Abstract
We evaluated the influence of cryoglobulinemic syndrome (CS) on the outcome of chronic hepatitis C virus (HCV) infection in a 15-year prospective study. We assessed a cohort of 950 chronically HCV-infected patients, collected from the beginning of 1990 to the end of 2010. All patients had received a liver histologic diagnosis. Mixed cryoglobulinemia (MC) was determined in 246 patients (25.8%), of whom 184 also had CS. They were assessed every 3 months for 15 years, at least; 141 patients with CS and 601 without MC completed the study.
No spontaneous clearance of cryoglobulins was noted. Type II to type III spontaneous switching was ascertained in 1.6% (0.08%/yr) patients. The estimated progression rate of liver fibrosis was lower in CS(+) than in MC(−) patients (p < 0.05). The 15-year cumulative probability of developing cirrhosis and/or hepatocellular carcinoma was higher in MC(−) than in CS(+) patients (24.9% vs. 14.2%, p < 0.005 and 20.3% vs. 7.5%, p = 0.003, respectively). Renal insufficiency, neurologic impairment, or B-cell non-Hodgkin lymphoma were significantly more frequent in CS(+) than in MC(−) patients (32.6% vs. 3%, p < 0.0001; 31.2% vs. 4.8%, p < 0.0001; and 15% vs. 7.1%, p = 0.003, respectively). However, in spite of different morbidity features and causes of death, the 15-year survival rate was similar in the 2 groups (70.2% vs. 71.7%). Antiviral therapy had an undisputable impact on patient outcome.
This 15-year prospective cohort study shows that, although CS has no influence on the overall survival of HCV-infected patients, it significantly modifies the natural history of chronically HCV-infected patients.
INTRODUCTION
Hepatitis C virus (HCV) infection is the leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), which in turn are the most common indications for liver transplantation in developed countries.2 An understanding of the natural history of HCV infection is essential to effectively manage, treat, and counsel patients with acute and chronic forms, yet this knowledge is fraught with controversy. It has been calculated that 60%–80% of HCV-infected patients will progress to chronic infection.25 The rate of progression to chronicity is influenced by several factors, namely, age, sex, race, insulin resistance, liver steatosis, and immune response.21
In addition, patients with chronic HCV infection are at risk of developing a number of extrahepatic disorders.1,9 Among these, the most frequent is mixed cryoglobulinemia (MC), an immune complex-mediated systemic vasculitis involving mostly small blood vessels. HCV plays a primary role in the formation of immune complexes and in the production of tissue damage.41 Although small amounts of cryoglobulins can be detected in approximately 40% of these patients, only 12%–15% will develop a full-blown cryoglobulinemic syndrome (CS), which includes cutaneous vasculitis (skin rush, palpable purpura, and chronic ulcers), fatigue, arthralgia, membranoproliferative glomerulonephritis, and peripheral neuropathy.29,33 Furthermore, MC is capable of a) evolving into more aggressive hematologic disorders, such as malignant lymphoproliferative diseases;11 and b) causing and/or complicating chronic kidney disease.34
Based on a meta-analysis of 19 studies comprising a total of 2323 patients with chronic hepatitis C, 1022 (44%) of whom had detectable cryoglobulins, and by combining odds ratios adjusted for age, sex, and estimated disease duration, a highly significant association was determined between cirrhosis and cryoglobulinemia. This conclusion provided evidence that in chronic hepatitis C, cryoglobulins are an important prognostic indicator for an increased risk of cirrhosis.23 However, the statistical association between cryoglobulins and advanced liver fibrosis is still controversial. In a subsequent study24 the same authors of the meta-analysis reported that cryoglobulins did not emerge as an independent factor for advanced liver fibrosis, attributing this difference mainly to the design of the studies which included many important clinical and laboratory variables. Furthermore, it was shown that fibrosis accumulation in the liver is not a linear process, in that impact of age on the progression of hepatic disease is remarkable.39 The risk of fibrosis progression in the fifth decade was estimated to be 3 times higher than in the second decade, supporting the concept that the aging liver is more susceptible to fibrosis.5 Compared to HCV-infected patients with cryoglobulins, those without have a median lower age and a shorter history of hepatitis.27,43 In a 10-year prospective cohort study, it was shown that MC did not influence the clinical course of HCV-related disease; rather, the rate of progression to cirrhosis was similar in patients with or without cryoglobulins.53 Due to the limited number of patients with overt CS evaluated in this and other studies,16,22,46 to our knowledge the long-term impact of active cryoglobulinemic vasculitis on the natural history of HCV infection has not been assessed. A retrospective analysis of MC patients emphasized that involvement of multiple organs obviously influences the severity of CS, thereby significantly reducing overall life expectancy.18 However, most studies exploring the natural history of HCV-infected MC patients have been biased,8,13,31 in that the variable outcome of MC patients can be at least partly explained by the spectrum of clinical course, ranging from spontaneous exacerbations and remissions to the occurrence of multiple signs and symptoms that may appear either together or separately. Thus, given their clinical heterogeneity, patients consult different subspecialists. An additional point is the geographic variability of MC, as its prevalence has been reported to vary in different areas.12
In this context, a critical point that remains to be clarified is whether and to what extent CS affects the course of HCV infection. A relatively benign course of CS was indeed suggested,30 although it could not be excluded that increases in morbidity and mortality may become apparent with longer follow-up. In addition, because the response rates of currently available therapies are largely variable, a precise assessment of their effectiveness in reducing morbidity and mortality is needed. To characterize the impact of CS on the course of chronically HCV-infected patients and to define the risk-factor profile capable of predicting clinical outcome, prospective long-term cohort studies are required.
This work presents the clinical features and progression rates of CS based on a 15-year prospective study carried out in patients with chronic hepatitis C.
