Abstract
Previous evidence suggests that higher circulating 25-hydroxyvitamin D (25[OH]D) levels are variably associated with lower breast cancer risk; however, prospective studies and clinical trials have been inconsistent, particularly between older and younger women of differing menopausal status. We conducted a quantitative nonlinear dose-response meta-analysis of prospective studies evaluating the association between circulating 25(OH)D and breast cancer risk, stratified by menopause. A systematic search of MEDLINE and EMBASE included studies published through May 2011. We reviewed references from retrieved articles and contacted relevant investigators for additional data from prospective studies on circulating 25(OH)D levels and incident breast cancers. Prospective studies of circulating vitamin D and breast cancer risk were reviewed, and no language restrictions were imposed. Information on study population, menopausal status, 25(OH)D levels, and relative risk (RR) estimates were extracted using a standardized protocol.
A total of 9 prospective studies were included, comprising 5206 cases and 6450 controls. Data were pooled using dose-response random-effects meta-regression models. Identifying nonlinear effects, spline models were optimized for thresholds. The relationship between circulating 25(OH)D and breast cancer risk differed by menopausal status (p = 0.05 for effect modification). While no association was found in premenopausal women, dose-response modeling revealed a nonlinear inverse association among postmenopausal women. Notably, a flat association was observed in the lowest range of 25(OH)D levels <27 ng/mL (RR = 1.01 per 5 ng/mL; 95% confidence interval [CI], 0.98–1.04). In contrast, postmenopausal breast cancer risk decreased with 25(OH)D levels 27-<35 ng/mL (p = 0.02 for nonlinear risk change), where a 5 ng/mL increase in 25(OH)D was associated with a 12% lower risk of breast cancer (RR = 0.88 per 5 ng/mL; 95% CI, 0.79–0.97), with suggestive flattening at higher doses >35 ng/mL. The significant inverse association did not appear to vary across strata of invasive/in-situ cases, body mass index adjustment, region, postmenopausal hormone use, or assay method.
In summary, this dose-response meta-analysis of prospective studies of plasma 25(OH)D suggested a breast cancer risk differential by menopause, whereby a step-wise inverse association was observed beyond a threshold of 27 ng/mL, but with flattening of effects above 35 ng/mL, in postmenopausal women. These findings help resolve prior inconsistent findings and may carry important clinical and public health implications.
INTRODUCTION
Breast cancer is a leading cause of mortality in women.3 Although a number of breast cancer risk factors are well established (for example, family history, breast density, parity, alcohol use), very few are readily modifiable. Low circulating vitamin D levels below 30 ng/mL were found in 77% of the United States population from 2000 to 2004, paralleling the increased trend of vitamin D deficiency in the last 2 decades.30 Factors associated with lower circulating 25-hydroxyvitamin D (25[OH]D) levels include obesity, low physical activity, higher geographic latitude (marker of ultraviolet-B exposure), age, race, skin type, and smoking.12,13,41,46,52 More importantly, circulating 25(OH)D, the best marker of vitamin D status,38,69 is easily modifiable with 1000 IU of daily vitamin D intake increasing circulating 25(OH)D by 10 ng/mL.37
Preclinical experimental evidence and previous retrospective studies have suggested that vitamin D intake and higher circulating vitamin D levels may be protective against cancer,7,8,31,66 potentially via regulation of cell division, apoptosis, and contact inhibition.39 Vitamin D may also partially mediate the observed association between physical activity and breast cancer risk through sunlight exposure.21,29,52 However, prospective studies in humans have been inconsistent. For example, in 2 recent studies, 1 study found no association for 25(OH)D and breast cancer risk,2 while another found a strong inverse association.58 Although an inverse association was also found in the Nurses’ Health Study,9 the largest prospective study to date from the Prostate, Lung, Colorectal and Ovarian Cancer Screening trial (PLCO) again found no association.24 Furthermore, results from 3 previous meta-analyses were also inconsistent, with 2 of the studies reporting no evidence for a dose-response relationship,14,26,68 and none of the previous studies accounted for menopause status.
Differences in study population, particularly menopausal status and the range of circulating 25(OH)D levels, may potentially account for some of these inconsistencies in observational studies. Moreover, most previous investigations only considered linear trends and compared extreme quantiles, without evaluating possible nonlinear dose-response relations or heterogeneity of baseline vitamin D levels across diverse populations.
Therefore, to assess the dose-response relationship between circulating 25(OH)D and breast cancer risk comprehensively, we conducted a systematic review and meta-analysis of the prospective literature, particularly focusing on differences between pre- and postmenopausal women as well as potential nonlinear associations for risk of breast cancer. (See also the accompanying commentary on this study by Stearns and Visvanathan62a in this same issue.)
METHODS
Study Selection
We conducted a comprehensive literature search of MEDLINE (National Library of Medicine, Bethesda, MD) and EMBASE (Elsevier, Amsterdam, The Netherlands) from 1966 through May 2011. We followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for searching and reporting. Search terms included MESH, EmTree, title/abstract, and synonyms of breast cancer combined with vitamin D, 25-hydroxyvitamin D, or calcifediol. Additional studies were searched for via references of retrieved articles, direct author contact for unpublished data, and referral by experts in the field. Studies were excluded if they did not fulfill the following criteria: a) human studies, b) prospective cohort and nested case-control studies, c) measured circulating (serum/plasma) 25(OH)D at baseline, d) reported a relative risk (RR) or odds ratio and confidence interval (CI) per vitamin D category, e) reported outcome of breast cancer risk. No language restrictions were imposed. Incident breast cancer was analyzed as the outcome of interest due to varying screening and treatments by country. In the first round of screening abstracts (n = 974), 938 articles were excluded by search criteria (Figure 1). In a second round of screening full text articles (n = 36), 27 articles were excluded: not prospective (11 articles), circulating 25(OH)D not measured (6 articles), survival among cancer cohort (5 articles), duplicate studies (3 articles), and case report (2 articles). Our search criteria yielded 9 total prospective case control studies, comprising 5206 incident cases and 6450 controls (Table 1).
FIGURE 1.
