Abstract
Whipple disease (WD) is a rare multisystemic infection with a protean clinical presentation. The central nervous system (CNS) is involved in 3 situations: CNS involvement in classic WD, CNS relapse in previously treated WD, and isolated CNS infection. We retrospectively analyzed clinical features, diagnostic workup, brain imaging, cerebrospinal fluid (CSF) study, treatment, and follow-up data in 18 patients with WD and CNS infection.
Ten men and 8 women were included with a median age at diagnosis of 47 years (range, 30–56 yr). The median follow-up duration was 6 years (range, 1–19 yr). As categorized in the 3 subgroups, 11 patients had classic WD with CNS involvement, 4 had an isolated CNS infection, and 3 had a neurologic relapse of previously treated WD. CNS involvement occurred during prolonged trimethoprim-sulfamethoxazole (TMP-SMX) treatment in 1 patient with classic WD.
The neurologic symptoms were various and always intermingled, as follows: confusion or coma (17%) related to meningo-encephalitis or status epilepticus; delirium (17%); cognitive impairment (61%) including memory loss and attention defects or typical frontal lobe syndrome; hypersomnia (17%); abnormal movements (myoclonus, choreiform movements, oculomasticatory myorhythmia) (39%); cerebellar ataxia (11%); upper motor neuron (44%) or extrapyramidal symptoms (33%); and ophthalmoplegia (17%) in conjunction or not with progressive supranuclear palsy. No specific pattern was correlated with any subgroup.
Brain magnetic resonance imaging (MRI) revealed a unique focal lesion (35%), mostly as a tumorlike brain lesion, or multifocal lesions (23%) involving the medial temporal lobe, midbrain, hypothalamus, and thalamus. Periventricular diffuse leukopathy (6%), diffuse cortical atrophy (18%), and pachymeningitis (12%) were observed. The spinal cord was involved in 2 cases. MRI showed ischemic sequelae at diagnosis or during follow-up in 4 patients. Brain MRI was normal despite neurologic symptoms in 3 cases. CSF cytology was normal in 62% of patients, whereas Tropheryma whipplei polymerase chain reaction (PCR) analysis was positive in 92% of cases with tested CSF. Periodic acid–Schiff (PAS)-positive cells were identified in cerebral biopsies of 4 patients.
All patients were treated with antimicrobial therapy for a mean duration of 2 years (range, 1–7 yr) with either oral monotherapy (TMP-SMX, doxycycline, third-generation cephalosporins) or a combination of antibiotics that sometimes followed parenteral treatment with beta-lactams and aminoglycosides. Eight patients also received hydroxychloroquine.
At the end of follow-up, the clinical outcome was favorable in 14 patients (78%), with mild to moderate sequelae in 9. Thirteen patients (72%) had stopped treatment for an average time of 4 years (range, 0.7–14 yr). Four patients had clinical worsening despite antimicrobial therapy; 2 of those died following diffuse encephalitis (n = 1) and lung infection (n = 1).
In conclusion, the neurologic manifestations of WD are diverse and may mimic almost any neurologic condition. Brain involvement may occur during or after TMP-SMX treatment. CSF T. whipplei PCR analysis is a major tool for diagnosis and may be positive in the absence of meningitis. Immune reconstitution syndrome may occur in the early months of treatment. Late prognosis may be better than previously reported, as a consequence of earlier diagnosis and a better use of antimicrobial therapy, including hydroxychloroquine and doxycycline combination.
INTRODUCTION
Whipple disease (WD) is a rare systemic chronic infection caused by the soil-borne gram-positive bacillus Tropheryma whipplei (T. whipplei).20 Clinical presentation is typically dominated by gastrointestinal symptoms, weight loss, and joint involvement.7,23 The diagnosis of WD involves a combination of pathology and molecular microbiology. T. whipplei-infected macrophages contain sickle-form particles that stain positive with periodic acid–Schiff (PAS). Immunochemistry with specific antibodies against T. whipplei may also be used. Polymerase chain reaction (PCR) analysis can be performed with any body tissue or fluid using broad-range primers for amplification of the 16S ribosomal ribonucleic acid (rRNA) gene or the intergenic region of the 16s-23s rRNA; PCR is followed by sequencing of the amplified product. Fluorescence in situ hybridization in tissue specimens and cultivation of T. whipplei on human fibroblast cell lines may also be performed in specialized laboratories.13
Central nervous system (CNS) involvement is a classic feature of WD.23 The neurologic manifestations of the disease are diverse and can mimic almost any neurologic condition.17,24 Neurologic manifestations occur in 3 circumstances: CNS relapse of previously treated classic WD (that is, with microscopic lesions in the gastrointestinal tract), neurologic involvement in untreated classic WD, and isolated neurologic symptoms (that is, without histologic evidence of intestinal involvement) due to T. whipplei.17,23–25
In the current study, we retrospectively analyzed clinical data, brain imaging, cerebrospinal fluid (CSF) analysis, and long-term follow-up in 18 cases of WD with CNS involvement.
