Abstract
Vitamin D deficiency is frequently found in patients with renal transplants (RTxs). Because vitamin D plays indispensable roles in the immune system, there may be an association between vitamin D deficiency and infection in these patients, but this has not been fully elucidated. Therefore, this study investigated the impact of pre-RTx vitamin D deficiency on urinary tract infection (UTI) development after RTx.
We measured 25-hydroxyvitamin D3 (25(OH)D3) levels in 410 patients 2 weeks before they underwent RTx. Vitamin D deficiency was defined as 25(OH)D3 <10 ng/mL. The primary outcome was UTI occurrence after RTx. Cox proportional hazard analysis determined whether vitamin D deficiency was independently associated with UTI.
The mean 25(OH)D3 level was 12.8 ± 6.9 ng/mL, and 171 patients (41.7%) were vitamin D deficient. During a median follow-up duration of 7.3 years, the UTI incidence was significantly higher in vitamin D-deficient patients (52 patients, 30.4%) compared with vitamin D-nondeficient patients (40 patients, 16.7%) (P = 0.001). Moreover, multivariate Cox proportional hazard analysis showed that vitamin D deficiency was an independent predictor of UTI after RTx (hazard ratio 1.81, 95% confidence interval 1.11–2.97, P = 0.02).
Vitamin D deficiency was an independent risk factor for UTI after RTx; hence, determining 25(OH)D3 levels might help to predict infectious complications after RTx.
INTRODUCTION
Urinary tract infection (UTI) is the most common infectious complication in renal transplant (RTx) patients. The UTI incidence in these patients is reportedly between 10% and 98%, and it is a significant source of posttransplant morbidity.1,2 Previous studies have demonstrated that posttransplant UTI is associated with posttransplant graft failure and mortality.3,4 Consequently, several studies have determined independent risk factors for posttransplant UTI, and investigators have found that being a woman, older age, diabetes mellitus, prolonged pretransplantation hemodialysis, cadaveric donors, acute rejection episodes, excessive immunosuppression, and urologic problems are significantly associated with UTI after RTx.4,5
Vitamin D may have pleiotropic effects on various organ systems that are related to the distribution of the vitamin D receptor (VDR) throughout the body.6 In addition to its effects on bone and mineral metabolism, vitamin D plays important roles in multiorgan systems, including the immune system. The VDR is expressed on monocytes and macrophages.7 Moreover, vitamin D promotes macrophage maturation and the secretion of lysosomal enzyme and hydrogen peroxide, which participate in macrophages’ antimicrobial activities.8 Vitamin D stimulates antibacterial peptide expression in monocytes and macrophages, including cathelicidin and β-defensin, which are directly involved in killing intracellular bacteria.9 In addition, vitamin D induces autophagy, an important process in the antibacterial response of macrophages to Mycobacterium tuberculosis.10 Hence, vitamin D deficiency might increase an individual's susceptibility to microbial infection. Indeed, previous studies have shown that vitamin D deficiency is associated with a higher risk of infection, especially respiratory tract infection.11,12
As glomerular filtration rates decline, vitamin D concentrations decrease proportionally; therefore, most patients with end-stage renal disease (ESRD) are deficient in or have insufficient vitamin D.13 Moreover, vitamin D concentrations are significantly lower in patients with malnutrition,14 which is prevalent in ESRD patients and is linked to a high risk of infection. Therefore, infection in ESRD patients might be attributable to vitamin D deficiencies, but this has not been extensively explored in these patients.
In this study, we investigated the impact of serum 25-hydroxyvitamin D3 (25(OH)D3) concentrations on posttransplant UTI development in RTx patients.
METHODS
Ethics
The institutional review board (IRB) of Yonsei University Health System Clinical Trial Center approved this study (IRB No. 4-2014-0366). All patients who participated in this study were aware of this investigation. However, because this was a retrospective medical record-based study, and the study subjects were anonymized, the IRB waived the need for written consent from the patients.
Patient Selection
We reviewed the medical records of 424 ESRD patients who received RTx at Yonsei University Health System between April 2002 and December 2008 and had their 25(OH)D3 levels measured 2 weeks before they underwent RTx. Among these patients, 14 were excluded for being aged <18 years (n = 1) or >70 years (n = 2), or having a follow-up duration <6 months (n = 11). Thus, the final analysis involved 410 RTx patients (Figure 1).
FIGURE 1.
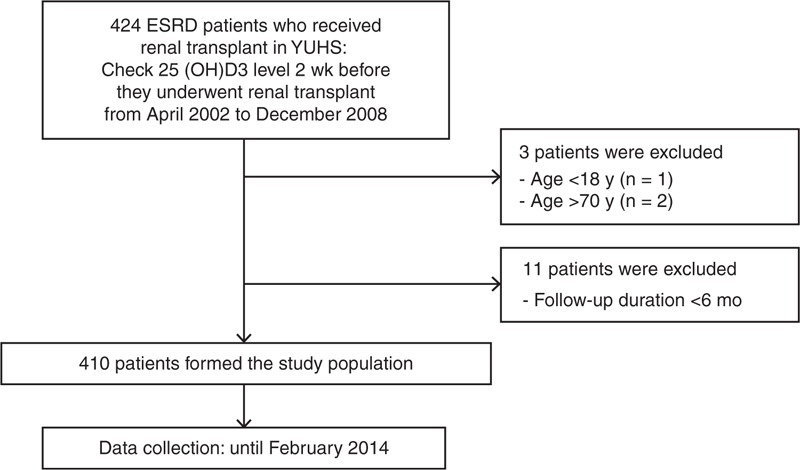
Flow diagram of the study. 25(OH)D3 = 25-hydroxyvitamin D3, ESRD = end-stage renal disease, YUHS = Yonsei University Health System.