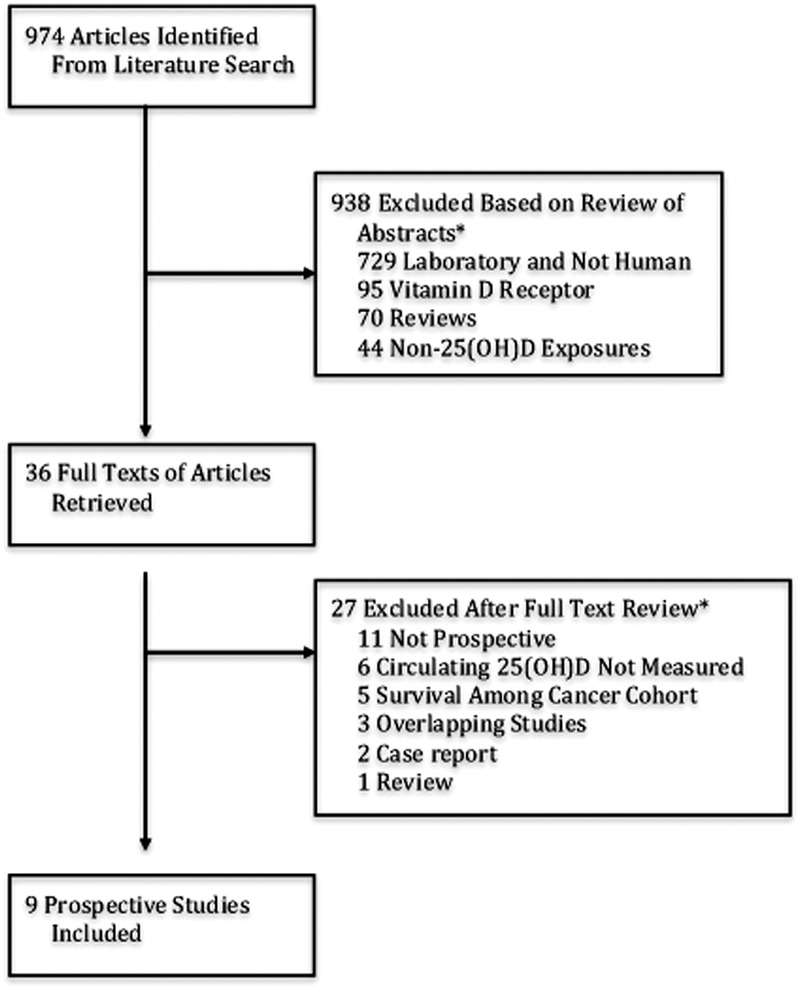
Summary of article selection process. *Studies belonging to multiple classifications were counted only once.
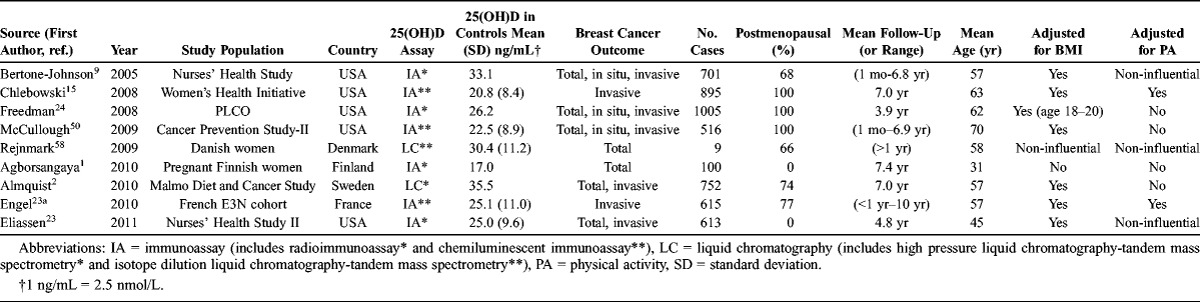
TABLE 1.
Characteristics of Studies Included in the Meta-Analysis of Circulating Vitamin D and Breast Cancer Risk, Ordered by Year of Publication and Alphabetically
Data Extraction
Data from these studies were tabulated using a standardized extraction form. Discrepancies were resolved via group discussion and review. Information extracted included lead author; publication year; population; country of origin; menopausal status; study design; average length and/or range of follow-up; number of cases and controls by quantile; adjustment for body mass index (BMI) or physical activity; mean age; 25(OHD) assay; mean/median/range of circulating 25(OH)D levels by quantile; RRs and standard error of breast cancer risk by quantile. When RR estimates were reported for more than 1 set of adjustments, we selected the most adjusted estimate.
We requested additional data via personal communications from authors of all studies in order to conduct thorough dose-response analysis and stratified analyses by menopausal status, current postmenopausal hormone use, and tumor characteristics (3 provided data by quantile, 1 provided stratified estimates and data by batch, 6 provided stratified data by menopausal status, and 5 provided stratified data by use of hormone replacement therapy). Only 1 author (Chlebowski) of the 9 contacted study authors did not provide additional de novo data. We further obtained detailed batch and subcohort data from the Nurses’ Health Study I cohort (Appendix 1). Follow-up in the originally published Rejnmark study averaged only 3 months, hence subclinical influences on vitamin D levels could not be ruled out in the original report. Thus, Rejnmark (personal communication) provided an updated analysis restricted to cases diagnosed >1 year after blood draw.
Vitamin D Measurements
Both immunoassay and liquid chromatography methods were used to assess circulating 25(OH)D levels. For stratified analyses, assay categories included radioimmunoassay (RIA) or chemiluminescent immunoassay (CIA) and high pressure liquid chromatography (HPLC) or isotope dilution liquid chromatography-tandem mass spectrometry. Plasma9,23,58 and serum1,2,15,24,50 are comparable mediums to measure circulating 25(OH)D, thus, we use circulating 25(OH)D to refer to both mediums.
Statistical Analysis
We calculated the RR as a pooled measure of the association between circulating 25(OH)D levels and breast cancer risk using both highest versus lowest category and a dose-response meta-regression analysis. A random-effects meta-regression trend estimation of summarized dose-response data, described by Greenland and Longnecker,34,54 was used to derive the incremental dose-response RRs between circulating 25(OH)D levels and breast cancer risk. The continuous linear scale increment for the trend-estimated RR was 5 ng/mL in circulating 25(OH)D. Apparent nonlinear associations were statistically analyzed using dose-response GLST (Generalized Least-Square Trend) meta-regression and spline analysis for change in slope at specified knot-points; splined variables were created using MKSPLINE in STATA (StataCorp, College Station, TX). Goodness of fit tests and comparative chi-square statistics were subsequently used to optimize the knot-points in spline regressions and to test robustness of spline knots. Based on prior literature, test of effect modification by menopausal status was determined a priori. Additionally, stratified meta-regressions were conducted to determine whether differences in tumor invasiveness, mean age, assay, country, mean 25(OH)D levels, or adjustment for BMI and physical activity influenced associations and explained heterogeneity across studies.64 Linear meta-regressions were conducted in sensitivity analyses using aggregate models, where effect estimates were combined from all studies before estimating the pooled linear dose-response. To assess the presence of publication bias, we assessed the symmetry of individual study linear dose-response slopes around the pooled estimate using Begg funnel plots.19 All analyses were conducted using STATA 10 (StataCorp, College Station, TX); p ≤ 0.05 was considered statistically significant.
