Abstract
Persistent Staphylococcus aureus bacteremia (SAB) that fails to respond to appropriate antibiotic therapy is associated with poor outcomes. Comprehensive prospective studies on risk factors and outcomes of persistent bacteremia are limited. We investigated outcomes and risk factors encompassing clinical, pharmacokinetic, microbiologic, and genotypic characteristics associated with persistent bacteremia using a case-control study nested in a prospective cohort of patients with SAB at a tertiary-care hospital from August 2008 through September 2010. We compared the clinical characteristics, management, and outcomes of patients with persistent bacteremia (≥7 d) with controls with resolving bacteremia (<3 d). To detect associations between microbiologic and genotypic characteristics of methicillin-resistant S. aureus (MRSA) isolates and persistent bacteremia, we determined the heteroresistance phenotype, SCCmec type, agr genotype and functionality, multilocus sequence typing, and presence of 41 virulence genes. Our cohort consisted of 483 patients; 76 (15.7%) had persistent bacteremia, 212 (43.5%) had resolving bacteremia. In the multivariate analysis, independent risk factors associated with persistent bacteremia were community-onset bacteremia (odds ratio [OR], 2.91; 95% confidence interval [CI], 1.24–6.87), bone and joint infection (OR, 5.26; 95% CI, 1.45–19.03), central venous catheter-related infection (OR, 3.36; 95% CI, 1.47–7.65), metastatic infection (OR, 36.22; 95% CI, 12.71–103.23), and methicillin resistance (OR, 16.99; 95% CI, 5.53–52.15). For patients with eradicable foci, delay (>3 d) in the removal of the infection focus was significantly associated with persistent bacteremia (OR, 2.18; 95% CI, 1.05–4.55). There were no significant associations of persistent bacteremia with high vancomycin minimal inhibitory concentration, vancomycin heteroresistance, and microbiologic/genotypic characteristics of MRSA isolates. However, initial vancomycin trough level <15 mg/L was an independent risk factor for persistent MRSA bacteremia (OR, 4.25; 95% CI, 1.51–11.96) in the multivariate analysis. Clinical outcomes were significantly worse for patients with persistent bacteremia. Relapse of bacteremia and attributable mortality within 12 weeks after SAB were significantly higher in patients with persistent bacteremia than in those with resolving bacteremia (9.2% [7/76] vs. 2.4% [5/212], p = 0.02 and 21.1% [16/76] vs. 9.4% [20/212], p = 0.009, respectively).
In conclusion, patients with SAB should be given early aggressive treatment strategies, including early source control and maintenance of a vancomycin trough level ≥15 mg/L, to reduce the risk of persistent bacteremia.
INTRODUCTION
Staphylococcus aureus bacteremia (SAB) is one of the most common serious bacterial infections, with an associated mortality of about 30%.49,57 SAB may persist in some patients despite several days of antibiotic therapy, a phenomenon called persistent bacteremia. Persistent bacteremia accounts for 6%–38% of all episodes of SAB, is particularly common in endovascular infections, and is associated with poor clinical outcomes.5,14,18,23,24,59 The prevalence of persistent bacteremia may differ depending on the duration of bacteremia used to define “persistent bacteremia.” Early aggressive treatment strategies may reduce the morbidity and mortality of patients with risk factors for persistent bacteremia. A comprehensive analysis is needed to investigate various factors associated with persistent bacteremia.
A 2007 retrospective study identified methicillin resistance, use of an intravascular catheter or other medical device, chronic renal failure, multiple sites of infection, and infective endocarditis as risk factors for persistent bacteremia.18 In other studies, vancomycin treatment, diabetes, retention of implicated devices, and methicillin-resistant S. aureus (MRSA) with vancomycin minimal inhibitory concentration (MIC) of 2 mg/L were also implicated.23,24,59 However, these studies are limited by their retrospective or noncomparative design and because they focused on clinical characteristics of the patients, but did not consider the various characteristics of S. aureus isolates.
Microbiologic and genotypic characteristics of S. aureus and pharmacokinetic and pharmacodynamic properties of antimicrobial agents may also be associated with persistent bacteremia. Several previous comparative studies have examined the microbiologic and genotypic characteristics of MRSA isolates associated with persistent bacteremia.14,48,58 However, these studies were performed with small numbers of MRSA isolates.
To the best of our knowledge, no prospective comparative studies have investigated the clinical risk factors and the pharmacokinetic, microbiologic, and genotypic factors associated with persistent bacteremia. In the present study, we performed a large case-control study nested in a prospective cohort of patients with SAB to evaluate these factors and outcomes associated with persistent bacteremia, using a definition of persistent bacteremia as ≥7 days of bacteremia.
PATIENTS AND METHODS
Study Population and Design
This case-control study, nested in a prospective observational cohort of patients with SAB, was conducted at the Asan Medical Center, a 2700-bed tertiary referral center that admits patients from throughout Korea. From August 2008 to September 2010, all adult patients with SAB were prospectively enrolled in the cohort and observed over a 12-week period. In our hospital, more than 90% of patients with SAB receive infectious disease consultation; follow-up blood cultures with 2- to 4-day intervals until negative conversion, echocardiography, and monitoring of vancomycin trough concentrations (at day 3 and then at 3- to 4-day intervals) are routinely recommended. It is also recommended that vancomycin trough concentrations always be maintained at >10 mg/L in patients with suspected S. aureus infection and at 15–20 mg/L in patients with S. aureus bacteremia.19,46 Patients were excluded if 1) they had polymicrobial bacteremia, 2) they died or were discharged before positive blood culture results, or 3) they had clinically insignificant SAB, which means that S. aureus was isolated from only 1 blood culture set and the patient did not have clinical findings consistent with bacteremia and did not received antibiotic treatment. Only the first episode of SAB in each patient was included for analysis.
Case patients were those who had an episode of persistent bacteremia, defined as bacteremia for ≥7 days while they were receiving appropriate antibiotic therapy.14,18 Control patients were those who had resolving bacteremia, defined as <3 days of bacteremia with all subsequent blood cultures documented to be negative after the initial positive blood culture (index blood culture). Patients with resolving bacteremia had to have at least 1 set of follow-up blood cultures 1–3 days after the index blood culture, and had to have no further positive blood culture results during antibiotic therapy.14 The duration of bacteremia was defined as the number of days between the first and last positive blood culture. Patients with intermediate duration of bacteremia were excluded from analysis to enable clear distinctions between persistent bacteremia and resolving bacteremia. This study was approved by the Asan Medical Center Institutional Review Board.
Data Collection and Study Definitions
Demographic characteristics, laboratory results, underlying diseases or conditions, presence or absence of a central venous catheter (CVC) or prosthetic device, severity of underlying disease, severity of bacteremia, site of infection, antibiogram results, patient management (including infection source control and antimicrobial therapy received), and clinical outcomes were recorded. The system of McCabe and Jackson was used to classify the severity of the underlying disease.36 The Charlson comorbidity index was used to provide a composite score of comorbid conditions.6 The severity of bacteremia at the time of the first positive blood culture was assessed using the Pitt bacteremia score.7
The type of infection causing SAB was defined by the criteria of the Centers for Disease Control and Prevention except for catheter-related infection and infective endocarditis.16 Catheter-related infection was defined as SAB in a patient who has an intravascular device and at least 1 positive blood culture result obtained from the peripheral vein, clinical features of infection, and no apparent source of SAB except for the catheter.37,38 Infective endocarditis was defined according to modified Duke criteria.30 Bacteremia was classified as nosocomial if a positive blood culture was obtained from patients who had been hospitalized for 48 hours or longer.16 Community-onset bacteremia was defined by a positive blood culture obtained at the time of hospital admission or within the 48 hours after hospital admission, and was further classified as health care-associated or community-acquired bacteremia.15 Septic shock was defined as sepsis associated with organ dysfunction and accompanied by persistent hypotension after adequate fluid resuscitation. Suppurative thrombophlebitis was defined as the presence of positive blood culture results for S. aureus plus the demonstration of a thrombus in the great central veins by radiographic testing (computed tomography, ultrasonography).