Laboratory and Clinical Data
The serum 25(OH)D3 concentrations were determined using a radioimmunoassay (DiaSorin Inc, Stillwater, MN). We defined vitamin D deficiency as serum 25(OH)D3 levels <10 ng/mL (25 nmol/L), vitamin D insufficiency as serum 25(OH)D3 levels of 10 to 29 ng/mL (25–72 nmol/L), and vitamin D sufficiency as serum 25(OH)D3 levels ≥30 ng/mL.14–16 Because only 8 patients had sufficient vitamin D levels, the patients were divided into group 1 that comprised patients with serum 25(OH)D3 levels <10 ng/mL and group 2 that comprised patients with serum 25(OH)D3 levels ≥10 ng/mL.
The demographic and clinical data at the time of RTx, including age, sex, body mass index (BMI), comorbidities, ESRD etiology, dialysis modality before RTx, donor type, the season at the time of RTx, and blood pressure, were recorded. The following biochemical laboratory test result data were also collected: hemoglobin, serum calcium, phosphate, intact parathyroid hormone, alkaline phosphatase, albumin, high-sensitivity C-reactive protein levels, and white blood cell counts. Furthermore, information about the immunosuppressant drugs received and acute rejection episodes after RTx was retrieved.
Follow-Up and Endpoints
All study participants were regularly assessed at the transplantation clinic, and all UTI events, hospitalizations, and mortalities were recorded in the events database. Data collection continued until February 28, 2014. The primary endpoint was UTI after RTx. A UTI diagnosis was defined as significant bacteriuria (≥100,000 colony forming units/mL) on urine culture with typical symptoms or signs, or fever (>38°C) and typical symptoms or signs without significant bacteriuria. The typical UTI symptoms or signs included dysuria, frequency, urgency, cloudy urine, suprapubic pain, pain over the graft, and costovertebral angle tenderness. The secondary endpoint was graft failure and all-cause mortality after RTx.
Statistical Analyses
All statistical analyses were performed using IBM SPSS software version 20 (IBM Corporation, Armonk, NY). Continuous variables are expressed as the means ± standard deviation (SD) or as the medians and interquartile ranges for skewed data. The Kolmogorov–Smirnov test was used to analyze the normality of the distribution of the measured parameters, and categorical variables are expressed as numbers and percentages. The patients were divided into 2 groups based on their serum 25(OH)D3 concentrations (<10 ng/mL and ≥10 ng/mL), and the differences between the groups were analyzed using Student t test or the Mann–Whitney U test for continuous variables and the χ2 test for categorical variables. Cumulative survival curves were generated using the Kaplan–Meier method to determine the impact of vitamin D deficiency on UTI occurrence, and between-group survival was compared using a log-rank test. In addition, the independent prognostic value of vitamin D deficiency in relation to posttransplant UTI was determined using multivariate Cox proportional hazards regression analysis, which only included the variables that were significant in the univariate analysis. P values <0.05 were considered statistically significant.
