Abstract
Cryoglobulinemia is characterized by a wide range of causes, symptoms, and outcomes. Hepatitis C virus (HCV) infection is detected in 30%–100% of patients with cryoglobulins. Although more than half the patients with cryoglobulinemic vasculitis present a relatively benign clinical course, some may present with potentially life-threatening situations. We conducted the current study to analyze the clinical characteristics and outcomes of HCV patients presenting with life-threatening cryoglobulinemic vasculitis. We evaluated 181 admissions from 89 HCV patients diagnosed with cryoglobulinemic vasculitis consecutively admitted to our department between 1995 and 2010. In addition, we performed a systematic analysis of cases reported to date through a MEDLINE search.
The following organ involvements were considered to be potentially life-threatening in HCV patients with cryoglobulinemic vasculitis: cryoglobulinemic, biopsy-proven glomerulonephritis presenting with renal failure; gastrointestinal vasculitis; pulmonary hemorrhage; central nervous system (CNS) involvement; and myocardial involvement. A total of 279 patients (30 from our department and 249 from the literature search) fulfilled the inclusion criteria: 205 presented with renal failure, 45 with gastrointestinal vasculitis, 38 with CNS involvement, 18 with pulmonary hemorrhage, and 3 with myocardial involvement; 30 patients presented with more than 1 life-threatening cryoglobulinemic manifestation. There were 146 (52%) women and 133 (48%) men, with a mean age at diagnosis of cryoglobulinemia of 54 years (range, 25–87 yr) and a mean age at life-threatening involvement of 55 years (range, 25–87 yr). In 232 (83%) patients, life-threatening involvement was the first clinical manifestation of cryoglobulinemia. Severe involvement appeared a mean of 1.2 years (range, 1–11 yr) after the diagnosis of cryoglobulinemic vasculitis. Patients were followed for a mean of 14 months (range, 3–120 mo) after the diagnosis of life-threatening cryoglobulinemia. Sixty-three patients (22%) died. The main cause of death was sepsis (42%) in patients with glomerulonephritis, and cryoglobulinemic vasculitis itself in patients with gastrointestinal, pulmonary, and CNS involvement (60%, 57%, and 62%, respectively). In conclusion, HCV-related cryoglobulinemia may result in progressive (renal involvement) or acute (pulmonary hemorrhage, gastrointestinal ischemia, CNS involvement) life-threatening organ damage. The mortality rate of these manifestations ranges between 20% and 80%. Unfortunately, this may be the first cryoglobulinemic involvement in almost two-thirds of cases, highlighting the complex management and very elevated mortality of these cases.
INTRODUCTION
Cryoglobulins are immunoglobulins that precipitate in vitro at temperatures less than 37°C and redissolve after rewarming.78 Cryoglobulinemia refers to the presence of cryoglobulins in serum, while the terms cryoglobulinemic disease or cryoglobulinemic vasculitis are used to describe patients with symptoms related to the presence of cryoglobulins, since many patients with cryoglobulinemia remain asymptomatic.78 Cryoglobulinemic vasculitis mainly affects the small and, less frequently, medium-size arteries and veins,29 which are thought to be damaged by the deposition of immune complexes on their walls, with the subsequent activation of the complement cascade.90 The distinctive etiopathogenic feature of cryoglobulinemia is an underlying B-cell clonal expansion that mainly involves rheumatoid factor-secreting cells.79,87 Cryoglobulins have been observed in a wide variety of diseases, principally infections, neoplasia, and systemic autoimmune diseases.1,30 A viral origin of cryoglobulinemia was long suspected, but it was not until the early 1990s that evidence emerged of a close relationship with the hepatitis C virus (HCV),1,30,76 which is responsible for more than 80% of cases. In 1966, Meltzer et al58 described the typical clinical symptoms associated with cryoglobulinemia (purpura, arthralgia, and weakness). Subsequent studies have described a broad spectrum of clinical features involving the skin, joints, kidneys, and nervous system.45,46,70,78,94,97
Although more than 50% of patients with cryoglobulinemia have a relatively benign clinical course with a good prognosis and survival,31 some may present with potentially life-threatening situations involving the internal organs and resulting in progressive (renal involvement) or acute (pulmonary hemorrhage, gastrointestinal ischemia, central nervous system [CNS] involvement) organ damage as occurs in other systemic vasculitides.37 Ferri et al31 found that 35% of patients with cryoglobulinemic vasculitis had a moderate-to-severe clinical course, with the prognosis being severely affected not only by cryoglobulinemic involvement, but also by associated processes such as HCV-related chronic liver disease. In a previous study,77 we found life-threatening cryoglobulinemia in 14% of patients with cryoglobulinemic syndrome, irrespective of the underlying etiologies, with a differentiated prognosis according to the organ involved. It remains unclear why some patients present with this severe form of vasculitis, and there is very limited information on the clinical presentation and prognosis of these patients. We conducted the current study to analyze the clinical characteristics and outcomes of HCV patients presenting with life-threatening cryoglobulinemic vasculitis.
METHODS
Definition of Life-Threatening Cryoglobulinemia
The following organ involvements were considered to be potentially life-threatening in HCV patients with cryoglobulinemic vasculitis according to previous reports:77,97
a) Renal failure: cryoglobulinemic glomerulonephritis presenting with raised serum creatinine > 1.5 mg/dL; glomerular disease was diagnosed by renal biopsy and classified as membranoproliferative glomerulonephritis, mesangial proliferative glomerulonephritis, or focal proliferative glomerulonephritis.
b)Gastrointestinal involvement of the esophagus, stomach, small and large intestine, or any intraabdominal viscera, presenting as gastrointestinal hemorrhage, intestinal ischemia, acute pancreatitis, or acute cholecystitis.
c) Pulmonary hemorrhage leading to respiratory failure, in the absence of pulmonary edema, adult respiratory distress syndrome, infectious pneumonia, lung cancer, or granulomatous disease.
d) CNS involvement: cerebral ischemia (in the absence of hypercoagulability or previously diagnosed cerebrovascular disease), cerebral hemorrhage, spinal cord or cranial nerve involvement.
e) Cardiac involvement: coronary involvement leading to myocardial infarction, in the absence of cardiovascular disease.
The 1996 Five-Factor Score (FFS) and the Birmingham Vasculitis Activity Score (BVAS), which are used to score the severity of systemic necrotizing vasculitides,40,53 were retrospectively measured at diagnosis of life-threatening involvement .
Selection of Cases
Patients
We evaluated 89 patients diagnosed with cryoglobulinemic vasculitis consecutively admitted to our department between 1995 and 2010. All patients fulfilled the 2010 classification criteria for cryoglobulinemic vasculitis.23 We reviewed clinical charts of the 181 admissions of these patients searching for life-threatening presentations of cryoglobulinemia according to the above-mentioned definitions. We identified 37 patients with 43 admissions due to life-threatening cryoglobulinemia, of whom 30 had HCV-related cryoglobulinemia (the remaining 7 had essential cryoglobulinemia).
Literature Review
In addition to the cases identified in our department, we systematically analyzed cases reported to date through a MEDLINE (National Library of Medicine, Bethesda, MD) and EMBASE (Elsevier) search.
a) Search strategy: We searched MEDLINE using the MeSH term cryoglobulinemia combined with the following MeSH terms: kidney failure, lung diseases, gastrointestinal diseases, central nervous system, and heart failure with these PubMed restrictions: date (January 1, 1990, to June 15, 2010), species (humans), and age (all adult).
b) Eligibility criteria: Studies were eligible when 1) the study population included adults with cryoglobulinemic vasculitis that fulfilled the definitions included in the clinical and laboratory items of the 2010 classification criteria;23 2) the patient/patients fulfilled the definition for at least 1 of the above-mentioned life-threatening presentations; 3) studies contained sufficient, clear information about the clinical characteristics and outcomes; and 4) patients had chronic HCV infection.
c) Study selection: Three authors (SR, CD-L, XB) read the titles and abstracts (if available) identified by the search and selected studies that might comply with the eligibility criteria. Five authors (SR, CD-L, XB, AB, PB-Z) fully reviewed the selected studies to determine criteria fulfillment, and disagreements were discussed among all authors until consensus was reached. We also searched the reference lists of relevant articles retrieved. Figure 1 shows a flow diagram of the MEDLINE literature search.
FIGURE 1.
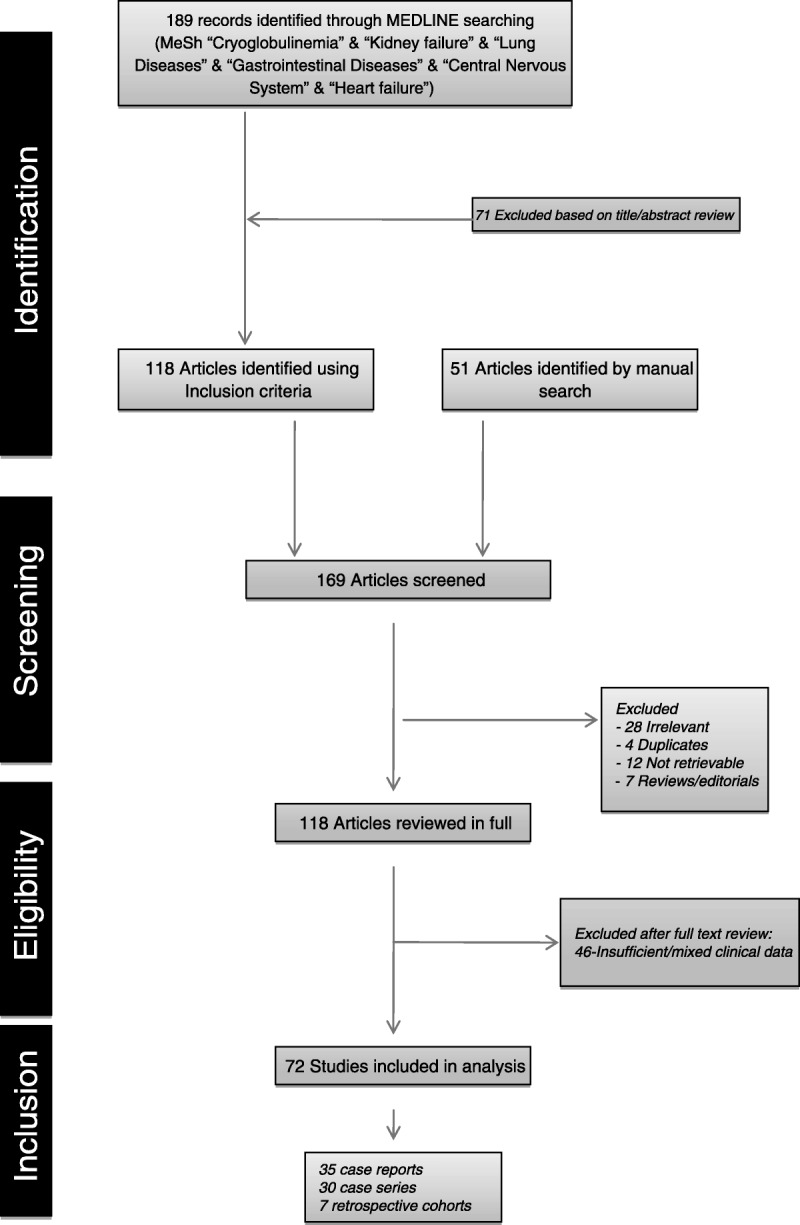
Flow chart of the MEDLINE literature search.