Initial appropriate antibiotic therapy was defined as the use of an antimicrobial agent with activity against the organism at the day of or 1 day after the index blood culture. Initial vancomycin trough level was defined as mean trough level during the first 7 days of vancomycin therapy.
Clinical outcome measures were duration of hospitalization after bacteremia, relapse of bacteremia, death during hospitalization, and death within 30 days or 12 weeks after the day of the index blood culture. Attributable mortality was defined as death due to S. aureus infection in a previously healthy individual or as the hastening of death by S. aureus infection in the presence of an underlying condition such as cancer.
Microbiologic Data and Genotypic Assays
The first blood isolate from each patient was used for microbiologic and molecular assessments. All S. aureus isolates were identified by standard methods. Antimicrobial susceptibilities were determined using the MicroScan system (Dade Behring, West Sacramento, CA) and standard criteria of the Clinical and Laboratory Standards Institute. Methicillin resistance was confirmed by polymerase chain reaction (PCR) detection of the mecA gene. Vancomycin MICs of MRSA isolates were determined by the vancomycin Etest (AB Biodisk, Piscataway, NJ) on Mueller-Hinton agar according to the manufacturer’s instructions. Identification of heteroresistant vancomycin-intermediate S. aureus (hVISA) was determined by modified population analysis.55,56
SCCmec type, agr genotype, and the presence of 41 putative virulence genes of MRSA were determined using previously described methods.4,10,21,42,45 We determined agr dysfunction by the level of δ-hemolysin production, measured by streaking the MRSA isolate adjacent to a β-hemolysin disk (Remel, Lenexa, KS).47 Triton-induced lysis was assessed to measure the propensity for autolysis in MRSA isolates (performed in triplicate), as described elsewhere;14 defective autolysis was defined as a decrease in OD630 less than 50% after 3 hours of exposure to Triton relative to the baseline reading. Multilocus sequence typing (MLST) was performed as described elsewhere.11,12 MLST allele names and sequence types were derived from the MLST database (http://www.mlst.net). Clonal complexes (CCs) were assigned to groups of isolates sharing 6 of 7 alleles by use of eBURST (http://eburst.mlst.net).
Statistical Analysis
We compared patients with persistent bacteremia and those with resolving bacteremia. Categorical variables were compared using the chi-square test or the Fisher exact test, as appropriate. Normally and non-normally distributed continuous variables were compared using the Student t test and the Mann-Whitney U test, respectively. Logistic regression was used to assess risk factors associated with persistent bacteremia and attributable mortality. All variables with p values less than 0.1 in the univariate analysis and other variables of clinical importance were included in the multiple logistic regression model to identify independent risk factors. The final model was constructed using the stepwise selection procedure. A 2-tailed p value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, v. 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Study Population and Follow-Up Blood Cultures
From August 1, 2008, to September 30, 2010, there were 631 consecutive episodes of SAB at our institution. A total of 148 of these episodes were excluded from the prospective study for the following reasons: polymicrobial bacteremia (n = 64), patients not admitted (n = 69), and clinically insignificant bacteremia (n = 15). Among a total of 483 episodes enrolled in the cohort, 372 (77%) had follow-up blood cultures 1–3 days after the index blood culture, and 464 (96.1%) had follow-up blood cultures that documented clearance of bacteremia. Of the 483 episodes of SAB, 76 (15.7%) episodes were persistent bacteremia, 212 (43.5%) episodes were resolving bacteremia, and 280 (58%) episodes were methicillin resistant (that is, MRSA bacteremia). Of the 280 episodes of MRSA bacteremia, 65 (23.2%) were persistent bacteremia and 102 (36.4%) episodes were resolving bacteremia. We excluded from the present analysis SAB episodes with an intermediate duration of bacteremia (3–6 d; n = 101 [59 in MRSA]), episodes with positive follow-up blood cultures 1–2 days after the index blood culture in patients with <3 days of bacteremia (n = 23 [15 in MRSA]), and episodes without adequate follow-up blood cultures to determine if they were persistent bacteremia or resolving bacteremia (n = 71 [39 in MRSA]).
The median duration of bacteremia in patients with persistent bacteremia was 11 days (interquartile range [IQR], 8.0–16.5 d). Patients with persistent MRSA bacteremia had a significantly longer duration of bacteremia than patients with persistent methicillin-susceptible S. aureus bacteremia (median, 13 d [IQR, 9–17 d] vs. 8 d [IQR, 7–9 d], p = 0.003). Among patients with resolving bacteremia, the first follow-up blood culture documented to be negative was obtained 1 day after the index blood culture in 16 (7.5%) patients, 2 days after in 57 (26.9%) patients, and 3 days after the index blood culture in 139 (65.6%) patients.
Risk Factors Associated With Persistent Bacteremia
Tables 1 and 2 show the results of our univariate analysis of risk factors associated with persistent bacteremia. The persistent bacteremia and resolving bacteremia groups were similar in age, sex, underlying conditions, recent antibiotic use, severity of underlying disease, and severity of bacteremia. Patients with persistent bacteremia were marginally more likely to have community-onset bacteremia (p = 0.07); less likely to have underlying liver cirrhosis (p = 0.03) and peripheral catheter-related infection (p = 0.003); and more likely to have a prosthetic device (p = 0.02), metastatic infection (p < 0.001), CVC-related infection (p < 0.001), infective endocarditis (p < 0.001), bone and joint infection (p = 0.02), and a methicillin-resistant isolate (p < 0.001), compared with patients with resolving bacteremia. The prevalence of prosthetic device infection was not significantly different between the 2 groups (6/76 patients with persistent bacteremia [7.9%] vs. 7/212 patients with resolving bacteremia [3.3%], p = 0.11). However, an infected prosthetic device was removed in 1 of 6 patients with persistent bacteremia, whereas the device was removed in all 7 patients with resolving bacteremia who had the device (p = 0.005). Among patients with prosthetic device infection, only 1 patient with resolving bacteremia had bone and joint infection.
TABLE 1.