RESULTS
Patients’ Baseline Characteristics
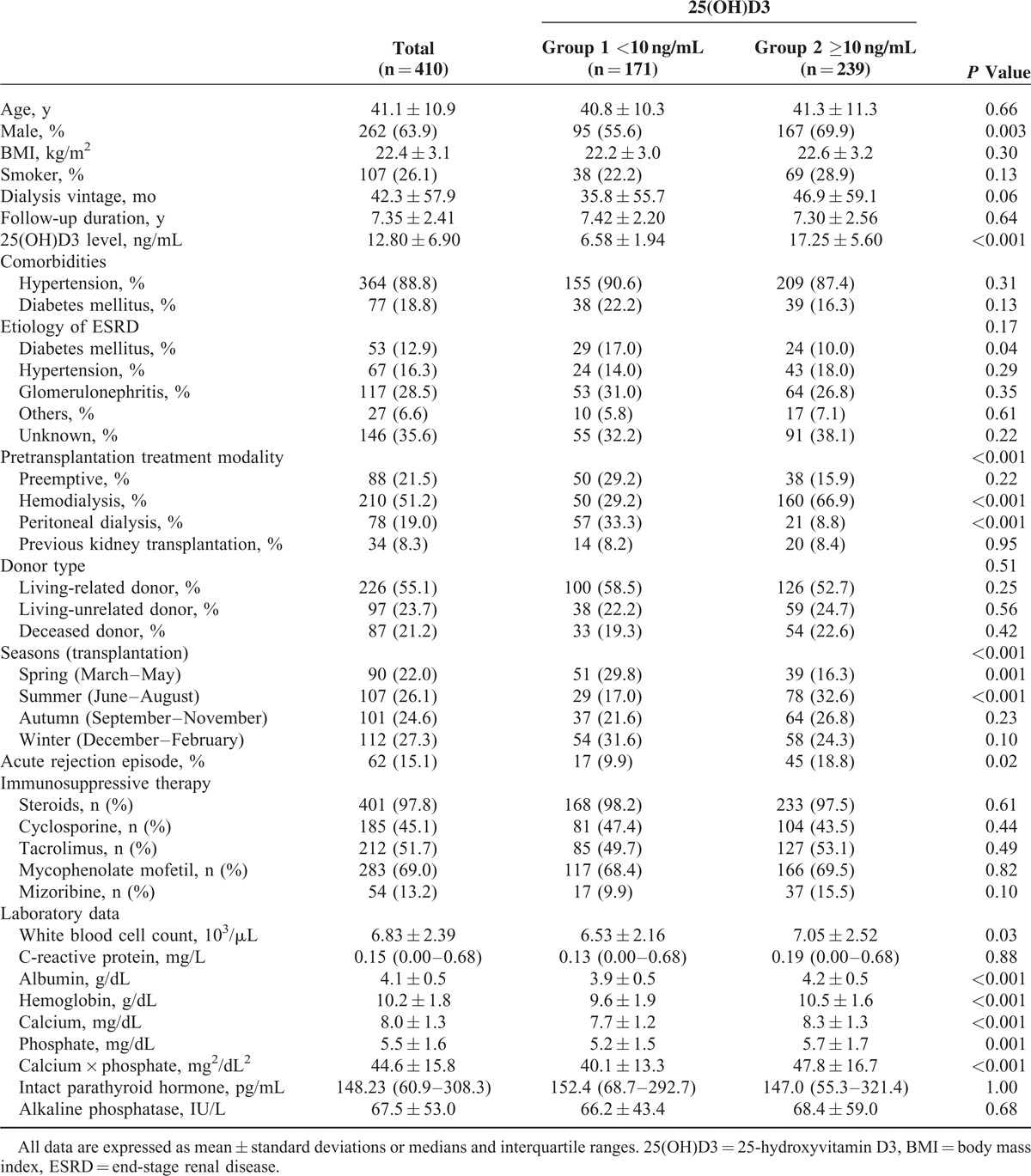
Of the 410 RTx patients evaluated, 171 patients (41.7%) were vitamin D deficient, 231 (56.3%) were vitamin D insufficient, and 8 (2.0%) had normal serum 25(OH)D3 concentrations (Table 1). Table 2 presents the patients’ demographic, clinical, and biochemical data. The mean ± SD age was 41.1 ± 10.9 years, and 262 patients (63.9%) were men. The mean ± SD serum 25(OH)D3 concentration was 12.80 ± 6.90 ng/mL, and the mean ± SD serum 25(OH)D3 levels were 6.58 ± 1.94 ng/mL in group 1 and 17.25 ± 5.60 ng/mL in group 2. No differences existed between the groups with respect to comorbidities, ESRD etiologies, donor type, and the immunosuppressive agents administered. However, the dialysis modality before RTx differed significantly between the groups. The proportion of hemodialysis patients was significantly higher in group 2 (66.9%) compared with group 1 (29.2%) (P < 0.001), and significantly more patients were on peritoneal dialysis in group 1 (33.3%) compared with group 2 (8.8%) (P < 0.001). Significantly more patients in group 2 (32.6%) underwent RTx in summer compared with group 1 (17.0%) (P < 0.001), whereas more patients in group 1 (29.8%) underwent RTx in spring compared with group 2 (16.3%) (P = 0.001). Acute rejection episodes were more frequent in group 2 (18.8%) than in group 1 (9.9%) (P = 0.02). Hemoglobin, serum calcium, and albumin concentrations were significantly lower in group 1 compared with group 2 (P < 0.001) (Table 2). Serum levels of intact parathyroid hormone and alkaline phosphatase did not differ significantly between the groups.
TABLE 1.
Prevalence of Vitamin D Deficiency in This Study Group
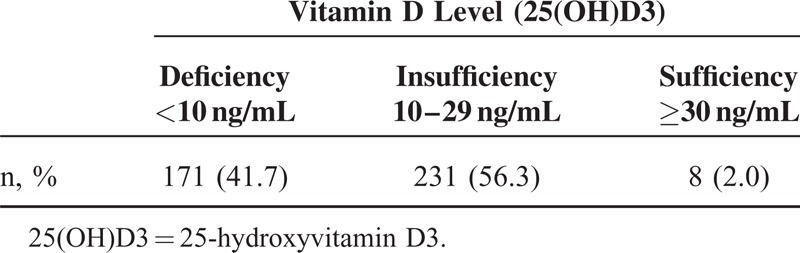
TABLE 2.