Statistical Methods
Categorical data were compared using the chi-square test. The Fisher exact test was used to confirm statistical differences where sample sizes were small. Continuous variables were analyzed using the Student t-test in large samples of similar variance, with results indicated as mean ± standard error of the mean (SEM), and the nonparametric Mann-Whitney U test for small samples, with results indicated as median and interquartiles. A 2-tailed value of p < 0.05 was taken to indicate statistical significance. When several independent variables appeared to have statistical significance in the univariate analysis, a multivariate Cox regression analysis was performed using a backward conditional stepwise method allowing adjustment for age, sex, and the variables that were statistically significant in the univariate analysis. The hazard ratios (HRs) and their 95% confidence intervals (CIs) obtained in the adjusted regression analysis were calculated. Kaplan–Meier survival curves were compared using the log-rank and Breslow tests. The statistical analysis was performed with the SPSS program (SPSS 18.0, Chicago, IL).
RESULTS
Clinical Description
A total of 279 patients (30 from our department and 249 from the literature search)2–9,11,12,15–19,24,25,27,32,33,35,36,38,39,42–52,54,55,59–75,81,82,84–86,89,91–95,98–100 fulfilled the inclusion criteria (Table 1). There were 146 (52%) female patients and 133 (48%) male, with a mean age at diagnosis of cryoglobulinemia of 54 years (range, 25–87 yr) and a mean age at life-threatening involvement of 55 years (range, 25–87 yr). In 232 (83%) patients, life-threatening involvement was the first clinical manifestation of cryoglobulinemia. In the remaining cases, severe involvement appeared a mean of 1.2 years (range, 1–11 yr) after the diagnosis of cryoglobulinemic vasculitis. In 207 (74%) patients, the clinical presentation required emergency room admission. Only 109 (39%) patients had positive cryoglobulins before the life-threatening presentation. In 92 (33%) patients, HCV infection was discovered due to the life-threatening presentation of cryoglobulinemic vasculitis.
TABLE 1.
Epidemiologic Features, Associated Processes, Mean Cryocrit, and Causes of Death in 279 HCV Patients With Life-Threatening Cryoglobulinemia
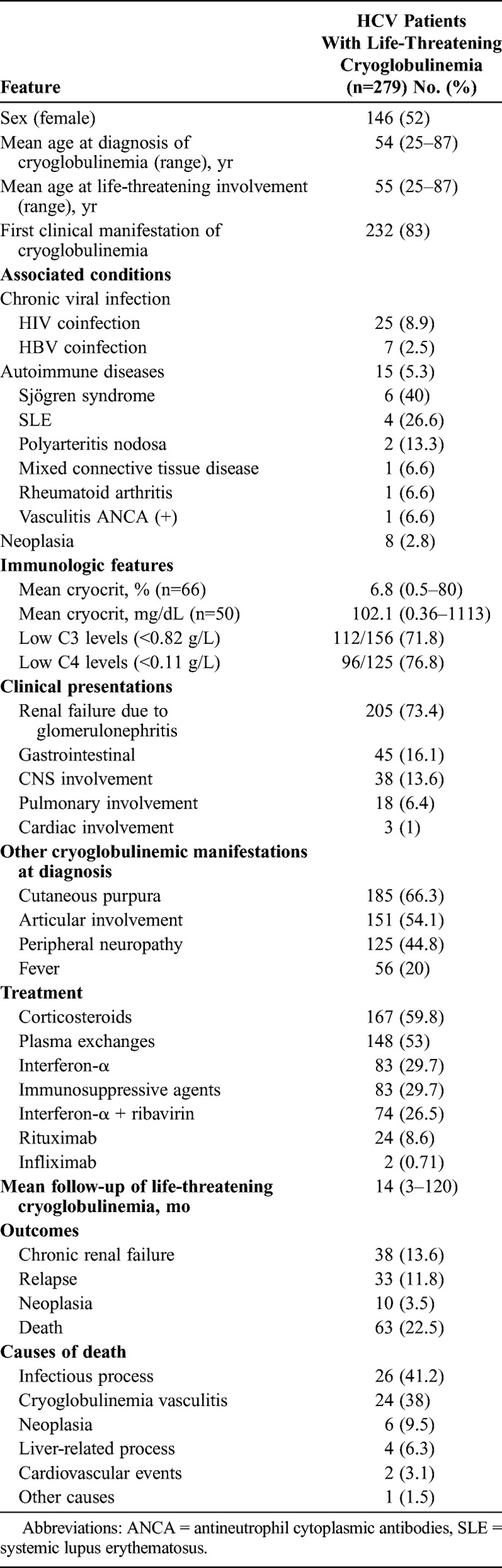
Fifty-five (20%) patients had other diseases associated with cryoglobulinemia in addition to chronic HCV infection, including human immunodeficiency virus (HIV) infection (n = 25), chronic hepatitis B virus (HBV) infection (n = 7), systemic autoimmune diseases (n = 15), and neoplasia (n = 8). Treatment of life-threatening cryoglobulinemia included corticosteroids in 167 cases, plasma exchanges in 148, immunosuppressive agents in 83, rituximab in 24, and infliximab in 2 cases. Antiviral therapy was administered in 157 patients (interferon [IFN-α] monotherapy in 83, and combined IFN-α and ribavirin in 74).
Renal Failure Due to Glomerulonephritis
Of the 205 patients with biopsy-proven cryoglobulinemic glomerulonephritis3–8,12,16,18,19,24,25,36,39,42–47,50–52,54,55,60–67,69–71,74,75,77,81,82,84–86,91–95,98,99 (Table 2), 106 were male and 99 were female, with a mean age of 50 years at diagnosis of cryoglobulinemic vasculitis and 52 years at diagnosis of glomerulonephritis. The clinical presentation was nephrotic syndrome in 96 (47%) patients and nephritic syndrome (proteinuria, hypertension, general edema) in 19 (9%), while the remaining 90 (44%) patients had an indolent presentation with asymptomatic raised creatinine levels. Renal biopsy disclosed membranoproliferative glomerulonephritis in 175 (85%) cases, mesangial proliferative glomerulonephritis in 15 (7%), focal proliferative glomerulonephritis in 10 (5%), and other histopathologic lesions in 5 (2%) patients.
TABLE 2.
Epidemiologic Features, Associated Processes, Mean Cryocrit, and Causes of Death in 205 HCV Patients With Renal Failure Caused by Biopsy-Proven Cryoglobulinemic Glomerulonephritis
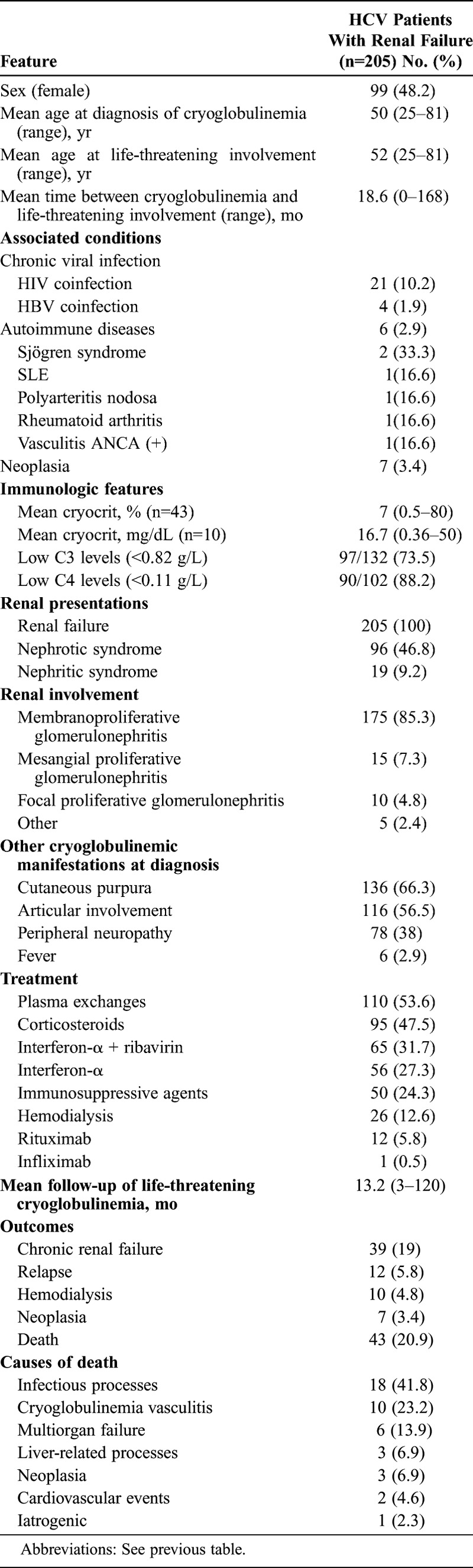
Specific treatment for cryoglobulinemia consisted of combined therapy including antiviral agents in 121 cases (59%), plasma exchange in 110 (54%), corticosteroids in 95 (47%), immunosuppressive agents in 50 (24%), hemodialysis in 26 (13%), and biological agents in 13 cases (6%) (rituximab in 12 and infliximab in 1). After a mean follow-up of 13.2 months (range, 3–120 mo) from the diagnosis of glomerulonephritis, 39 (19%) patients developed chronic renal failure and 10 (5%) evolved to end-stage renal disease. Baseline renal function was associated with survival. In comparison with survivors, patients who died had higher baseline levels of serum creatinine (2.03 ± 0.76 mg/dL vs. 2.40 ± 0.20 mg/dL; p = 0.044) and glomerular filtration rate (GFR) estimated by the Modification of Diet in Renal Disease (MDRD) formula (38.37 ± 1.01 vs. 30.67 ± 1.48; p < 0.001). We also analyzed the influence of each therapy on the main outcomes (chronic renal failure defined as serum creatinine > 2 mg/dL at the end of follow-up, and death). With respect to renal outcome, patients with chronic renal failure were treated less frequently with antiviral therapies (p = 0.008). Patients who died had more frequently received corticosteroids (p = 0.001), immunosuppressants (p = 0.024), plasma exchanges (p = 0.043) and hemodialysis (p = 0.043), results that may have been related to a more severe clinical presentation. In contrast, patients who died had less frequently received antiviral therapies (p < 0.001).
