Abstract
Current Staphylococcus aureus bacteremia (SAB) practice guidelines stratify treatment duration according to the likelihood of complications and recommend transesophageal echocardiography (TEE) in all cases. The benefit of TEE in uncomplicated SAB has not been validated. We performed a retrospective analysis of TEE and transthoracic echocardiography (TTE) among hospitalized adults with SAB in 3 prior observational studies (2002–2003, 2005–2006, and 2008–2009). Echocardiograms were ordered at the attending physician’s discretion. SAB cases were stratified into the following types: complicated (persistent bacteremia [duration ≥3 d], relapse, and/or secondary foci); device-associated (intracardiac prosthetic devices); suspected endocarditis (the presence of murmurs or emboli); and uncomplicated (bacteremia duration ≤2 d, no device and/or secondary foci). We encountered 960 SAB cases; 83 were excluded (57 death/transfer/discharge within 48 h; 19 contaminants/no treatment; 7 care withdrawn). TEE and TTE were performed within 0–28 days of SAB onset in 177 (20.2%) and 321 (36.6%) instances, respectively. TEE was positive (with signs of endocarditis) in 42/177 (23.7%) cases: 7/39 (17.9%) community associated and 35/138 (25.4%) health care associated. It was positive in 29/120 (24.2%) complicated, 3/11 (27.3%) device-associated, 9/15 (60.0%) suspected endocarditis, and 1/31 (3.2%) uncomplicated cases of SAB. TTE was positive in 25/321 (7.8%) cases of SAB, 1 was uncomplicated; it was negative in 20/30 (66.7%) TEE-positive cases. Follow-up of ≥100 days was possible in 282/361 (78.1%) uncomplicated SAB; many (46.8%) received ≤15 days of therapy. None of them had relapses or secondary foci.
These findings suggest that echocardiography is dispensable in cases of uncomplicated community-associated and health care-associated SAB. It should be limited to subsets with clinical findings of endocarditis, persistence, intracardiac devices, secondary foci, and relapse. The cost effectiveness of TTE prior to TEE among these patients is unknown.
INTRODUCTION
Staphylococcus aureus bacteremia (SAB) remains a common and serious infection. It is associated with a high risk for secondary foci and infective endocarditis.1,2,4,11,17,25 Therefore, extended treatment duration was often used.11,17 With the widespread use of echocardiography, transesophageal echocardiogram (TEE) was advocated to optimize treatment duration. Subsequently, the need for extended treatment duration in uncomplicated intravenous catheter-associated SAB was challenged.15,18 In an attempt to support short-term treatment for uncomplicated intravascular catheter-associated bacteremia, Rosen et al22 proposed TEE as a cost-effective measure to avoid prolonged treatment. Since then, TEE was often advocated in SAB.3,7,24 Kern11 recommended echocardiography in patients with community-onset disease, intravenous drug users, those with persistent bacteremia, patients with vascular implants, and hemodialysis-dependent patients. Mortara et al16 proposed limiting echocardiography to community-associated disease. The most recent Infectious Diseases Society of America practice guidelines for the treatment of SAB recommend echocardiography, preferably TEE, in all patients.14 The benefit of this approach in uncomplicated SAB has not been clearly documented. The risk for endocarditis and distant complications is known to be higher in patients with prolonged bacteremia.2,8,17,25 Whether echocardiography is dispensable in patients without these risk factors is unclear. Kaasch et al10 recently recommended the use of criteria to select cases for echocardiography among patients with nosocomial SAB. An accompanying editorial supported this approach.23 We conducted the current study to review the results of echocardiography in community- and health care-associated SAB and to define clinical criteria that may optimize TEE predictive values.
PATIENTS AND METHODS
Patients
Adult (aged ≥18 yr) inpatients with SAB identified during 3 prior observational studies (2002–2003, 2005–2006, and 2008–2009) were included.12 Selection methods were similar in all studies. All patients with ≥1 positive blood culture (BC) for S. aureus were screened. All patients with SAB (positive BC and clinical signs of bacteremia) were included. Patients who died or were discharged within 48 hours and who did not receive any treatment (care withdrawn or considered contaminant) were excluded. SAB cases separated by ≥100 days were counted as a new bacteremia.
The following information was gathered prospectively during each of the prior observational studies: demographics, underlying conditions, presence of cardiac devices (prosthetic valve, pacemakers/defibrillators), source of infection, onset mode, duration of bacteremia, TTE and TEE performance with the date and the results, treatment, and outcome measures that included the development of secondary foci (anatomically unrelated to the primary focus and became apparent after the onset of bacteremia), relapse, and 100-day all-cause mortality. Comorbidity score was retrospectively calculated according to the modified Charlson weighted index.19
Definitions
The onset of SAB was classified into 3 mutually exclusive categories according to the Centers for Disease Control definition.18 Hospital onset: SAB after 3 days of inpatient hospitalization. Health care-associated community-onset: SAB in patients with invasive devices or exposure to a health care setting within the preceding 12 months. Community-associated: SAB within 3 days of admission without invasive devices or health care exposure during the preceding 12 months.