Univariate Analysis of Demographic and Clinical Characteristics of Patients With Persistent Bacteremia and Resolving Bacteremia
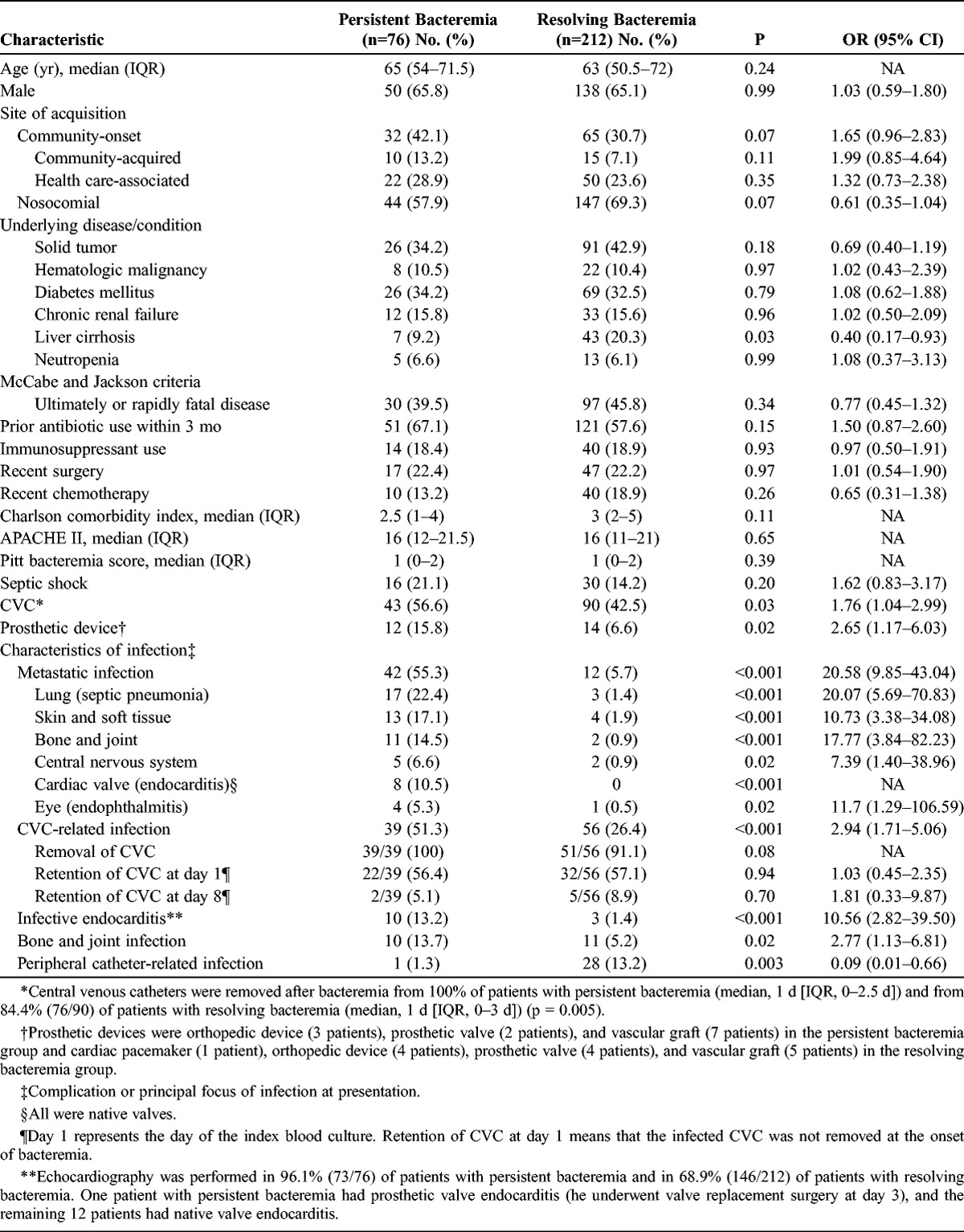
TABLE 2.
Univariate Analysis of Microbiologic Characteristics and Clinical Management of Patients With Persistent Bacteremia and Resolving Bacteremia
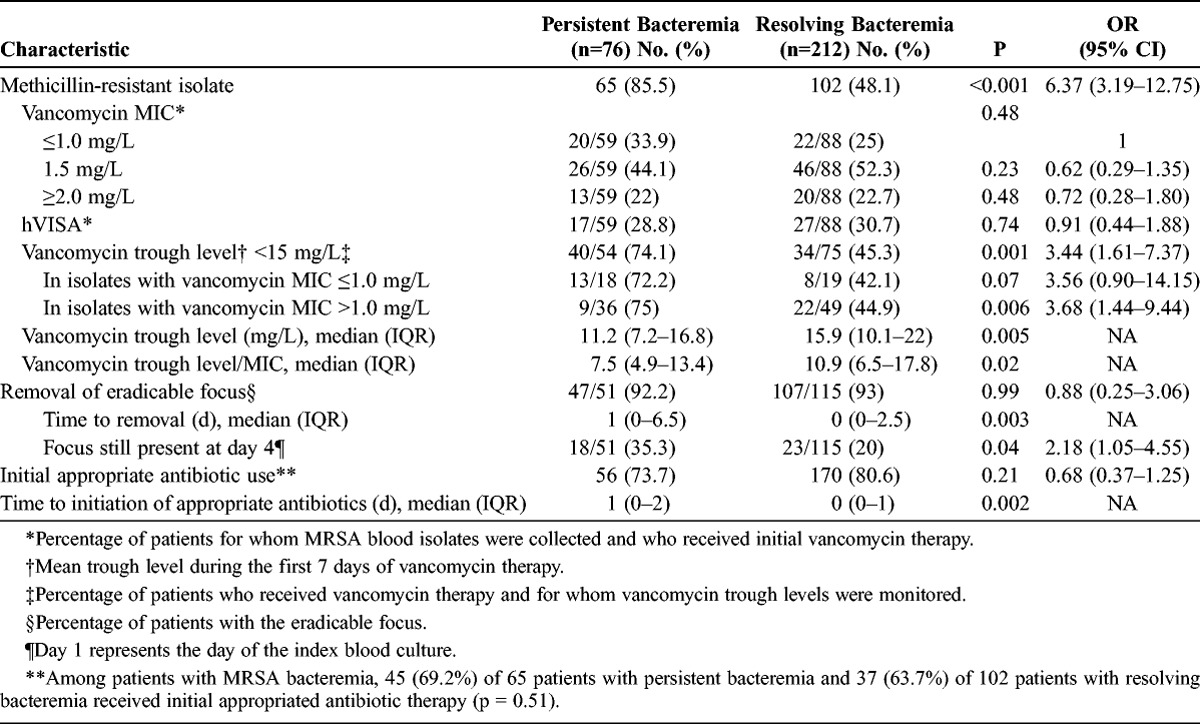
Patients with persistent bacteremia were more likely to have a CVC at the onset of bacteremia than those with resolving bacteremia (43/76 [56.6%] vs. 90/212 [42.5%], p = 0.03). Regardless of the presence of CVC-related infection, a CVC was more frequently removed from patients with persistent bacteremia than from patients with resolving bacteremia (43/43 [100%] vs. 76/90 [84.4%], p = 0.005). Time to removal of CVC after bacteremia was similar in the 2 groups (median, 1 d [IQR, 0–2.5 d] vs. 1 d [IQR, 0–3 d], p = 0.55). Among the 39 patients with CVC-related infection in the persistent bacteremia group, 34 (87.2%) had persistent bacteremia (≥7 d) even after removal of CVC, 14 (35.9%) also had suppurative thrombophlebitis in the great central veins, and 6 (15.4%) had infective endocarditis as secondary complications.
The rates of removal of the eradicable focus were similar in the 2 groups (p = 0.99), but the time to removal was significantly longer in the persistent bacteremia group (p = 0.003) (Table 2). Delay (>3 d) in the removal of the eradicable focus was significantly associated with persistent bacteremia (odds ratio [OR], 2.18; 95% confidence interval [CI], 1.05–4.55). In addition, patients with persistent bacteremia had significantly longer delays to appropriate antibiotic therapy (p = 0.002) than those with resolving bacteremia.
Significant univariate variables with p values < 0.1 and patient age were included in the multivariate analysis. Because only 57.6% of patients had an eradicable focus, variables regarding time to the removal of the eradicable focus had missing values in at least 42.4% of patients and were not included in the multivariate analysis. Independent risk factors associated with persistent bacteremia were community-onset bacteremia, bone and joint infection, CVC-related infection, metastatic infection, and methicillin resistance (Table 3).
TABLE 3.