Visual Assessments of Dose-Response Relations: Ding Spaghetti Plot
A novel meta-analytic visual representation method was developed by Eric L. Ding to aid in detecting nonlinear relationships between circulating 25(OH)D levels and breast cancer risk among postmenopausal women (Figure 2). The Ding Spaghetti Plot consists of connected study-series line plots of individual study RRs, where each “spaghetti noodle” represents a RR series from the same study; and data points are represented by circles, in which the relative size of each circle reflects the analytic weight of each RR estimate (although weighting does not affect the shape of the connected line plots). Thus, RRs with smaller standard errors (that is, relatively larger sample sizes) are represented by larger data points. The aggregate graphical visual representation, via the Ding Spaghetti Plot of all studies’ dose-response “noodle” plots together, allows investigators to visually identify potential nonlinear associations and different dose-response curves from multiple data series across various studies. The centrally averaged pooled dose-response curve, highlighted as the main “noodle” in the Spaghetti Plot, represents the aggregate slope between knot-points. It is accompanied by upper and lower 95% CI bands that represent the uncertainty of the central pooled dose response curve.
FIGURE 2.
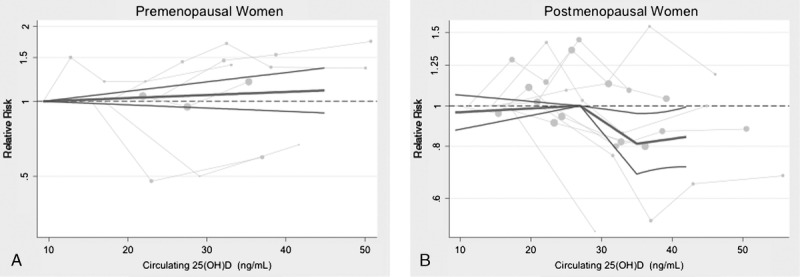
Ding Spaghetti Plot and pooled dose-response relationship between circulating 25(OH)D Levels and breast cancer risk, stratified by menopausal status (A, premenopausal, and B, postmenopausal women). The solid dark gray line represents the central pooled dose-response estimate, and the surrounding black lines represent 95% confidence interval bands. Each light gray “spaghetti noodle” represents a relative risk series from the same study; data points are represented by circles, with the relative size of each circle reflecting the analytic weight of each RR estimate.
Note: Quantitative RR for Figure 2:
Postmenopausal p value for nonlinear dose effect modification:
• at 27 ng/mL: p for nonlinear slope change = 0.02
• at 35 ng/mL: p for nonlinear slope change = 0.05
Point-specific RRs compared to 27 ng/mL (reference) among postmenopausal women:
• 35 ng/mL: RR = 0.81 (95% CI, 0.69–0.96), p = 0.01
• 40 ng/mL: RR = 0.83 (95% CI, 0.71–0.97), p = 0.02
Dose-response nonlinear slope RRs per 5 ng/mL increase in circulating 25(OH)D in postmenopausal women:
• <27 ng/mL range: RR per 5 ng/mL increase = 1.01 (95% CI, 0.98–1.04)
• 27–34 ng/mL range: RR per 5 ng/mL increase = 0.88 (95% CI, 0.79–0.97)
• 35–40 ng/mL range: RR per 5 ng/mL increase = 1.03 (95% CI, 0.94–1.12)
RESULTS
A total of 9 prospective studies with 11 study sets were included, comprising 5206 incident cases of breast cancer and 6450 controls (see Table 1). Mean 25(OH)D concentrations ranged from 17.0 to 33.1 ng/mL. BMI was evaluated as a potential confounder in 8 of 9 studies, although adjustment for physical activity was considered less often (4 of 9 studies).
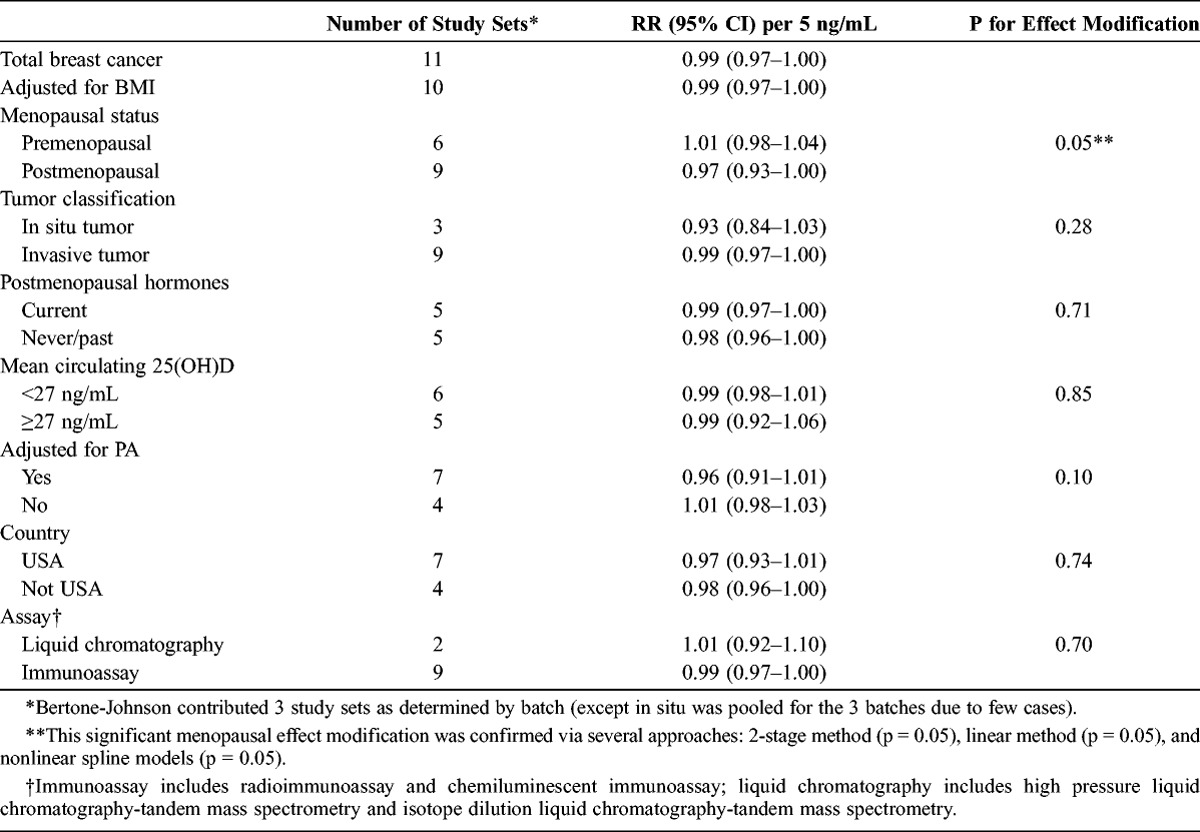