Baseline Characteristics of the Study Population by Vitamin D Levels
Outcomes
During the median follow-up period of 7.3 years, posttransplant UTI was significantly more prevalent in patients with vitamin D deficiencies (52 patients, 30.4%) compared with patients without vitamin D deficiencies (40 patients, 16.7%) (P = 0.001), and the follow-up periods were comparable between the groups. Additionally, we compared the occurrence of early UTI, which developed within 6 months from RTx, and there was no difference between the groups (group 1, 4.7% vs group 2, 2.9%, P = 0.35). The frequency of UTI events during follow-up was calculated, and 68.6 UTI events per 1000 patient-years occurred in group 1 and 33.8 UTI events per 1000 patient-years occurred in group 2. Graft failure and patient mortality did not differ significantly between the groups (Table 3). The Kaplan–Meier curves showed that UTI-free survival rates were significantly lower in vitamin D-deficient patients (P = 0.001) (Figure 2). Additionally, we apportioned the patients into tertiles according to their serum 25(OH)D3 levels to compare the incidence of UTI among the 3 groups, which were defined as follows: tertile 1, 25(OH)D3 <8.5 ng/mL; tertile 2, 8.5 ≤25(OH)D3≤15.1; and tertile 3, 25(OH)D3 > 15.1 ng/mL. The incidence of UTI after RTx was 33.8% in tertile 1, 21.9% in tertile 2, and 11.7% in tertile 3 (Figure 3).
TABLE 3.
Outcomes According to Baseline 25(OH)D3 Levels
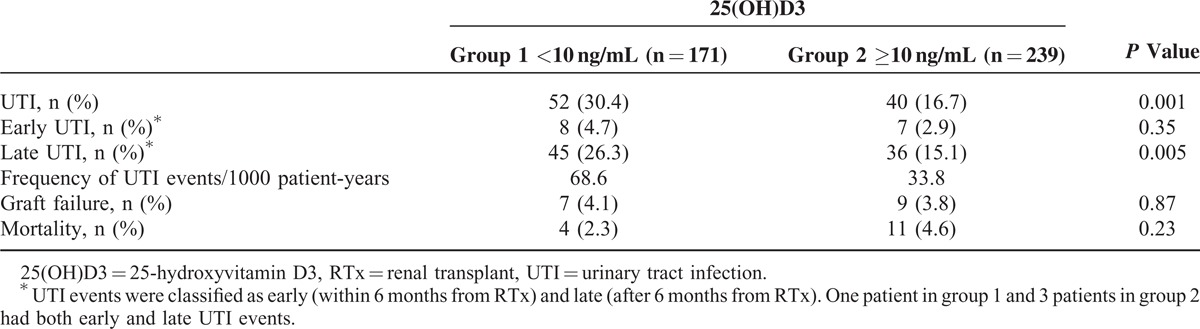
FIGURE 2.
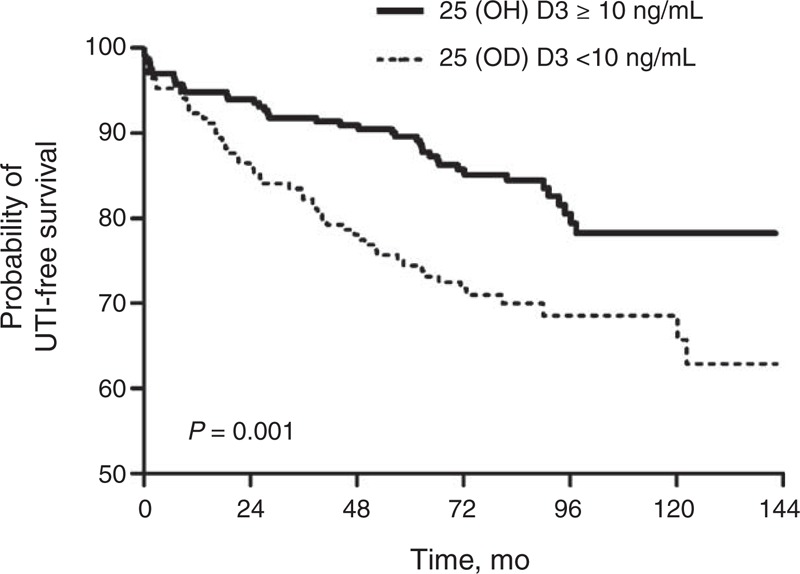
UTI-free survival rates based on vitamin D levels. UTI = urinary tract infection.
FIGURE 3.