Gastrointestinal Vasculitis
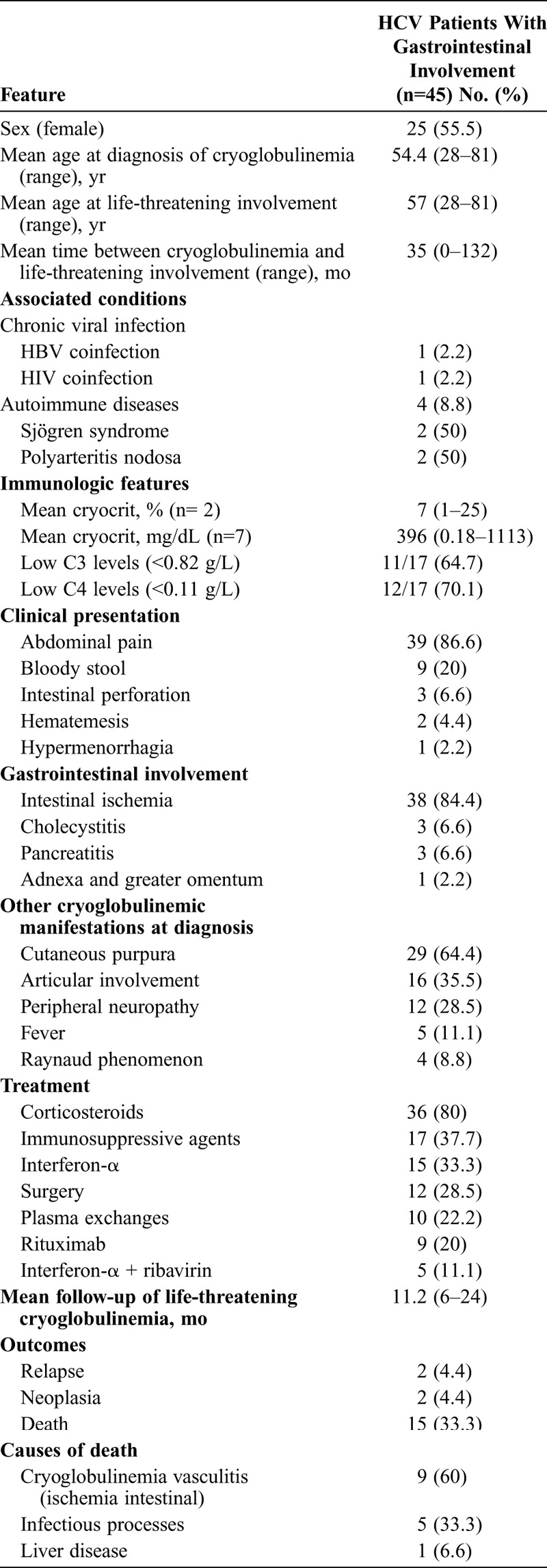
Gastrointestinal involvement was the second most frequently reported life-threatening cryoglobulinemic presentation (45 cases)11,19,24,33,42,43,48,49,51,59,69,73,77,81,83,89,93,98 (Table 3). There were 25 female patients and 20 male, with a mean age of 54 years at diagnosis of cryoglobulinemic vasculitis and 57 years at diagnosis of gastrointestinal vasculitis. The mean time between diagnosis of cryoglobulinemia and life-threatening involvement was 35 months. The clinical presentation included intestinal ischemia in 38 (84%) patients, abdominal pain in the upper right quadrant suggestive of cholecystitis in 3 (7%), cryoglobulinemic pancreatitis in 3 (7%), and cryoglobulinemic vasculitis of the adnexa and greater omentum in 1 (2%) patient. Clinical symptoms included severe abdominal pain and general malaise in 39 patients, bloody stool in 9, intestinal perforation in 3, hematemesis in 2, and hypermenorrhagia in 1 patient. Intestinal vasculitis was confirmed histopathologically in 23 patients; in 6 patients, gastrointestinal involvement was confirmed by endoscopy, in 2 by endoscopic retrograde cholangiopancreatography, and in the remaining 14 patients the diagnosis was based on the clinical and laboratory features. Treatment included corticosteroids (n = 36), immunosuppressive agents (n = 17), antiviral agents (n = 20), surgery (n = 12), plasma exchanges (n = 10), and rituximab (n = 9).
TABLE 3.
Epidemiologic Features, Associated Processes, Mean Cryocrit, and Causes of Death in 45 HCV Patients With Cryoglobulinemic Gastrointestinal Involvement
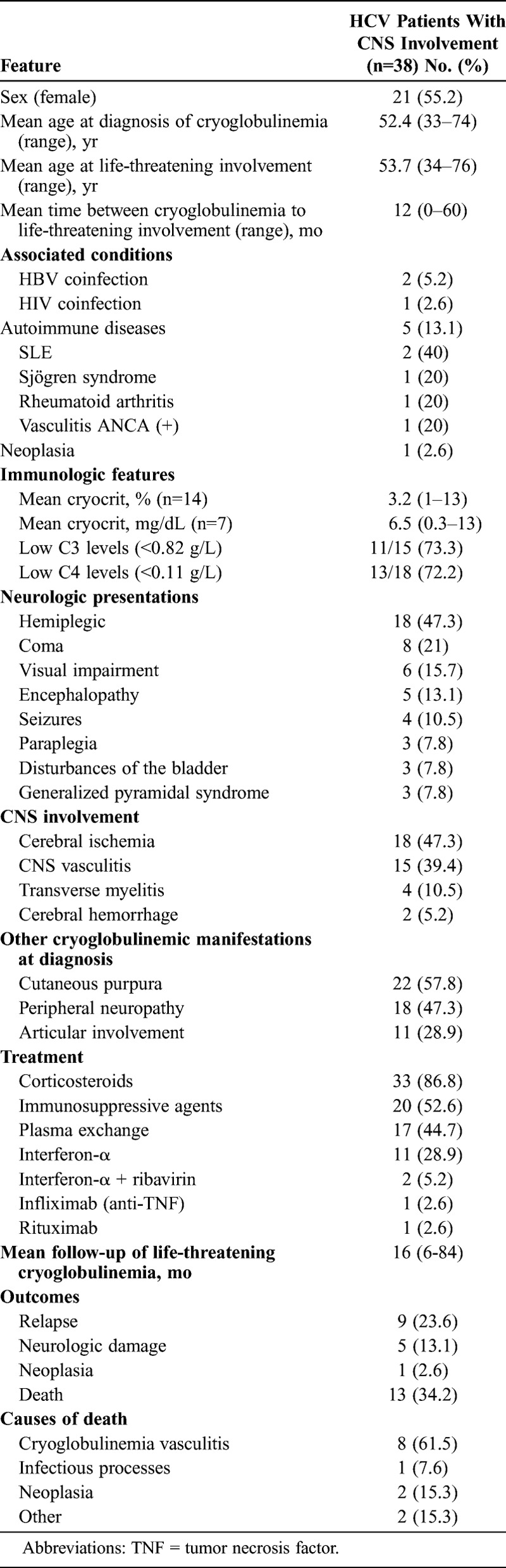
CNS Involvement
Thirty-eight patients had cryoglobulinemic CNS involvement2,3,6,8,9,15,17,27,32,35,39,42,47,51,59,68,70,72,77,81,95,100 (Table 4). There were 21 female patients and 17 male, with a mean age of 52 years at diagnosis of cryoglobulinemic vasculitis and 54 years at diagnosis of CNS cryoglobulinemic involvement. The clinical presentation consisted of hemiplegia in 18 (47%) patients, coma in 8 (21%), visual impairment in 6 (16%), encephalopathy in 5 (13%), seizures in 4 (10%), paraplegia in 3 (8%), disturbances of the bladder in 3 (8%), and generalized pyramidal syndrome in 3 patients (8%). CNS involvement consisted of cerebral ischemia in 18 (47%) cases, CNS vasculitis in 15 (40%) (demonstrated by magnetic resonance imaging [MRI] in all patients and postmortem studies in 4), transverse myelitis in 4 (10%), and cerebral hemorrhage in 2 (5%) patients. Treatment included corticosteroids (n = 33), immunosuppressive agents (n = 20), plasma exchange (n = 17), antiviral agents (n = 13), and biological therapies (n = 2). After a mean follow-up of 16 months (range, 6–84 mo) from the diagnosis of CNS involvement, 5 patients had established neurologic impairment (pyramidal syndrome and paraplegia in 2; pyramidal syndrome in 1; dysbasia, dysarthria, and spasticity in 1; and sensory alteration in 1).