Evaluating the presence of a linear dose-response relationship, we observed a borderline statistically significant inverse association between circulating 25(OH)D and breast cancer risk (RR per 5 ng/mL = 0.99; 95% CI, 0.97–1.00; Table 2). However, menopausal status was a statistically significant effect modifier of this relationship (pinteraction = 0.05), where the inverse association between circulating 25(OH)D and breast cancer risk was limited to postmenopausal women (RR per 5 ng/mL = 0.97; 95% CI, 0.93–1.00). No dose-response relationship was observed among premenopausal women (RR per 5 ng/mL = 1.01; 95% CI, 0.98–1.04). This significant menopausal effect modification was confirmed via several analytic approaches: 2-stage pooling method (p = 0.05 for menopause effect), linear aggregate method (p = 0.05 for menopause effect), and nonlinear spline models (p = 0.05 for menopause effect).
TABLE 2.
Stratified, Pooled Linear Dose-Response Relative Risks per 5 ng/mL Circulating 25(OH)D
In our primary analysis, analyzing 25(OH)D levels to carefully assess a dose-response, results indicated a significant inverse, nonlinear association between circulating 25(OH)D and breast cancer risk among postmenopausal women, with apparent thresholds of 27 ng/mL (67 nmol/L) and 35 ng/mL (see Figure 2 and 3). Notably, while no dose-response relationship was observed among the lowest range of 25(OH)D levels <27 ng/mL (RR slope = 1.01 per 5 ng/mL; 95% CI, 0.98–1.04), higher 25(OH)D levels were associated with a reduced risk of breast cancer between 27 ng/mL and 35 ng/mL (RR slope = 0.88 per 5 ng/mL; 95% CI, 0.79–0.97), with a p for nonlinear risk change of 0.02 at 27 ng/mL. Furthermore, the reduction in risk somewhat flattened (p = 0.05 for nonlinear risk change) at highest levels ≥35 ng/mL (RR slope = 1.03 per 5 ng/mL; 95% CI, 0.94–1.12), yet remained at lower risk compared to 27 ng/mL. The nonlinear results were robust and relatively insensitive to changes in knot location. The point-specific RRs among postmenopausal women compared to a reference risk level of 27 ng/mL were RR = 0.81 (95% CI, 0.69–0.96) at 35 ng/mL, and RR = 0.83 (95% CI, 0.71–0.97) at 40 ng/mL. Moreover, effect modification by menopause was also confirmed in these spline models (p = 0.05), with no association in premenopausal women.
FIGURE 3.
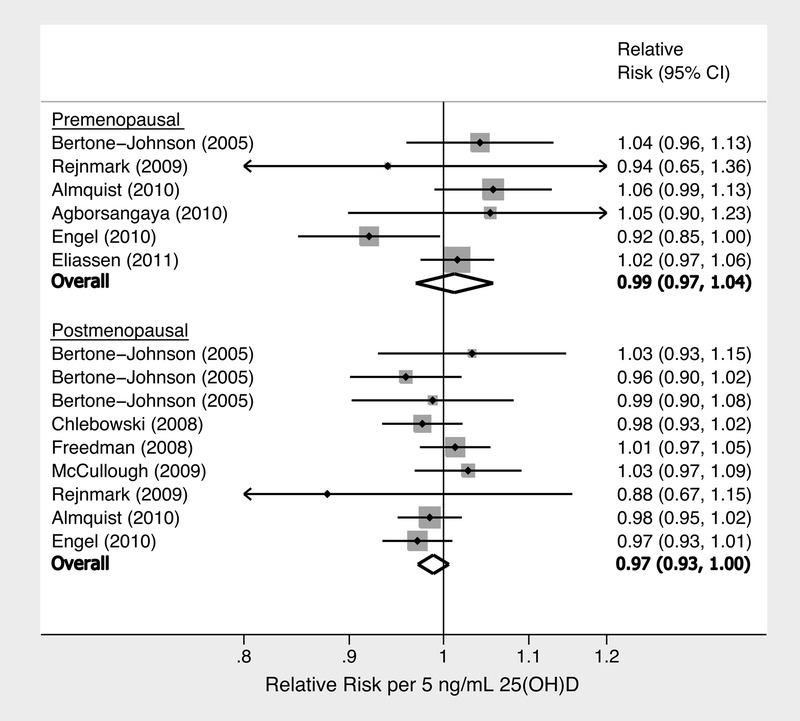
Forest plot of linear dose-response of circulating 25(OH)D and breast cancer risk, stratified by menopausal status, listed by first author and date of study. (P for menopause effect modification = 0.05.) Note: Bertone-Johnson et al contributed 3 study sets as determined by batch (except in situ was pooled for the 3 batches due to few cases).
Parsimoniously modeling linear dose-response in subgroup analyses, the association did not appear to be modified by tumor classification, study mean circulating 25(OH)D, geographic region of the study cohort, assay type, or current postmenopausal hormone use (see Table 2), although these factors were assessed among all women (since data further stratified by menopausal status were not available). Restricting the analysis to studies that adjusted for BMI did not alter the results. Physical activity was a suggestive effect modifier of the linear dose-response relationship among all women, where specifically, studies that adjusted for physical activity observed a somewhat stronger inverse association (RR per 5 ng/mL = 0.96; 95% CI, 0.91–1.01), compared to studies that did not adjust for physical activity (RR per 5 ng/mL = 1.01; 95% CI, 0.98–1.03), with pinteraction = 0.10.
