Abstract
Type I cryoglobulinemia vasculitis (CryoVas) is considered a life-threatening condition; however, data on the characteristics and outcome are scarce. To analyze the presentation, prognosis, and efficacy and safety of treatments of type I CryoVas, we conducted a French nationwide survey that included 64 patients with type I CryoVas between January 1995 and July 2010: 28 patients with monoclonal gammopathy of unknown significance (MGUS) and 36 with hematologic malignancy.
Type I monoclonal CryoVas was characterized by severe cutaneous involvement (necrosis and ulcers) in almost half the patients and high serum cryoglobulin levels, contrasting with a lower frequency of glomerulonephritis than expected. The 1-, 3-, 5-, and 10-year survival rates were 97%, 94%, 94%, and 87%, respectively. Compared to MGUS, type I CryoVas related to hematologic malignancy tended to be associated with a poorer prognosis. Therapeutic regimens based on alkylating agents, rituximab, thalidomide or lenalinomide, and bortezomib showed similar efficacy on vasculitis manifestations, with clinical response rates from 80% to 86%.
Data from the CryoVas survey show that the prognosis of type I CryoVas does not seem to be as poor as previously suggested. Besides alkylating agents, the use of regimens based on rituximab, thalidomide or lenalinomide, and bortezomib are interesting alternative options, although the exact role of each strategy remains to be defined.
INTRODUCTION
Cryoglobulinemia is defined as the presence of circulating immunoglobulins that precipitate with cold temperature and dissolve with rewarming. After immunochemical typing, cryoglobulins are sorted according to the classification of Brouet et al:2 type I cryoglobulinemia comprises single monoclonal immunoglobulins, and types II and III are mixed cryoglobulinemias (MCs), with a monoclonal component in type II and only polyclonal immunoglobulins in type III. Cryoglobulinemia may cause systemic vasculitis (CryoVas), with manifestations ranging from MC syndrome (purpura, arthralgia, and asthenia) to more serious lesions with skin, neurologic, and renal involvement.9 Skin is the most frequently involved target organ, with palpable purpura, but chronic cutaneous ulcers may occur. Neurologic manifestations range from pure sensory axonopathy to mononeuritis multiplex, but the most frequently described form is distal sensory or sensory-motor polyneuropathy. Finally, the most frequent clinical and histologic picture of renal involvement is acute or chronic type I membranoproliferative glomerulonephritis with subendothelial deposits. Two major mechanisms are at play to varying degrees across the different types of cryoglobulinemia: cryoglobulin precipitation in the microcirculation, and immune complex-mediated inflammation of blood vessels. Vascular occlusion is more frequent in type I cryoglobulinemia, which is usually accompanied by high cryoglobulin concentrations, and can be associated with hyperviscosity syndrome and cold-induced acral necrosis. In contrast, immune complex–mediated vasculitis is more frequent in mixed cryoglobulinemia.18
The prevalence and incidence of CryoVas are unknown, in particular because of the heterogeneity in the cause, clinical presentation, and geographical distribution of the disease. However, the prevalence was initially reported as approximately 1:100,000 individuals.9 CryoVas appears more commonly in patients aged 45 to 65 years, with a maximum incidence in women (sex ratio women to men, 2–3 to 1).7,20 Type I cryoglobulins are always linked to a B-cell lymphoproliferative disorder, that is, multiple myeloma, Waldenström macroglobulinemia, chronic lymphocytic leukemia, B-cell non-Hodgkin lymphoma, and hairy cell leukemia. In contrast, MCs (type II or III) are associated with systemic autoimmune diseases, lymphoproliferative disorders, and chronic infections, hepatitis C virus (HCV) infection representing 80% of the CryoVas cases. When MC is found in the absence of well-defined disease, the syndrome is designated “essential MC.” Since the discovery of HCV infection in 1989, it has become clear that HCV is associated with most cases of MC. Among patients with MC, 60%–90% are infected with HCV.3,7,20,27
Type I cryoglobulinemia accounts for 10%–15% of patients with cryoglobulinemia.14 Data on type I CryoVas are scarce in the literature, therefore the characteristics and outcome are poorly known. To our knowledge the largest series of type I CryoVas, recently reported, included only 6 patients.17 Despite the lack of data and large case series of patients, type I CryoVas is considered a life-threatening disorder because of the severity of cutaneous and renal involvement and the underlying hemopathy.13,27 In the absence of prospective or randomized controlled trials, the management of type I CryoVas is based on empirical data. In patients with malignant hematologic disorders, the treatment of vasculitis is that of the hemopathy, while in those without overt B-cell lymphoma, the treatment of vasculitis may include plasma exchange, corticosteroids, alkylating agents, rituximab, or iloprost.