PATIENTS AND METHODS
Study Design
We conducted the current cohort study to provide data from a large population of chronic HCV-infected individuals on the occurrence of cold-precipitating proteins in order to clarify the natural history of cryoglobulin-related HCV-positive patients and their long-term outcome. We applied the following inclusion criteria: 1) positivity for anti-HCV antibodies and polymerase chain reaction (PCR)-based assay to detect HCV RNA in serum of patients with/without palpable purpura; 2) detection of serum cryoglobulins; 3) liver biopsy showing chronic hepatitis performed within 3 months from enrollment; 4) negativity for hepatitis B surface antigen and human immunodeficiency virus (HIV); and 5) no previous administration of interferons (IFNs) or immunosuppressive drugs. Starting with enrollment, each patient’s data were computerized in real-time. Patients were identified by a unique central identification code, which was used only for study purposes. After providing informed consent, each patient was assigned a study code followed by a city code and a patient number. For the duration of the study and afterwards, only the patient’s physician was able to identify the patient based on his or her identification number. We prospectively analyzed the records of all patients followed at the Liver Unit of our Department from 1990 to 2010 (Table 1).
TABLE 1.
Baseline Clinical, Laboratory, Virologic and Histologic Parameters of Chronically HCV-Infected Patients With and Without Cryoglobulins

We considered the clinical and laboratory findings and the underlying diseases of 950 HCV-infected patients. Chronic liver damage ascribable to drugs, alcohol, iron, copper, autoimmune damage, and α-1-antitrypsin deficiency was excluded based on the patient’s history, appropriate serologic studies, and findings on liver biopsy. Patients with a history of organ transplantation were also excluded.
Two expert pathologists blinded to both the patients’ clinical records and their graded liver histologic specimens participated in the study. Liver biopsies were scored for grade and stage according to METAVIR score.4,19 The stage of fibrosis was assessed as a 5-point scale, with 0 defined as no fibrosis and 4 as frank cirrhosis. Necro-inflammatory activity was graded according to a 4-point scale, in which 0 indicated no inflammation and 3 indicated a heavy inflammatory cell infiltration and severe hepatocyte necrosis. Steatosis was estimated and scored according to a 4-point scale7: 0 = steatosis; 1 = 1%–33% of involved hepatocytes; 2 = 34%–66% of involved hepatocytes; and 3 = >66% of involved hepatocytes. In terms of interobserver reliability, the percentage of agreement for disease activity and staging in addition to the pairwise comparison, weighted kappa score were measured. The percentage agreement for histologic variable and the categorical deviance among the observers were performed for each sample in a slide by slide analysis.49
To calculate lifetime drinking habits and total lifetime alcohol consumption, an accurate history of alcohol use was deduced from a detailed questionnaire. Only patients with a daily alcohol consumption below 40 g were enrolled. The rate of fibrosis progression was estimated in paired liver biopsy samples from patients who had received no specific antiviral treatment and had no evidence of cirrhosis. Fibrosis progression/year was calculated as the ratio between the fibrosis stage (in METAVIR units) and the estimated time elapsed between the 2 bioptic evaluations (in years).35
Study Objectives
Because the present study was designed to assess the impact of CS on the outcome of patients with chronic hepatitis C, the primary endpoint was overall survival, defined as the time from enrollment until death. Death was defined as liver-related if it was closely linked to end-stage liver disease and/or to the progression of HCC. A progression of chronic hepatitis to cirrhosis, clinical decompensation, and HCC development were all carefully monitored. An additional endpoint was cryoglobulin-related extrahepatic complications, including chronic kidney damage, neurologic impairment, autoimmune disorders, and the development of lymphoma.
Baseline and Follow-Up Evaluations
Baseline evaluation included demographic data, medical history, physical examination, evaluation of comorbidities, and use of concomitant medications. In addition to routine laboratory parameters, HCV RNA, HCV genotypes, immunochemical typing, and cryoglobulin levels were measured and ultrasonography with or without contrast-enhancement was obtained. Neurologic assessment included electromyography, motor and sensory nerve conduction velocity, and short-latency somatosensory-evoked potentials.
Lymphocytes were phenotyped by single/double immunofluorescence on a FACScan instrument (Becton Dickinson, Franklin Lakes, NJ) using fluorochrome-conjugated antibodies against CD19, CD20, and CD5, and CD3, CD4, and CD8 (Becton Dickinson).
During follow-up, each patient was assessed approximately every 3 months for 15 years at least. Follow-up included physical examination, cryocrit assay, laboratory measurements, performance status, and ultrasonography study. Each patient provided written consent. The study was in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the ethical review board of the University of Bari.
Laboratory Parameters
All patients were tested for HCV antibodies with second-/third-generation enzyme-linked immunosorbent assay (ELISA) (Abbott Laboratories, Chicago, IL). Serum HCV RNA was determined by RT-PCR (Roche Diagnostic System, Branchburg, NJ). HCV RNA levels were measured with a branched-chain DNA assay (bDNA 3.0, Bayer Health Care, Tarrytown, NY). HCV genotyping was performed by INNO-LiPA (Innogenetics NV, Ghent, Belgium).
Routine tests included rheumatoid factor (RF), serum IgG, IgA, and IgM levels, and complement fractions C3 and C4, which were measured by immuno-turbidimetric assay. Cryoglobulins were measured as described elsewhere.12 The immunochemical composition of the cryoprecipitates was determined by immunofixation. Cryoglobulins were classified according to Brouet et al6: type I cryoglobulins consisted of a single monoclonal component, whereas mixed cryoglobulins comprised 2 immunoglobulin isotypes, with (type II) or without (type III) a monoclonal component.
Statistical Analysis
The McNemar chi-square or the Wilcoxon signed-rank test was used to compare qualitative and quantitative variables. The Cochran-Mantel-Haenszel test or the Fisher exact test was used to compare categorical variables. The influence of clinical characteristics on the response was determined by logistic regression analyses. All statistical tests were 2-tailed, with p < 0.05 as the significance cutoff. The probability of patient survival was calculated by life-table analysis. The difference between the cumulative curves was tested by the log-rank test.
RESULTS
Among the 950 patients enrolled in this study, 246 (25.8%) were shown to have circulating cryoglobulins. In these 246 patients, an overt CS was recorded in 115 (46.7%) at study referral, whereas in 69 patients (28%) symptoms appeared at variable times after enrollment, with a median period of 14 months (range, 6–18 mo). In the remaining 62 patients (25.2%), there was no occurrence of CS throughout the follow-up; these patients were not considered further in this study.