The duration of bacteremia was defined as the number of days between the first and last positive BC or resolution of symptoms in patients without follow-up BC as previously described.12 Persistent bacteremia was defined as bacteremia for ≥3 days. Positive echocardiogram was defined as echocardiographic evidence of infective endocarditis such as an intracardiac oscillating mass, abscess, new valvular regurgitation, or new dehiscence of a prosthetic valve, according to the criteria proposed by Fowler et al.7
Echocardiograms were ordered according to the discretion of the attending physician or the consultant. TEE was performed and evaluated by an experienced echocardiographer. TTE was performed by an experienced technician and evaluated by an experienced echocardiographer. For echocardiogram utility assessment we included all patients with TTE or TEE within 0–28 days of the first positive BC. Echocardiograms performed after 28 days were excluded because they were often ordered for reasons other than the SAB. SAB was classified into 4 exclusive categories: complicated (bacteremia duration ≥3 d, relapse, and/or secondary foci); the presence of cardiac devices (prosthetic valves, pacemakers, defibrillators) without complicated bacteremia, suspected endocarditis (the presence of murmurs or emboli) without complicated bacteremia, and uncomplicated bacteremia (bacteremia duration ≤2 d and without devices, secondary foci, or relapse within 100 d). TEE and TTE results were then stratified according to SAB type. Follow-up for 100 days or more was attempted by reviewing subsequent medical encounters in all patients with uncomplicated bacteremia who survived. Information sought in follow-up included recurrence of bacteremia and the development of distant foci of infection such as endocarditis or osteomyelitis.
The study was approved by the St John Hospital institutional review board.
Statistical Methods