A survey was initiated in France in 2010 to describe the presentation, prognosis, and efficacy and safety of treatments of patients with type I CryoVas. We report here data for 64 cases of type I CryoVas included in the survey.
METHODS
Patients
The French CryoVas survey is a nationwide retrospective study that was initiated in February 2010 to describe the presentation and the efficacy and safety of treatments in patients with nonviral CryoVas. Inclusion criteria for the study were as follows: 1) detectable cryoglobulinemia in the serum; 2) systemic vasculitis; and 3) diagnosis of CryoVas between January 1995 and July 2010. Exclusion criteria were the presence of anti-HCV and anti-human immunodeficiency virus (HIV) antibodies and hepatitis B surface antigen. French hospital-based and community-based units of internal medicine, nephrology, rheumatology, dermatology, and hematology were invited to take part in the survey. The medical centers were informed that patient inclusion in this observational registry would not interfere with their current practice and would not involve the specific collection of laboratory data.
Patients were considered to have type I monoclonal CryoVas after detection and immunochemical typing; systemic vasculitis was diagnosed if they had the following in association with clinical manifestations of vasculitis (purpura, cutaneous ulcers or necrosis, arthralgia, myalgia, peripheral neuropathy, renal involvement, cerebral vasculitis, gastrointestinal involvement): 1) histologically proven vasculitis (n = 43), and/or 2) detectable cryoglobulinemia with at least purpura or cutaneous ulcers as a clinical manifestation in the absence of histology (n = 21). Patients without histologically proven vasculitis but with purpura or cutaneous ulcers and detectable MC were considered to have small-vessel vasculitis based on previously defined clinical and laboratory criteria.9,11 Finally, of the 324 patients included in the CryoVas survey, 64 patients from 29 centers had type I CryoVas (Figure 1). The study was performed in accordance with the ethical standards of the Helsinki Declaration and was approved by the local institutional review board.
FIGURE 1.

Flow chart of the CryoVas survey.
Clinical and laboratory data were recorded for each patient at the time of the initial evaluation (that is, diagnosis of cryoglobulinemia vasculitis), during follow-up, and at the end of follow-up (that is, last visit, or date of death for the 4 patients who died), by the practitioners in charge of the patients using a standardized form. The following data were collected: sex, age at diagnosis of CryoVas, dates of CryoVas, cutaneous involvement (acrosyndrome, purpura, distal ulcers, necrosis), neurologic involvement (polyneuropathy, multiple mononeuritis, central nervous system), renal involvement (proteinuria, hematuria, serum creatinine level), rheumatologic involvement (arthralgia, arthritis, myalgia), gastrointestinal involvement, type of underlying hematologic disorders, and outcome. Laboratory evaluation included determination of the serum C3 and C4 fraction of complement, cryoglobulin, serum creatinine level, urinalysis to screen for hematuria, and a 24-hour urine protein collection. Because of the retrospective design of the study, some patients were considered to have a significant cryoglobulin level when it was > 0.05 g/L, whereas some laboratories quantified the cryoglobulin level by determining the cryocrit.
The diagnosis of B-cell non-Hodgkin lymphoma was made on the basis of evidence of bone marrow, nodal, or extranodal lymphoproliferative disease with pathologic features that were compatible with the World Health Organization classification of neoplastic diseases.10 The diagnosis of monoclonal gammopathy of unknown significance (MGUS) was defined by a monoclonal immunoglobulin concentration in serum of 30 g/L or less; the absence of lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the proliferation of monoclonal plasma cells; and a proportion of plasma cells in the bone marrow of 10% or less.1
Time of therapeutic management and treatment-related data were also recorded. An alkylating agent-based regimen (n = 25) was defined by the use of cyclophosphamide (n = 12), chlorambucil (n = 7), or melphalan (n = 6), in association with corticosteroids (n = 19) or alone (n = 6). A rituximab-based regimen (n = 23) was defined by the use of rituximab in association with corticosteroids (n = 13) or alone (n = 10). A bortezomib-based regimen (n = 7) was defined by the use of bortezomib in association with dexamethasone in all cases. A thalidomide/lenalinomide-based regimen (n = 6) was defined by the use of thalidomide (n = 4) or lenalinomide (n = 2), in association with dexamethasone (n = 5) or not (n = 1). Polychemotherapy (n = 15) was defined as the combination of different cytotoxic agents, including rituximab-fludarabine-cyclophosphamide (n = 4), fludarabine-cyclophosphamide (n = 2), rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone (n = 3), cyclophosphamide-doxorubicin-vincristine-prednisone (n = 1), vincristine-Adriamycin-dexamethasone (n = 3), and rituximab-cyclophosphamide (n = 2).