The study began in 1990 and had a median duration of 182 months, with no difference in duration between CS(+) and MC(−) patients (178 vs. 166 mo, respectively). Forty-three CS(+) and 103 MC(−) patients were lost to the study for reasons including denial of informed consent to proceed with the study, logistical problems, and lack of compliance.
As shown in Table 1, comparable median levels of circulating HCV RNA were detected in the 141 CS(+) patients and 601 MC(−) patients. Genotype 1 occurred in 76 (54%) of the CS(+) and in 306 (51%) of the MC(−) patients. The 2 groups did not differ in the mean age at study referral (59 ± 7.6 yr vs. 57.6 ± 12.2 yr for CS(+) and MC(−) patients, respectively); however, CS occurred more frequently in female patients (p = 0.003).
The transmission of HCV is primarily by exposure to infected blood. In the present series, the transfusion of blood or blood products was determined to be the cause in 30 (21.3%) CS(+) and 150 (24.9%) MC(−) patients. Needle-stick puncture was confirmed in 20 (14.2%) CS(+) and 91 (15.2%) MC(−) patients. However, in almost 60% of the patients, no recognized source of infection could be identified. In this group, intravenous drug use, high-risk sexual activity, occupational exposure, hemodialysis, and birth to an infected mother were excluded.
The mean cryocrit was 7.2% ± 10.5%, ranging from 1% to 80%. Cryoglobulinemic patients were tested for the presence or absence of a monoclonal component by the immunofixation assay. Both serum and purified cryoprecipitates were simultaneously tested. Among 141 patients, a monoclonal component was found in 124 cryoprecipitates and in 116 corresponding serum samples, showing that use of cold precipitates is most suitable for cryoglobulin phenotyping in clinical practice. Thus, immunochemically there was a remarkable prevalence of type II MC, which occurred in 88.6%, as compared with type III MC, which was typed in the remaining 11.4% of patients.
The cryoglobulins never cleared spontaneously and persisted throughout the follow-up period, with fluctuating cryocrit values in all untreated patients. We noted that in 2 patients (1.6%), there was a switch from type II to type III MC over a median time of 5.5 ± 0.7 years and at an annual rate of 0.08%. This immunochemical change was accompanied by a reduction of IgM-RF titers and a normalization of serum C4 levels, whereas the serum viral load remained largely unchanged and clinical symptoms of vasculitis spontaneously subsided.
A symptoms review was carried out during clinical assessment on the first and subsequent follow-up visits. Palpable purpura was the most specific symptom, with 90% of the patients experiencing recurring episodes. Conversely, purpuric vasculitis could not be documented in any of the MC(−) patients (p < 0.0001). Weakness and arthralgia were the other main symptoms reported at diagnosis and during follow-up. As expected, both at initial diagnosis and subsequently, CS(+) patients had significantly more complaints than MC(−) patients (86.5% vs. 47%, p < 0.0001 and 63.1% vs. 16%, p < 0.0001, respectively). At the time of study admission, only 6 (4.2%) of the CS(+) patients had chronic leg ulcers, whereas during follow-up 41 patients (29%) developed nonhealing cutaneous ulcers requiring treatment in numerous hospital admissions. In sharp contrast, only 4 MC(−) patients (0.6%) developed leg ulcers (p < 0.0001). In terms of liver involvement at referral, the occurrence of cirrhosis was 8% in CS(+) and 6.5% in MC(−) groups.
Laboratory parameters were regularly monitored. Serum IgM levels were significantly higher in CS(+) (p = 0.0002) patients, whereas serum IgG concentrations were higher in MC(−) patients (p < 0.0001). In addition, the former group had higher levels of RF activity (p = 0.02) and lower concentrations of the complement C4 fraction (p < 0.0001). Because interactions between lymphocytes and HCV may result in a direct modulation of B and T cells, with consequent phenotypic changes of circulating cell populations, we analyzed peripheral blood lymphocytes by flow cytometry. Comparable total lymphocyte counts were determined in CS(+) and MC(−) patients (1390 ± 305 cells/μL vs. 1460 ± 291 cells/μL, respectively). The numbers of CD19+/CD20+ B cells and CD4+ T cells were significantly higher in CS(+) patients (p < 0.0001 and p < 0.001, respectively).
Fibrosis Progression
Fibrosis progression was defined as the ratio between fibrosis stage (in METAVIR units) and estimated duration of infection (in years), calculated as the time elapsed between 2 subsequent liver biopsies. Twenty-five of the 30 CS(+) patients and 60 of the 85 MC(−) patients who refused IFN-α ± ribavirin (RBV) antiviral therapy agreed to undergo a second liver biopsy. The mean time between the 2 biopsies was 6.7 ± 0.6 years in CS(+) patients and 7.1 ± 0.9 years in MC(−) patients. Fibrosis progression, evaluated as a 1-stage increase in the METAVIR grade, was assessed independently by 2 expert pathologists. Results of the agreement percentage was 92% with a weighted kappa of 0.67 ± 0.12 value. Liver disease progression was documented in 16 (53%) of the CS(+) and in 51 (85%) of the MC(−) patients, corresponding to an estimated annual progression rate of 0.13 units and 0.20 units, respectively (p < 0.05).