The chi-square test and student t test were used to assess the significance of differences in categorical and continuous variables, respectively. Sensitivity, specificity, positive and negative predictive value for TEE evidence of infective endocarditis in SAB were calculated for each selected clinical criteria as follows: sensitivity (positive TEE in patients with the selected criteria/all TEE performed in patients with the selected criteria), specificity (negative TEE without the selected criteria/negative TEE without the selected criteria + positive TEE without the selected criteria), positive predictive value (positive TEE in patients with the selected criteria/all positive TEE), and negative predictive value (negative TEE in patients without the selected criteria/all negative TEE).
Statistical analyses were performed using SPSS v 12 and a web-based open source epidemiologic statistics software for public health (openepi.com). P value < 0.05 was considered to indicate statistical significance.
RESULTS
We encountered 960 episodes of SAB in patients during the 3 study periods; 83 were excluded because of death within 48 h (n = 45), discharge within 48 h (n = 12), care withdrawn within 48 h (n = 7), and suspected contamination (n = 19). The remaining 877 cases of SAB occurred among 805 patients. Thirty-three patients had 2 cases of bacteremia, 33 had 3 cases, and 6 patients had 4 cases of bacteremia. The majority of SAB cases (87.2%) were health care associated; 30.7% were hospital-onset and 56.5% were health care-associated community-onset cases. Follow-up BC was obtained in 86.4% of cases. The first follow-up culture was obtained within a median of 2 days. Additional follow-up BC were obtained within a median of 3 days. The time between last positive and first negative BC, available in 677 patients, was 1–17 days with a median = 3 days. A summary of all patients and the frequency of endocarditis based on clinical and/or TEE findings is shown in Table 1. An echocardiogram was performed in 379 (43.2%) episodes (Fig. 1).
TABLE 1.
Characteristics of Patients With Staphylococcus aureus Bacteremia and Frequency of Infective Endocarditis Stratified According to the Characteristics
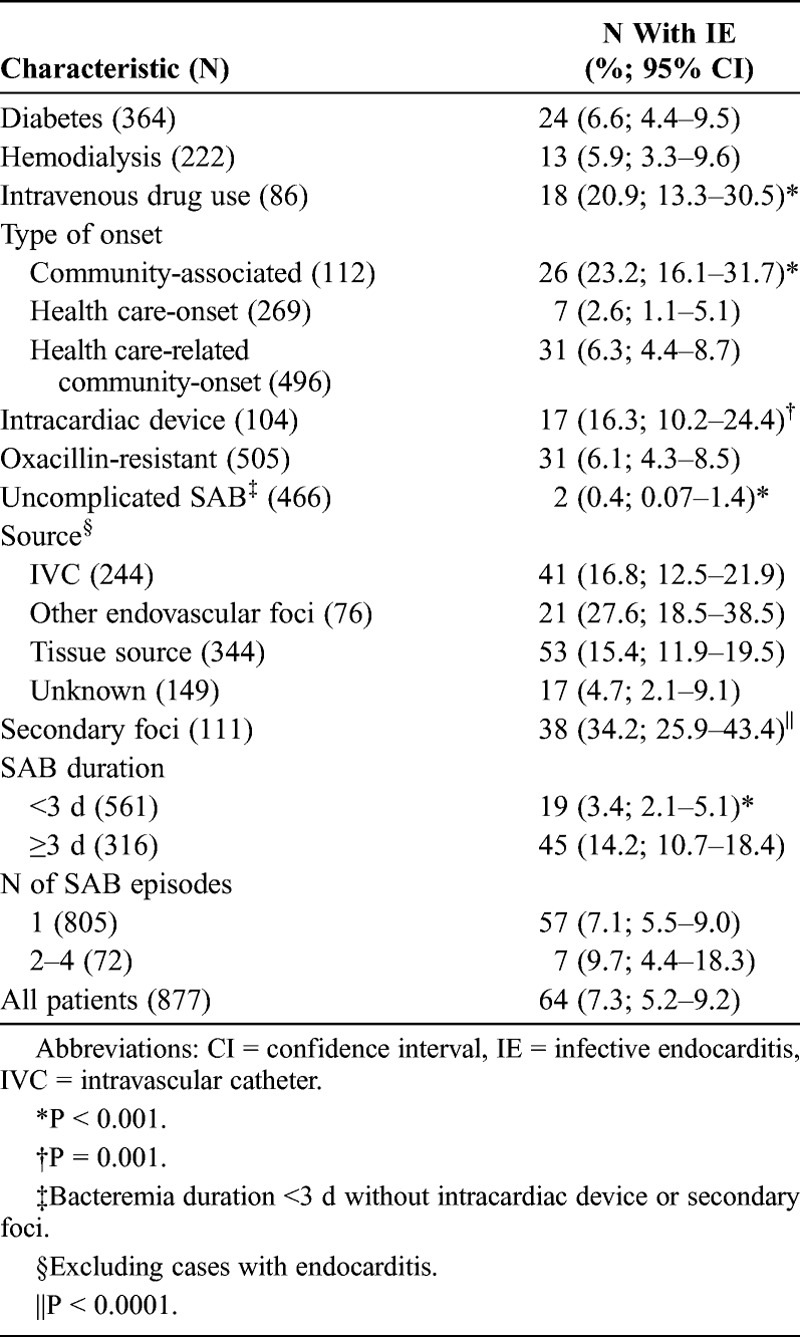
FIGURE 1.
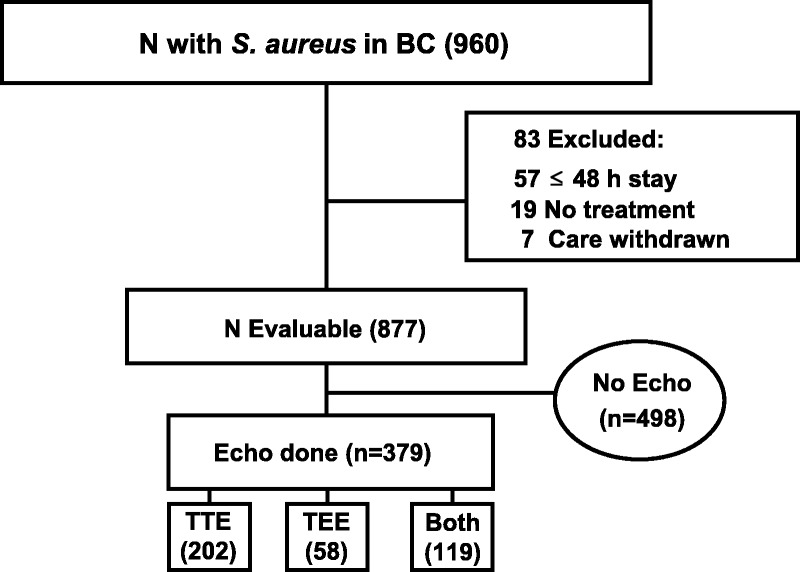
Flow diagram of the patients with Staphylococcus aureus in 1 or more blood culture (BC) included in echocardiography (Echo) assessment. Abbreviations: TTE = transthoracic echocardiogram; TEE = transesophageal echocardiogram.