PATIENTS AND METHODS
We included 18 patients with WD who were followed between 1989 and 2007 in the departments of Internal Medicine, Infectious Diseases, Rheumatology, and Neurology in France and Belgium. Six cases have already been published, mostly as single case reports with a short follow-up.1,6,8,14,17,19 All patients had 1) microscopic lesions characteristic of the disease on tissues stained with PAS and/or the presence of T. whipplei DNA, as assessed by positive PCR of tissues or body fluids; and 2) clinical neurologic symptoms or proven meningeal involvement, with or without other manifestations of the disease.5,10,12
We analyzed demographic characteristics, clinical symptoms and signs associated with WD, imaging, diagnostic workup, treatment, and follow-up data. Notably, various PCR assays targeting the 16S bacterial RNA gene and other specific T. whipplei sequences were applied following technical improvements over time.12
Approval from an institutional review board was not required for this human noninterventional study. For ethical considerations, however, patients were informed that data collected in their medical records might be used for research study in accordance with privacy rules and ethical guidelines of the 1975 Declaration of Helsinki.
CASE PRESENTATION
Case 1: CNS Relapse in Previously Treated Classic WD
A 65-year-old man presented with unilateral uveitis and diarrhea. A vitrectomy was performed and revealed foamy macrophages with slender, rod-shaped structures that tested positive on PAS staining. WD was confirmed by immunochemistry and PCR in vitreous and duodenal biopsy.6 The CSF was normal, and T. whipplei PCR in CSF was negative. The patient was treated with trimethoprim-sulfamethoxazole (TMP-SMX) and recovered completely.
Four years later, the patient displayed cognitive disorder, hypersomnia, and extrapyramidal symptoms including tremor, dyskinesia, postural instability, and facial bradykinesia. At that time he was still receiving TMP-SMX. Laboratory findings were normal. Brain magnetic resonance imaging (MRI) revealed diffuse cortical atrophy. CSF cytology was normal but T. whipplei PCR was positive, proving the neurologic relapse of WD despite antibiotic treatment.14
TMP-SMX was switched for doxycycline and hydroxychloroquine. PCR in CSF was controlled negative after 6 months of treatment. Duodenal biopsy done after 1 year of treatment still showed PAS-positive macrophages despite a negative PCR assay. Hydroxychloroquine was stopped after 1 year. Tremor and dyskinesia slowly improved, but mild cognitive impairment and postural instability persisted after 4 years of doxycycline treatment.
Case 2: Isolated CNS T. whipplei Infection
A 44-year-old man, without previous medical history, presented with cerebellar ataxia and upper motor neuron symptoms. He had no fever, weight loss, or gastrointestinal or joint symptoms. Laboratory tests, thoraco-abdominal computed tomography (CT), and brain MRI were normal. A lumbar puncture was performed and revealed lymphocytic meningitis with 25 cells/mm3 and mild protein level (0.6 g/L). As his neurologic condition rapidly worsened with cognitive impairment, memory loss, and attention deficit, antituberculosis therapy was started. Because neurologic symptoms had not improved after 4 months, lumbar puncture was repeated and revealed 2 cells/mm3. T. whipplei PCR was positive in CSF. The patient refused digestive endoscopy.
Treatment with TMP-SMX, doxycycline, and hydroxychloroquine was started. After 1 month of treatment, the patient’s walking and motor coordination slowly improved. One year later, CSF analysis was normal and T. whipplei PCR was negative. After 3 years of treatment, neurologic status had globally improved, although the patient was still unable to work because of persistent attention deficit and cerebellar symptoms.
RESULTS
Patient Characteristics
The study included 18 patients with WD with neurologic involvement. Ten patients (56%) were male. The age range at diagnosis was 30–56 years (median age, 47 yr).
Eleven patients had classic WD with neurologic involvement. Four patients had isolated brain infections without intestinal tract involvement (including Case 2). Three patients had a neurologic relapse of previously treated classic WD (including Case 1). In 2 of the relapse cases, the relapse was observed while antimicrobial therapy—including TMP-SMX—had been stopped for more than 1 year. One patient relapsed under TMP-SMX at 4 years after treatment initiation (Case 1).11
Clinical Manifestations
All 14 patients with classic WD (11 classic patients plus 3 relapse patients) had weight loss (100%), and 5 (35%) had fever (mean temperature, 38°C; range, 37.8–38.5°C). In the 4 patients with isolated CNS infections, mild weight loss was present in 2 cases (50%).