Multivariate Analysis of Risk Factors for Persistent Bacteremia
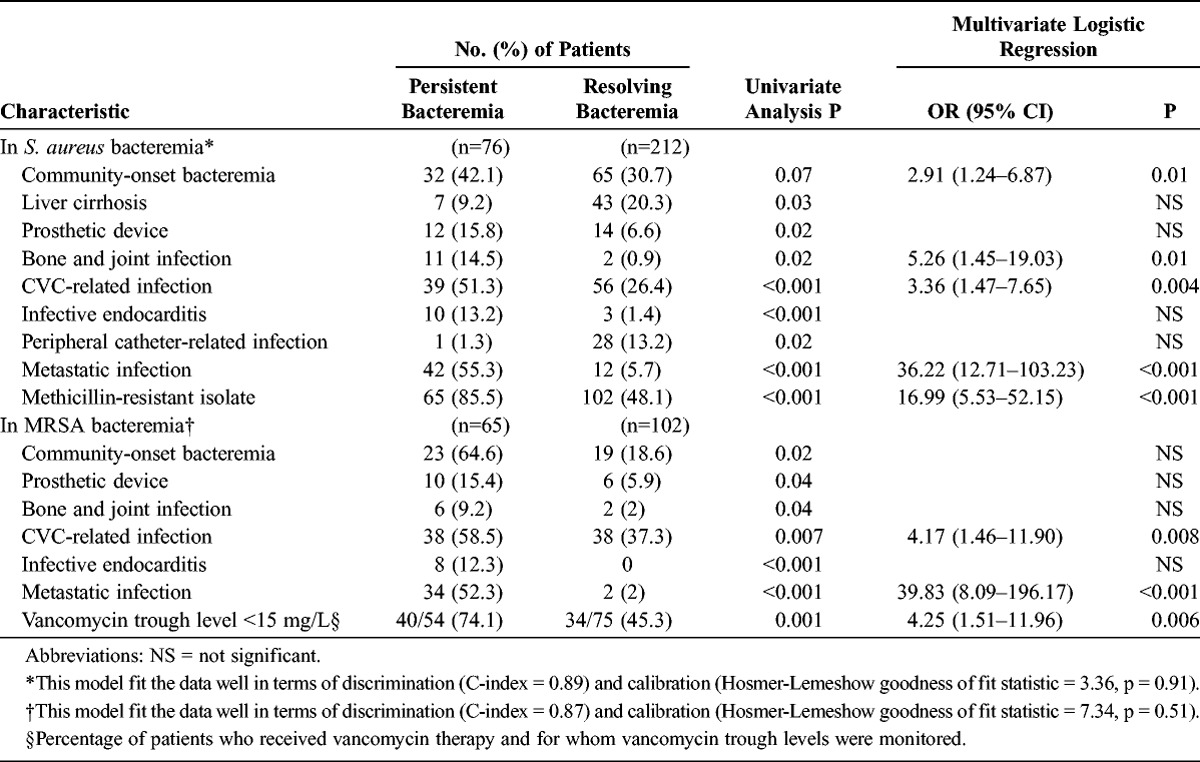
When the analysis was restricted to patients with MRSA bacteremia, patients with persistent bacteremia were more likely than those with resolving bacteremia to have community-onset infection (p = 0.02), a prosthetic device (p = 0.04), metastatic infection (p < 0.001), CVC-related infection (p = 0.007), bone and joint infection (p = 0.04), infective endocarditis (p < 0.001), and longer delays in the removal of eradicable focus (median, 1 d [IQR, 0–4 d] vs. 0 d [IQR, 0–2 d], p = 0.03) in the univariate analysis. All MRSA isolates were susceptible to daptomycin, linezolid, and teicoplanin by the Microscan automated system. Of 167 patients with MRSA bacteremia, 155 (92.8%) were initially treated with vancomycin, 6 (3.6%) with teicoplanin, and 6 (3.6%) with linezolid. Vancomycin MIC determination and hVISA tests were performed on 147 (94.8%) of the 155 MRSA isolates from patients initially treated with vancomycin. The overall vancomycin MIC distribution, determined by the Etest, was as follows: MIC of 0.75 mg/L, 9 isolates (6.1%); MIC of 1 mg/L, 33 isolates (22.4%); MIC of 1.5 mg/L, 72 isolates (49%); MIC of 2 mg/L, 31 isolates (21.1%); and MIC of 3 mg/L, 2 isolates (1.4%). There were no significant differences in vancomycin MICs and heterogeneous vancomycin resistance between MRSA isolates in the persistent bacteremia and resolving bacteremia groups. However, patients with persistent bacteremia were significantly less likely to have an initial vancomycin trough level of ≥15 mg/L (p = 0.001) and were more likely to have lower trough level/MIC, compared with patients with resolving bacteremia (p = 0.02). Results of the multivariate analysis indicated that independent risk factors associated with persistent MRSA bacteremia included CVC-related infection, metastatic infection, and vancomycin trough level <15 mg/L (see Table 3).
To evaluate the applicability of these risk factors for persistent bacteremia to intermediate duration of bacteremia, we compared patients with intermediate duration of bacteremia (excluded from the study) and patients with resolving bacteremia. In the univariate analysis, patients with intermediate duration of bacteremia were significantly more likely than patients with resolving bacteremia to have community-onset bacteremia, a prosthetic device, metastatic infection, and infective endocarditis; less likely to have peripheral catheter-related infection; and marginally more likely to have CVC-related infection and a methicillin-resistant isolate. Among patients with MRSA bacteremia, vancomycin MIC, hVISA, and initial vancomycin trough level were not significantly associated with intermediate duration of bacteremia. Results of the multivariate analysis indicated that independent risk factors associated with intermediate duration of bacteremia were the presence of a prosthetic device (OR, 3.36; 95% CI, 1.46–7.73), metastatic infection (OR, 10.49; 95% CI, 4.68–23.54), CVC-related infection (OR, 2.27; 95% CI, 1.23–4.18), and methicillin resistance (OR, 2.25; 95% CI, 1.24–4.09). There was considerable overlap in risk factors for persistent bacteremia and intermediate duration of bacteremia.
Outcomes of Patients With Persistent Bacteremia
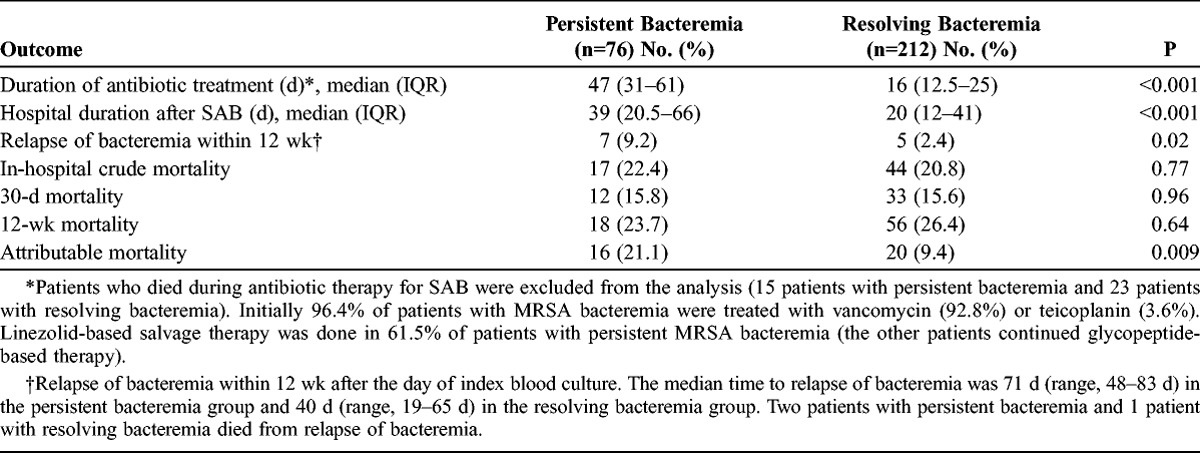
Patients with persistent bacteremia had significantly longer hospital stays after SAB and received longer antibiotic treatment than patients with resolving bacteremia (Table 4). Although there were no significant differences in crude mortality rates between the 2 groups, relapse rates of bacteremia and attributable mortality rates within 12 weeks after the day of the index blood culture were significantly higher in patients with persistent bacteremia (see Table 4).