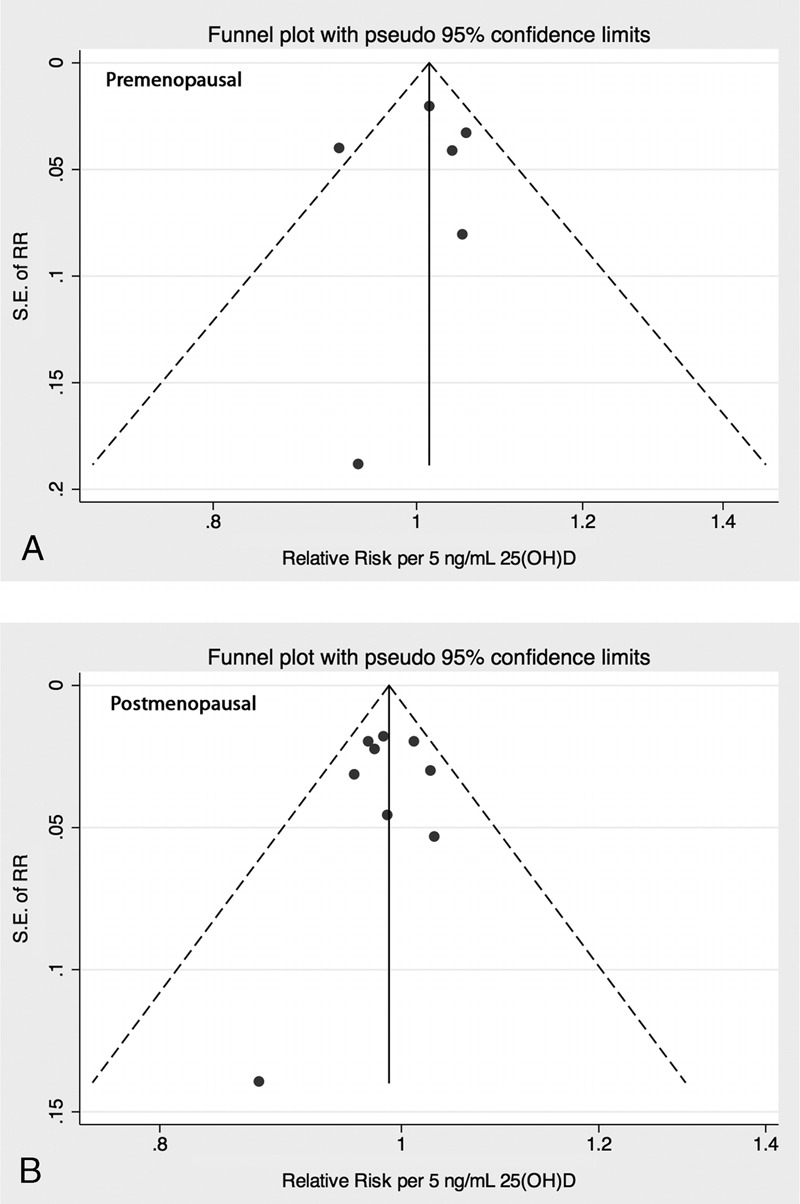
Finally, we conducted a sensitivity analysis of 25(OH)D cutpoints for the 3 laboratory batches of the Nurses’ Health Study, with no evidence for an effect of specific cutpoints on the results (see Appendix 1). As for assessing publication bias, the Begg test (premenopausal: p = 0.71, postmenopausal: p = 0.92), the Egger test (premenopausal: p = 0.83, postmenopausal: p = 0.88), and a funnel plot of linear dose-response slopes provided no evidence of publication bias (Appendix 2).
DISCUSSION
In the current dose-response meta-regression of prospective studies examining the association between circulating vitamin D and breast cancer risk, we observed an apparent nonlinear inverse association where higher 25(OH)D levels at or above a 27 ng/mL threshold were associated with a 12% lower risk of postmenopausal breast cancer per 5 ng/mL increase in 25(OH)D. However, no further reductions in risk of breast cancer were observed above 35 ng/mL 25(OH)D. Increases of 5 ng/mL circulating 25(OH)D will typically occur when vitamin D intake is increased 500 IU/d.37 In contrast, no association was observed among premenopausal women. These results were consistent across multiple disease definitions and population characteristics. Data indicated that apparent inconsistencies from previous individual studies may have been due to inadequate assessment of effect modification by menopausal status and lack of spline dose-response analysis to account for a nonlinear relationship between circulating vitamin D and postmenopausal breast cancer risk. Previous conflicting reviews did not account for these dose-response and menopausal issues.14,26,68
Our nonlinear results are supported by other congruent findings and indications of a threshold effect, most notably in studies of dietary vitamin D and breast cancer risk. As discussed by Garland et al,28 many earlier studies of vitamin D and breast cancer risk may have offered null results given that the mean 25(OH)D levels in the majority of those studies were below the spline threshold that we observed. A recent meta-analysis of dietary vitamin D intake and breast cancer risk supports the potential threshold effect. Although no association was found in the crude linear analysis, an inverse trend was observed comparing highest versus lowest intake when limited to vitamin D intakes greater than 400 IU/d (RR = 0.92; 95% CI, 0.87–0.97).32 Evidence of a nonlinear relationship for the protective effect of circulating 25(OH)D has also been shown in other cancers. Notably, a prospective analysis of circulating 25(OH)D and colon cancer risk found a 3-fold decrease in risk of colon cancer above a threshold of 20 ng/mL.27 Similarly, a possible threshold effect was observed in the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, where circulating 25(OH)D levels in women were associated with a significantly decreased risk of kidney cancer above 30 ng/mL (RR = 0.31; 95% CI, 0.12–0.85); however, as this was an unexpected finding in their subgroup analyses,25 it warrants further replication.