Response to Therapy
The clinical response of vasculitis was defined by analyzing the course of the following main clinical signs: skin involvement (absence of purpura or distal ulcers), peripheral neuropathy (clinical and/or electrophysiologic improvement at 2 successive examinations), renal involvement (normalization or improvement of serum creatinine level, proteinuria, and hematuria) and absence of arthralgia. A complete clinical response was defined as improvement in all baseline clinical manifestations. A partial response was defined as improvement in at least half of the baseline clinical manifestations. All other patients were classified as nonresponders. Sustained remission was defined as complete or partial clinical response and the absence of relapse during the patient’s follow-up. Relapse was defined as the reappearance of clinical signs of vasculitis.
Statistical Analysis
Descriptive statistics included the mean (SD) or median (minimum-maximum) as appropriate for continuous variables and frequency (percentage) for categorical variables. Univariate analysis included the chi-square or Fisher exact test as appropriate to compare categorical variables, and the nonparametric Mann-Whitney test to compare continuous variables. The Kaplan-Meier curve was plotted to describe the survival rate according to the type of CryoVas, and the log-rank test compared both curves. Differences were considered significant when the p values were < 0.05.
RESULTS
Patient Characteristics
Sixty-four patients met the inclusion criteria (Table 1). The mean age at diagnosis was 65.4 ± 11.4 years (range, 38–89 yr), and 36 patients (56%) were female. B-cell lymphoproliferative disorders were present in all patients, including MGUS in 28 patients (44%) and hematologic malignancy in 36 patients (56%).
TABLE 1.
Main Characteristics of the 64 Patients at Diagnosis of CryoVas

Clinical manifestations at diagnosis included purpura in 44 patients (69%), acrocyanosis in 19 (30%), skin necrosis in 18 (28%), skin ulcers in 17 (27%), livedo reticularis in 8 (13%), cold urticaria in 3 (5%), peripheral neuropathy in 28 (44%), renal involvement in 19 (30%), and arthralgia/arthritis in 18 patients (28%). Peripheral neuropathy included sensory polyneuropathy in 15 patients, sensory-motor polyneuropathy in 7 patients, and sensory-motor multiple mononeuropathy in 6 patients. Renal involvement was documented by kidney biopsy in 18 of 19 cases, showing type I membranoproliferative glomerulonephritis in 17 cases and glomerulonephritis with isolated C3 deposits in 1 case. The patient without kidney biopsy had a urine protein excretion of 1.12 g/d with hematuria. None of the patients had central nervous system, pulmonary, gastrointestinal, or cardiac involvement. Fifty patients (78%) had severe manifestations of vasculitis, defined as the presence of extensive cutaneous ulcers and/or necrosis, sensory-motor multiple mononeuropathy, and/or glomerulonephritis. The median cryoglobulin level at diagnosis was 1.55 g/L (range, 0.1–10.4 g/L), and the median C3 and C4 complement levels were 0.87 (range, 0.30–1.93) and 0.09 g/L (range, 0.01–0.34), respectively. Sixteen of 45 patients (36%) had decreased C3 complement levels (<0.80 g/L), while 38 of 47 patients (81%) had decreased C4 complement levels (<0.14 g/L). Histologic confirmation of vasculitis was available in 43 patients (67%). Clinical manifestations and laboratory features were similar between patients with MGUS and hematologic malignancy, except for lower C3 complement levels in patients with hematologic malignancy (see Table 1).
Therapeutic Management
Fifty-six patients (88%) received at least 1 course of treatment for the management of type I CryoVas, while 2 patients were lost to follow-up within the first 3 months after diagnosis, and 6 patients with mild vasculitis did not receive treatment. The median number of treatments in treated patients was 2 (range, 1–6).
We analyzed the therapeutic efficacy according to 1) the line of treatment (that is, naive patients vs. refractory or relapsing patients), 2) the therapeutic regimen used, and 3) the underlying B-cell lymphoproliferative disorders.
Therapeutic Management According to Line of Treatment
First-line therapy: Treatment characteristics according to the underlying B-cell lymphoproliferative disorder are shown in Table 2. For the management of MGUS-related CryoVas, 20 patients (87%) received corticosteroids at an initial median dose of 60 mg of prednisone per day, associated with plasmapheresis in 3 (13%), alkylating agents in 5 (22%; including chloraminophene in 3 and cyclophosphamide in 2), rituximab in 2 (9%), and azathioprine in 1 (4%); 8 cases (35%) resulted in a sustained remission. Nine patients (39%) relapsed after an initial response, whereas 6 patients (26%) were considered refractory to first-line treatment.
TABLE 2.