TABLE 4.
Outcomes of Patients With Persistent Bacteremia and Resolving Bacteremia
Risk Factors for Attributable Mortality
We performed univariate and multivariate analysis of risk factors associated with attributable mortality to evaluate whether persistent bacteremia was independently associated with attributable mortality of SAB. Univariate analysis indicated that risk factors for attributable mortality were a high Charlson comorbidity index score, rapidly fatal or ultimately fatal disease, a high APACHE II score (Acute Physiology and Chronic Health Evaluation II), metastatic infection, infective endocarditis, and persistent bacteremia. Multivariate analysis indicated that rapidly fatal or ultimately fatal disease (OR, 7.31; 95% CI, 2.94–18.17; p < 0.001), infective endocarditis (OR, 4.35; 95% CI, 1.07–17.65; p = 0.04), and persistent bacteremia (OR, 2.78; 95% CI, 1.26–6.12; p = 0.01) were independent risk factors for attributable mortality. When we performed univariate and multivariate analysis of risk factors for attributable mortality as a cohort study (including all 483 patients with SAB), persistent bacteremia was still an independent risk factor (OR, 4.19; 95% CI, 1.81–9.68, p = 0.001).
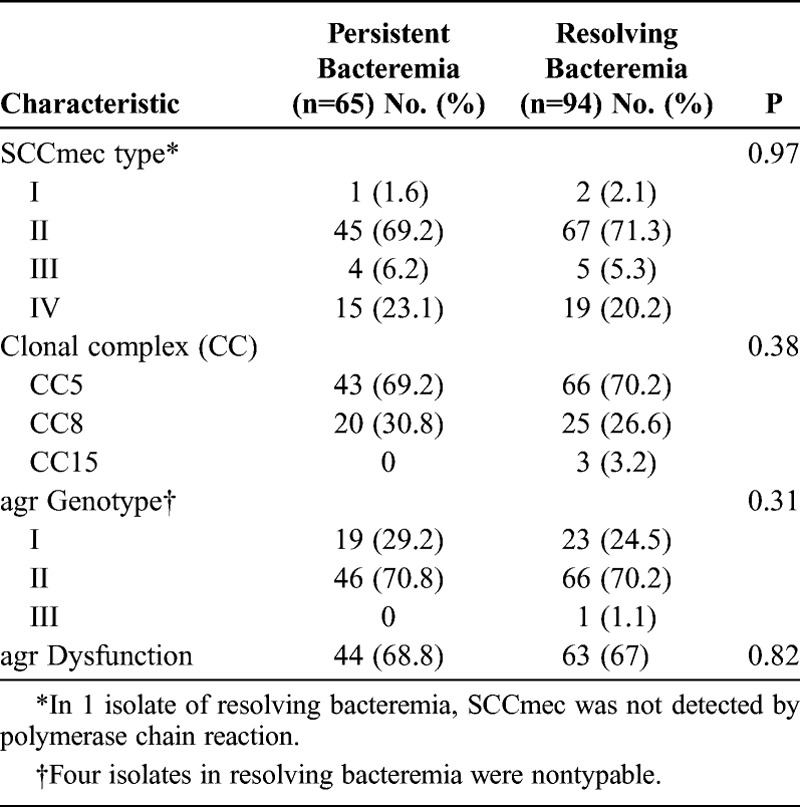
Genetic Determinants Associated With Persistent MRSA Bacteremia
MRSA isolates were analyzed in 159 (95.2%) of 167 patients with MRSA bacteremia (65 [100%] of 65 patients with persistent bacteremia and 94 [90.3%] of 103 patients with resolving bacteremia). Table 5 shows the genotypic characteristics of MRSA isolates responsible for persistent bacteremia and resolving bacteremia. Of 112 MRSA isolates with SCCmec type II, 110 (98.2%) had agr group II genotype; in contrast, of 34 MRSA isolates with SCCmec type IV, 32 (94.1%) had agr group I genotype. We found that agr dysfunction, as measured by the δ–hemolysin assay, was present in 14.3% (6/42) and 90.9% (101/111) of MRSA isolates with agr group I and II, respectively (p < 0.001). A total of 7 sequence types contained in 3 CCs was present, and CC5 (present in 111 [69.8%, all of which were ST5] of 159 isolates) was the most common CC. There were no significant differences in genotype distribution and agr dysfunction in MRSA isolates between the 2 groups. Defective autolysis was similar in the 2 groups (26.2% [17/65] vs. 29.8% [28/94], respectively; p = 0.62).
TABLE 5.
Genotypes and agr Dysfunction Distribution in MRSA Isolates Responsible for Persistent Bacteremia and Resolving Bacteremia
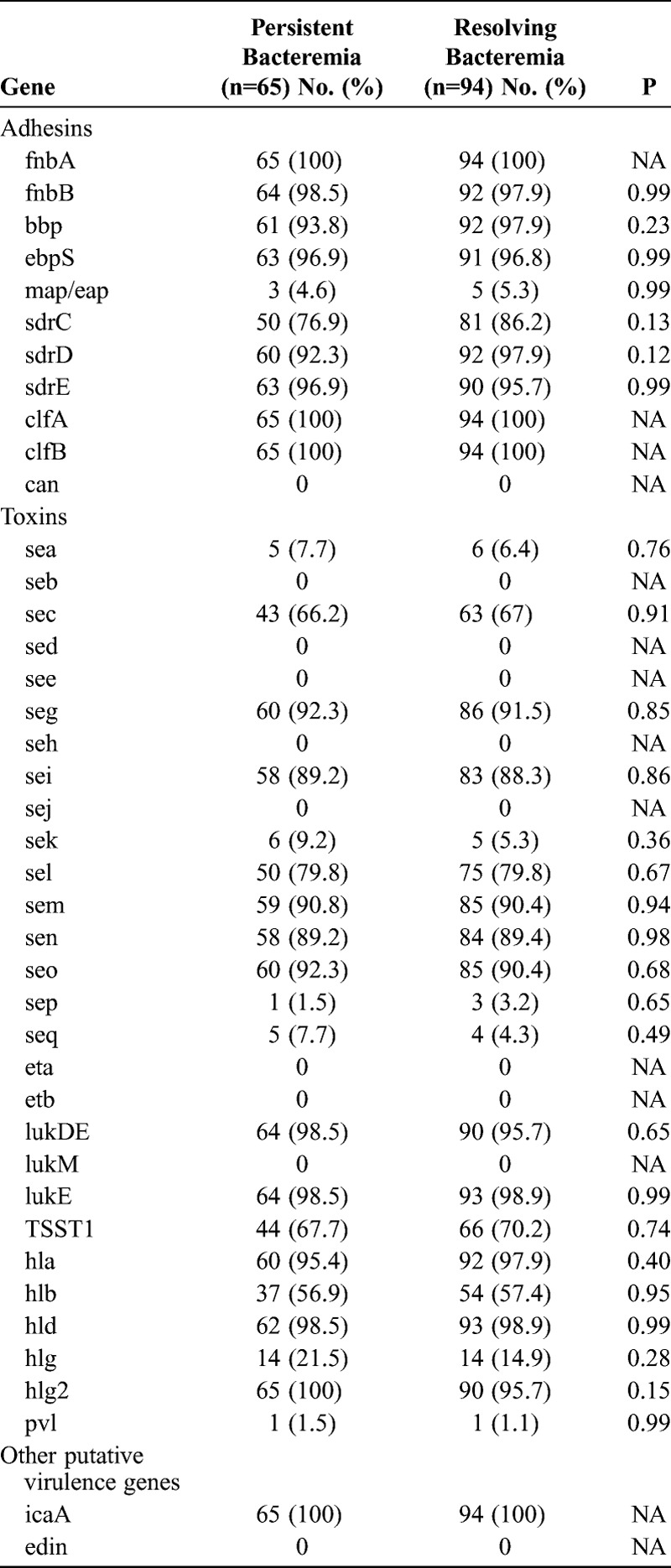
Finally, we determined the distribution of putative virulence genes in our available MRSA isolates (Table 6). The results indicated that some of these putative virulence genes (fnbA, fnbB, bbp, ebpS, sdrD, sdrE, clfA, clfB, lukDE, lukE, hla, hld, hlg2, icaA) were present in almost all isolates of both groups and that there were no significant differences in the presence of virulence genes of MRSA isolates causing persistent bacteremia and resolving bacteremia.