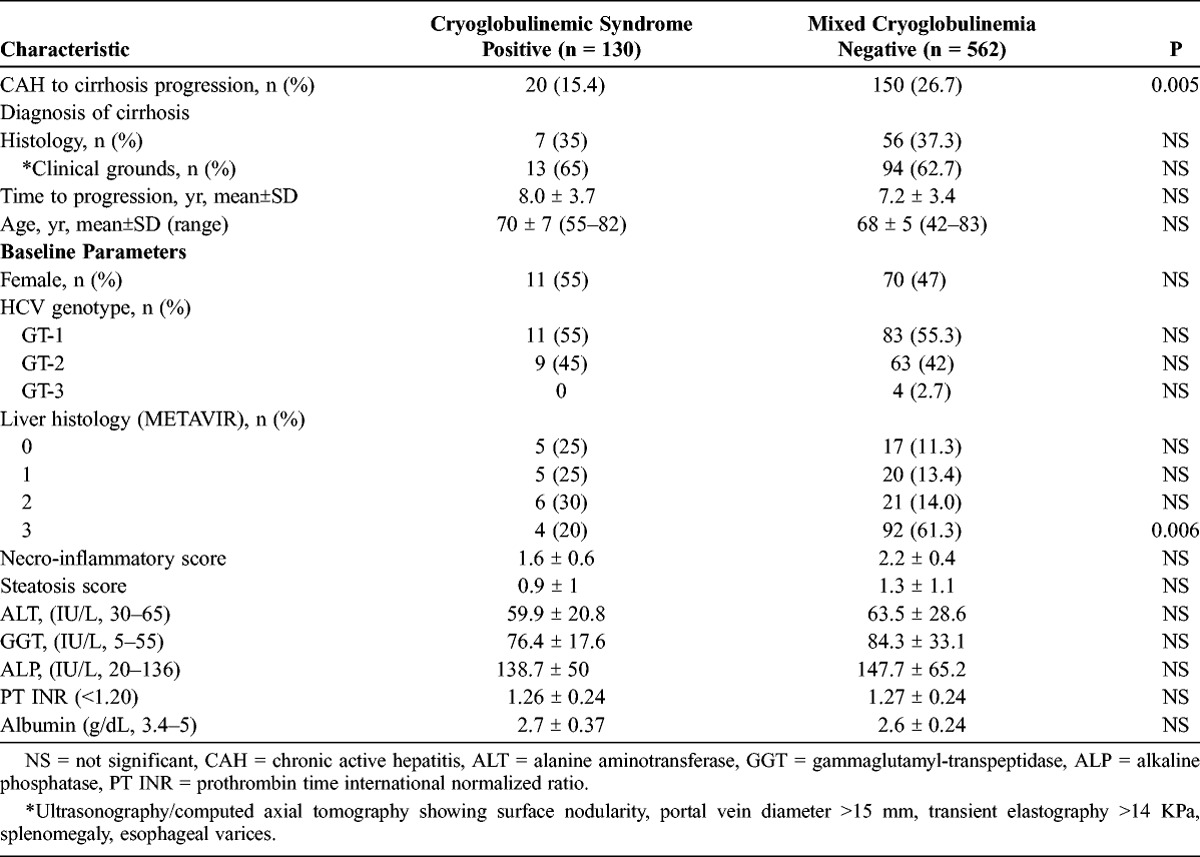
The 15-year cumulative probability of developing cirrhosis is diagrammed in Figure 1. Progression from chronic hepatitis to cirrhosis was detected by histology in 7 CS(+) patients and in 56 MC(−) patients. In the remaining patients, cirrhosis was diagnosed on clinical grounds and by imaging techniques, including ultrasonography with or without contrast enhancement, computed axial tomography, and elastography. Progression to cirrhosis was shown in 20 CS(+) and 150 MC(−) patients, with the cumulative probability rate reaching 15.4% in the former group and 26.7% in the latter group (odds ratio, 0.65; 95% confidence interval, 0.29-0.82; p < 0.005). This finding supports the notion that in CS(+) patients progression of liver fibrosis was slower than that occurring in MC(−) patients. As shown in Table 2, the only independent predictor was METAVIR stage 3 at baseline (p = 0.006).
FIGURE 1.

Cumulative probability of histologic/clinical progression to cirrhosis in 130 CS(+) and 562 MC(−) chronically HCV-infected patients.
TABLE 2.
Chronic Active Hepatitis to Cirrhosis Progression in Chronically HCV-Infected Patients With or Without Cryoglobulins
Outcome
Clinical outcome is reported in Table 3. Kidney involvement was the most frequent clinical outcome in the present series of CS(+) patients. Notably, at the time of admission none of the CS(+) patients and only 2 (0.3%) of the MC(−) patients had abnormal renal function. However, within a period ranging from 5 to 12 years, 46 CS(+) patients (32.6%) and 18 MC(−) patients (3%) developed acute and/or chronic renal insufficiency (p < 0.0001). Renal biopsies were performed in the 32 CS(+) and in the 4 MC(−) patients with overt functional abnormalities; histology identified membranoproliferative glomerulonephritis as the prevalent pattern. Other forms of glomerulopathies included membranous nephropathy, focal/segmental glomerulosclerosis, and IgA nephropathy. The clinical course was characterized by remission as well as by relapsing phases. No specific symptoms or clinical manifestations occurred. Renal signs included proteinuria and microscopic hematuria with mild to moderate renal insufficiency. In some cases, glomerular disease was ultimately diagnosed as acute oliguria associated with arterial hypertension.
TABLE 3.
Clinical Outcome According to the Presence or Absence of Cryoglobulins

Monoclonal gammopathy of undetermined significance (MGUS) of IgM isotype occurred with a significantly higher frequency in CS(+) patients. Its mean time to appearance was 4.5 ± 3.7 years in 17 CS(+) patients (12%) and 10 ± 8.5 years in 5 MC(−) patients (0.8%) (p < 0.0001). Conversely, there was no difference in the distribution of MGUS of IgG and IgA isotypes between the 2 groups of patients.
Neurologic disorders were significantly more frequent in CS(+) patients (p < 0.0001). Among the 44 (31.2%) with peripheral neuropathy, 36 underwent electromyography, which evidenced symmetrical, distal sensory polyneuropathy, and mononeuritis multiplex in most of them. Characteristically, there was a slowly progressive involvement of the legs. By contrast, neuropathy in the 29 MC(−) patients (4.8%) tended to remain stable.
Unexpectedly, arterial hypertension occurred mostly in the MC(−) patients. In the course of the study, 93 MC(−) patients (15.5%) and 10 CS(+) patients (7%) developed arterial hypertension (p < 0.001).
There was no difference in the distribution of thyroid disorders, including nodular disease and autoimmune thyroiditis, between the 2 groups at referral. Thyropathies became evident during follow-up in 38 CS(+) (27%) and 91 MC(−) (15.1%) patients (p = 0.002). Similarly, the distribution of autoimmune diseases was essentially the same in the 2 groups. At the end of the study, 5 (3.5%) CS(+) and 12 MC(−) (2%) patients developed autoimmune disorders, including Sjögren syndrome, autoimmune thrombocytopenia, and Raynaud syndrome.