First-Line Treatments in Patients With Type I CryoVas According to the Underlying B-Cell Disorder

For the management of hematologic malignancy-related CryoVas, 29 patients (87%) received corticosteroids at an initial median dose of 60 mg of prednisone per day, associated with plasmapheresis in 6 (18%); alkylating agents in 11 (37%; including chloraminophene in 4, cyclophosphamide in 4, melphalan in 3); multidrug chemotherapy in 9 (27%; including rituximab plus cyclophosphamide/doxorubicin/vincristine/prednisone in 2, rituximab plus fludarabine and cyclophosphamide in 2, vincristine/Adriamycin/dexamethasone followed by high-dose melphalan in 2, rituximab plus chloraminophene and dexamethasone in 2, and cyclophosphamide/doxorubicin/vincristine/prednisone in 1); rituximab in 5 (17%), bortezomib-based regimen in 2 (6%); and azathioprine, mycophenolate mofetil, and fludarabine in 1 case each. Six patients (19%) had a sustained remission, 16 patients (48%) relapsed after an initial response, and 7 patients (21%) were considered refractory to first-line therapy.
Treatment of refractory and relapsing vasculitis: In total, 36 (56%) patients received at least 1 “second-line” therapy, detailed in Table 2. Eighteen patients (51%) had a sustained remission, 6 patients (17%) relapsed after an initial response, and 11 patients (32%) were considered refractory to second-line treatment (including 5 patients who were also refractory to first-line therapy). Six patients received 3 or more courses of treatment.
Therapeutic Management According to Therapeutic Regimen
Given the heterogeneity of the treatments used, we also analyzed the different regimens separately.
Alkylating agent-based regimen: Twenty-five patients received at least 1 course of alkylating agents (in the absence of associated multidrug chemotherapy) for the treatment of type I CryoVas, as first-line (n = 16) or second-line (n = 9) treatment. The initial response rate, which was assessable in 23 patients, was 83% (17 complete response [CR] and 2 partial response [PR]), although 13 of 19 (68%) responding patients relapsed (Figure 2).
FIGURE 2.

Efficacy of alkylating agents-based regimen in patients with type I CryoVas according to its use in first or second line. Abbreviations: B-NHL = B-cell non-Hodgkin lymphoma, Bor = bortezomib, CR = complete response, CS = corticosteroids, Dexa = dexamethasone, MGUS = monoclonal gammopathy of unknown significance, MM = multiple myeloma, NR = nonresponder, PR = partial response, RTX = rituximab, Thal = thalidomide.
Rituximab-based regimen: Twenty-three patients received 1 course of rituximab (in the absence of associated multidrug chemotherapy) as first-line (n = 7), second-line (n = 12), third-line (n = 3), or fourth-line (n = 1) treatment. The initial response rate, which was assessable in 20 patients, was 80% (11 CR and 5 PR) and was comparable in first-line and second-line therapy (Figure 3). Eight of these patients relapsed, after a median time of 12.5 months (range, 6–29 mo).
FIGURE 3.

Efficacy of rituximab in patients with type I CryoVas according to its use in first or second line. Abbreviations: See Figure 2; AA = alkylating agents, HD MLP = high-dose melphalan, VAD = vincristine/Adriamycin/dexamethasone.
Bortezomid- and thalidomide/lenalidomide-based regimens: Seven patients (all with multiple myeloma) received at least 1 course of a bortezomib-based regimen as first-line (n = 2), second-line (n = 2), third-line (n = 2), or fourth-line (n = 1) treatment. The overall response rate to the bortezomib-based regimen was 86% (5 CR and 1 PR). Six patients (including 4 patients with multiple myeloma and 2 with MGUS) received at least 1 course of a thalidomide- and/or lenalidomide-based regimen as second-line (n = 4) or fifth-line (n = 2) treatment. The initial response rate to the thalidomide- and/or lenalidomide-based regimen was 83% (4 CR and 1 PR).
Therapeutic Management According to Underlying B-cell Lymphoproliferative Disorder
Therapeutic management of patients with MGUS-related CryoVas is summarized in Table 2 and Figure 4. First-line therapy in patients with MGUS-related CryoVas was mainly based on corticosteroids alone, while second-line therapy included mainly rituximab- and alkylating agents-based regimens. Both of these regimens seem to provide good efficacy, but no comparison can be made between these therapeutic strategies.
FIGURE 4.