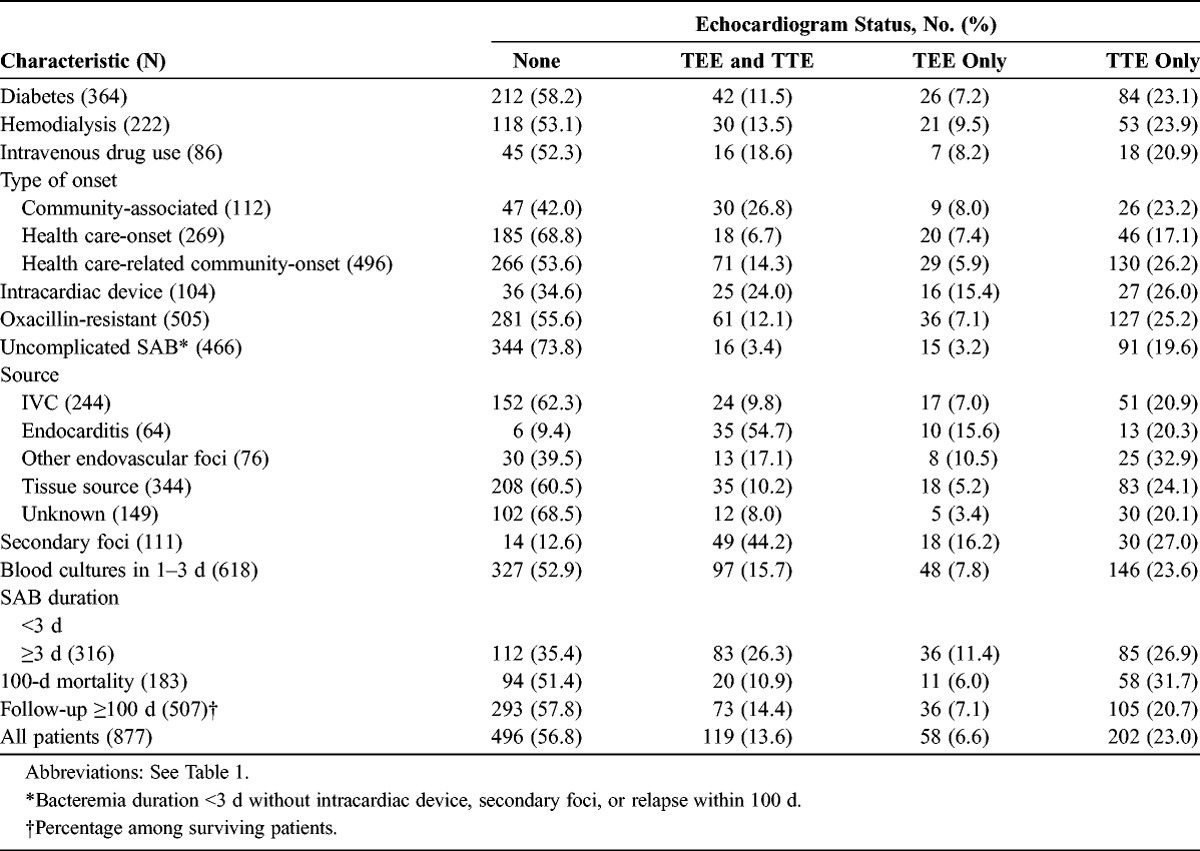
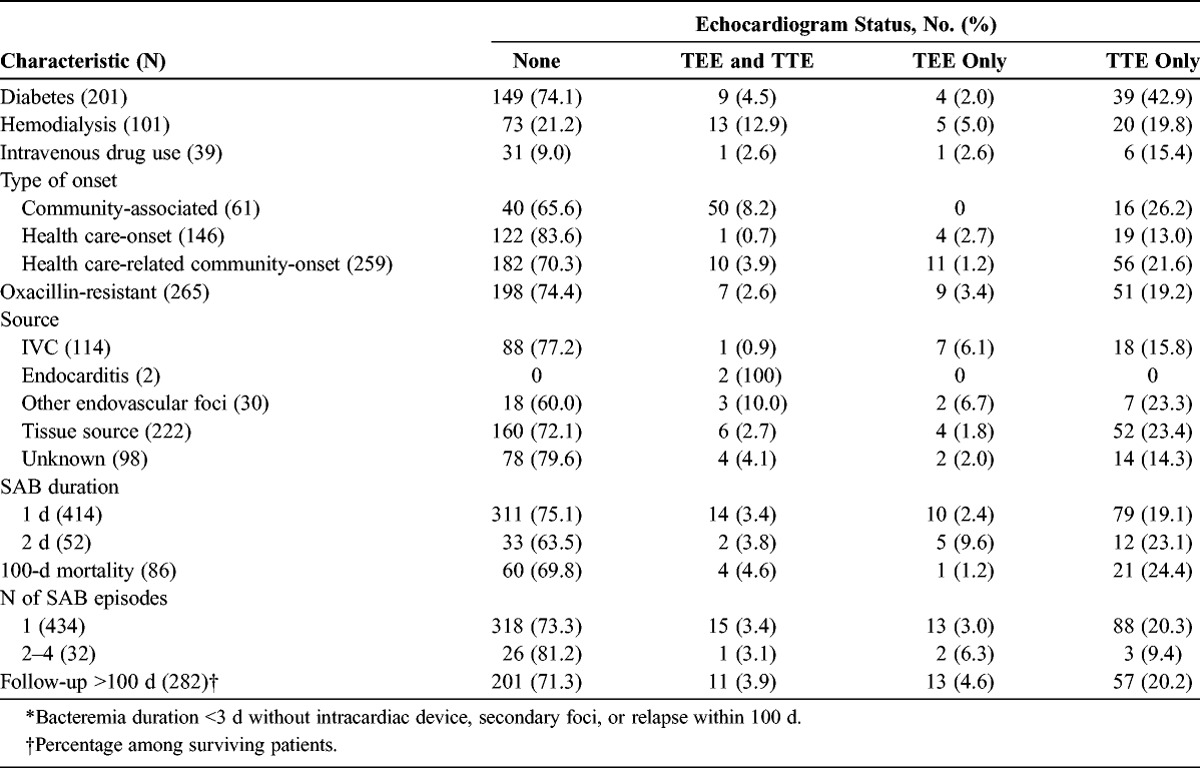
The frequency of TEE and TTE performance was stratified according to the type of bacteremia (Fig. 2). The characteristics of patients with and without echocardiograms were compared (Table 2). Clinicians were more likely to resort to echocardiography in community-associated infections (p < 0.001), the presence of intracardiac devices (p < 0.001), secondary foci (p < 0.001), and with bacteremia duration ≥3 days (p < 0.001).