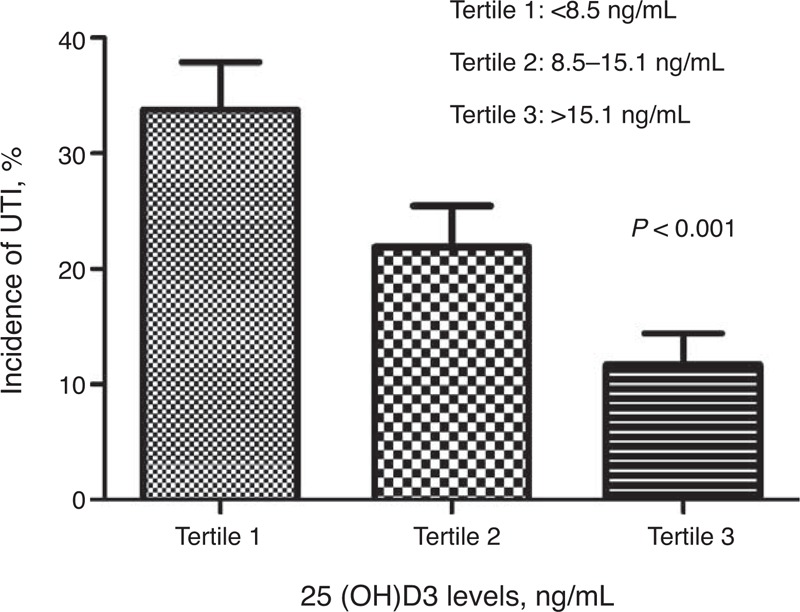
Incidence of UTIs among the tertiles based on vitamin D levels. 25(OH)D3 = 25-hydroxyvitamin D3, UTI = urinary tract infection.
Risk Factors for Posttransplant UTI
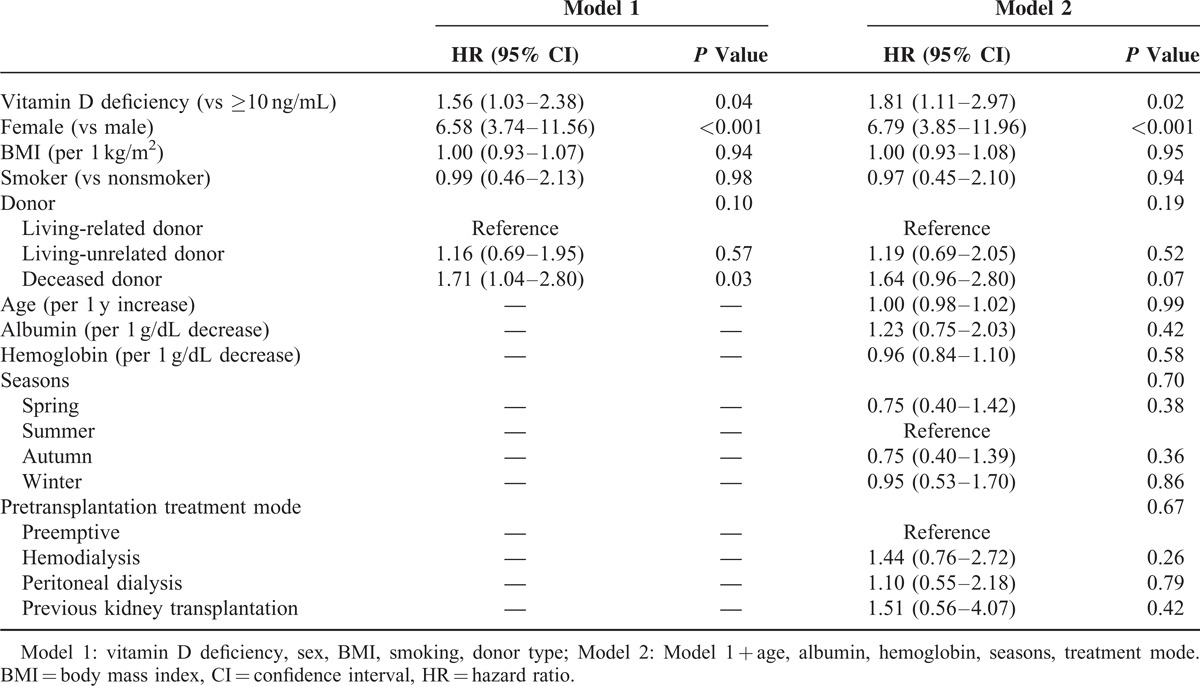
Cox proportional hazards regression analysis determined the independent risk factors for posttransplant UTI development. Univariate Cox regression analysis determined that vitamin D deficiency (hazard ratio [HR] 1.99; 95% confidence interval [CI] 1.32–3.00, P = 0.001), being a woman (HR 7.19, 95% CI 4.48–11.56, P < 0.001), BMI (HR 0.92, 95% CI 0.86–0.98, P = 0.01), smoking (HR 0.31, 95% CI 0.16–0.60, P = 0.001), and a deceased donor (HR 1.79, 95% CI 1.13–2.85, P = 0.01) were statistically significant factors associated with posttransplant UTI. Multivariate Cox regression analysis determined that vitamin D deficiency was independently associated with posttransplant UTI after adjusting for gender, BMI, smoking status, and donor type (HR 1.56, 95% CI 1.03–2.38, P = 0.04). When the variables clinically associated with vitamin D deficiency, including age, pre-RTx dialysis modality, hemoglobin, and serum albumin concentrations, and the season at the time of the RTx, were included in the multivariate model, vitamin D deficiency remained an independent risk factor for posttransplant UTI (HR 1.81, 95% CI 1.11–2.97, P = 0.02) (Table 4).
TABLE 4.