TABLE 6.
Association Between Putative Virulence Genes of MRSA Isolates and Persistent Bacteremia
DISCUSSION
Persistent SAB that fails to respond to appropriate antibiotic therapy is a well-documented problem that is frequently encountered in the management of patients with SAB. Various definitions of persistent bacteremia have been proposed, but it is most often described as bacteremia that persists for 3–7 days.14,18,20,23,24,40,50,58 SAB that persists for 7 days rather than 3 days of appropriate antibiotic therapy presents a greater challenge to physicians;17 hence, many recent studies considered persistence as bacteremia ≥7 days.28,32,33,48,59 We therefore classified bacteremia that persists for ≥7 days as persistent bacteremia.
Various factors may contribute to the development of persistent bacteremia: 1) clinical factors such as infection site or removal of infection focus,18,23,24,40,59 2) pharmacokinetic and pharmacodynamic characteristics of the antibiotic,28,40 and 3) microbiologic and genotypic characteristics of S. aureus.14,20,26,48 Several studies have investigated risk factors for persistent bacteremia, but most were retrospective analyses and focused on either clinical factors or microbiologic factors,18,20,26,40,48,58,59 not both. A previous prospective comparative study14 examined persistent bacteremia caused by MRSA, but enrolled a small number of patients and focused on microbiologic factors of MRSA isolates. Khatib et al23,24 performed prospective observational analyses of persistent bacteremia, but they did not perform comparative analyses of patients with persistent bacteremia and resolving bacteremia, and they investigated clinical risk factors only for persistent bacteremia. The present study is a large case-control study nested in a prospective cohort of patients with SAB, where we investigated the association of persistent bacteremia with clinical factors, vancomycin pharmacokinetics, microbiologic, and genotypic factors of the isolates.
The results of the present study indicate that the independent risk factors for persistent bacteremia were community-onset bacteremia, bone and joint infection, CVC-related infection, metastatic infection, and methicillin resistance. In patients with an eradicable focus, delay (>3 d) in the removal of the infection focus was also significantly associated with persistent bacteremia. In patients with persistent bacteremia due to CVC-related infection, all infected CVCs were removed, and the median time to removal of CVC after bacteremia was 1 day (IQR, 0–2.5d). Nonetheless, bacteremia persisted for ≥7 days even after removal of CVC in 87.2% of patients. In these patients, complications of CVC-related infection such as metastatic infection, suppurative thrombophlebitis, and infective endocarditis could have contributed to persistent bacteremia. Our univariate analysis indicated that infective endocarditis was significantly associated with persistent bacteremia, but this association was not significant in our multivariate analysis, probably because of its significant relationship with community-onset bacteremia and metastatic infection (p = 0.01 and p < 0.001, respectively). In contrast to previous studies,18,23,24,40 our results indicated that community-onset bacteremia was a significant risk factor for persistent bacteremia. In the present study, patients with community-onset bacteremia were significantly more likely to have metastatic infection, infective endocarditis, and bone and joint infection than patients with nosocomial bacteremia. Therefore, these risk factors for persistent bacteremia could contribute to the association between community-onset bacteremia and persistent bacteremia. An additional possible explanation is that community-onset bacteremia could have more deep-seated or extensive infection due to a longer interval from infection to detection of bacteremia and adequate treatment, compared with nosocomial bacteremia.13,22,41
Among patients in the current study with MRSA bacteremia, the independent risk factors for persistent bacteremia were CVC-related infection, metastatic infection, and vancomycin trough level <15 mg/L. Among patients with an eradicable focus, longer delays in the removal of the infection focus were also significantly associated with persistent MRSA bacteremia (p = 0.03). Our results indicated that vancomycin MICs, hVISA phenotype, genotypic characteristics, and agr dysfunction of MRSA isolates were not significantly associated with persistent bacteremia.
There has been a controversy as to whether high vancomycin MIC (MIC >1 mg/L) and hVISA phenotype of MRSA are associated with worse clinical outcomes, including mortality and vancomycin treatment failure.2,25,28,34,39,51,54 Similarly, there has been a controversy as to whether high vancomycin MIC and hVISA phenotype are associated with persistent bacteremia.2,14,18,25,48,59 A 2012 meta-analysis of 22 studies reported that high vancomycin MIC (especially by Etest methodology) was significantly associated with a higher mortality rate and vancomycin treatment failure in MRSA bacteremia.53 However, when the analysis was limited to studies that examined persistent bacteremia, high vancomycin MIC was not significantly associated with persistence (OR, 2.44; 95% CI, 0.72–8.24; p = 0.15).53 Because high vancomycin MIC is associated with vancomycin treatment failure, it would be expected that high vancomycin MIC would be associated with persistent bacteremia. However, this association was not found in the meta-analysis or in the current study. There are several possible explanations for this discrepancy. First, clinical factors such as site of infection, severity of infection, and non-removal of the eradicable focus could be more important risk factors for persistence than high vancomycin MIC. Second, because the definitions of treatment failure are usually composite endpoint including persistent bacteremia,53 the impact of high vancomycin MIC could be more evident in treatment failure. Third, the high proportion (about 60%–70%) of MRSA isolates with high vancomycin MIC in our study and previous studies,2,34 could make no significant difference for persistent bacteremia according to vancomycin MIC.
Several small comparative studies have examined phenotypic and genotypic characteristics of MRSA isolates causing persistent bacteremia, and the results have been inconsistent.14,48,58 In these studies, hVISA phenotype was not detected, and factors associated with persistent bacteremia were specific agr genotype, agr dysfunction, some virulence genes, and resistance to host defense cationic peptides, not high vancomycin MIC. After comparing the presence of 33 virulence genes among 39 MRSA isolates causing persistent and resolving bacteremia, Xiong et al58 found that the presence of tst was significantly associated with persistent bacteremia, and the presence of sdrD and sdrE was significantly associated with resolving bacteremia. In another small comparative study, Seidl et al48 found that except for sspA, there were no significant differences between the persistent bacteremia and resolving bacteremia strain sets with regard to the presence or absence of the other 29 virulence genes. Although not a comparative study, Lalani et al29 reported that the presence of seg was significantly associated with persistent bacteremia. However, we did not find any virulence genes associated with persistent bacteremia despite comparing a relatively large number of MRSA isolates. Our comparative study suggests that clinical factors, including infection site and management of bacteremia, contribute more to the development of persistent MRSA bacteremia than previously known microbiologic factors.