FIGURE 2.
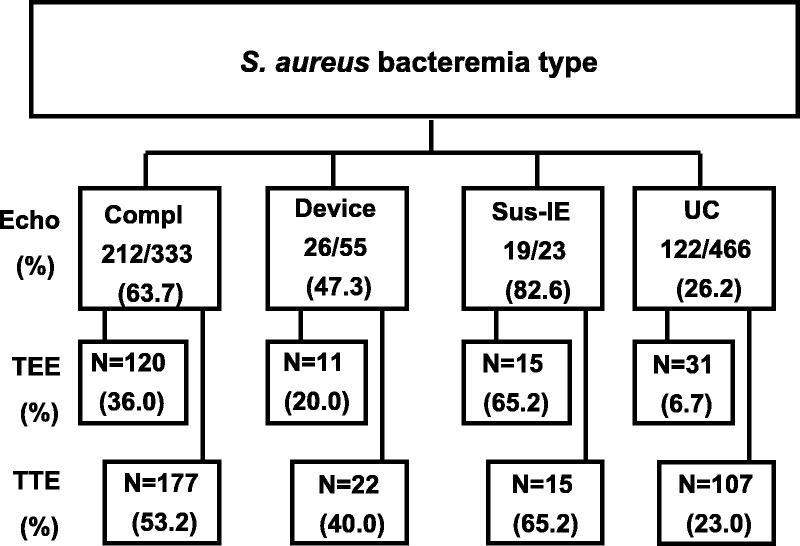
Echocardiography in Staphylococcus aureus bacteremia stratified according to bacteremia category. Abbreviations: Compl = complicated; Sus-IE = suspected infective endocarditis; UC = uncomplicated.
TABLE 2.
Characteristics of Patients With SAB, by Echocardiogram Status
TTE was performed within 0–28 days (median, 4.2 d) in 321 (36.6%) cases. TEE was performed within 1–28 d (median, 7.2 d) in 177 (20.2%) instances.
TTE and TEE showed findings consistent with infective endocarditis in 25/321 (7.8%) and 42/177 (23.7%) instances, respectively. Echocardiogram results were stratified according to SAB category (Fig. 3). TEE was positive in 1 (3.2%) case of uncomplicated methicillin-susceptible S. aureus (MSSA) bacteremia in a hemodialysis patient with a hemodialysis graft infection. TTE was positive in a hemodialysis patient with methicillin-resistant S. aureus (MRSA) bacteremia suspected to be due to access infection. TEE performed 2 days later did not verify TTE findings.
FIGURE 3.
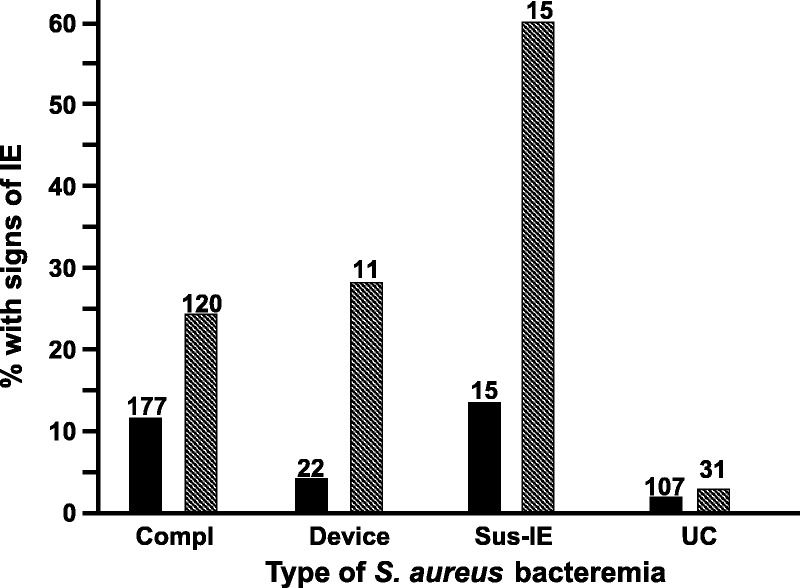
Echocardiogram with signs of infective endocarditis (IE) in Staphylococcus aureus bacteremia: Transthoracic (solid bars) and transesophageal (shaded bars) echocardiogram results, stratified according to the category of bacteremia. Abbreviations: Compl = complicated; Sus-IE = suspected infective endocarditis; UC = uncomplicated.
TEE was negative in 6/15 patients with clinically suspected endocarditis without meeting the criteria for complicated bacteremia. One with a stroke and SAB of unknown source, 1 with meningitis plus septic knee and a murmur, 1 with multiple traumatic soft tissue injuries and pulmonary contusions plus a murmur, 1 intravenous drug user with multiple pulmonary infiltrates, 1 with a known valvular heart disease, and 1 hemodialysis patient with access infection and a murmur. The remaining patients with suspected endocarditis who did not have TEE included 3 patients who died within 3–7 days, 2 who left against medical advice, 1 with a negative TEE performed for unrelated reasons before SAB was diagnosed, and 1 patient who refused the procedure.