Multivariate Cox Regression Analysis for the Incidence of UTIs With Adjustments for Various Factors
DISCUSSION
Vitamin D plays an important role in the antimicrobial action of macrophages, but its impact on UTI has not been extensively explored in RTx patients. In this study, the prevalence of vitamin D deficiency was extremely high among Korean ESRD patients at the time of RTx. Furthermore, we demonstrated that vitamin D deficiency was an independent predictor of posttransplant UTI in these patients.
Vitamin D deficiency is prevalent in not only ESRD patients but also the general population.17,18 Almost all ESRD patients are vitamin D deficient because of reduced mobility and sun exposure, defects in skin synthesis, malnutrition, and increases in vitamin D catabolism. Sadlier and Magee15 found that vitamin D deficiency (28.6%) and insufficiency (58.9%) were prevalent in 112 ESRD patients at the time of RTx. However, an Australian study of 257 patients who were about to undergo kidney or kidney–pancreas transplantations showed that serum 25(OH)D3 levels <10 ng/mL were present in only 6% of the patients.19 In this study, vitamin D deficiency was present in 41.7% of the patients, which was significantly higher compared with the results from previous studies. Old age, being a woman, diabetes, peritoneal dialysis, malnutrition, winter, and high latitudes are associated with low serum vitamin D concentrations in ESRD patients. Hence, it is unclear why the proportion of vitamin D-deficient ESRD patients was rather high at the time of RTx in this study, but poor dietary and supplementary vitamin D intakes, sunscreen use, and the dislike of ultraviolet B radiation in Korea may contribute to this phenomenon. An international epidemiological study investigated 2589 community-dwelling, postmenopausal women with osteoporosis from 18 countries to determine the risk factors for vitamin D insufficiency.17 Interestingly, Korean participants had the lowest mean serum vitamin D levels (17.6 ng/mL), and >85% and 95% of the participants had vitamin D insufficiency (<30 ng/mL) in summer and winter, respectively, which supports the results of this study.
Vitamin D exerts its calciotropic action by binding to VDR in the intestine, parathyroid gland, and bones, thereby increasing calcium reabsorption in the small intestine, suppressing the synthesis and secretion of parathyroid hormone, and enhancing calcium resorption in the bone.7 However, accumulating evidence suggests that VDRs are distributed throughout the body, and that they are present within the brain, cardiac muscle, kidney, prostate, breast, lymphocytes, and macrophages, suggesting that vitamin D plays important roles in these organs and tissues, in addition to its roles in calcium homeostasis and bone metabolism.6 Several laboratory investigations have shown that vitamin D controls multiple genes that are involved in cellular proliferation, differentiation, and apoptosis. Furthermore, numerous clinical studies have demonstrated that vitamin D deficiency is associated with different chronic diseases, including colorectal, prostate, breast, and pancreatic cancers, multiple sclerosis, systemic lupus erythematosus, diabetes, hypertension, congestive heart failure, myocardial infarction, and depression.20–25
Vitamin D is a potent immunomodulator, and it prevents macrophages from releasing too many inflammatory cytokines and chemokines,26 promotes their maturation and lysosomal enzyme secretion, and their production of antimicrobial peptides, including cathelicidin and β-defensin.27 Cathelicidin and β-defensin are also synthesized and secreted by cells other than macrophages, including airway and urogenital epithelial cells.28,29 Moreover, these peptides inactivate influenza virus, suggesting that they also have antiviral properties.30 Human and mouse urinary tract epithelial cells produce cathelicidin, and bacterial contact with epithelial cells results in the rapid production and secretion of cathelicidin.31 In vivo experiments using cathelin-related antimicrobial peptide-deficient and neutrophil-depleted mice showed that epithelially derived cathelicidin substantially contributed to the protection of the urinary tract against infection, suggesting that cathelicidin is a key factor in the mucosal immunity of the urinary tract.31 After the cathelicidin and β-defensin gene promoters were shown to contain vitamin D response elements, it was inferred that vitamin D could reinforce host defenses by inducing these peptides. Although vitamin D coadministered with a commercially available influenza vaccine failed to enhance humoral immunity in 175 human subjects, the coadministration of vitamin D with a trivalent influenza vaccine augmented mucosal and systemic antibody responses in mice.32 Moreover, the activation of Toll-like receptors, which trigger direct antimicrobial activity against intracellular bacteria, upregulated VDR and vitamin D-1-hydroxylase expression in human macrophages, inducing cathelicidin production and killing intracellular M tuberculosis.33 Based on these findings, vitamin D deficiency may increase susceptibility to microbial infection, but this has not been extensively explored in RTx patients. The results from this study found, for the first time, that vitamin D deficiency was independently associated with a higher UTI risk in RTx patients. Furthermore, the independent predictive value of vitamin D deficiency and being a woman for post-RTx UTI remained significant, even after adjusting for donor type, which has been shown to be independently associated with post-RTx UTI development. Unfortunately, we did not determine cathelicidin and β-defensin expression in the 2 groups; hence, it was difficult to clarify whether the UTI risk was attributable to defects in the antimicrobial peptides of the urogenital epithelium or macrophages, or other factors.