TABLE 4.
Epidemiologic Features, Associated Processes, Mean Cryocrit, and Causes of Death in 38 HCV Patients With Cryoglobulinemic CNS Involvement
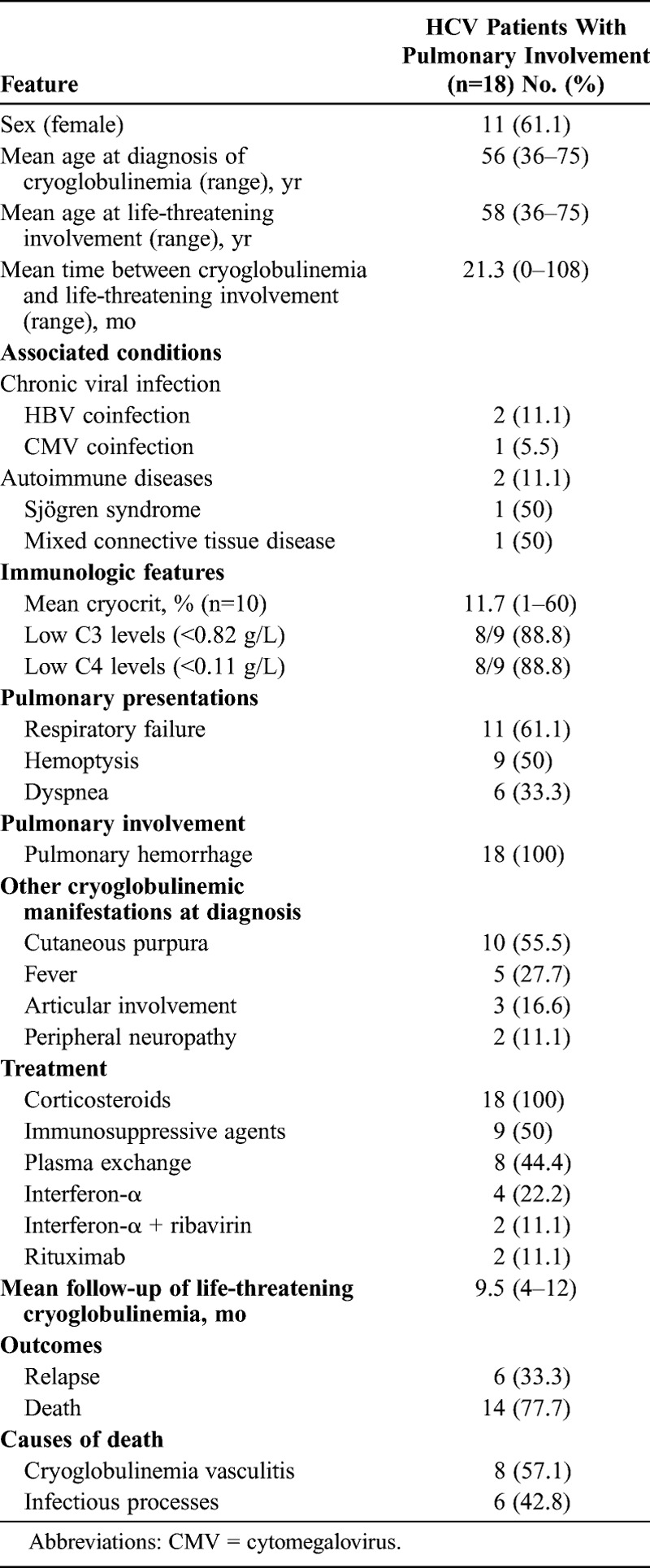
Pulmonary Hemorrhage
Eighteen patients presented with pulmonary hemorrhage 3,38,43,47,69,71,77,84,85,92,93 (Table 5). There were 11 female patients and 7 male, with a mean age of 56 years at diagnosis of cryoglobulinemic vasculitis and 58 years at diagnosis of pulmonary hemorrhage. The clinical features included respiratory failure in 11 (61.1%) patients, hemoptysis in 9 (50%), and dyspnea in 6 (33.3%) patients. All patients showed pulmonary infiltrates in the chest X-ray and required admission to the intensive care unit. Thirteen (72%) patients concomitantly had glomerulonephritis. Treatment included intravenous methylprednisolone (n = 18), immunosuppressive agents (n = 9), plasma exchanges (n = 8), antiviral agents (n = 6), and biological therapies (n = 2).
TABLE 5.
Epidemiologic Features, Associated Processes, Mean Cryocrit, and Causes of Death in 18 HCV Patients With Cryoglobulinemic Pulmonary Hemorrhage
Cardiac Involvement
Three cases of cryoglobulinemic cardiac involvement have been reported.81 All 3 were male, with a mean age of 60 years at diagnosis of cryoglobulinemic vasculitis and 63 years at diagnosis of myocardial involvement. All 3 patients presented with ischemic heart attack demonstrated by arteriography. No patient had associated cardiovascular risk factors. Other cryoglobulinemic manifestations included purpura, peripheral neuropathy, joint involvement, and Raynaud phenomenon in all patients. Treatment consisted of high-dose corticosteroids (1–1.5 mg/kg per d) and plasma exchange in all patients.
Activity, Survival Analysis, and Risk Factors
At diagnosis, the mean FFS score was 1.58 ± 0.31 and the mean BVAS score was 15.00 ± 0.30. Patients were followed for a mean of 14 months (range, 3–120 mo) after the diagnosis of life-threatening cryoglobulinemia. Sixty-three patients (22%) died. The stated cause of death was sepsis in 26 (41%) cases, cryoglobulinemic vasculitis (multisystem vasculitis in 8, chronic renal failure in 5, ischemic colitis in 4, pulmonary hemorrhage in 4, CNS vasculitis in 2, and cerebral hemorrhage in 1 patient) in 24 (38%), neoplasia in 6 (10%), chronic liver disease in 4 (6%), cardiovascular disease in 2 (3%), and post-renal biopsy bleeding complication in 1 (1%) patient. The main cause of death was sepsis (42%) in patients with glomerulonephritis, and cryoglobulinemic vasculitis itself in patients with gastrointestinal, pulmonary, and CNS involvement (60%, 57%, and 62%, respectively). No patient with myocardial involvement died. Survivors had a lower baseline FFS score (1.57 ± 0.03 vs. 1.62 ± 0.07; p = 0.548) and a lower BVAS score (14.63 ± 0.30 vs. 16.27 ± 0.84; p = 0.025) in comparison with patients who died.
Figure 2 shows the survival rate of the entire cohort (77%). Kaplan–Meier survival plots of patients classified according to the organ involved (renal, gastrointestinal, pulmonary, cardiac, CNS, and multiple organ life-threatening involvement) are shown in Figure 3. The poorest survival rates were observed in patients presenting with multiple involvement and those with pulmonary involvement (log rank and Breslow tests <0.001). Antiviral therapy was used in 157 patients (83 received monotherapy with IFN-α and 74 received combined therapy with IFN-α and ribavirin); life-threatening involvement occurred after or during the administration of antiviral therapy in 25 patients. Adjusted multivariate Cox regression analysis identified age (HR, 1.036; 95% CI, 1.007–1.067; p = 0.016) and use of antiviral therapy (HR, 0.296; 95% CI, 0.162–0.540; p < 0.001) as the baseline risk factors at diagnosis associated with survival. Figure 4 shows the Kaplan–Meier survival plots of patients classified according to the use of antiviral therapy.
FIGURE 2.
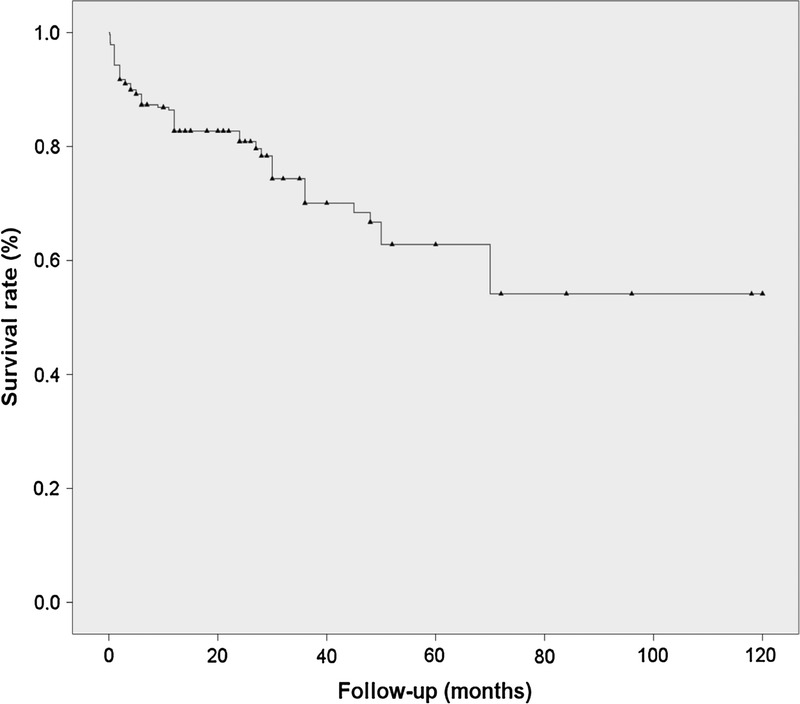
Kaplan-Meier survival curve in 279 patients with HCV-related life-threatening cryoglobulinemia.
FIGURE 3.