Comparison of uncomplicated SAB cases with and without echocardiography is shown in Table 3.
TABLE 3.
Characteristics of Patients With Uncomplicated* SAB, by Echocardiogram Status
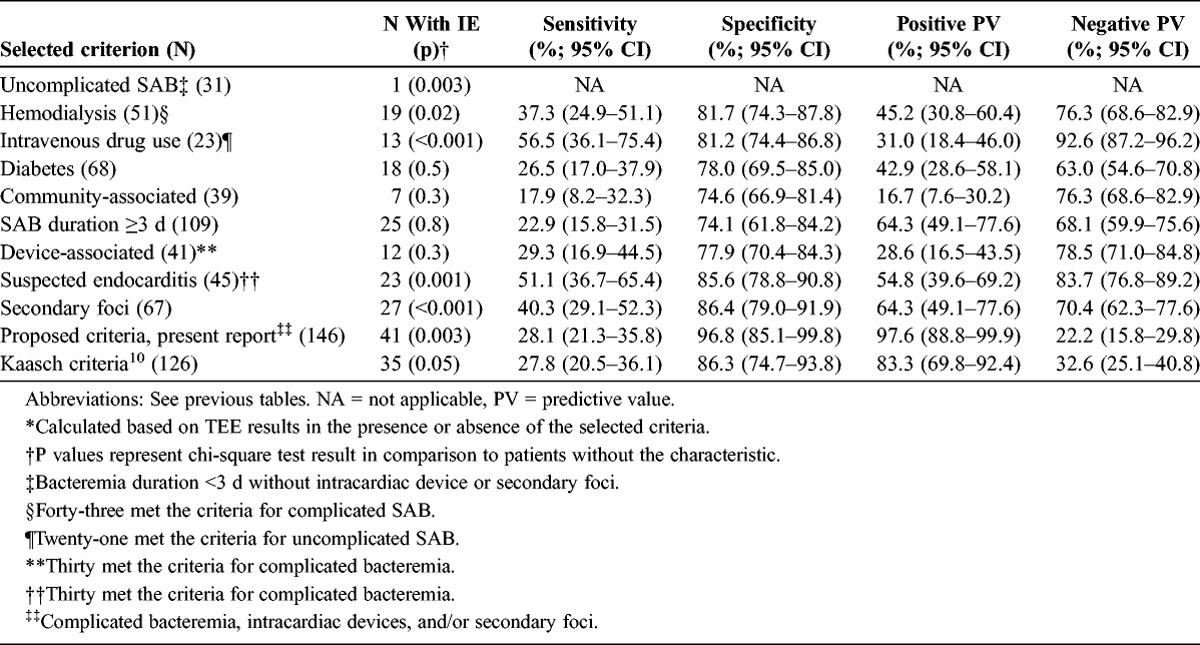
The sensitivity, specificity, and positive and negative predictive values of selected clinical criteria for TEE evidence of infective endocarditis are shown in Table 4. TEE was more often positive among hemodialysis-dependent patients, intravenous drug users, and those with clinically suspected endocarditis. It was significantly less likely to be positive in patients with uncomplicated bacteremia. No difference was noted in mean comorbidity score between patients with and without endocarditis (2.8 ± 2.2 [with endocarditis] vs. 2.6 ± 2.1 [without endocarditis]; p = 0.5).
TABLE 4.
Sensitivity, Specificity, Positive and Negative Predictive Value* for TEE Evidence of IE in SAB, Stratified by Selected Clinical Criteria
Among hemodialysis cases, TEE was positive in 11/34 (32.4%) complicated, 1/2 (50.0%) device-associated, 6/7 (85.7%) suspected endocarditis, and 1/8 uncomplicated bacteremia cases. Among intravenous drug users, TEE was positive in 10/15 (66.7%) complicated, 0/2 device-associated, 3/4 (75.0%) suspected endocarditis, and 0/2 uncomplicated bacteremia cases.
We applied the criteria of Kaasch et al10 to our patients who had TEE. Both TTE and TEE were performed in 119 cases. TEE was done within 1–2, 3–5, and ≥6 days of TTE in 34 (28.6%), 41 (34.4%), and 44 (37.0%) cases, respectively. TTE was negative in 20/30 TEE-positive cases, and was positive in 2 cases with negative TEE. The 2 cases with TTE-positive/TEE-negative results were a patient with positive TTE on the first day of bacteremia and negative TEE 2 days after the TTE, and a second patient with a negative TEE 4 days after the onset of bacteremia and a positive TTE 7 days after TEE.
Follow-up for 100 days or more was possible in 507/669 patients who survived. Follow-up was accomplished in 282/361 patients with uncomplicated bacteremia including 81/92 (88.0%) with echocardiography. Duration of therapy was >15 days in 71.6% and 46.0% of patients with and without follow-up, respectively. None had a relapse or secondary complications.