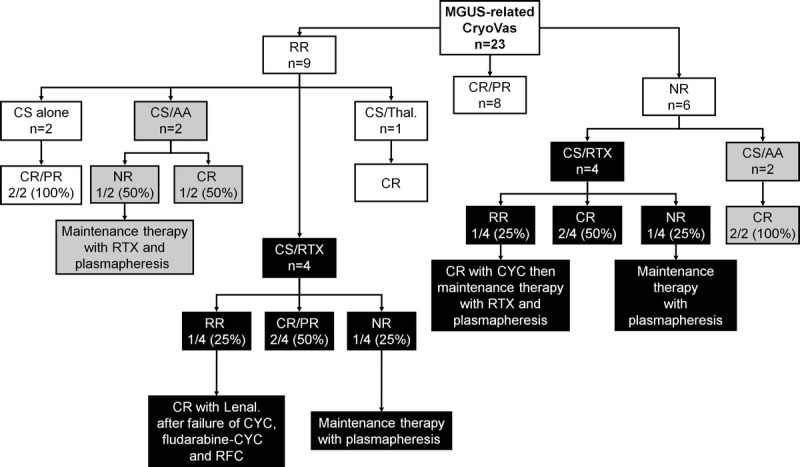
Therapeutic management and outcome of patients with MGUS-related CryoVas. Abbreviations: See previous figures; CYC = cyclophosphamide, Lenal = lenalinomide, RFC = RTX/fludarabine/CYC, RR = responder-relapser.
Treatment of patients with hematologic malignancy-related CryoVas is summarized in Table 2 and Figure 5. First-line therapy in these patients was mainly based on alkylating agents-based regimen and polychemotherapy, while second-line therapy included mainly rituximab-, bortezomib-, and thalidomide/lenalinomide-based regimens. Both of these strategies provided good efficacy.
FIGURE 5.

Therapeutic management and outcome of patients with hematologic malignancy-related CryoVas. Abbreviations: See previous figures; Plasm. = plasmapheresis, WM = Waldenström macroglobulinemia. †Patients with B-cell non-Hodgkin lymphoma included WM in 4 patients, marginal zone lymphoma in 4 patients, and lymphocytic lymphoma in 1 patient.
Outcome
After a mean follow-up of 46.2 ± 42.0 months, 8 patients (13%) were found to have severe infections, including bacterial septicemia in 3 patients, bacterial pneumonia in 3 patients, herpes virus infection in 2 patients, and cutaneous infection in 1 patient. Three of the 23 patients (13%) receiving rituximab experienced worsening vasculitis within 48 hours following the rituximab infusion. All 3 patients had lymphoplasmacytic lymphoma associated with high cryoglobulin levels (5.20, 2.75, and 2.14 g/L, respectively). In 1 of these patients, retreatment with rituximab was associated with a new episode of vasculitis flare. Four deaths (7%) were noted and were attributed to sepsis (n = 2), hemopathy (n = 1), and an unknown cause (n = 1). All patients who died had hematologic malignancy, including Waldenström macroglobulinemia in 3 patients and multiple myeloma in 1 patient. The 1-year, 3-year, 5-year, and 10-year survival rates were 97%, 94%, 94%, and 87%, respectively (Figure 6A). Compared to MGUS, type I CryoVas related to hematologic malignancy tended to be associated with a poorer prognosis (p = 0.06) (Figure 6B).
FIGURE 6.

Kaplan-Meier survival curve in 64 patients with type I CryoVas included in the survey (A), and according to the type of B-cell lymphoproliferative disorder (B). Survival rates were compared using the log-rank test in univariate analysis.
DISCUSSION
Type I CryoVas has long been considered a rare and life-threatening condition, despite the absence of published series in the literature. To better define the clinical spectrum of type I monoclonal CryoVas, we analyzed the data from 64 patients included in the French multicenter and transdisciplinary CryoVas survey. This analysis provides new insights into the presentation, outcome, and management of type I monoclonal CryoVas.
Regarding demographics, both the mean age at the time of diagnosis and the sex ratio (predominance of females) were in keeping with those usually observed in HCV- and non-HCV-related mixed CryoVas.20,26 Type I monoclonal CryoVas was characterized by severe cutaneous involvement (necrosis and ulcers) in almost half the patients, high serum cryoglobulin levels, and a lower frequency of glomerulonephritis than in mixed CryoVas. Compared with previous data in HCV- and non-HCV-related mixed CryoVas,20,26 there was an approximate 2-fold increase in frequency of severe cutaneous involvement in type I CryoVas, while renal involvement seemed to occur 1.5 times less frequently. Serum cryoglobulin levels that are approximately 2-fold higher are classically observed in type I CryoVas compared to mixed CryoVas. These differences compared to mixed CryoVas could be related to the absence of rheumatoid factor activity of type I cryoglobulins and their decreased ability to activate the complement pathway. It is usually proposed that vasculitis cutaneous manifestations in type I cryoglobulinemia may be the consequence of hyperviscosity-related rather than immune complex-induced vasculopathy. However, no classical manifestations of hyperviscosity syndrome were reported in our patients. Finally, type I CryoVas was always associated with B-cell lymphoproliferative disorders, including malignant hemopathy in 56%, confirming the need to systematically screen these patients for an underlying B-cell malignancy.