These results suggest that higher-dose vitamin D interventions may yield a benefit for postmenopausal, but not premenopausal, breast cancer. One previous 4-year randomized trial of vitamin D supplementation and cancer does appear to suggest that daily supplementation with 1000 IU vitamin D plus calcium reduced total cancer mortality (RR = 0.40; 95% CI, 0.20–0.82), albeit there were few breast cancer cases.47 Although the Women’s Health Initiative (WHI) vitamin D plus calcium trial was, to our knowledge, the first randomized trial to specifically study vitamin D supplementation and risk of invasive breast cancer among postmenopausal women, the trial population had low baseline 25(OH)D levels and used a supplemental dose of only 400 IU/d. In concordance with the high-dose nonlinear hypotheses, the WHI trial found no reduction in breast cancer risk (RR = 0.96; 95% CI, 0.85–1.09).15 Furthermore, in a recent reanalysis, vitamin D and calcium supplementation was associated with a significant reduction in risk of breast cancer among women who were not taking personal calcium and vitamin D supplements at randomization (RR = 0.82; 95% CI, 0.70–0.97).11
However, many studies and reports have conflicting evidence regarding dietary vitamin D. The recently released Institute of Medicine (IOM) guidelines for dietary intake of vitamin D and calcium stated that circulating 25(OH)D concentrations of 20 ng/mL are sufficient for 97% of the population, primarily based on bone health.4,60 The IOM committee cited, at the time of their report, a lack of sufficient evidence supporting higher circulating 25(OH)D concentrations for protection against nonskeletal outcomes, even though this has been a controversial topic among national experts.10
Although the effect of menopausal status on the association between circulating 25(OH)D and breast cancer risk has not been previously studied in detail, menopause is an important effect modifier of the relationship between obesity and breast cancer.63 In postmenopausal women, both obesity and adult weight gain are associated with an increased risk of breast cancer, primarily through increasing concentrations of circulating estrogens.20,44 Conversely, obesity is inversely associated with risk of premenopausal breast cancer.51 Higher estrogen concentrations are associated with an increased risk of breast cancer in postmenopausal and possibly premenopausal women.22,36,42,43 However, higher concentrations of circulating estrogens in postmenopausal women are primarily driven by secretion of estrogen from adipose tissue, whereas ovarian production is the primary driver of estrogen concentrations in premenopausal women. Vitamin D may also inhibit growth of breast cancer cells through down-regulation of estrogen receptor expression and attenuation of estrogen signaling and synthesis.45 Vitamin D supplementation may have interacted with concurrent estrogen treatments in the WHI, as suggested in a reanalysis of vitamin D and estrogen with colorectal cancer risk, but not breast cancer risk, in the WHI.18 Variation in the association between 25(OH)D and breast cancer risk by menopausal status, similar to the relationship between obesity and breast cancer, may potentially be due to competitive binding of vitamin D and estrogen at lower levels of circulating 25(OH)D.
The exact mechanism behind a specific threshold is unclear; however, there are several molecular mechanisms that may account for an inverse association between circulating 25(OH)D and postmenopausal breast cancer risk. There are 3 primary pathways through which vitamin D, via the converted and tightly regulated form of 1,25(OH)D (calcitriol), may prevent breast cancer risk, including cell division, apoptosis, and contact inhibition.39 1,25(OH)D and a functional vitamin D receptor control cell growth and division through regulation of cyclins, cyclin-dependent kinases, and cell cycle checkpoints.16,35,67 In addition to regulating cell division, calcitriol is needed for cells to undergo apoptosis.6,17,40,48,49,62 Failure to undergo apoptosis following DNA damage can lead to continued proliferation and eventual malignancy. Lastly, calcitriol regulates E-cadherin, a cell adhesion molecule that is partially responsible for cellular contact inhibition.53,57,59,61 Loss of contact inhibition is common in neoplastic cells and often predicts a poor prognosis.55 Higher levels of prognostic circulating 25(OH)D may also be associated with increased survival among breast cancer patients.33,56,65 These mechanisms support the biological plausibility of an inverse association between circulating 25(OH)D and breast cancer risk, although more work is needed to establish potential mechanisms of a nonlinear threshold effect.
The current study has several potential clinical implications. Most importantly, since low vitamin D levels are safely and inexpensively reversed by supplementation, low vitamin D may be one of the few modifiable risk factors for postmenopausal breast cancer. Indeed, low vitamin D status is remarkably common, particularly in older and non-white populations, which are known to have an increased risk of breast cancer.46,52 From the national average circulating 25(OH)D level of 24 ng/mL,30 daily supplementation of 1000 IU/d vitamin D would be needed to reach the approximate threshold of 35 ng/mL.37,38,69 Our results highlight and reinforce the importance of ongoing higher-dose vitamin D intervention studies, such as the VITamin D and OmegA-3 TriaL (VITAL) (2000 IU/d).5 This level of supplementation corresponds to an increase in circulating 25(OH)D levels of approximately 20 ng/mL among treatment arm participants.37,38,69 Furthermore, our results may support ongoing efforts to increase vitamin D levels in selected populations, specifically postmenopausal women, and help refine the indications for clinical measurement of circulating vitamin D.
Although to our knowledge this is the most comprehensive meta-analysis to date of the association between circulating 25(OH)D and breast cancer risk, there are limitations. First, it is not possible to know to what degree the differences in 25(OH)D levels between study populations are due to true differences in exposure versus varying assay methods and batch-to-batch variation in laboratory results. Further, due to the nature of the published data on circulating 25(OH)D and breast cancer risk, RRs were reported by category of 25(OH)D levels rather than as a continuous variable. Thus, inconsistent assays of circulating 25(OH)D may potentially lead to some misclassification, thus reducing precision in the exact value of the optimal 25(OH)D spline knot thresholds. However, assay misclassification would be non-differentially random with respect to breast cancer, and seems unlikely to explain the significant nonlinear spline association. A future pooled analysis of individual patient-level data and circulating 25(OH)D as a continuous variable, with an embedded recalibration study to determine true differences in levels between studies, would be helpful in confirming the nonlinear inverse association as well as refine the spline thresholds.
The current meta-analysis was limited to published data, and further adjustment for individual BMI and physical activity was not possible, thus residual confounding remains a possibility. However, almost all the studies included adjusted or considered adjusting for BMI, and the results were not altered when excluding studies that did not adjust for BMI. Furthermore, stratified analyses of adjustment for physical activity suggested that studies that adjusted for physical activity observed a stronger inverse association between circulating 25(OH)D and breast cancer. Thus, residual confounding by physical activity is likely to attenuate the results, and is unlikely to explain observed associations. Not all studies reported breast cancer endpoints by tumor classification (in situ or invasive); however, authors of studies that assessed different endpoints were contacted, and stratified results were retrieved for all studies queried, which reported similar associations. Lastly, the systematic review was limited to published results or additional data provided by study investigators, and although the possibility cannot be excluded, we observed no publication bias.