DISCUSSION
These findings illustrate that endocarditis is rare in cases of uncomplicated community-associated, hospital-onset, and health care-associated community-onset SAB, and question the need for echocardiography in these cases. Current Infectious Diseases Society of America practice guidelines advocates echocardiography, preferably TEE, in all cases of SAB.14 These guidelines were probably generated based on the concerns for SAB complications and the positive predictive value of TEE.10,24 Going back to the history of SAB, a high incidence of endocarditis among patients with SAB without an identifiable source was often noted.17 Because of these concerns, and the acknowledged insensitivity of clinical findings for the diagnosis of endocarditis,7,24 treatment was often extended in all patients with SAB. Subsequently, treatment for 10–14 days was recommended for uncomplicated intravascular catheter-associated SAB,15,18 although this short-term therapy was not universally accepted.9,20
Then, additional studies attempted to identify patients at high risk for complications who warrant extended therapy.5 Fowler et al8 proposed that patients with positive surveillance BC, 3 days or more after the first positive culture, have higher incidence of complications. The risk for complications among patients with positive follow-up BC on day 3 after the initial set was also noted in other studies.2 Around the same time, the Duke criteria6 for the diagnosis of infective endocarditis, which use echocardiography, were proposed. Consequently, echocardiography was advocated in SAB, and treatment stratification was suggested based on echocardiography results.14,22 Most of these studies, however, were observational and may have selection bias favoring TEE performance to patients with high risk for complications.23 Additionally, cost-effectiveness analysis was based on decreasing treatment duration in cases with negative TEE.22 This analysis did not assess the feasibility of 10-14 days of therapy without resorting to echocardiography in uncomplicated bacteremia. Nevertheless, the widespread use of echocardiography, the modified Duke criteria for the diagnosis of infective endocarditis,13 and the reported complications in SAB without identifiable risk factors probably influenced the formulation of treatment guidelines.
The utility of echocardiogram in SAB remains debatable. Kaasch et al10 recently recommended clinical/microbiologic criteria to aid in selecting cases for TEE. The criteria included bacteremia for >4 days’ duration, the presence of a permanent intracardiac device, hemodialysis dependency, spinal infection, and nonvertebral osteomyelitis. Our criteria were slightly different. We chose bacteremia for 3 days or more because it has been shown to be associated with higher complications.8 We included all secondary foci, whereas Kaasch et al included only spinal infection and osteomyelitis. We did not include hemodialysis dependency as a criterion. Our proposed criteria had excellent predictive values.
Our findings support their recommendation, and we propose to extend it to all uncomplicated SAB, including community-associated cases. Kaasch et al10 limited their analysis to health-care related cases.
Our observations also suggest that most clinicians in our hospital do not follow current practice guidelines and do not routinely resort to echocardiography. Additionally, they often order TTE only. The approach of screening with TTE and preserving TEE for cases with negative TTE had been suggested, probably because of potential complications due to anesthesia or the endoscopies.3,16,26 The cost-effectiveness of this approach among patients who meet the criteria for echocardiography has not been thoroughly evaluated. With the known low TTE sensitivity,7,21 TEE is probably pursued in most cases. Our data are insufficient to assess the benefit of TTE because of the wide variation in the time-lag between TTE and TEE.
The current study has many limitations. First, although the data were obtained prospectively, the analysis was retrospective with potential bias. Second, most of our attending physicians and consultants appear to have a bias for selecting cases for echocardiography and do not follow current practice guidelines. However, this bias is likely to select patients with suspected complications and overestimates TEE utility. Additionally, the timing of echocardiography was not standardized. It is possible that some patients may develop vegetations several days after the onset of bacteremia. The numbers of patients with uncomplicated bacteremia among drug users and hemodialysis cases were too small to assess the TEE benefit in these patients. We believe that the high frequency of positive TEE in these patients could be explained by their propensity for having complications. Furthermore, we did not have follow-up in about 22% of patients with uncomplicated bacteremia who survived. Lastly, most patients received prolonged therapy that may have prevented the development of complications.
In summary, our findings suggest that TEE is dispensable in cases with uncomplicated community-associated, hospital-onset, and health care-associated community-onset SAB. We recommend limiting echocardiography to patients with positive follow-up BC 3 days after the initial set, prior history of endocarditis, clinical signs of endocarditis, device-associated cases, secondary foci, and relapse. Additional studies are needed to validate the use of TEE in other risk factors, such as community-associated disease, intravenous drug users, and hemodialysis dependence. Other remaining issues that need to be defined include the optimal time for echocardiography and the cost-benefit of TTE before TEE.
ACKNOWLEDGMENT
The authors are grateful to Ms. Alice Mar for editing the figures.
