were representative of at least three independent experiments.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health National Cancer Institute [CA78039] and the Fiske Drug Discovery Fund.
Abbreviations
- ERK
extracellular signal-regulated kinases
- FAC
focal adhesion complex
- PRL
phosphatase of regenerating liver
- PTP
protein tyrosine phosphatase
- siRNA
short interference RNAs
- shRNA
short hairpin RNAs.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Achiwa H, Lazo JS. PRL-1 tyrosine phosphatase regulates c-Src levels, adherence, and invasion in human lung cancer cells. Cancer Res. 2007;67:643–650. doi: 10.1158/0008-5472.CAN-06-2436. [DOI] [PubMed] [Google Scholar]
- 2.Basak S, Jacobs SB, Krieg AJ, Pathak N, Zeng Q, Kaldis P, et al. The metastasis-associated gene Prl-3 is a p53 target involved in cell-cycle regulation. Mol Cell. 2008;30:303–314. doi: 10.1016/j.molcel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cates CA, Michael RL, Stayrook KR, Harvey KA, Burke YD, Randall SK, et al. Prenylation of oncogenic human PTP(CAAX) protein tyrosine phosphatases. Cancer Lett. 1996;110:49–55. doi: 10.1016/s0304-3835(96)04459-x. [DOI] [PubMed] [Google Scholar]
- 4.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Diamond RH, Cressman DE, Laz TM, Abrams CS, Taub R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994;14:3752–3762. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiordalisi JJ, Keller PJ, Cox AD. PRL tyrosine phosphatases regulate rho family GTPases to promote invasion and motility. Cancer Res. 2006;66:3153–3161. doi: 10.1158/0008-5472.CAN-05-3116. [DOI] [PubMed] [Google Scholar]
- 7.Forte E, Orsatti L, Talamo F, Barbato G, De Francesco R, Tomei L. Ezrin is a specific and direct target of protein tyrosine phosphatase PRL-3. Biochim Biophys Acta. 2008;1783:334–344. doi: 10.1016/j.bbamcr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Guo K, Li J, Tang JP, Koh V, Gan BQ, Zeng Q. Catalytic domain of PRL-3 plays an essential role in tumor metastasis: formation of PRL-3 tumors inside the blood vessels. Cancer Biol Ther. 2004;3:945–951. doi: 10.4161/cbt.3.10.1111. [DOI] [PubMed] [Google Scholar]
- 9.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- 10.Hardy S, Wong NN, Muller WJ, Park M, Tremblay ML. Overexpression of the Protein Tyrosine Phosphatase PRL-2 Correlates with Breast Tumor Formation and Progression. Cancer Res. 2010;70:8959–8967. doi: 10.1158/0008-5472.CAN-10-2041. [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Semba S, Miskad UA, Seo Y, Kasuga M, Yokozaki H. High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: a predictive molecular marker of metachronous liver and lung metastases. Clin Cancer Res. 2004;10:7318–7328. doi: 10.1158/1078-0432.CCR-04-0485. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Kook S, Kim DJ, Teodorof C, Song WK. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem. 2004;279:8333–8342. doi: 10.1074/jbc.M312026200. [DOI] [PubMed] [Google Scholar]
- 13.Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai SR, et al. Association of tyrosine PRL-3 phosphatase protein expression with peritoneal metastasis of gastric carcinoma and prognosis. Surg Today. 2007;37:646–651. doi: 10.1007/s00595-006-3437-9. [DOI] [PubMed] [Google Scholar]
- 14.Liang F, Liang J, Wang WQ, Sun JP, Udho E, Zhang ZY. PRL3 promotes cell invasion and proliferation by down-regulation of Csk leading to Src activation. J Biol Chem. 2007;282:5413–5419. doi: 10.1074/jbc.M608940200. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Liang F, Zhang ZY. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry. 2009;48:1838–1846. doi: 10.1021/bi8020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min SH, Kim DM, Heo YS, Kim YI, Kim HM, Kim J, et al. New p53 target, phosphatase of regenerating liver 1 (PRL-1) downregulates p53. Oncogene. 2009;28:545–554. doi: 10.1038/onc.2008.409. [DOI] [PubMed] [Google Scholar]