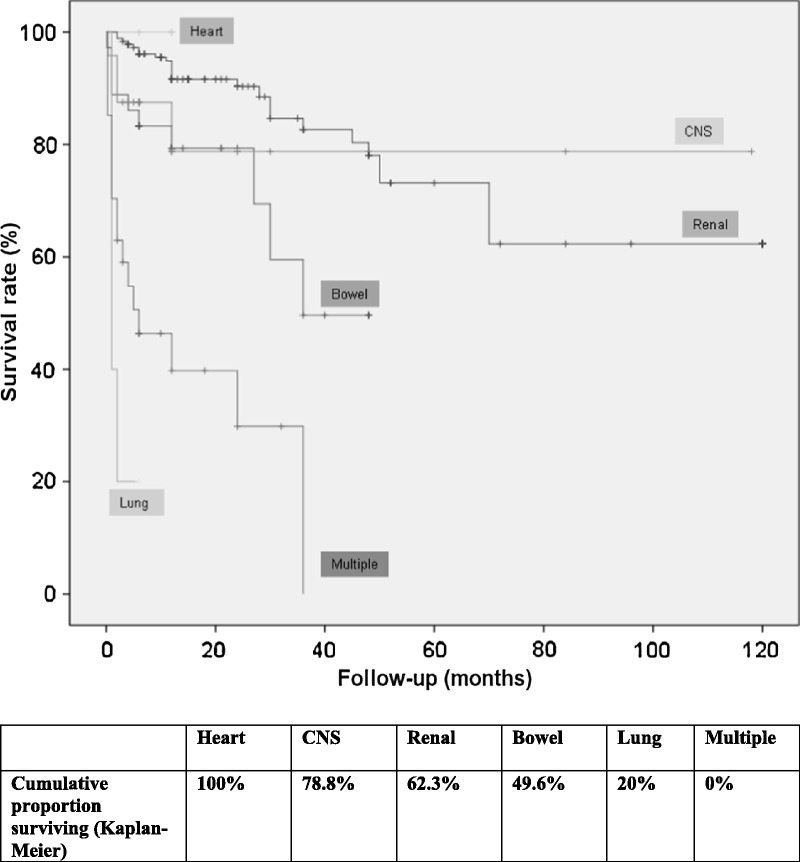
Kaplan-Meier survival curves in patients with HCV-related life-threatening cryoglobulinemia by organ involvement in clinical presentation (renal, gastrointestinal, pulmonary, cardiac, CNS, and multiple organ life-threatening involvement).
FIGURE 4.
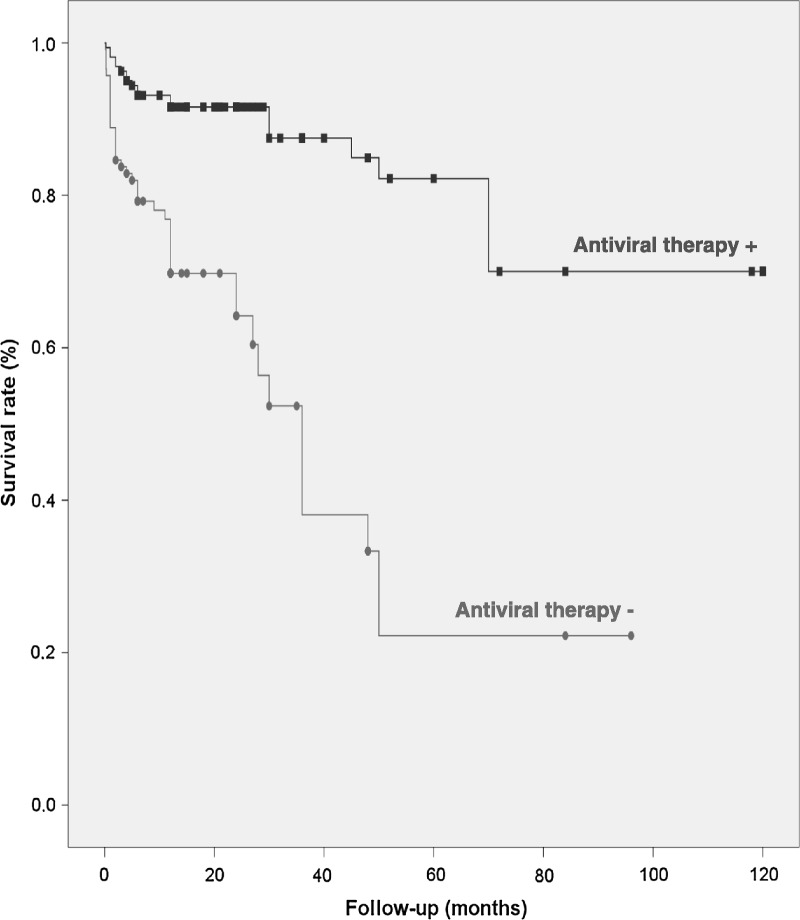
Kaplan-Meier survival curves in patients with HCV-related life-threatening cryoglobulinemia treated (“Antiviral therapy +”) and not treated (“Antiviral therapy −”) with antiviral therapy.
DISCUSSION
The prevalence of HCV infection in cryoglobulinemic patients ranges between 30% and 100%, with the highest prevalence found in Mediterranean countries.78 Conversely, between 12% and 56% of HCV-infected patients have circulating cryoglobulins, with the frequency also being highest in Mediterranean patients.78 However, only 5%–10% of HCV patients with cryoglobulinemia are estimated to have symptomatic disease.87 The severity of the cryoglobulinemic disease varies widely, with nearly half the cases having chronic disease with no vital organ involvement, one-third having moderate-to-severe disease, and less than 15% presenting with sudden, life-threatening disease.78
The most frequent type of life-threatening presentation identified in the current study was renal failure caused by cryoglobulinemic glomerulonephritis, with 20% of patients developing chronic renal failure and 5% progressing to end-stage renal disease. Studies have suggested that cryoglobulinemic glomerulonephritis significantly affects the prognosis and survival and is a major cause of death, either directly or secondary to infection or cardiovascular disease, with series from the 1990s showing 10-year survival rates ranging between 33% and 49%.31,94 However, authors of a 2007 multicenter Italian study83 including 146 patients with cryoglobulinemic glomerulonephritis reported a better survival rate of nearly 80%, probably due to improved therapeutic management. We found a survival rate of 70% in HCV patients presenting with renal failure, with cryoglobulinemic involvement contributing directly to death in less than 10% of cases, and with cardiovascular disease and infection being the most frequent causes of death, as found by the largest reported series of patients with cryoglobulinemic glomerulonephritis.83,94 In 2002 Beddhu et al6 reported that all their cryoglobulinemic patients whose serum creatinine doubled or who progressed to end-stage renal disease were HCV positive, suggesting that HCV-related cryoglobulinemic glomerulonephritis seems to have a poor prognosis compared with non-HCV cryoglobulinemia. However, more recently, Matignon et al56 found that 10% of non-HCV patients with cryoglobulinemic glomerulonephritis entered end-stage renal failure, a percentage double that found in our study in HCV patients presenting with renal failure, suggesting that the prognosis may have improved in HCV patients, possibly related to the progressive standardization of the use of antiviral therapies.
Gastrointestinal vasculitis was the second most-frequently reported life-threatening situation, with more than 80% of patients presenting with intestinal vasculitis and with a mortality rate of 40%. A 2010 single-center study found a mortality rate of nearly 20% in 12 HCV patients presenting with gastrointestinal involvement.96 This range of 20%–40% is markedly better than the 87% we found in 2006 in patients with cryoglobulinemia of all etiologies,77 which may have been due to various reasons: the majority of deaths were reported before the standardization of antiviral therapies; some cases were probably nonviral cryoglobulinemia, in which severe vasculitic involvement seems to be more frequent;34 and possible delays in the diagnosis may have influenced the poor prognosis, as reported in systemic lupus erythematosus patients with intestinal vasculitis.57
We found nearly 40 cases of CNS involvement in HCV patients, a similar number to that reported for gastrointestinal involvement and twice that reported for pulmonary hemorrhage, suggesting that CNS vasculitic cryoglobulinemia may be more frequent than previously supposed. The main clinical presentations include stroke (overwhelmingly cerebral ischemia, with only 2 cases of cerebral hemorrhage) and CNS vasculitis, with a mortality rate of 34%. Casato et al13 described a higher frequency of impaired cognitive function and MRI abnormalities in patients with HCV-related cryoglobulinemia compared with healthy controls or HCV patients without cryoglobulinemia, suggesting a potential role for cryoglobulins in CNS vasculitic damage. CNS cryoglobulinemic vasculitis should be included in the differential diagnosis of HCV patients presenting with focal neurologic deficits.80
Although we found only 18 cases of pulmonary hemorrhage in HCV-related cryoglobulinemia, this vasculitic presentation had the highest mortality rate (80%). Some cryoglobulinemic patients survived the first episode of pulmonary hemorrhage but died after a second episode. These results confirm that cryoglobulinemic pulmonary hemorrhage, which has a very poor prognosis, as do other types of systemic vasculitis, is one of the main challenges in dealing with HCV patients.14
The uncontrolled design of the current study is a limitation when generalizing the results to all HCV patients. We included only the most severe presentation of HCV-related cryoglobulinemia, thus creating a patient selection bias, and the clinical and therapeutic data were retrospectively collected. This makes it impossible to compare our results with those of controlled studies, a bias that, in our opinion, is very difficult to avoid due to the rarity of the life-threatening presentation of cryoglobulinemia. In addition, the heterogeneous therapeutic approaches may limit the accurate evaluation of the therapeutic response in these patients. Nevertheless, in spite of these limitations, we believe that the recruitment of 279 cases with this rare, very severe presentation represents a significant, representative number that provides useful information on the management of these patients.