UTI is the most common infectious complication after RTx, accounting for 40% to 50% of all infectious complications. It is also responsible for about 30% of the sepsis in RTx patients.2 Early UTI was often associated with acute pyelonephritis and bacteremia, and these could be complicated by acute rejection and the patients’ morbidity and mortality.1 However, some studies debated whether late UTI had an impact on graft and patient outcomes.34,35 In the present study, UTI was more prevalent in patients with vitamin D deficiency, but there was no difference between the 2 groups in relation to early UTI occurrence (patients with vitamin D deficiency 4.7%, patients without vitamin D deficiency 2.9%, P = 0.35). However, there was no difference in all-cause mortality and graft failure between the 2 groups because of a limited number of mortality and graft failure cases with limited follow-up duration. Further studies with a longer follow-up duration are needed to determine the impact of frequent late UTI on graft failure and mortality in vitamin D-deficient patients.
There are several limitations to this study. First, because all of the subjects were Korean ESRD patients, the associations between vitamin D and post-RTx UTI may not extend to other populations. Further studies will be necessary to clarify the relationship of vitamin D deficiency with UTI in patients with other chronic diseases as well as in the general population. Second, serum vitamin D concentrations were only measured once; therefore, it is difficult to elucidate whether serum vitamin D level changes had any influence on post-RTx UTI development. Third, even though some previous studies showed that 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) correlated more accurately with estimated glomerular filtration rate than 25(OH)D3,36,37 only 25(OH)D3 levels were used to determine vitamin D status in the current study. The reasons are as follows: 25(OH)D3 has a longer half-life of 3 weeks than 1,25(OH)2D3 of 4 hours.38,39 In addition, a single measurement of 1,25(OH)2D3 levels does not represent vitamin D storage status correctly because of its high variability, whereas 25(OH)D3 has been validated in many studies as a reliable marker of vitamin D status.11,12,15,18,40 Moreover, we focused on the impact of vitamin D deficiency on UTI development in RTx patients rather than the correlation between vitamin D levels and renal function. Fourth, because asymptomatic UTI cannot be detected by physicians, the post-RTx UTI prevalence might be underestimated. Fifth, even though serious urologic problems were not present at the time of RTx, the data on newly developed urologic abnormality or the duration of catheter indwelling during the follow-up period after RTx were not clearly available. Lastly, even though standard dietary advice including balanced nutritional intake and essential vitamin supplement was provided to all patients close to the time of discharge after RTx, there was still a possibility that individual difference in not only dietary habits but also sunlight exposure after RTx had some influences on the results of the current study.
In conclusion, vitamin D deficiency was prevalent among ESRD patients undergoing RTx, and it was an independent risk factor for post-RTx UTI, suggesting that determining 25(OH)D3 levels might predict infectious complications after RTx. Further studies are needed to verify the association between vitamin D deficiency and infection risk after RTx by examining the effects of vitamin D replacement in these patients.
Footnotes
Abbreviations: 1,25(OH)2D3 = 1,25-dihydroxyvitamin D3, 25(OH)D3 = 25-hydroxyvitamin D3, BMI = body mass index, CI = confidence interval, ESRD = end-stage renal disease, HR = hazard ratio, IRB = institutional review board, RTx = renal transplant, SD = standard deviation, UTI = urinary tract infection, VDR = vitamin D receptor.
This work was supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, and a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020)
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Schmaldienst S, Dittrich E, Horl WH. Urinary tract infections after renal transplantation. Curr Opin Urol 2002; 12:125–130. [DOI] [PubMed] [Google Scholar]
- 2.Castaneda DA, Leon K, Martin R, et al. Urinary tract infection and kidney transplantation: a review of diagnosis, causes, and current clinical approach. Transplant Proc 2013; 45:1590–1592. [DOI] [PubMed] [Google Scholar]
- 3.Muller V, Becker G, Delfs M, et al. Do urinary tract infections trigger chronic kidney transplant rejection in man? J Urol 1998; 159:1826–1829. [DOI] [PubMed] [Google Scholar]
- 4.Chuang P, Parikh CR, Langone A. Urinary tract infections after renal transplantation: a retrospective review at two US transplant centers. Clin Transplant 2005; 19:230–235. [DOI] [PubMed] [Google Scholar]
- 5.Abbott KC, Oliver JD, 3rd, Hypolite I, et al. Hospitalizations for bacterial septicemia after renal transplantation in the United States. Am J Nephrol 2001; 21:120–127. [DOI] [PubMed] [Google Scholar]
- 6.Maalouf NM. The noncalciotropic actions of vitamin D: recent clinical developments. Curr Opin Nephrol Hypertens 2008; 17:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 8.Gavison R, Bar-Shavit Z. Impaired macrophage activation in vitamin D3 deficiency: differential in vitro effects of 1,25-dihydroxyvitamin D3 on mouse peritoneal macrophage functions. J Immunol 1989; 143:3686–3690. [PubMed] [Google Scholar]