The evaluation of efficacy and safety of different therapeutic regimens was a major goal of the CryoVas survey. In patients with malignant hemopathy, the heterogeneity of disease subtypes and treatments used makes it difficult to draw any conclusions on the efficacy of the different regimens. In this context, the treatment of vasculitis is primarily that of the underlying hematologic malignancy using the optimal combined chemotherapy. In patients with MGUS-related CryoVas, the initiation of therapy is only justified by the severity of the vasculitis. Treatment options in these patients include corticosteroids with or without alkylating agents, plasma exchange, and, more recently, rituximab6 and other new biologic agents. The current study provides additional information on the use of these new agents, in particular rituximab-, thalidomide-, lenalidomide-, and bortezomib-based regimens.
The efficacy of rituximab in type I CryoVas remains controversial.15,16,19,23,25 The absence of CD20 expression on plasma cells was supposed to explain its lack of efficacy. In the present study, a response to rituximab was reported in 80% of patients in first- or second-line treatment, supporting its use in naive or relapsing/refractory patients. Vasculitis flares were noted within 48 hours following rituximab infusion in 13% of patients. Similar exacerbations of the disease have been previously reported in association with increased cryoglobulin levels. It has been suggested that CD20 cross-linking could provoke cryoglobulin release through massive B-cell apoptosis or activation, raising some concerns as to the use of rituximab in these patients.15 In HCV-related MC vasculitis, severe early flares of vasculitis after rituximab have been reported, especially in patients with high levels of serum cryoglobulin and with the use of 1 g infusions on days 1 and 15.22 An increase in the cryoglobulin level after rituximab was also reported in a patient with type II MC associated with Waldenström macroglobulinemia, without a vasculitis flare but with a complete clinical response.8
We established the efficacy of thalidomide and lenalinomide in 6 patients, with an efficacy rate of 83%. In the literature, the efficacy of thalidomide and lenalinomide was reported in 4 patients with type I CryoVas, either related to multiple myeloma5,12 or to MGUS.4,21 A bortezomib-based regimen was shown to be effective in 86% of our patients, while its efficacy has been an ecdotally reported in the literature.23,24 Thalidomide, lenalinomide, and bortezomib were well tolerated. Overall, these findings suggest that in patients with severe and/or refractory MGUS-related type I CryoVas, rituximab-, thalidomide- or lenalinomide-, and bortezomib-based regimens could be interesting alternative options when combined with corticosteroids. The patients’ profiles and the type of organ involvement could help practitioners choose between these different therapeutic regimens. In patients with peripheral neuropathy, the use of thalidomide, lenalinomide, and bortezomib should probably be avoided. Rituximab should be administered with caution in elderly patients with renal failure receiving high-dose corticosteroids due to the increased risk of severe infections.25
The prognosis of type I CryoVas did not seem to be as poor as previously thought. Although the small number of patients and deaths in the current series did not allow us to identify prognostic factors of survival, all patients who died had hematologic malignancy. The long-term prognosis in type I CryoVas seemed to be better than that in HCV-related mixed CryoVas (the most frequent type of CryoVas), with 5- and 10-year survival rates of 94% and 87%, respectively, compared with 75% and 63% in HCV-related mixed CryoVas.26 As found in non-HCV mixed CryoVas,20 severe infections accounted for half of the deaths, suggesting that the therapeutic management and prevention of severe infection should be improved in these patients.
We acknowledge some limitations of the present study, primarily the retrospective design and the heterogeneity of the treatments used. In addition, because of our inclusion criteria—detectable serum cryoglobulinemia and systemic vasculitis—our patients did not have manifestations of hyperviscosity syndrome, which represents a complication of type I cryoglobulinemia. However, despite these limitations, the current study provides interesting data on type I monoclonal CryoVas.
In conclusion, the CryoVas survey describes the presentation, prognosis, and therapeutic management of the largest series of type I CryoVas patients reported, to our knowledge. We observed the frequent occurrence of severe cutaneous involvement and high cryoglobulin levels, whereas the long-term prognosis does not appear to be as poor as in mixed CryoVas. Rituximab-, thalidomide- or lenalinomide-, and bortezomib-based regimens seem to be beneficial alternative therapeutic options when combined with corticosteroids.