The current results, which demonstrated a global mortality rate of about 25% (and of >50% in some types of involvement), emphasize that the optimal therapeutic strategy for life-threatening cryoglobulinemic vasculitis remains to be defined. The clinical scenario is much more complicated when we consider that, for the majority of HCV patients, this life-threatening involvement presentation was the first clinical manifestation of cryoglobulinemia. This suggests that some cryoglobulinemic HCV patients may have a higher risk of developing a catastrophic presentation of cryoglobulinemic vasculitis: in these patients, we found a more profound autoimmune response (higher levels of serum cryoglobulins and complement consumption).77 In addition, we found significant differences according to the different life-threatening involvements. Pulmonary hemorrhage and multiple organ involvement should be considered as devastating situations with a mortality rate of 63%–80%, with the majority of deaths caused by the vasculitic disease. In contrast, patients with renal cryoglobulinemic failure had a mortality rate of 15%, with the causes of death being unrelated to cryoglobulinemic vasculitis in most cases. Although the retrospective character of this study does not permit a differentiated analysis of the different therapeutic regimens used, the adjusted Cox regression model showed that the use of antiviral therapy was independently associated with survival, emphasizing the key role of antiviral therapy in HCV-related cryoglobulinemia.10,28,88 In addition, we found a positive trend for the use of rituximab in the adjusted multivariate analysis, reinforcing the usefulness of B cell-depleting agents in cryoglobulinemia22,26 together with plasma exchange, as proposed for other systemic vasculitides.41 In spite of the limited data and the lack of case-control studies, the high mortality rate of HCV-related life-threatening cryoglobulinemia suggests the use of an aggressive therapeutic schedule including a combination of immunosuppressive agents and plasma exchanges or rituximab, followed by antiviral therapy,78 together with an exhaustive follow-up, especially of the development of nonvasculitic complications including infection, liver cirrhosis, and cardiovascular disease.20,21,51
Abbreviations
- BVAS
Birmingham Vasculitis Activity Score
- CI
confidence interval
- CNS
central nervous system
- FFS
Five-Factor Score
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- IFN-α
interferon
- GFR
glomerular filtration rate
- MDRD
Modification of Diet in Renal Disease
- MRI
magnetic resonance imaging
Footnotes
Financial support and conflicts of interest: Pilar Brito-Zerón was supported by Grant “Ajut per a la Recerca Josep Font” from Hospital Clinic-Barcelona (2012). Maria C. Cid was funded by Ministerio de Ciencia e Innovación (SAF11/30073). The other authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1. Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992; 327: 1490– 1495. [DOI] [PubMed] [Google Scholar]
- 2. Aktipi KM, Ravaglia S, Ceroni M, Nemni R, Debiaggi M, Bastianello S, Alfonsi E, Zardini E, Minoli L, Tavazzi E, Marchioni E. Severe recurrent myelitis in patients with hepatitis C virus infection. Neurology. 2007; 68: 468– 469. [DOI] [PubMed] [Google Scholar]
- 3. Amital H, Rubinow A, Naparstek Y. Alveolar hemorrhage in cryoglobulinemia an indicator of poor prognosis. Clin Exp Rheumatol. 2005; 23: 616– 620. [PubMed] [Google Scholar]
- 4. Bartolucci P, Ramanoelina J, Cohen P, Mahr A, Godmer P, Le Hello C, Guillevin L. Efficacy of the anti-TNF-alpha antibody infliximab against refractory systemic vasculitides: an open pilot study on 10 patients. Rheumatology (Oxford). 2002; 41: 1126– 1132. [DOI] [PubMed] [Google Scholar]
- 5. Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F, Esposito L, Guitard J, Lavayssiere L, Oksman F, Durand D, Rostaing L. Rituximab therapy for de novo mixed cryoglobulinemia in renal transplant patients. Transplantation. 2005; 80: 1560– 1564. [DOI] [PubMed] [Google Scholar]
- 6. Beddhu S, Bastacky S, Johnson JP. The clinical and morphologic spectrum of renal cryoglobulinemia. Medicine (Baltimore). 2002; 81: 398– 409. [DOI] [PubMed] [Google Scholar]
- 7. Bestard O, Cruzado JM, Ercilla G, Goma M, Torras J, Seron D, Rama I, Ibernon M, Vinas O, Carrera M, Grinyo JM. Rituximab induces regression of hepatitis C virus-related membranoproliferative glomerulonephritis in a renal allograft. Nephrol Dial Transplant. 2006; 21: 2320– 2324. [DOI] [PubMed] [Google Scholar]
- 8. Bruchfeld A, Lindahl K, Stahle L, Soderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant. 2003; 18: 1573– 1580. [DOI] [PubMed] [Google Scholar]
- 9. Buccoliero R, Gambelli S, Sicurelli F, Malandrini A, Palmeri S, De Santis M, Stromillo ML, De Stefano N, Sperduto A, Musumeci SA, Federico A. Leukoencephalopathy as a rare complication of hepatitis C infection. Neurol Sci. 2006; 27: 360– 363. [DOI] [PubMed] [Google Scholar]
- 10. Cacoub P, Saadoun D, Limal N, Sene D, Lidove O, Piette JC. PEGylated interferon alfa-2b and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2005; 52: 911– 915. [DOI] [PubMed] [Google Scholar]
- 11. Cai FZ, Ahern M, Smith M. Treatment of cryoglobulinemia associated peripheral neuropathy with rituximab. J Rheumatol. 2006; 33: 1197– 1198. [PubMed] [Google Scholar]
- 12. Canada R, Chaudry S, Gaber L, Waters B, Martinez A, Wall B. Polyarteritis nodosa and cryoglobulinemic glomerulonephritis related to chronic hepatitis C. Am J Med Sci. 2006; 331: 329– 333. [DOI] [PubMed] [Google Scholar]
- 13. Casato M, Saadoun D, Marchetti A, Limal N, Picq C, Pantano P, Galanaud D, Cianci R, Duhaut P, Piette JC, Fiorilli M, Cacoub P. Central nervous system involvement in hepatitis C virus cryoglobulinemia vasculitis: a multicenter case-control study using magnetic resonance imaging and neuropsychological tests. J Rheumatol. 2005; 32: 484– 488. [PubMed] [Google Scholar]
- 14. Casian A, Jayne D. Management of alveolar hemorrhage in lung vasculitides. Semin Respir Crit Care Med. 2011; 32: 335– 345. [DOI] [PubMed] [Google Scholar]
- 15. Chandesris MO, Gayet S, Schleinitz N, Doudier B, Harle JR, Kaplanski G. Infliximab in the treatment of refractory vasculitis secondary to hepatitis C-associated mixed cryoglobulinaemia. Rheumatology (Oxford). 2004; 43: 532– 533. [DOI] [PubMed] [Google Scholar]
- 16. Cheng JT, Anderson HL, Jr, Markowitz GS, Appel GB, Pogue VA, D’Agati VD. Hepatitis C virus-associated glomerular disease in patients with human immunodeficiency virus coinfection. J Am Soc Nephrol. 1999; 10: 1566– 1574. [DOI] [PubMed] [Google Scholar]
- 17. Chepyala P, Velchala N, Brown D, Olden K. Encephalopathy, a rare initial presentation of HCV related cryoglobulinemia. Am J Gastroenterol. 2009; 104: S289. [Google Scholar]
- 18. D’Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998; 54: 650– 671. [DOI] [PubMed] [Google Scholar]
- 19. Daoud MS, El-Azhary RA, Gibson LE, Lutz ME, Daoud S. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. Clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996; 34: 219– 223. [DOI] [PubMed] [Google Scholar]
- 20. Della Rossa A, Marchi F, Catarsi E, Tavoni A, Bombardieri S. Mixed cryoglobulinemia and mortality: a review of the literature. Clin Exp Rheumatol. 2008; 26 (5 Suppl 51): S105– S108. [PubMed] [Google Scholar]
- 21. Della Rossa A, Tavoni A, D’Ascanio A, Catarsi E, Marchi F, Bencivelli W, Salvadori S, Migliorini P, Bombardieri S. Mortality rate and outcome factors in mixed cryoglobulinaemia: the impact of hepatitis C virus. Scand J Rheumatol. 2010; 39: 167– 170. [DOI] [PubMed] [Google Scholar]
- 22. De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M, Ferri C, Mascia MT, Masolini P, Zabotti A, Maset M, Roccatello D, Zignego AL, Pioltelli P, Gabrielli A, Filippini D, Perrella O, Migliaresi S, Galli M, Bombardieri S, Monti G. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012; 64: 843– 853. [DOI] [PubMed] [Google Scholar]
- 23. De Vita S, Soldano F, Isola M, Monti G, Gabrielli A, Tzioufas A, Ferri C, Ferraccioli GF, Quartuccio L, Corazza L, De Marchi G, Ramos Casals M, Voulgarelis M, Lenzi M, Saccardo F, Fraticelli P, Mascia MT, Sansonno D, Cacoub P, Tomsic M, Tavoni A, Pietrogrande M, Zignego AL, Scarpato S, Mazzaro C, Pioltelli P, Steinfeld S, Lamprecht P, Bombardieri S, Galli M. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis. 2011; 70: 1183– 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhib M, Francois A, Godin M. Gallbladder vasculitis and mixed cryoglobulinemia. Histopathology. 1994; 25: 399– 400. [DOI] [PubMed] [Google Scholar]