In the United States, the predominant strain of community-associated MRSA (CA-MRSA) is a Panton-Valentine leukocidin (PVL)-positive SCCmec type IV stain (pulsed-field type USA300), whereas in Korea, the most common CA-MRSA strain is ST72-SCCmec type IV without PVL genes.8,27 In the current study, there were no PVL-positive SCCmec type IV MRSA isolates, and only 2 MRSA isolates carrying SCCmec type I had PVL genes. One might say that these differences in the presence of PVL genes could affect interpretation of our data. However, according to recent studies,3,31 PVL is not an important virulence factor, but appears to be a marker of CA-MRSA strain. Therefore, the absence or presence of PVL is probably not an important factor for persistent MRSA bacteremia.
Limited data are available on the association of vancomycin trough concentrations and clinical efficacy,19,28 although recent guidelines recommend targeting a trough level of 15–20 mg/L in patients with serious MRSA infections such as bacteremia, infective endocarditis, osteomyelitis, and pneumonia.33,46 The ratio of the area under the curve to the MIC (AUC/MIC) ≥400 is the pharmacodynamic parameter that best predicts the efficacy of vancomycin.28,33 By performing a series of Monte Carlo simulations, Patel et al43 found that when vancomycin trough levels were between 15 and 20 mg/L, the probability of achieving an AUC/MIC ratio ≥400 was 100% in various dosing regimens when the vancomycin MIC was ≤1 mg/L, but was <50% with a MIC of 2 mg/L. The probability of nephrotoxicity was 14%–34% with a daily vancomycin dose of 4 g. Therefore, they suggested that vancomycin trough levels in excess of 15 mg/L are not always necessary to provide an AUC/MIC ratio ≥400 if the vancomycin MIC is ≤1 mg/L, and that vancomycin may not be optimal for serious MRSA infections with the MIC >1 mg/L. However, clinical data to support vancomycin trough levels between 10–15 mg/L if the vancomycin MIC is ≤1 mg/L are limited. Kullar et al28 reported that independent predictors of vancomycin failure were initial vancomycin trough levels <15 mg/L and vancomycin MIC >1 mg/L by Etest in 320 patients with MRSA bacteremia. They did not evaluate the association with vancomycin treatment failure and initial vancomycin trough levels stratified by vancomycin MIC. In their study, 13% of patients with vancomycin trough levels of 15–20 mg/L experienced nephrotoxicity. Hidayat et al19 demonstrated that vancomycin doses targeting an unbound trough level/MIC of ≥4 (trough level/MIC of ≥8 based on 50% protein binding of vancomycin) significantly improved initial response rates in patients with MRSA infections and concluded that vancomycin dose should be adjusted so that the trough level is at least 15 mg/L. Similar to that study,19 our results indicate that lower initial trough level/MIC and trough levels <15 mg/L are associated with persistent bacteremia. Higher loading doses of vancomycin achieve appropriate levels, but at the risk of nephrotoxicity (12%–35%).19,28,35,43,46 Based on the studies mentioned above, in patients with persistent MRSA bacteremia despite vancomycin therapy at recommended dosing and adequate source control, an early change to alternative anti-MRSA agents should be considered for isolates with vancomycin MIC ≥2 mg/L by Etest.9,33,53 Recent practice guidelines recommend high-dose daptomycin (10 mg/kg per d) if the isolate is susceptible, sometimes in combination with another agent, or other anti-MRSA agents (single or in combination) as an alternate regimen in the setting of persistent MRSA bacteremia and vancomycin treatment failure.33 Other alternative anti-MRSA agents include linezolid, telavancin, ceftaroline, trimethoprim-sulfamethoxazole, and quinupristin-dalfopristin. Because clinical data supporting these recommendations are limited, clinical studies comparing these alternative regimens for persistent MRSA bacteremia are needed.
We found that persistent bacteremia was significantly associated with worse clinical outcomes. Patients with persistent bacteremia had significantly longer hospitalizations, higher relapse rates, and higher attributable mortality rates than patients with resolving bacteremia. However, despite the increase in morbidity due to persistent bacteremia, we found no significant increase in crude mortality, as Yoon et al59 previously reported. Patients with SAB are usually elderly and commonly have 1 or more comorbidities that could affect mortality, including liver cirrhosis, chronic renal failure requiring hemodialysis, and malignancy,18,22,40,44,51,54 so persistent bacteremia would be expected to have less impact on crude mortality in these patients. Although the proportion of patients with malignancy was higher in the current study (50%) than in previous studies (about 20%–25%),18,22,44,51 the McCabe and Jackson score (reflecting the prognosis of underlying disease) and the Charlson comorbidity index (reflecting the severity of underlying disease) in our study were comparable to those in previous studies.1,40,51,52 Hawkins et al18 reported that persistent bacteremia was significantly associated with crude and attributable mortality, although their multivariate analysis indicated that persistent bacteremia was not an independent risk factor for crude mortality. Yoon et al59 also reported that persistent bacteremia was not associated with crude mortality but was associated with infection-caused mortality. Our multivariate analysis indicated that persistent bacteremia was an independent risk factor for attributable mortality. Therefore, when SAB develops in a patient with risk factors for persistent bacteremia, we suggest implementing aggressive interventions, such as early infection source control, extensive workup and control for metastatic infections, and assurance of adequate vancomycin trough level, to reduce morbidity and mortality.
The current study has several limitations. Despite the use of a prospective cohort, patients with persistent bacteremia were more likely to undergo further diagnostic testing, including echocardiography, and this may have resulted in a bias toward the detection of increased numbers of sites of infection and infective endocarditis. In addition, because our study was performed in a tertiary center, microbiologic and genotypic characteristics of MRSA isolates would be expected to be different from those of previous studies due to clonal and geographic issues. Finally, for the assessment of genotypic characteristics of MRSA isolates, we tested only the presence of virulence genes, rather than the expression of these genes. The level of expression of certain virulence genes may be associated with persistent bacteremia, and further research is warranted.
In summary, our results indicated that many patients (15.7%) with SAB, particularly those with community-onset bacteremia, bone and joint infection, CVC-related infection, metastatic infection, MRSA isolates, and late source control, have persistent bacteremia despite 7 days of antibiotic treatment. Persistent bacteremia causes high rates of morbidity and attributable mortality, so early aggressive treatment strategies, such as early source control and higher vancomycin trough levels (≥15 mg/L), should be implemented in patients with risk factors for persistent bacteremia. We found no phenotypic or genotypic characteristics of MRSA to be associated with persistent bacteremia, although more research on this topic is needed.