- 17.Ming J, Liu N, Gu Y, Qiu X, Wang EH. PRL-3 facilitates angiogenesis and metastasis by increasing ERK phosphorylation and up-regulating the levels and activities of Rho-A/C in lung cancer. Pathology. 2009;41:118–126. doi: 10.1080/00313020802579268. [DOI] [PubMed] [Google Scholar]
- 18.Miskad UA, Semba S, Kato H, Yokozaki H. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology. 2004;71:176–184. doi: 10.1159/000078671. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, Ning J, Meng L, Shou C. The association of the expression level of protein tyrosine phosphatase PRL-3 protein with liver metastasis and prognosis of patients with colorectal cancer. J Cancer Res Clin Oncol. 2004;130:521–526. doi: 10.1007/s00432-004-0563-x. [DOI] [PubMed] [Google Scholar]
- 20.Peng L, Xing X, Li W, Qu L, Meng L, Lian S, et al. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 2009;8:110. doi: 10.1186/1476-4598-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radke I, Gotte M, Kersting C, Mattsson B, Kiesel L, Wulfing P. Expression and prognostic impact of the protein tyrosine phosphatases PRL-1, PRL-2, and PRL-3 in breast cancer. Br J Cancer. 2006;95:347–354. doi: 10.1038/sj.bjc.6603261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- 23.Stephens B, Han H, Hostetter G, Demeure MJ, Von Hoff DD. Small interfering RNA-mediated knockdown of PRL phosphatases results in altered Akt phosphorylation and reduced clonogenicity of pancreatic cancer cells. Mol Cancer Ther. 2008;7:202–210. doi: 10.1158/1535-7163.MCT-07-0542. [DOI] [PubMed] [Google Scholar]
- 24.Stephens BJ, Han H, Gokhale V, Von Hoff DD. PRL phosphatases as potential molecular targets in cancer. Mol Cancer Ther. 2005;4:1653–1661. doi: 10.1158/1535-7163.MCT-05-0248. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007a;67:2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Kirby CE, Herbst R. The tyrosine phosphatase PRL-1 localizes to the endoplasmic reticulum and the mitotic spindle and is required for normal mitosis. J Biol Chem. 2002a;277:46659–46668. doi: 10.1074/jbc.M206407200. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Peng L, Dong B, Kong L, Meng L, Yan L, et al. Overexpression of phosphatase of regenerating liver-3 in breast cancer: association with a poor clinical outcome. Ann Oncol. 2006;17:1517–1522. doi: 10.1093/annonc/mdl159. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Holmes DI, Powell SM, Lu QL, Waxman J. Analysis of stromal-epithelial interactions in prostate cancer identifies PTPCAAX2 as a potential oncogene. Cancer Lett. 2002b;175:63–69. doi: 10.1016/s0304-3835(01)00703-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Li ZF, He J, Li YL, Zhu GB, Zhang LH. Expression of the human phosphatases of regenerating liver (PRLs) in colonic adenocarcinoma and its correlation with lymph node metastasis. Int J Colorectal Dis. 2007b;22:1179–1184. doi: 10.1007/s00384-007-0303-1. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Zeng H, Zhang X, Zhao Y, Sha H, Ge X, et al. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am J Pathol. 2004;164:2039–2054. doi: 10.1016/S0002-9440(10)63763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi T, Morimoto A, Eguchi M, Hibi S, Sako M, Ishii E, et al. Identification of a gene expression signature associated with pediatric AML prognosis. Blood. 2003;102:1849–1856. doi: 10.1182/blood-2003-02-0578. [DOI] [PubMed] [Google Scholar]
- 32.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Q, Si X, Horstmann H, Xu Y, Hong W, Pallen CJ. Prenylation-dependent association of protein-tyrosine phosphatases PRL-1, -2, and -3 with the plasma membrane and the early endosome. J Biol Chem. 2000;275:21444–21452. doi: 10.1074/jbc.M000453200. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Q, Dong JM, Guo K, Li J, Tan HX, Koh V, et al. PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Res. 2003;63:2716–2722. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.