Abbreviations
- CR
complete response
- CryoVas
cryoglobulinemia vasculitis
- HCV
hepatitis C virus
- MC
mixed cryoglobulinemia
- MGUS
monoclonal gammopathy of unknown significance
- PR
partial response
APPENDIX
The authors acknowledge the physicians who participated in the CryoVas Study Group:
Laurent Aaron, Sébastien Abad, Redouane Bakir, Pauline Belenotti, Lucas Benarous, Nathalie Beneton, Gilles Blaison, Claire Blanchard-Delaunay, Fabrice Bonnet, Frank Bridoux, Patrice Cacoub, Pascal Cathébras, Fabrice Carrat, Laurent Chiche, Olivier Chosidow, Bernard Combe, Christian Combe, Nathalie Costedoat-Chalumeau, Pierre Cougoul, Bernard Cribier, Gilles Defuentes, Elisabeth Diot, Isabelle Durieu, Philippe Evon, Thibault Fraisse, Camille Francès, Hélène Francois, Thierry Frouget, Loik Geffray, Helder Gil, Jean Gutnecht, Eric Hachulla, Olivier Hermine, Olivier Hinschberger, Arnaud Hot, Antoine Huart, Aurélie Hummel, Jean-Emmanuel Kahn, Gilles Kaplanski, Alexandre Karras, Evguenia Krastinova, Adeline Lacraz, Claire Larroche, David Launay, Estibaliz Lazaro, Guillaume Le Guenno, Véronique Leblond, Jean-Marc Léger, Nicolas Limal, Eric Liozon, Catherine Lok, Véronique Loustau-Ratti, Kim Ly, Hervé Maillard, Raifah Makdassi, Isabelle Marie, Xavier Mariette, Olivier Meyer, Catherine Michel, Philippe Modiano, David Noel, Raphaele Nove-Josserand, Céline Onno, Christian Pagnoux, Caroline Pajot, Géraldine Perceau, Antoinette Perlat, Anne-Marie Piette, Emmanuelle Plaisier, Jean-François Pouget-Abadie, Thomas Quemeneur, Viviane Queyrel, Joseph Rivalan, Eric Rondeau, Patricia Rullier, Luc de Saint-Martin, Elisabeth Salles-Thomasson, Françoise Sarrot-Reynauld, Thierry Schaeverbeke, Gwendoline Sebille, Patricia Senet, Raphaele Seror, Jacques Serratrice, Alexandre Somogyi, Pascale Soria, Benjamin Terrier, Marie-Francoise Thiercelin-Legrand, François Truchetet, Jean-Paul Vagneur, Philippe Vanhille, Bérengère Vivet, Pierre-Jean Weiller, Bernadette Woehl-Kremer, Jean-Marie Woehl, Thierry Zénone.
Footnotes
Dr. Kahn reports previous financial support for work outside this study from GlaxoSmithKline (GSK) (board membership and grant to his institution). The other authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Anonymous. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003; 121: 749– 757. [PubMed] [Google Scholar]
- 2. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974; 57: 775– 788. [DOI] [PubMed] [Google Scholar]
- 3. Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999; 42: 2204– 2212. [DOI] [PubMed] [Google Scholar]
- 4. Calabrese C, Faiman B, Martin D, Reu F, Calabrese LH. Type 1 cryoglobulinemia: response to thalidomide and lenalidomide. J Clin Rheumatol. 2011; 17: 145– 147. [DOI] [PubMed] [Google Scholar]
- 5. Cem Ar M, Soysal T, Hatemi G, Salihoglu A, Yazici H, Ulku B. Successful management of cryoglobulinemia-induced leukocytoclastic vasculitis with thalidomide in a patient with multiple myeloma. Ann Hematol. 2005; 84: 609– 613. [DOI] [PubMed] [Google Scholar]
- 6. Dimopoulos MA, Merlini G, Leblond V, Anagnostopoulos A, Alexanian R. How we treat Waldenstrom’s macroglobulinemia. Haematologica. 2005; 90: 117– 125. [PubMed] [Google Scholar]
- 7. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002; 55: 4– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghobrial IM, Uslan DZ, Call TG, Witzig TE, Gertz MA. Initial increase in the cryoglobulin level after rituximab therapy for type II cryoglobulinemia secondary to Waldenstrom macroglobulinemia does not indicate failure of response. Am J Hematol. 2004; 77: 329– 330. [DOI] [PubMed] [Google Scholar]
- 9. Gorevic PD, Kassab HJ, Levo Y, Kohn R, Meltzer M, Prose P, Franklin EC. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980; 69: 287– 308. [DOI] [PubMed] [Google Scholar]