In conclusion, findings from the current systematic review comprising 5206 incident cases of breast cancer and 6450 control cases suggest that the association of circulating 25(OH)D with breast cancer risk differed a) by menopausal status, and b) nonlinearly by dose. Notably, a modest inverse association between 25(OH)D and breast cancer risk was observed among postmenopausal women, whereas no association was observed among premenopausal women. Furthermore, there is suggestive evidence of a nonlinear inverse association between circulating 25(OH)D and postmenopausal breast cancer risk, specifically at or above a threshold of 27 ng/mL. These findings highlight the potential importance of attaining a target threshold of circulating 25(OH)D levels for vitamin D among postmenopausal women to exert possible protective effects on breast cancer risk. Additional detailed dose-response assessments in large prospective studies are needed to confirm these findings. Ultimately, the benefit of vitamin D supplementation for postmenopausal women will need to be validated in large clinical trials, such as the on-going VITAL trial,5 with adequate doses that sufficiently modify circulating 25(OH)D levels.
ACKNOWLEDGMENTS
The authors thank the following people for their assistance: Lars Rejnmark, Michael Freedman and staff of the PLCO trial, Martin Almquist, Pierre Engel, and Calypse Agborsangaya. We also thank Edward Giovannucci (Harvard School of Public Health) for his thoughtful comments and suggestions.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CI
confidence interval
- IOM
Institute of Medicine
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening trial
- RR
relative risk
- VITAL
VITamin D and OmegA-3 TriaL
- WHI
Women’s Health Initiative
APPENDIX 1.
Data From the Nurses’ Health Study and 25(OH)D Batch Cutpoints
Due to variation in mean and standard deviation of circulating 25(OH)D levels between 3 different batches of distinct cases and controls in the Nurses’ Health Study, the study data were extracted and analyzed with 3 sets of RRs and 25(OH)D levels by quantile for dose-response analyses.20 Additional information from the original author (Bertone-Johnson, personal communication) facilitated the extraction of accurate information for each batch independently; thus data from the Nurses’ Health Study were analyzed as 3 study sets instead of 1. No cases or controls belonged to more than 1 of these independent batches, and batches represented distinctly different person-time, which makes the 3-batches analysis identical to pooling HRs from Cox proportional hazard models stratifying on time. A sensitivity analysis was conducted using different 25(OH)D cutpoints since the variation in levels was likely due largely to laboratory batch-to-batch variation.
To assess the effect of batch-to-batch variation in 25(OH)D cutpoints of the 3 batches used from the Nurses’ Health Study, we conducted a sensitivity analysis using the lowest and highest 25(OH)D level for all 3 batches. Since the variation between batches is likely due to lab differences rather than true differences among the participants, we wanted to ensure that the different batch 25(OH)D cutpoints were not influencing the results. Accordingly, the batch cutpoint did not influence the results when the lowest or highest 25(OH)D level was used for all 3 batches (p = 0.02, p = 0.02, and p = 0.04 for dose-interaction).
APPENDIX 2.
Figures A and B
Funnel plot of linear dose-response slopes, by menopausal status (A, premenopausal, and B, postmenopausal women). Note there was no evidence of publication bias.
No captions available.
Footnotes
Financial support and conflicts of interest: No direct funding was provided for this project. Eric Ding was supported by a grant from the American Heart Association and American Diabetes Association. Susan Hankinson was supported by a grant from the National Institutes of Health (CA49449, CA67262). The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Agborsangaya CB, Surcel HM, Toriola AT, Pukkala E, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M. Serum 25-hydroxyvitamin D at pregnancy and risk of breast cancer in a prospective study. Eur J Cancer. 2010; 46: 467– 470. [DOI] [PubMed] [Google Scholar]
- 2. Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk—a prospective nested case-control study. Int J Cancer. 2010; 127: 2159– 2168. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 4.Anonymous. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: Institute of Medicine; 2011. [Google Scholar]
- 5.Anonymous. VITamin D and OmegA-3 TriaL (VITAL) website. 2010; http://www.vitalstudy.org/. [Google Scholar]
- 6. Audo I, Darjatmoko SR, Schlamp CL, Lokken JM, Lindstrom MJ, Albert DM, Nickells RW. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Invest Ophthalmol Vis Sci. 2003; 44: 4192– 4199. [DOI] [PubMed] [Google Scholar]
- 7. Bertone-Johnson ER. Prospective studies of dietary vitamin D and breast cancer: more questions raised than answered. Nutr Rev. 2007; 65: 459– 466. [DOI] [PubMed] [Google Scholar]
- 8. Bertone-Johnson ER. Vitamin D and breast cancer. Ann Epidemiol. 2009; 19: 462– 467. [DOI] [PubMed] [Google Scholar]
- 9. Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14: 1991– 1997. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Willett WC. Comment on the IOM Vitamin D and Calcium Recommendations. Harvard School of Public Health: The Nutrition Source [serial on the Internet]. 2010. [Google Scholar]
- 11. Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011; 94: 1144– 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brock KE, Graubard BI, Fraser DR, Weinstein SJ, Stolzenberg-Solomon RZ, Lim U, Tangrea JA, Virtamo J, Ke L, Snyder K, Albanes D. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers in Finland. Eur J Clin Nutr. 2010; 64: 280– 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan J, Jaceldo-Siegl K, Fraser GE. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010; 21: 501– 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010; 121: 469– 477. [DOI] [PubMed] [Google Scholar]
- 15. Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O’Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008; 100: 1581– 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai X, Yamasaki K, Yang L, Sayama K, Shirakata Y, Tokumara S, Yahata Y, Tohyama M, Hashimoto K. Keratinocyte G2/M growth arrest by 1,25-dihydroxyvitamin D3 is caused by Cdc2 phosphorylation through Wee1 and Myt1 regulation. J Invest Dermatol. 2004; 122: 1356– 1364. [DOI] [PubMed] [Google Scholar]
- 17. Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther. 2006; 5: 2786– 2797. [DOI] [PubMed] [Google Scholar]
- 18. Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women’s Health Initiative randomized trial. Int J Cancer. 2008; 122: 1690– 1694. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Altman DG, Smith GD, eds. Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Books; 2001. [Google Scholar]
- 20. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006; 296: 193– 201. [DOI] [PubMed] [Google Scholar]
- 21. Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010; 170: 1758– 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006; 98: 1406– 1415. [DOI] [PubMed] [Google Scholar]