Type 2 diabetes mellitus, solid tumors (breast, lung, stomach, prostate, colon, bladder), chronic ischemic heart disease, chronic lymphocytic leukemia, Hodgkin lymphoma, and primary amyloidosis were diagnosed with similar frequencies in the 2 groups. Gastrointestinal involvement was also equally distributed. Several episodes of diffuse abdominal pain were reported, but gastrointestinal bleeding was not.
B-Cell Non-Hodgkin Lymphoma
B-cell non-Hodgkin lymphoma (B-NHL), a potential late complication of cryoglobulinemia, occurred in both groups of patients. The cumulative probability of developing B-NHL is provided in Figure 2. Its prevalence in CS(+) and MC(−) patients was 5.3% vs. 1.2% (p = 0.006); 8.9% vs. 3.6% (p = 0.01); 13% vs. 6.2% (p = 0.02); and 15% vs. 7.1% (p = 0.003) at 3, 5, 10, and 15 years from the initial diagnosis, respectively.
FIGURE 2.

Cumulative probability of type B non-Hodgkin lymphoma in 141 CS(+) and 601 MC(−) chronically HCV-infected patients.
As shown in Table 4, among the patient characteristics, female sex, mean age, mean concentration of HCV RNA, and HCV genotype were similar between the 2 groups. A nodal localization of B-NHL was seen in 10 (45.4%) and 25 (58.1%) of CS(+) and MC(−) patients, respectively. Among the extranodal localizations, the spleen was the most frequent site in both groups. Concerning histotype, diffuse large B cells had a higher prevalence in both CS(+) and MC(−) patients. Concomitant cirrhosis occurred in 22.7% of CS(+) and in 30.2% of MC(−), a difference that was not significant. Mean survival was also comparable between the 2 groups: 4.4 ± 2.2 years and 5.6 ± 3.1 years in CS(+) and MC(−) patients, respectively.
TABLE 4.
Clinical, Histologic, and Virologic Characteristics of Non-Hodgkin Lymphoma Development in Chronically HCV-Infected Patients With or Without Cryoglobulins

Hepatocellular Carcinoma
The 15-year cumulative probability of developing HCC in the presence or absence of CS is reported in Figure 3. The rate of occurrence of HCC in CS(+) and MC(−) patients was 0% vs. 6% (p = 0.007), 1.8% vs. 8.2% (p = 0.006), 7.5% vs. 18.5% (p = 0.0003), and 10.6% vs. 20.3% (p = 0.001) at 3, 5, 10, and 15 years from the initial diagnosis, respectively.
FIGURE 3.

Cumulative probability of hepatocellular carcinoma in 141 CS(+) and 601 MC(−) chronically HCV-infected patients.
The main features of HCC patients are summarized in Table 5. Females prevailed in the CS(+) patients, whereas the mean age was comparable in the 2 groups. No differences were found regarding mean levels of circulating HCV RNA, clinical stage of the tumor, and frequency of HCC nodules. A comparable mean survival was also confirmed.
TABLE 5.
HCC Development in Chronically HCV-Infected Patients With or Without Cryoglobulins

Mortality Rate and Causes of Death
During the 15-year follow-up, 212 patients died (28.6%). The mortality rate was similar between the 2 groups: 29.7% (42/141) in CS(+) and 28.3% (170/601) in MC(−) patients.
As shown in Figure 4, liver-related death was ascertained in 104 patients (50.5%). Liver decompensation, including ascites, encephalopathy, bleeding of esophageal varices, and HCC complications, was the final cause of liver-related mortality in 14 of 42 CS(+) (33%) and in 90 of 170 MC(−) (52.9%) patients (p = 0.02). Notably, HCC and B-NHL were concomitantly present in 2 CS(+) and 2 MC(−) patients. Among the extrahepatic-related deaths, renal failure and septic shock following systemic infection significantly prevailed in CS(+) patients compared with MC(−) patients: 33% vs. 4.3% and 14.3% vs. 4.3%; p = 0.0001 and p = 0.03, respectively. The 2 groups did not differ with respect to cardiac- or respiratory-related deaths.
FIGURE 4.

Cumulative incidence of liver-related and non-liver-related events in 141 CS(+) and 601 MC(−) chronically HCV-infected patients.
Overall Survival
The 15-year cumulative survival was similar in CS(+) and MC(−) patients (70.2% vs. 71.7%, respectively) (Fig. 5A). However, the length of survival significantly differed between patients with and without antiviral therapy. Figure 5B illustrates the change in the proportion of treated patients with CS and those without MC. Antiviral therapy included IFN-α monotherapy (n = 56), pegylated-IFN-α (pIFN-α) monotherapy (n = 90), IFN-α plus RBV (n = 85), and pIFN-α plus RBV (n = 108). Treatment provided sustained virologic response in 44% and 53% of CS(+) and MC(−) patients respectively.
FIGURE 5.

A. Overall patient survival in 141 CS(+) and 601 MC(−) chronically HCV-infected patients. B. Overall patient survival according to antiviral therapy.
DISCUSSION
The main advantage of the current study is that we were able to enroll and follow-up a large number of untreated chronically HCV-infected patients, all of whom had undergone liver biopsy. To reduce the risk of selection bias, patients with different sources of infection were included. Conversely, the main limitation is that the estimated duration of HCV infection mostly relies on the history; however, patients frequently consider the day of ascertainment of abnormal liver enzymes as the starting point, whereas the true duration is difficult to establish due to the lack of an acute phase of HCV infection, or to the fact that it was silent and therefore overlooked, coupled with the absence of symptoms during the early phase of chronic infection.26 In sharp contrast, cryoglobulinemic vasculitis, which complicates HCV infection, develops from the very beginning with exuberant clinical symptoms that are thought to result from the deposition of immune complexes in various organs.41 In these patients, CS is a function of the host inflammatory response to the cryoprecipitating immune complexes.45
A few studies have attempted to measure the time interval from HCV infection to the detection of MC and from MC onset to the occurrence of CS.8,52,55 Because only a proportion of cryoglobulinemic patients develop CS, the diagnosis of MC is frequently delayed and underestimated. In the present study, CS was clinically overt in almost half the patients at recruitment, developed later in about 30% of them, and remained totally absent throughout the period of observation in the remainder. Therefore, given the close time range between MC and CS, screening for cryoglobulins is appropriate in all chronically HCV-infected patients. It is also advisable to search for cryoglobulins when patients are found to be anti-HCV positive rather than when they become symptomatic.17
Type II MC occurred in more than 80% of our CS(+) patients. This high rate emphasizes the relatively poor sensitivity of immunofixation in detecting a monoclonal component in serum. It also importantly suggests that cryoprecipitates should be redissolved before being tested by immunofixation, to more efficiently reveal monoclonality.47 In only 2 instances did we observe a switch from type II to type III MC in the absence of any specific treatment. This immunochemical shift occurred in step with the loss of clinical manifestations of vasculitis, the normalization of circulating C4 levels, and the significant lowering of RF activity. These observations suggest that cryoglobulinemic damage is strictly related to cryoglobulin structure, in particular to the presence of monoclonal IgM-RF molecules, which are capable of activating the complement cascade.44 Indeed, according to our data, 51 of the 62 (82.2%) patients with MC, who remained CS(−) throughout the observation period, had type III MC.