- 10. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. ASH Education Book. Hematology Am Soc Hematol Educ Program. 2009; 2009 (1): 523– 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997; 337: 1512– 1523. [DOI] [PubMed] [Google Scholar]
- 12. Lin RJ, Curran JJ, Zimmerman TM, Song J, Niewold TB, Sweiss NJ. Lenalidomide for the treatment of cryoglobulinemia and undifferentiated spondyloarthropathy in a patient with multiple myeloma. J Clin Rheumatol. 2010; 16: 90– 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, Pietrogrande M, Renoldi P, Bombardieri S, Bordin G, Candela M, Ferri C, Gabrielli A, Mazzaro C, Migliaresi S, Mussini C, Ossi E, Quintiliani L, Tirri G, Vacca Aand the GISC. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC. Italian Group for the Study of Cryoglobulinaemias. QJM. 1995; 88: 115– 126. [PubMed] [Google Scholar]
- 14. Morra E. Cryoglobulinemia. ASH Education Book. Hematology Am Soc Hematol Educ Program. 2005; 2005 (1): 368– 372. [DOI] [PubMed] [Google Scholar]
- 15. Nehme-Schuster H, Korganow AS, Pasquali JL, Martin T. Rituximab inefficiency during type I cryoglobulinaemia. Rheumatology (Oxford). 2005; 44: 410– 411. [DOI] [PubMed] [Google Scholar]
- 16. Pandrangi S, Singh A, Wheeler DE, Rossi NF. Rituximab treatment for a patient with type I cryoglobulinemic glomerulonephritis. Nat Clin Pract Nephrol. 2008; 4: 393– 397. [DOI] [PubMed] [Google Scholar]
- 17. Payet J, Livartowski J, Kavian N, Chandesris O, Dupin N, Wallet N, Karras A, Salliot C, Suarez F, Avet-Loiseau H, Alyanakian MA, Nawakil CA, Park S, Tamburini J, Roux C, Bouscary D, Sparsa L. Type I cryoglobulinemia in multiple myeloma: a rare entity. Analysis of clinical and biological characteristics of 7 cases and review of the literature. Leuk Lymphoma. 2012; [April 19, 2012; Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012; 379: 348– 360. [DOI] [PubMed] [Google Scholar]
- 19. Rosenthal E, Pesce A, Karsenti JM, Allieri-Rosenthal MA, Cassuto JP. Polyneuropathy and vasculitis associated with IgG type I cryoglobulinemia can be treated with the anti-CD20 monoclonal antibody (rituximab). Blood. 2001; 98 (Suppl): 292b. [Google Scholar]
- 20. Saadoun D, Sellam J, Ghillani-Dalbin P, Crecel R, Piette JC, Cacoub P. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med. 2006; 166: 2101– 2108. [DOI] [PubMed] [Google Scholar]
- 21. Sampson A, Callen JP. The cutting edge: thalidomide for type 1 cryoglobulinemic vasculopathy. Arch Dermatol. 2006; 142: 972– 974. [DOI] [PubMed] [Google Scholar]
- 22. Sene D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab may form a complex with iGmkappa mixed cryoglobulin and induce severe systemic reactions in patients with hepatitis C virus-induced vasculitis. Arthritis Rheum. 2009; 60: 3848– 3855. [DOI] [PubMed] [Google Scholar]
- 23. Spizzo G, Mitterer M, Gunsilius E. Bortezomib for the treatment of refractory type-1 cryoglobulinaemia. Br J Haematol. 2010; 150: 235– 237. [DOI] [PubMed] [Google Scholar]
- 24. Talamo G, Claxton D, Tricot G, Fink L, Zangari M. Response to bortezomib in refractory type I cryoglobulinemia. Am J Hematol. 2008; 83: 883– 884. [DOI] [PubMed] [Google Scholar]
- 25. Terrier B, Launay D, Kaplanski G, Hot A, Larroche C, Cathebras P, Combe B, de Jaureguiberry JP, Meyer O, Schaeverbeke T, Somogyi A, Tricot L, Zenone T, Ravaud P, Gottenberg JE, Mariette X, Cacoub P. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res (Hoboken). 2010; 62: 1787– 1795. [DOI] [PubMed] [Google Scholar]
- 26. Terrier B, Semoun O, Saadoun D, Sene D, Resche-Rigon M, Cacoub P. Prognostic factors in patients with hepatitis C virus infection and systemic vasculitis. Arthritis Rheum. 2011; 63: 1748– 1757. [DOI] [PubMed] [Google Scholar]
- 27. Trejo O, Ramos-Casals M, Garcia-Carrasco M, Yague J, Jimenez S, de la Red G, Cervera R, Font J, Ingelmo M. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001; 80: 252– 262. [DOI] [PubMed] [Google Scholar]