- 25. Dussol B, Moal V, Daniel L, Pain C, Berland Y. Spontaneous remission of HCV-induced cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2001; 16: 156– 159. [DOI] [PubMed] [Google Scholar]
- 26. Engel P, Gomez-Puerta JA, Ramos-Casals M, Lozano F, Bosch X. Therapeutic targeting of B cells for rheumatic autoimmune diseases. Pharmacol Rev. 2011; 63: 127– 156. [DOI] [PubMed] [Google Scholar]
- 27. Erro Aguirre ME, Ayuso Blanco T, Tunon Alvarez T, Herrera Isasi M. Brain hemorrhage as a complication of chronic hepatitis C virus-related vasculitis. J Neurol. 2008; 255: 944– 945. [DOI] [PubMed] [Google Scholar]
- 28. Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005; 436: 967– 972. [DOI] [PubMed] [Google Scholar]
- 29. Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E, Fosella PV, Pasero G, Bombardieri S. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991; 34: 1606– 1610. [DOI] [PubMed] [Google Scholar]
- 31. Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004; 33: 355– 374. [DOI] [PubMed] [Google Scholar]
- 32. Filippini D, Colombo F, Jann S, Cornero R, Canesi B. Central nervous system involvement in patients with HCV-related cryoglobulinemia: literature review and a case report. Reumatismo. 2002; 54: 150– 155. [DOI] [PubMed] [Google Scholar]
- 33. Fine GD, Trainer TD, Krawitt EL. Gastrointestinal bleeding, cryoglobulinemia, and hepatitis C. Am J Gastroenterol. 2004; 99: 964– 965. [DOI] [PubMed] [Google Scholar]
- 34. Foessel L, Besancenot JF, Blaison G, Magy-Bertrand N, Jaussaud R, Etienne Y, Maurier F, Audia S, Martin T. Clinical spectrum, treatment, and outcome of patients with type II mixed cryoglobulinemia without evidence of hepatitis C infection. J Rheumatol. 2011; 38: 716– 722. [DOI] [PubMed] [Google Scholar]
- 35. Fragoso M, Carneado J, Tuduri I, Jimenez-Ortiz C. Essential mixed cryoglobulinemia as a cause of ischemic cerebrovascular accident. Rev Neurol. 2000; 30: 444– 446. [PubMed] [Google Scholar]
- 36. Garini G, Allegri L, Carnevali ML, Iannuzzella F, Buzio C. Successful treatment of severe/active cryoglobulinaemic membranoproliferative glomerulonephritis associated with hepatitis C virus infection by means of the sequential administration of immunosuppressive and antiviral agents. Nephrol Dial Transplant. 2006; 21: 3333– 3334. [DOI] [PubMed] [Google Scholar]
- 37. Geetha D, Seo P. Life-threatening presentations of ANCA-associated vasculitis. In: Khamashta MA, Ramos-Casals M, eds. Autoimmune Diseases. Acute and Complex Situations. London: Springer-Verlag; 2011: 101– 118. [Google Scholar]
- 38. Gomez-Tello V, Onoro-Canaveral JJ, de la Casa Monje RM, Gomez-Casero RB, Moreno Hurtrez JL, Garcia-Montes M, Armas LC. Diffuse recidivant alveolar hemorrhage in a patient with hepatitis C virus-related mixed cryoglobulinemia. Intensive Care Med. 1999; 25: 319– 322. [DOI] [PubMed] [Google Scholar]
- 39. Gournay J, Ferrell LD, Roberts JP, Ascher NL, Wright TL, Lake JR. Cryoglobulinemia presenting after liver transplantation. Gastroenterology. 1996; 110: 265– 270. [DOI] [PubMed] [Google Scholar]
- 40. Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, Thibult N, Casassus P. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996; 75: 17– 28. [DOI] [PubMed] [Google Scholar]
- 41. Guillevin L, Pagnoux C. Indication for plasma exchange for systemic necrotizing vasculitides. Transfus Apher Sci. 2007; 36: 179– 185. [DOI] [PubMed] [Google Scholar]
- 42. Heckmann JG, Kayser C, Heuss D, Manger B, Blum HE, Neundorfer B. Neurological manifestations of chronic hepatitis C. J Neurol. 1999; 246: 486– 491. [DOI] [PubMed] [Google Scholar]
- 43. Iusova OI, Krivosheev OG, Semenkova EN, Kogan EA. [Cryoglobulinemic vasculitis.]. Arkh Patol. 2000; 62: 51– 54. [PubMed] [Google Scholar]
- 44. Izzedine H, Sene D, Cacoub P, Jansen H, Camous L, Brocheriou I, Bourry E, Deray G. Kidney diseases in HIV/HCV-co-infected patients. AIDS. 2009; 23: 1219– 1226. [DOI] [PubMed] [Google Scholar]
- 45. Johnson R, Gretch D, Couser WG, Alpers CE, Wilson J, Chung M, Hart J, Willson R. Hepatitis C virus-associated glomerulonephritis. Effect of α-interferon therapy. Kidney Int. 1994; 46: 1700– 1704. [DOI] [PubMed] [Google Scholar]
- 46. Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, Willson R. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993; 328: 465– 470. [DOI] [PubMed] [Google Scholar]
- 47. Johnson SL, Bander J. Pulmonary hemorrhage in a patient with hepatitis C induced essential mixed cryoglobulinemia. Chest. 1998; 114: 422S– 425S. [Google Scholar]
- 48. Lamprecht P, Lerin-Lozano C, Merz H, Dennin RH, Gause A, Voswinkel J, Peters SO, Gutzeit O, Arlt AC, Solbach W, Gross WL. Rituximab induces remission in refractory HCV associated cryoglobulinaemic vasculitis. Ann Rheum Dis. 2003; 62: 1230– 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lamprecht P, Moubayed P, Donhuijsen K, Gause A, Gross WL. Vasculitis of adnexa, greater omentum and gallbladder as abdominal manifestations of cryoglobulinemic vasculitis. Clin Exp Rheumatol. 2001; 19: 112– 113. [PubMed] [Google Scholar]
- 50. Lamprecht P, Schmitt WH, Gross WL. Mixed cryoglobulinaemia, glomerulonephritis, and ANCA: essential cryoglobulinaemic vasculitis or ANCA-associated vasculitis? Nephrol Dial Transplant. 1998; 13: 213– 221. [DOI] [PubMed] [Google Scholar]
- 51. Landau DA, Scerra S, Sene D, Resche-Rigon M, Saadoun D, Cacoub P. Causes and predictive factors of mortality in a cohort of patients with hepatitis C virus-related cryoglobulinemic vasculitis treated with antiviral therapy. J Rheumatol. 2010; 37: 615– 621. [DOI] [PubMed] [Google Scholar]
- 52. Lozano Maya M, Aldeguer Martinez M, Banares Canizares R, Yepes Barreto I, Ponferrada Diaz A, Rodriguez Benitez P, Niembro de Rasche E, Cos Arregui E. Mixed cryoglobulinemia associated with hepatitis C virus. Diagnosis by transjugular renal biopsy. Rev Esp Enferm Dig. 2009; 101: 658– 659. [DOI] [PubMed] [Google Scholar]
- 53. Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994; 87: 671– 678. [PubMed] [Google Scholar]
- 54. Markowitz GS, Cheng JT, Colvin RB, Trebbin WM, D’Agati VD. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998; 9: 2244– 2252. [DOI] [PubMed] [Google Scholar]
- 55. Massari M, Catania A, Magnani G. Efficacy and risk of rituximab in type II mixed cryoglobulinemia: a significant case report. Dig Liver Dis. 2007; 39: S134– S135. [DOI] [PubMed] [Google Scholar]
- 56. Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, Roudot-Thoraval F, Vanhille P, Moranne O, Hachulla E, Hatron PY, Fermand JP, Fakhouri F, Ronco P, Plaisier E, Grimbert P. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore). 2009; 88: 341– 348. [DOI] [PubMed] [Google Scholar]
- 57. Medina F, Ayala A, Jara LJ, Becerra M, Miranda JM, Fraga A. Acute abdomen in systemic lupus erythematosus: the importance of early laparotomy. Am J Med. 1997; 103: 100– 105. [DOI] [PubMed] [Google Scholar]
- 58. Meltzer M, Franklin EC. Cryoglobulinemia—a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966; 40: 828– 836. [DOI] [PubMed] [Google Scholar]
- 59. Mendez P, Saeian K, Reddy KR, Younossi ZM, Kerdel F, Badalamenti S, Jeffers LJ, Schiff ER. Hepatitis C, cryoglobulinemia, and cutaneous vasculitis associated with unusual and serious manifestations. Am J Gastroenterol. 2001; 96: 2489– 2493. [DOI] [PubMed] [Google Scholar]
- 60. Misiani R, Bellavita P, Baio P, Caldara R, Ferruzzi S, Rossi P, Tengattini F. Successful treatment of HCV-associated cryoglobulinaemic glomerulonephritis with a combination of interferon-alpha and ribavirin. Nephrol Dial Transplant. 1999; 14: 1558– 1560. [DOI] [PubMed] [Google Scholar]
- 61. Mohan S, Jaitly M, Cheng JT, D’Agati VD, Pogue VA. Unusual biopsy findings in a hepatitis C-infected white man with cryoglobulinemia, purpuric rash, and renal failure. Am J Kidney Dis. 2006; 48: 513– 517. [DOI] [PubMed] [Google Scholar]
- 62. Montagna G, Piazza V, Banfi G, Bellotti V, Segagni S, Picardi L, Mangione P, Giorgetti S, Zorzoli I, Cerino A, Salvadeo A. Hepatitis C virus-associated cryoglobulinaemic glomerulonephritis with delayed appearance of monoclonal cryoglobulinaemia. Nephrol Dial Transplant. 2001; 16: 432– 434. [DOI] [PubMed] [Google Scholar]
- 63. Monti G, Saccardo F. Emergency in cryoglobulinemic syndrome: what to do? Dig Liver Dis. 2007; 39 (Suppl 1): S112– S115. [DOI] [PubMed] [Google Scholar]