Because the production of high RF levels in MC is strictly associated with oligoclonal/monoclonal expansions of B cells,36,42 it has been inferred that the initial immune response to HCV results in the synthesis of IgM molecules devoid of monoclonal RF activity, a property that is subsequently acquired through somatic mutations accompanying cell proliferation due to the persistence of HCV. One would therefore expect that the production of IgM-RF leads to the formation of type III MC, which eventually switches into type II MC when the RF becomes monoclonal.14 However, there is no experimental or clinical support to this mechanism, despite many years of close observation. Thus, based on our findings, it can be hypothesized that type II MC originates as such and seldom changes to type III MC because of spontaneous exhaustion of the involved B-cell clonotype. In addition, since according to this study a type II to type III conversion occurred in the presence of a roughly unchanged viral load, it seems reasonable to assume that the production of monoclonal RF is not directly dependent on HCV, but instead can arise in a subgroup of patients who possibly lack the physiologic mechanism(s) needed to inhibit the production of high-affinity IgM-RF molecules with high pathogenetic potential.51,54
We analyzed liver fibrosis to identify independent risk factors for both patient groups. The major finding that emerged from this study was somewhat surprising, in that the grade of fibrosis was significantly lower in CS(+) than in MC(−) patients, despite the similar prevalence of factors commonly associated with an increased risk of liver fibrosis, including age, duration of HCV infection, and steatosis.35 Overall, while there were no differences between CS(+) and MC(−) patients with respect to HCV genotypes, viral load, or transaminases, prothrombin, or albumin levels, a higher fibrosis score and, in parallel, an increased incidence of cirrhosis was determined in the MC(−) patients.
These data suggest that cryoglobulins only slightly impact liver fibrosis, and rule out their clinical significance as a prognostic indicator for an increased risk of cirrhosis, in contrast to previous reports.27,48 The reasons for these discrepancies are unclear. However, previous studies were biased by the selection of patients with specific signs and symptoms17 and had a cross-sectional design37 or were of relatively short mean duration, (20 ± 12 months).16 In another report, the apparent lack of association between cryoglobulins and fibrosis was attributed to sample size and/or to the inclusion of patients with alcohol abuse.24
The risk profile for HCC development differed between the 2 groups of patients. Virtually all HCV-related HCC develops in patients with established cirrhosis. Accordingly, we found that HCC significantly prevailed in MC(−) patients. The 15-year cumulative probability of developing HCC was estimated to be 20.3% and 10.6% in MC(−) and CS(+) patients (p = 0.003), respectively.
A prerequisite for MC development is HCV-driven B-cell deregulation, which accounts for another important difference between the 2 groups that is likely to have contributed to the worse outcome of CS(+) patients in the current study. The risk of developing B-NHL was significantly higher in the presence of cryoglobulins: in fact, it occurred in 15.6% of the CS(+) and in 7.1% of MC(−) patients (p = 0.003). We did not find any difference in the specific histotype or in the predominant site between the 2 groups, as already reported.10,15,28 Low- and high-grade B-NHLs as well as nodal and extranodal localizations had similar frequencies in the patient groups. This likely reflected the design of the present long-term cohort prospective study, which allowed assessment of the associations of multiple outcomes.
To our knowledge, no studies have considered the time-period required to develop kidney involvement. In the present series, kidney function was affected in 32.6% of CS(+) but in only 3% of MC(−) patients (p < 0.001). It should be emphasized that renal failure develops slowly, over a period of years, during which the only signs are usually slight proteinuria and/or microhematuria. Arterial hypertension, indicative of impaired renal function, may appear during the course of CS. A periodic surveillance of renal function is therefore warranted to exclude cryoglobulin-related renal complications.
Involvement of the nervous system is an obviously important determinant of the outcome of patients with cryoglobulinemia. Its incidence varies and may reach almost 90% of cases.3,20,50 Nervous system involvement was, in fact, significantly prevalent in the CS(+) patients (31.2%) vs. only 4.8% of the MC(−) cases (p < 0.001). Sensory-motor neuropathy, especially paresthesias of the lower limbs, were often reported as painful and were associated with a loss of strength. Conversely, the central nervous system is known to be rarely affected.
HCV-infected populations face a reduction in their overall life expectancy of approximately 8–12 years.38 The increased mortality can be explained by the complications of cirrhosis 20–25 years after HCV infection.32 The present 15-year prospective cohort study clearly shows that the appearance of CS in HCV-related MC does not affect overall patient survival. There were, however, differences regarding mortality, in that liver-related deaths were prevalent in HCV-infected MC(−) patients whereas extrahepatic causes predominated in CS(+) patients. However, antiviral therapy and viral clearance positively influenced morbidity and mortality. Antiviral therapy is indeed a crucial factor that has been shown to affect outcome. In the current study, about half of the patients were treated with antiviral therapy. In treated patients who achieved a sustained virologic response, their long-term benefits and better outcome were unquestionable.
In conclusion, this long-term study provides strong evidence that the occurrence of CS profoundly affects the natural history of HCV chronic infection. However, in spite of different morbidity features and causes of death, CS has no influence on the overall survival of these patients.
ACKNOWLEDGMENT
The authors thank Mr. Vito De Gennaro for his skillful assistance.