Abbreviations
- BC
blood culture
- SAB
Staphylococcus aureus bacteremia
- TEE
transesophageal echocardiogram
- TTE
transthoracic echocardiogram
Footnotes
Financial support and conflicts of interest: The study was supported by St John Hospital Medical Education Fund. The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1. Calfee DP, Salgada CD, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Coffin SE, Dubberke ER, Fraser V, Gerding DN, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle L, Pegues DA, Perl TM, Saint S, Weinstein RA, Wise DS. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect Contr Hosp Epidemiol. 2008; 29 (S1): S62– S80. [DOI] [PubMed] [Google Scholar]
- 2. Chang FY, MacDonald BB, Peacock JE, Jr, Musher DM, Triplett P, Mylotte JM, O’Donnell A, Wagener MM, Yu VL. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin-resistance. Medicine (Baltimore). 2003; 82: 322– 332. [DOI] [PubMed] [Google Scholar]
- 3. Chu VH, Bayer AS. Use of echocardiography in the diagnosis and management of infective endocarditis. Curr Infect Dis Rep. 2007; 9: 283– 290. [DOI] [PubMed] [Google Scholar]
- 4. Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis. 2009; 48 (S4): S254– S259. [DOI] [PubMed] [Google Scholar]
- 5. del Rio A, Cervera C, Moreno A, Moreillon P, Miro JM. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis. 2009; 48 (S4): S246– S253. [DOI] [PubMed] [Google Scholar]
- 6. Durack DT, Lukes AS, Bright DK. New criteria for the diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994; 96: 200– 209. [DOI] [PubMed] [Google Scholar]
- 7. Fowler VG, Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, Kong LK, Gottlieb G, Donovan CL, Sexton DJ, Ryan T. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. Am J Coll Cardiol. 1997; 30: 1072– 1078. [DOI] [PubMed] [Google Scholar]
- 8. Fowler VG, Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, Cheng AC, Dudley T, Oddone EZ. Clinical identifies of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003; 163: 2066– 2072. [DOI] [PubMed] [Google Scholar]
- 9. Ghanem GA, Boktour M, Warneke C, Pham-Williams T, Kassis C, Bahna P, Aboufaycal H, Hachem R, Raad I. Catheter-related Staphylococcus aureus bacteremia in cancer patients: high rate of complications with therapeutic implications. Medicine (Baltimore). 2007; 86: 54– 60. [DOI] [PubMed] [Google Scholar]
- 10. Kaasch AJ, Fowler VG, Jr, Rieg S, Peyer-Hoffmann G, Birkholz H, Hellmich M, Kern WV, Seifer H. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis. 2011; 53: 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progress and challenges. Curr Opin Infect Dis. 2010; 23: 346– 358. [DOI] [PubMed] [Google Scholar]
- 12. Khatib R, Jose J, Musta A, Sharma M, Fakih MG, Johnson LB, Riederer K, Shemes S. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011; 66: 1594– 1599. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, Bashore T, Corey R. Proposed modification to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000; 30: 633– 638. [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011; 52: 285– 292. [DOI] [PubMed] [Google Scholar]
- 15. Malanoski GJ, Samore MH, Pefanis A, Karchmer AW. Staphylococcus aureus catheter-associated bacteremia: minimal effective therapy and unusual infectious complications associated with arterial sheath catheters. Arch Intern Med. 1995; 155: 1161– 1166. [DOI] [PubMed] [Google Scholar]
- 16. Mortara LA, Bayer AS. Staphylococcus aureus bacteremia and endocarditis. New diagnostic and therapeutic concepts. Infect Dis Clin North Am. 1993; 7: 53– 68. [PubMed] [Google Scholar]
- 17. Nolan CM, Beaty HN. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med. 1976; 60: 495– 500. [DOI] [PubMed] [Google Scholar]
- 18. Pigrau C, Rodriguez D, Planes AM, Almirante B, Larrosa N, Ribera E, Gavalda J, Pahissa A. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis. 2003; 22: 218– 221. [DOI] [PubMed] [Google Scholar]
- 19. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173: 676– 682. [DOI] [PubMed] [Google Scholar]
- 20. Raad I, Narro J, Khan A, Tarrand J, Vartivarian S, Bodey GP. Serious complications of vascular catheter-related Staphylococcus aureus bacteremia in cancer patients. Eur J Clin Microbiol Infect Dis. 1992; 11: 675– 682. [DOI] [PubMed] [Google Scholar]
- 21. Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiograph. 2003; 16: 67– 70. [DOI] [PubMed] [Google Scholar]
- 22. Rosen AB, Fowler VG, Jr, Corey GR, Downs SM, Biddle AK, Li J, Jollis JG. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med. 1999; 130: 810– 820. [DOI] [PubMed] [Google Scholar]
- 23. Soriano A, Mensa J. Is transesophageal echocardiography dispensable in hospital-acquired Staphylococcus aureus bacteremia? Clin Infect Dis. 2011; 53: 10– 12. [DOI] [PubMed] [Google Scholar]
- 24. Sullenberger AL, Avedissian LS, Kent SM. Importance of transesophageal echocardiography in the evaluation of Staphylococcus aureus bacteremia. J Heart Valve Dis. 2005; 14: 23– 28. [PubMed] [Google Scholar]
- 25. Thwaites GE, Edgeworth JS, Gkrania-Kotsas E, Kirby A, Tilley R, Torok ME, Walker S, Wertheimer HFL, Wilson S, Llewlyn MJ. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011; 11: 208– 222. [DOI] [PubMed] [Google Scholar]
- 26. Van Hall SJ, Mathur G, Kelly J, Aronis C, Cranney GB, Jones PD. The role of transthoracic echocardiography in excluding left sided infective endocarditis in Staphylococcus aureus bacteraemia. J Infect. 2005; 51: 218– 221. [DOI] [PubMed] [Google Scholar]