- 64. Morales E, Alegre R, Herrero J, Morales J, Ortuno T, Praga M. Hepatitis-C-virus-associated cryoglobulinaemic membranoproliferative glomerulonephritis in patients infected by HIV. Nephrol Dial Transplant. 1997; 12: 1980– 1984. [DOI] [PubMed] [Google Scholar]
- 65. Morosetti M, Sciarra G, Meloni C, Palmieri G, Palombo G, Taccone Gallucci M, Casciani CU. Membranoproliferative glomerulonephritis and hepatitis C: effects of interferon-α therapy on clinical outcome and histological pattern. Nephrol Dial Transplant. 1996; 11: 532– 534. [PubMed] [Google Scholar]
- 66. Moses P, Krawitt E, Aziz W, Corwin H. Renal failure associated with hepatitis C virus infection. Improvement in renal function after treatment with interferon-alpha. Dig Dis Sci. 1997; 42: 443– 446. [DOI] [PubMed] [Google Scholar]
- 67. Myers JP, Di Bisceglie AM, Mann ES. Cryoglobulinemia associated with Purtscher-like retinopathy. Am J Ophthalmol. 2001; 131: 802– 804. [DOI] [PubMed] [Google Scholar]
- 68. Origgi L, Vanoli M, Carbone A, Grasso M, Scorza R. Central nervous system involvement in patients with HCV-related cryoglobulinemia. Am J Med Sci. 1998; 315: 208– 210. [DOI] [PubMed] [Google Scholar]
- 69. Perello Carbonel R, Supervia Caparros A, Nolla Salas J, Vazquez Sanchez A, Torrente Segarra V, Gutierrez Cebollada J. [Alveolar haemorrhage and hepatitis virus C related mixed cryoglobulinemia. Report of three cases]. An Med Interna. 2005; 22: 529– 531. [DOI] [PubMed] [Google Scholar]
- 70. Petty GW, Duffy J, Houston J., III Cerebral ischemia in patients with hepatitis C virus infection and mixed cryoglobulinemia. Mayo Clin Proc. 1996; 71: 671– 678. [DOI] [PubMed] [Google Scholar]
- 71. Prasad M, Buller G, Mena CI, Sofair AN. Sum of the parts. N Engl J Med. 2006; 355: 2468– 2473. [DOI] [PubMed] [Google Scholar]
- 72. Propst T, Propst A, Nachbauer K, Graziadei I, Willeit H, Margreiter R, Vogel W. Papillitis and vasculitis of the arteria spinalis anterior as complications of hepatitis C reinfection after liver transplantation. Transpl Int. 1997; 10: 234– 237. [DOI] [PubMed] [Google Scholar]
- 73. Quartuccio L, Petrarca A, Mansutti E, Pieroni S, Calcabrini L, Avellini C, Zignego A, De Vita S. Efficacy of rituximab in severe and mild abdominal vasculitis in the course of mixed cryoglobulinemia. Clin Exp Rheumatol. 2010; 28: S84– S87. [PubMed] [Google Scholar]
- 74. Quartuccio L, Soardo G, Romano G, Zaja F, Scott CA, De Marchi G, Fabris M, Ferraccioli G, De Vita S. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford). 2006; 45: 842– 846. [DOI] [PubMed] [Google Scholar]
- 75. Quigg RJ, Brathwaite M, Gardner DF, Gretch DR, Ruddy S. Successful cyclophosphamide treatment of cryoglobulinemic membranoproliferative glomerulonephritis associated with hepatitis C virus infection. Am J Kidney Dis. 1995; 25: 798– 800. [DOI] [PubMed] [Google Scholar]
- 76. Ramos-Casals M, Font J. Extrahepatic manifestations in patients with chronic hepatitis C virus infection. Curr Opin Rheumatol. 2005; 17: 447– 455. [DOI] [PubMed] [Google Scholar]
- 77. Ramos-Casals M, Robles A, Brito-Zeron P, Nardi N, Nicolas JM, Forns X, Plaza J, Yague J, Sanchez-Tapias JM, Font J. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthritis Rheum. 2006; 36: 189– 196. [DOI] [PubMed] [Google Scholar]
- 78. Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012; 379: 348– 360. [DOI] [PubMed] [Google Scholar]
- 79. Ramos-Casals M, Trejo O, Garcia-Carrasco M, Cervera R, Font J. Mixed cryoglobulinemia: new concepts. Lupus. 2000; 9: 83– 91. [DOI] [PubMed] [Google Scholar]
- 80. Retamozo S, Diaz-Lagares C, Bosch X, De Vita S, Ramos-Casals M. Life-threatening cryoglobulinemia. In: Khamashta MA, Ramos-Casals M, eds. Autoimmune Diseases. Acute and Complex Situations. London: Springer-Verlag; 2011: 133– 162. [Google Scholar]
- 81. Rieu V, Cohen P, Andre MH, Mouthon L, Godmer P, Jarrousse B, Lhote F, Ferriere F, Deny P, Buchet P, Guillevin L. Characteristics and outcome of 49 patients with symptomatic cryoglobulinaemia. Rheumatology (Oxford). 2002; 41: 290– 300. [DOI] [PubMed] [Google Scholar]
- 82. Roccatello D, Baldovino S, Rossi D, Mansouri M, Naretto C, Gennaro M, Cavallo R, Alpa M, Costanzo P, Giachino O, Mazzucco G, Sena LM. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2004; 19: 3054– 3061. [DOI] [PubMed] [Google Scholar]
- 83. Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banfi G, Confalonieri R, Tarantino A, Pasquali S, Amoroso A, Savoldi S, Colombo V, Manno C, Ponzetto A, Moriconi L, Pani A, Rustichelli R, Di Belgiojoso GB, Comotti C, Quarenghi MI. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007; 49: 69– 82. [DOI] [PubMed] [Google Scholar]
- 84. Rodriguez-Vidigal FF, Roig Figueroa V, Perez-Lucena E, Ledesma Jurado V, Ramirez Gurruchaga P, Aguilar Escobar FJ, Relea Calatayud MF, Baz MJ. [Alveolar hemorrhage in mixed cryoglobulinemia associated with hepatitis C virus infection]. An Med Interna. 1998; 15: 661– 663. [PubMed] [Google Scholar]
- 85. Roithinger FX, Allinger S, Kirchgatterer A, Prischl F, Balon R, Haidenthaler A, Knoflach P. A lethal course of chronic hepatitis C, glomerulonephritis, and pulmonary vasculitis unresponsive to interferon treatment. Am J Gastroenterol. 1995; 90: 1006– 1008. [PubMed] [Google Scholar]
- 86. Rossi P, Bertani T, Baio P, Caldara R, Luliri P, Tengattini F, Bellavita P, Mazzucco G, Misiani R. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int. 2003; 63: 2236– 2241. [DOI] [PubMed] [Google Scholar]
- 87. Saadoun D, Landau DA, Calabrese LH, Cacoub PP. Hepatitis C-associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology (Oxford). 2007; 46: 1234– 1242. [DOI] [PubMed] [Google Scholar]
- 88. Saadoun D, Resche-Rigon M, Thibault V, Piette JC, Cacoub P. Antiviral therapy for hepatitis C virus associated mixed cryoglobulinemia vasculitis: a long-term follow-up study. Arthritis Rheum. 2006; 54: 3696– 3706. [DOI] [PubMed] [Google Scholar]
- 89. Salamone F, Puzzo L. Intestinal HCV-related mixed cryoglobulinemia. Gastroenterology. 2010; 138: e9– e10. [DOI] [PubMed] [Google Scholar]
- 90. Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005; 5: 227– 236. [DOI] [PubMed] [Google Scholar]
- 91. Shibazaki K, Iguchi Y, Kimura K, Wada K, Ueno Y, Sunada Y. Paradoxical brain embolism associated with HCV-related type II mixed cryoglobulinemia. J Clin Neurosci. 2007; 14: 780– 782. [DOI] [PubMed] [Google Scholar]
- 92. Suzuki R, Morita H, Komukai D, Hasegawa T, Nakao N, Ideura T, Yoshimura A. Mixed cryoglobulinemia due to chronic hepatitis C with severe pulmonary involvement. Intern Med. 2003; 42: 1210– 1214. [DOI] [PubMed] [Google Scholar]
- 93. Tada M, Naruse S, Arai A, Sato A, Tanaka K, Piao YS, Kakita A, Takahashi H, Nishizawa M, Tsuji S. An autopsy case of systemic vasculitis associated with hepatitis C virus-related mixed cryoglobulinemia presenting severe peripheral neuropathy. Rinsho Shinkeigaku. 2004; 44: 686– 690. [PubMed] [Google Scholar]
- 94. Tarantino A, Campise M, Banfi G, Confalonieri R, Bucci A, Montoli A, Colasanti G, Damilano I, D’Amico G, Minetti L, Ponticelli C. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995; 47: 618– 623. [DOI] [PubMed] [Google Scholar]
- 95. Tembl JI, Ferrer JM, Sevilla MT, Lago A, Mayordomo F, Vilchez JJ. Neurologic complications associated with hepatitis C virus infection. Neurology. 1999; 53: 861– 864. [DOI] [PubMed] [Google Scholar]
- 96. Terrier B, Saadoun D, Sene D, Scerra S, Musset L, Cacoub P. Presentation and outcome of gastrointestinal involvement in hepatitis C virus-related systemic vasculitis: a case-control study from a single-centre cohort of 163 patients. Gut. 2010; 59: 1709– 1715. [DOI] [PubMed] [Google Scholar]
- 97. Trejo O, Ramos-Casals M, Garcia-Carrasco M, Yague J, Jimenez S, de la Red G, Cervera R, Font J, Ingelmo M. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001; 80: 252– 262. [DOI] [PubMed] [Google Scholar]
- 98. Visentini M, Granata M, Veneziano ML, Borghese F, Carlesimo M, Pimpinelli F, Fiorilli M, Casato M. Efficacy of low-dose rituximab for mixed cryoglobulinemia. Clin Immunol. 2007; 125: 30– 33. [DOI] [PubMed] [Google Scholar]
- 99. Zaja F, De Vita S, Russo D, Michelutti A, Fanin R, Ferraccioli G, Baccarani M. Rituximab for the treatment of type II mixed cryoglobulinemia. Arthritis Rheum. 2002; 46: 2252– 2254. [DOI] [PubMed] [Google Scholar]
- 100. Zandman-Goddard G, Levy Y, Weiss P, Shoenfeld Y, Langevitz P. Transverse myelitis associated with chronic hepatitis C. Clin Exp Rheumatol. 2003; 21: 111– 113. [PubMed] [Google Scholar]