Abbreviations
- B-NHL
B-cell non-Hodgkin lymphoma
- CS
cryoglobulinemic syndrome
- HCV
hepatitis C virus
- MC
mixed cryoglobulinemia
- HCC
hepatocellular carcinoma
- IFN-α
interferon-α
- MGUS
monoclonal gammopathy of undetermined significance
- PCR
polymerase chain reaction
- RBV
ribavirin
- RF
rheumatoid factor
Footnotes
Financial support and conflicts of interest: The study was supported in part by grants from Italian Medicines Agency (AIFA), funds for independent studies, 2007, contract no. FARM7SJX (to DS); Fondazione Cassa di Risparmio di Puglia; University of Bari. The funders had no role in the design, conduct, or analysis of the study. The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992; 327: 1490– 1495. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad J, Eng FJ, Branch AD. HCV and HCC: clinical update and a review of HCC-associated viral mutations in the core gene. Semin Liver Dis. 2011; 31: 347– 355. [DOI] [PubMed] [Google Scholar]
- 3. Ammendola A, Sampaolo S, Migliaresi S, Ambrosone L, Ammendola E, Ciccone G, Di Iorio G. Autonomic neuropathy in mixed cryoglobulinemia. J Neurol. 2007; 254: 215– 219. [DOI] [PubMed] [Google Scholar]
- 4. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996; 24: 289– 293. [DOI] [PubMed] [Google Scholar]
- 5. Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999; 30: 1054– 1058. [DOI] [PubMed] [Google Scholar]
- 6. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974; 57: 775– 788. [DOI] [PubMed] [Google Scholar]
- 7. Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander-Tetri BA, Janney CG, Bacon BR, Di Bisceglie AM. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol. 2003; 16: 49– 56. [DOI] [PubMed] [Google Scholar]
- 8. Bryce AH, Kyle RA, Dispenzieri A, Gertz MA. Natural history and therapy of 66 patients with mixed cryoglobulinemia. Am J Hematol. 2006; 81: 511– 518. [DOI] [PubMed] [Google Scholar]
- 9. Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009; 76: 818– 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006; 15: 2078– 2085. [DOI] [PubMed] [Google Scholar]
- 11. Dammacco F, Gatti P, Sansonno D. Hepatitis C virus infection, mixed cryoglobulinemia, and non-Hodgkin’s lymphoma: an emerging picture. Leuk Lymphoma. 1998; 31: 463– 476. [DOI] [PubMed] [Google Scholar]
- 12. Dammacco F, Sansonno D, Piccoli C, Tucci FA, Racanelli V. The cryoglobulins: an overview. Eur J Clin Invest. 2001; 31: 628– 638. [DOI] [PubMed] [Google Scholar]
- 13. Della Rossa A, Tavoni A, D’Ascanio A, Catarsi E, Marchi F, Bencivelli W, Salvadori S, Migliorini P, Bombardieri S. Mortality rate and outcome factors in mixed cryoglobulinaemia: the impact of hepatitis C virus. Scand J Rheumatol. 2010; 39: 167– 170. [DOI] [PubMed] [Google Scholar]
- 14. De Rosa FG, Abel G, Agnello V. Observations on cryoglobulin testing: II. The association of oligoclonal mixed cryoglobulinemia with cirrhosis in patients infected with hepatitis C virus. J Rheumatol. 2009; 36: 1956– 1957. [DOI] [PubMed] [Google Scholar]
- 15. de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, Spinelli JJ, Zheng T, Zhang Y, Franceschi S, Talamini R, Holly EA, Grulich AE, Cerhan JR, Hartge P, Cozen W, Boffetta P, Brennan P, Maynadie M, Cocco P, Bosch R, Foretova L, Staines A, Becker N, Nieters A. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008; 6: 451– 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donada C, Crucitti A, Donadon V, Tommasi L, Zanette G, Crovatto M, Santini GF, Chemello L, Alberti A. Systemic manifestations and liver disease in patients with chronic hepatitis C and type II or III mixed cryoglobulinaemia. J Viral Hepat. 1998; 5: 179– 185. [DOI] [PubMed] [Google Scholar]
- 17. El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002; 36: 1439– 1445. [DOI] [PubMed] [Google Scholar]
- 18. Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, Puccini R, Michelassi C, Zignego AL. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004; 33: 355– 374. [DOI] [PubMed] [Google Scholar]
- 19.The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994; 20: 15– 20. [PubMed] [Google Scholar]
- 20. Germignani F, Melli G, Inglese C, Marbini A. Cryoglobulinemia is a frequent cause of peripheral neuropathy in undiagnosed referral patients. Peripher Nerv Syst. 2002; 7: 59– 64. [DOI] [PubMed] [Google Scholar]
- 21. Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, Park Y, Liang TJ, Hoofnagle JH. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003; 124: 97– 104. [DOI] [PubMed] [Google Scholar]
- 22. Joshi S, Kuczynski M, Heathcote EJ. Symptomatic and virological response to antiviral therapy in hepatitis C associated with extrahepatic complications of cryoglobulimia. Dig Dis Sci. 2007; 52: 2410– 2417. [DOI] [PubMed] [Google Scholar]
- 23. Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002; 36: 978– 985. [DOI] [PubMed] [Google Scholar]
- 24. Kayali Z, Tan S, Shinkunas L, Voigt M, LaBrecque DR, Stapleton JT, Brown KE, Schmidt WN. Risk factors for hepatitis C fibrosis: a prospective study of United States veterans compared with nonveterans. J Viral Hepat. 2007; 14: 11– 21. [DOI] [PubMed] [Google Scholar]
- 25. Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000; 132: 296– 305. [DOI] [PubMed] [Google Scholar]
- 26. Loomba R, Rivera MM, McBurney R, Park Y, Haynes-Williams V, Rehermann B, Alter HJ, Herrine SK, Liang TJ, Hoofnagle JH, Heller T. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther. 2011; 33: 559– 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lunel F, Musset L, Cacoub P, Frangeul L, Cresta P, Perrin M, Grippon P, Hoang C, Piette JC, Huraux JM, Opolon P. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994; 106: 1291– 1300. [DOI] [PubMed] [Google Scholar]