- 23. Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011; 13: R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a. Engel P, Fagherazzi G, Boutten A, Dupre T, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010; 19: 2341– 2350. [DOI] [PubMed] [Google Scholar]
- 24. Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008; 17: 889– 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallicchio L, Moore LE, Stevens VL, Ahn J, Albanes D, Hartmuller V, Setiawan VW, Helzlsouer KJ, Yang G, Xiang YB, Shu XO, Snyder K, Weinstein SJ, Yu K, Zeleniuch-Jacquotte A, Zheng W, Cai Q, Campbell DS, Chen Y, Chow WH, Horst RL, Kolonel LN, McCullough ML, Purdue MP, Koenig KL. Circulating 25-hydroxyvitamin D and risk of kidney cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010; 172: 47– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011; 128: 1414– 1424. [DOI] [PubMed] [Google Scholar]
- 27. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989; 2: 1176– 1178. [DOI] [PubMed] [Google Scholar]
- 28. Garland CF, Gorham ED, Baggerly CA, Garland FC. Re: Prospective study of vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2008; 100: 826– 827. [DOI] [PubMed] [Google Scholar]
- 29. Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990; 19: 614– 622. [DOI] [PubMed] [Google Scholar]
- 30. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009; 169: 626– 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005; 16: 83– 95. [DOI] [PubMed] [Google Scholar]
- 32. Gissel T, Rejnmark L, Mosekilde L, Vestergaard P. Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol. 2008; 111: 195– 199. [DOI] [PubMed] [Google Scholar]
- 33. Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009; 27: 3757– 3763. [DOI] [PubMed] [Google Scholar]
- 34. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135: 1301– 1309. [DOI] [PubMed] [Google Scholar]
- 35. Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN, Choi I. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin [sic] D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003; 22: 4035– 4046. [DOI] [PubMed] [Google Scholar]
- 36. Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998; 90: 1292– 1299. [DOI] [PubMed] [Google Scholar]
- 37. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003; 77: 204– 210. [DOI] [PubMed] [Google Scholar]
- 38. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266– 281. [DOI] [PubMed] [Google Scholar]
- 39. Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008; 24: 139– 149. [DOI] [PubMed] [Google Scholar]
- 40. James SY, Mackay AG, Colston KW. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J Steroid Biochem Mol Biol. 1996; 58: 395– 401. [DOI] [PubMed] [Google Scholar]
- 41. Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromso study. Eur J Nutr. 2010; 49: 401– 407. [DOI] [PubMed] [Google Scholar]
- 42. Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quiros JR, Roddam A, Thiebaut A, Tjonneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2005; 97: 755– 765. [DOI] [PubMed] [Google Scholar]
- 43. Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002; 94: 606– 616. [DOI] [PubMed] [Google Scholar]
- 44. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003; 95: 1218– 1226. [DOI] [PubMed] [Google Scholar]
- 45. Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010; 121: 343– 348. [DOI] [PubMed] [Google Scholar]
- 46. Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009; 29: 3713– 3720. [PubMed] [Google Scholar]
- 47. Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007; 85: 1586– 1591. [DOI] [PubMed] [Google Scholar]
- 48. Maruyama R, Aoki F, Toyota M, Sasaki Y, Akashi H, Mita H, Suzuki H, Akino K, Ohe-Toyota M, Maruyama Y, Tatsumi H, Imai K, Shinomura Y, Tokino T. Comparative genome analysis identifies the vitamin D receptor gene as a direct target of p53-mediated transcriptional activation. Cancer Res. 2006; 66: 4574– 4583. [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto T, Sowa Y, Ohtani-Fujita N, Tamaki T, Takenaka T, Kuribayashi K, Sakai T. p53-independent induction of WAF1/Cip1 is correlated with osteoblastic differentiation by vitamin D3. Cancer Lett. 1998; 129: 61– 68. [DOI] [PubMed] [Google Scholar]
- 50. McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009; 11: R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006; 166: 2395– 2402. [DOI] [PubMed] [Google Scholar]
- 52. Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, Robbins J, LaCroix AZ, LeBoff MS, Jackson RD. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010; 91: 1324– 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muehlemann M, Miller KD, Dauphinee M, Mizejewski GJ. Review of growth inhibitory peptide as a biotherapeutic agent for tumor growth, adhesion, and metastasis. Cancer Metastasis Rev. 2005; 24: 441– 467. [DOI] [PubMed] [Google Scholar]
- 54. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006; 6: 40– 57. [Google Scholar]
- 55. Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001; 154: 369– 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palmieri C, MacGregor T, Girgis S, Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006; 59: 1334– 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pendas-Franco N, Gonzalez-Sancho JM, Suarez Y, Aguilera O, Steinmeyer A, Gamallo C, Berciano MT, Lafarga M, Munoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007; 75: 193– 207. [DOI] [PubMed] [Google Scholar]
- 58. Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, Mosekilde L. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2009; 18: 2655– 2660. [DOI] [PubMed] [Google Scholar]
- 59. Rosenau W. The nature and mechanism of metastasis. Oncology. 1970; 24: 21– 25. [DOI] [PubMed] [Google Scholar]
- 60. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96: 53– 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004; 1692: 77– 102. [DOI] [PubMed] [Google Scholar]
- 62. Sebag M, Gulliver W, Kremer R. Effect of 1,25 dihydroxyvitamin D3 and calcium on growth and differentiation and on c-fos and p53 gene expression in normal human keratinocytes. J Invest Dermatol. 1994; 103: 323– 329. [DOI] [PubMed] [Google Scholar]
- 62a. Stearns V, Visvanathan K. Optimizing vitamin D concentrations for breast cancer risk reduction [Commentary]. Medicine (Baltimore). 2013; 92: 132– 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stephenson GD, Rose DP. Breast cancer and obesity: an update. Nutr Cancer. 2003; 45: 1– 16. [DOI] [PubMed] [Google Scholar]
- 64. van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002; 21: 589– 624. [DOI] [PubMed] [Google Scholar]
- 65. Vrieling A, Hein R, Abbas S, Schneeweiss A, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011; 13: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004; 80: 1721S– 4S. [DOI] [PubMed] [Google Scholar]
- 67. Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997; 15: 1555– 1563. [DOI] [PubMed] [Google Scholar]
- 68. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010; 46: 2196– 2205. [DOI] [PubMed] [Google Scholar]
- 69. Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008; 87: 1087S– 1091S. [DOI] [PubMed] [Google Scholar]