- 9.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004; 173:2909–2912. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Li C, Hong W, et al. Autophagy during Mycobacterium tuberculosis infection and implications for future tuberculosis medications. Cell Signal 2013; 25:1272–1278. [DOI] [PubMed] [Google Scholar]
- 11.Hong JY, Kim SY, Chung KS, et al. Association between vitamin D deficiency and tuberculosis in a Korean population. Int J Tuberc Lung Dis 2014; 18:73–78. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case–control study. Lancet 2000; 355:618–621. [DOI] [PubMed] [Google Scholar]
- 13.Rickers H, Christiansen C, Christensen P, et al. Serum concentrations of vitamin D metabolites in different degrees of impaired renal function. Estimation of renal and extrarenal secretion rate of 24,25-dihydroxyvitamin D. Nephron 1985; 39:267–271. [DOI] [PubMed] [Google Scholar]
- 14.LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis 2005; 45:1026–1033. [DOI] [PubMed] [Google Scholar]
- 15.Sadlier DM, Magee CC. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin Transplant 2007; 21:683–688. [DOI] [PubMed] [Google Scholar]
- 16.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007; 72:1004–1013. [DOI] [PubMed] [Google Scholar]
- 17.Rizzoli R, Eisman JA, Norquist J, et al. Risk factors for vitamin D inadequacy among women with osteoporosis: an international epidemiological study. Int J Clin Pract 2006; 60:1013–1019. [DOI] [PubMed] [Google Scholar]
- 18.Clayton P, Singer R. 25-Hydroxyvitamin D levels in prevalent Australian dialysis patients. Nephrology (Carlton) 2009; 14:554–559. [DOI] [PubMed] [Google Scholar]
- 19.Elder GJ. Vitamin D levels, bone turnover and bone mineral density show seasonal variation in patients with chronic kidney disease stage 5. Nephrology (Carlton) 2007; 12:90–94. [DOI] [PubMed] [Google Scholar]
- 20.Gupta D, Vashi PG, Trukova K, et al. Prevalence of serum vitamin D deficiency and insufficiency in cancer: review of the epidemiological literature. Exp Ther Med 2011; 2:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon KC, Munger KL, Ascherio A. Vitamin D and multiple sclerosis: epidemiology, immunology, and genetics. Curr Opin Neurol 2014; 25:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Irastorza G, Egurbide MV, Olivares N, et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008; 47:920–923. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Chen M, Hankins SR, et al. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol 2012; 110:834–839. [DOI] [PubMed] [Google Scholar]
- 24.Deleskog A, Piksasova O, Silveira A, et al. Serum 25-hydroxyvitamin D concentration, established and emerging cardiovascular risk factors and risk of myocardial infarction before the age of 60 years. Atherosclerosis 2014; 223:223–229. [DOI] [PubMed] [Google Scholar]
- 25.Annweiler C, Rastmanesh R, Richard-Devantoy S, et al. The role of vitamin D in depression: from a curious idea to a therapeutic option. J Clin Psychiatry 2013; 74:1121–1122. [DOI] [PubMed] [Google Scholar]
- 26.Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFkappaB and MAPK signalling and chemokine release in human adipocytes. PLoS One 2013; 8:e61707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins RR, Yamshchikov AV, Lemonovich TL, et al. The role of vitamin D deficiency in sepsis and potential therapeutic implications. J Infect 2011; 63:321–326. [DOI] [PubMed] [Google Scholar]
- 28.Tecle T, Tripathi S, Hartshorn KL. Review: defensins and cathelicidins in lung immunity. Innate Immun 2010; 16:151–159. [DOI] [PubMed] [Google Scholar]
- 29.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol 2007; 18:2810–2816. [DOI] [PubMed] [Google Scholar]
- 30.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol 2011; 50:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 2006; 12:636–641. [DOI] [PubMed] [Google Scholar]
- 32.Kriesel JD, Spruance J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine 1999; 17:1883–1888. [DOI] [PubMed] [Google Scholar]
- 33.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 34.Cuvelier R, Pirson Y, Alexandre GP, et al. Late urinary tract infection after transplantation: prevalence, predisposition and morbidity. Nephron 1985; 40:76–78. [DOI] [PubMed] [Google Scholar]
- 35.Abbott KC, Swanson SJ, Richter ER, et al. Late urinary tract infection after transplantation in the United States. Am J Kidney Dis 2004; 44:353–362. [DOI] [PubMed] [Google Scholar]
- 36.Rouached M, El Kadiri Boutchich S, Al Rifai AM, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2008; 74:389–390. [DOI] [PubMed] [Google Scholar]
- 37.Petchey WG, Johnson DW, Hawley CM, et al. Predictors of vitamin D status in predialysis chronic kidney disease patients: a cross-sectional analysis in a high ultraviolet climate. J Ren Nutr 2012; 22:400–408. [DOI] [PubMed] [Google Scholar]
- 38.Clements MR, Davies M, Hayes ME, et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf) 1992; 37:17–27. [DOI] [PubMed] [Google Scholar]
- 39.Gray RW, Caldas AE, Wilz DR, et al. Metabolism and excretion of 3H-1,25-(OH)2-vitamin D3 in healthy adults. J Clin Endocrinol Metab 1978; 46:756–765. [DOI] [PubMed] [Google Scholar]
- 40.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008; 87:1087S–1091S. [DOI] [PubMed] [Google Scholar]