- 28. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011; 117: 1792– 1798. [DOI] [PubMed] [Google Scholar]
- 29. Meltzer M, Franklin EC. Cryoglobulinemia—a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966; 40: 828– 836. [DOI] [PubMed] [Google Scholar]
- 30. Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G, Candela M, Ferri C, Gabrielli A, Mazzaro C, Migliaresi S, Mussini C, Ossi E, Quintiliani L, Tirri G, Vacca AGISC. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995; 88: 115– 126. [PubMed] [Google Scholar]
- 31. Monti G, Saccardo F, Pioltelli P, Rinaldi G. The natural history of cryoglobulinemia: symptoms at onset and during follow-up. A report by the Italian Group for the Study of Cryoglobulinemias (GISC). Clin Exp Rheumatol. 1995; 13: S129– S133. [PubMed] [Google Scholar]
- 32. Neal KR, Ramsay S, Thomson BJ, Irving WL. Excess mortality rates in a cohort of patients infected with the hepatitis C virus: a prospective study. Gut. 2007; 56: 1098– 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pawlotsky JM, Ben Yahia M, Andre C, Voisin MC, Intrator L, Roudot-Thoraval F, Deforges L, Duvoux C, Zafrani E, Duval J, Dhumeaux D. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994; 19: 841– 848. [PubMed] [Google Scholar]
- 34. Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009; 4: 207– 220. [DOI] [PubMed] [Google Scholar]
- 35. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997; 349: 825– 832. [DOI] [PubMed] [Google Scholar]
- 36. Racanelli V, Sansonno D, Piccoli C, D’Amore FP, Tucci FA, Dammacco F. Molecular characterization of B cell clonal expansions in the liver of chronically hepatitis C virus-infected patients. J Immunol. 2001; 167: 21– 29. [DOI] [PubMed] [Google Scholar]
- 37. Rieu V, Cohen P, Andre MH, Mouthon L, Godmer P, Jarrousse B, Lhote F, Ferriere F, Deny P, Buchet P, Guillevin L. Characteristics and outcome of 49 patients with symptomatic cryoglobulinaemia. Rheumatology (Oxford). 2002; 41: 290– 300. [DOI] [PubMed] [Google Scholar]
- 38. Ryder SD. Outcome of hepatitis C infection: bleak or benign? J Hepatol. 2007; 47: 4– 6. [DOI] [PubMed] [Google Scholar]
- 39. Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004; 53: 451– 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saadoun D, Asselah T, Resche-Rigon M, Charlotte F, Bedossa P, Valla D, Piette JC, Marcellin P, Cacoub P. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology. 2006; 43: 1337– 1345. [DOI] [PubMed] [Google Scholar]
- 41. Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005; 5: 227– 236. [DOI] [PubMed] [Google Scholar]
- 42. Sansonno D, De Vita S, Iacobelli AR, Cornacchiulo V, Boiocchi M, Dammacco F. Clonal analysis of intrahepatic B cells from HCV-infected patients with and without mixed cryoglobulinemia. J Immunol. 1998; 160: 3594– 3601. [PubMed] [Google Scholar]
- 43. Sansonno D, Lauletta G, Nisi L, Gatti P, Pesola F, Pansini N, Dammacco F. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinaemia. Clin Exp Immunol. 2003; 133: 275– 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sansonno D, Tucci FA, Ghebrehiwet B, Lauletta G, Peerschke EI, Conteduca V, Russi S, Gatti P, Sansonno L, Dammacco F. Role of the receptor for the globular domain of C1q protein in the pathogenesis of hepatitis C virus-related cryoglobulin vascular damage. J Immunol. 2009; 183: 6013– 6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sansonno L, Tucci FA, Sansonno S, Lauletta G, Troiani L, Sansonno D. B cells and HCV: an infection model of autoimmunity. Autoimmun Rev. 2009; 9: 93– 94. [DOI] [PubMed] [Google Scholar]
- 46. Schott P, Hartmann H, Ramadori G. Hepatitis C virus-associated mixed cryoglobulinemia. Clinical manifestations, histopathological changes, mechanisms of cryoprecipitation and options of treatment. Histol Histopathol. 2001; 16: 1275– 1285. [DOI] [PubMed] [Google Scholar]
- 47. Shihabi ZK. Cryoglobulins: an important but neglected clinical test. Ann Clin Lab Sci. 2006; 36: 395– 408. [PubMed] [Google Scholar]
- 48. Siagris D, Christofidou M, Tsamandas A, Lekkou A, Thomopoulos K, Labropoulou-Karatza C. Cryoglobulinemia and progression of fibrosis in chronic HCV infection: cause or effect? J Infect. 2004; 49: 236– 241. [DOI] [PubMed] [Google Scholar]
- 49. Silcocks P. Some issues in observer error studies in pathology. J Pathol. 1992; 168: 255– 256. [DOI] [PubMed] [Google Scholar]
- 50. Tedeschi A, Barate C, Minola E, Morra E. Cryoglobulinemia. Blood Rev. 2007; 21: 183– 200. [DOI] [PubMed] [Google Scholar]
- 51. Tighe H, Warnatz K, Brinson D, Corr M, Weigle WO, Baird SM, Carson DA. Peripheral deletion of rheumatoid factor B cells after abortive activation by IgG. Proc Natl Acad Sci USA. 1997; 94: 646– 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trejo O, Ramos-Casals M, Garcia-Carrasco M, Yague J, Jimenez S, de la Red G, Cervera R, Font J, Ingelmo M. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001; 80: 252– 262. [DOI] [PubMed] [Google Scholar]
- 53. Vigano M, Lampertico P, Rumi MG, Folli C, Maggioni L, Morabito A, Del Ninno E, Cicardi M, Colombo M. Natural history and clinical impact of cryoglobulins in chronic hepatitis C: 10-year prospective study of 343 patients. Gastroenterology. 2007; 133: 835– 842. [DOI] [PubMed] [Google Scholar]
- 54. Williams RC, Malone CC, Kao KJ. IgM rheumatoid factors react with human class I HLA molecules. J Immunol. 1994; 156: 1684– 1694. [PubMed] [Google Scholar]
- 55. Willson RA. Extrahepatic manifestations of chronic viral hepatitis. Am J Gastroenterol. 1997; 92: 3– 17. [PubMed] [Google Scholar]