Abbreviations
- AUC
area under the curve
- CCs
clonal complexes
- CI
confidence interval
- CVC
central venous catheter
- hVISA
heteroresistant vancomycin-intermediate Staphylococcus aureus
- IQR
interquartile range
- MIC
minimal inhibitory concentration
- MLST
multilocus sequence typing
- MRSA
methicillin-resistant Staphylococcus aureus
- OR
odds ratio
- SAB
Staphylococcus aureus bacteremia
Footnotes
Financial support and conflicts of interest: This study was supported by Future-based Technology Development Program (grant numbers 2010-0028746 and 2010-0028747) through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science and Technology. The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M, Kluytmans J, Bonten M. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009; 49: 997– 1005. [DOI] [PubMed] [Google Scholar]
- 2. Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009; 200: 1355– 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008; 198: 1166– 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, Tonthat GT, Rude TH, Barriere SL, Corey R, Fowler VG. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008; 46: 678– 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang FY, MacDonald BB, Peacock JE, Musher DM, Triplett P, Mylotte JM, O’Donnell A, Wagener MM, Yu VL. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore). 2003; 82: 322– 332. [DOI] [PubMed] [Google Scholar]
- 6. Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373– 383. [DOI] [PubMed] [Google Scholar]
- 7. Chow J, Fine M, Shlaes D, Quinn J, Hooper D, Johnson M, Ramphal R, Wagener M, Miyashiro D, Yu V. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991; 115: 585– 590. [DOI] [PubMed] [Google Scholar]
- 8. David MZ, Glikman D, Crawford SE, Peng J, King KJ, Hostetler MA, Boyle-Vavra S, Daum RS. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008; 197: 1235– 1243. [DOI] [PubMed] [Google Scholar]
- 9. Deresinski S. Methicillin-resistant Staphylococcus aureus and vancomycin: minimum inhibitory concentration matters. Clin Infect Dis. 2012; 54: 772– 774. [DOI] [PubMed] [Google Scholar]
- 10. Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2006; 193: 1495– 1503. [DOI] [PubMed] [Google Scholar]
- 11. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000; 38: 1008– 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fowler VG, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, Federspiel J, Naidich S, Remortel B, Rude T, Brown P, Reller LB, Corey GR, Gill SR. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007; 196: 738– 747. [DOI] [PubMed] [Google Scholar]
- 13. Fowler VG, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Cheng AC, Dudley T, Oddone EZ. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003; 163: 2066– 2072. [DOI] [PubMed] [Google Scholar]
- 14. Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004; 190: 1140– 1149. [DOI] [PubMed] [Google Scholar]
- 15. Friedman N, Kaye K, Stout J, McGarry S, Trivette S, Briggs J, Lamm W, Clark C, MacFarquhar J, Walton A, Reller L, Sexton D. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137: 791– 797. [DOI] [PubMed] [Google Scholar]
- 16. Garner J, Jarvis W, Emori T, Horan T, Hughes J. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16: 128– 140. [DOI] [PubMed] [Google Scholar]
- 17. Hageman JC, Liedtke LA, Sunenshine RH, Strausbaugh LJ, McDonald LC, Tenover FC. Management of persistent bacteremia caused by methicillin-resistant Staphylococcus aureus: a survey of infectious diseases consultants. Clin Infect Dis. 2006; 43: e42– e45. [DOI] [PubMed] [Google Scholar]
- 18. Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med. 2007; 167: 1861– 1867. [DOI] [PubMed] [Google Scholar]
- 19. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006; 166: 2138– 2144. [DOI] [PubMed] [Google Scholar]
- 20. Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006; 50: 3039– 3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002; 70: 631– 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhøj P, Frimodt-Møller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med. 2002; 162: 25– 32. [DOI] [PubMed] [Google Scholar]
- 23. Khatib R, Johnson LB, Fakih MG, Riederer K, Khosrovaneh A, Shamse Tabriz M, Sharma M, Saeed S. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006; 38: 7– 14. [DOI] [PubMed] [Google Scholar]
- 24. Khatib R, Johnson LB, Sharma M, Fakih MG, Ganga R, Riederer K. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand J Infect Dis. 2009; 41: 4– 9. [DOI] [PubMed] [Google Scholar]
- 25. Khatib R, Jose J, Musta A, Sharma M, Fakih MG, Johnson LB, Riederer K, Shemes S. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011; 66: 1594– 1599. [DOI] [PubMed] [Google Scholar]
- 26. Khosrovaneh A, Riederer K, Saeed S, Tabriz MS, Shah AR, Hanna MM, Sharma M, Johnson LB, Fakih MG, Khatib R. Frequency of reduced vancomycin susceptibility and heterogeneous subpopulation in persistent or recurrent methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2004; 38: 1328– 1330. [DOI] [PubMed] [Google Scholar]
- 27. Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, Park WB, Kim SH, Bang JH, Kim DM, Park KU, Shin S, Lee MS, Choi HJ, Kim NJ, Kim EC, Oh MD, Kim HB, Choe KW. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007; 60: 1108– 1114. [DOI] [PubMed] [Google Scholar]
- 28. Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011; 52: 975– 981. [DOI] [PubMed] [Google Scholar]
- 29. Lalani T, Federspiel JJ, Boucher HW, Rude TH, Bae IG, Rybak MJ, Tonthat GT, Corey GR, Stryjewski ME, Sakoulas G, Chu VH, Alder J, Steenbergen JN, Luperchio SA, Campion M, Woods CW, Fowler VG. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol. 2008; 46: 2890– 2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000; 30: 633– 638. [DOI] [PubMed] [Google Scholar]
- 31. Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010; 202: 1866– 1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin SH, Liao WH, Lai CC, Liao CH, Tan CK, Wang CY, Huang YT, Hsueh PR. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J Antimicrob Chemother. 2010; 65: 1792– 1798. [DOI] [PubMed] [Google Scholar]
- 33. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011; 52: e18– e55. [DOI] [PubMed] [Google Scholar]
- 34. Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008; 52: 3315– 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008; 52: 1330– 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCabe WR, Jackson GG. Gram-negative bacteremia: I. Etiology and ecology. Arch Intern Med. 1962; 110: 847– 855. [Google Scholar]
- 37. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 49: 1– 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, Craven DE. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001; 32: 1249– 1272. [DOI] [PubMed] [Google Scholar]
- 39. Musta AC, Riederer K, Shemes S, Chase P, Jose J, Johnson LB, Khatib R. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol. 2009; 47: 1640– 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2010; 67: 228– 233. [DOI] [PubMed] [Google Scholar]
- 41. Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med. 1976; 60: 495– 500. [DOI] [PubMed] [Google Scholar]
- 42. Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002; 46: 2155– 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can’t get there from here. Clin Infect Dis. 2011; 52: 969– 974. [DOI] [PubMed] [Google Scholar]
- 44. Paul M, Kariv G, Goldberg E, Raskin M, Shaked H, Hazzan R, Samra Z, Paghis D, Bishara J, Leibovici L. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2010; 65: 2658– 2665. [DOI] [PubMed] [Google Scholar]
- 45. Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, O’Neill G, Day NP. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002; 70: 4987– 4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66: 82– 98. [DOI] [PubMed] [Google Scholar]
- 47. Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2011; 55: 1082– 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seidl K, Bayer AS, Fowler VG, McKinnell JA, Abdel Hady W, Sakoulas G, Yeaman MR, Xiong YQ. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother. 2011; 55: 575– 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006; 34: 2588– 2595. [DOI] [PubMed] [Google Scholar]
- 50. Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand J Infect Dis. 2005; 37: 572– 578. [DOI] [PubMed] [Google Scholar]
- 51. Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008; 46: 193– 200. [DOI] [PubMed] [Google Scholar]
- 52. Soriano A, Martinez JA, Mensa J, Marco F, Almela M, Moreno-Martinez A, Sanchez F, Munoz I, Jimenez de Anta MT, Soriano E. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000; 30: 368– 373. [DOI] [PubMed] [Google Scholar]
- 53. van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012; 54: 755– 771. [DOI] [PubMed] [Google Scholar]
- 54. Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011; 66: 2386– 2392. [DOI] [PubMed] [Google Scholar]
- 55. Walsh TR, Bolmstrom A, Qwarnstrom A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J Clin Microbiol. 2001; 39: 2439– 2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001; 47: 399– 403. [DOI] [PubMed] [Google Scholar]
- 57. Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997–2003: cohort study. BMJ. 2006; 333: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis. 2009; 199: 201– 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother. 2010; 65: 1015– 1018. [DOI] [PubMed] [Google Scholar]