Gastrointestinal signs were noted in 11 of the 14 patients (78%), all with classic WD: diarrhea (9/11; 82%), hepatomegaly (4/11; 36%), moderate abdominal pain (1/11; 9%), and splenomegaly (1/11; 9%).
Rheumatic symptoms were noted in 8 of the 14 patients (57%): transient, recurring and grossly symmetric polyarthralgia (6/8; 75%), nonerosive polyarthritis (4/8; 50%), monoarthritis of the knee (1/8; 12,5%), lumbar pain (1/8; 12,5%), and bilateral sacroiliitis (3/8; 37,5%).
Unilateral uveitis occurred in 2 patients (including Case 1). Dry cough was present in 2 patients. Hyperpigmentation was noted in 2 cases.
Neurologic Manifestations
The CNS symptoms were various and always intermingled (Table 1). Only 2 patients had no neurologic symptoms; both patients had classic WD.
TABLE 1.
Central Nervous System Symptoms in 18 Patients With Predominant Neurologic Involvement of Whipple Disease*
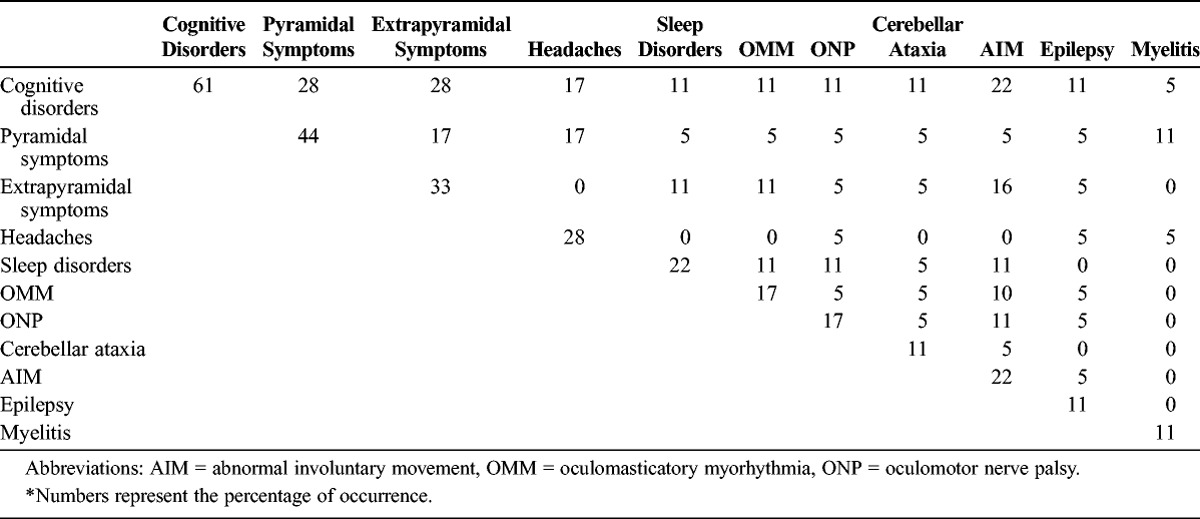
For 1 patient, CNS symptoms occurred 3 months before the other features of the disease. Neurologic symptoms occurred as an early-onset manifestation of the disease in 5 cases, or 1–10 years after the first manifestation of untreated classic WD in 5 cases. The average time between the first symptom and diagnosis of WD was 2 years (range, 1 mo to 11 yr).
Confusion or coma occurred in 3 patients (17%) and was related to acute meningo-encephalitis (n = 1) or status epilepticus (n = 2). Delirium was observed in 3 cases (17%). Eleven patients (61%) had cognitive impairment including memory loss and attention defects, and 3 patients had typical frontal lobe syndrome. Three patients had hypersomnia (17%).
Abnormal movements were observed in 7 cases (39%) and included myoclonus (n = 2), choreiform movements (n = 2), and oculomasticatory myorhythmia (n = 3). No patient had oculo-facial-skeletal myorhythmia. Two patients presented with cerebellar ataxia. Upper motor neuron disorder was noted in 8 patients (44%) with motor deficiency in 4 patients (22%) including hemiplegia (n = 2) and paraplegia (n = 2). Extrapyramidal symptoms were observed in 6 patients (33%).
Ophthalmoplegia occurred in 3 patients (17%), in conjunction with progressive supranuclear palsy in 2 cases. No patient had peripheral nervous system involvement. The distribution of CNS symptoms in the classic WD, WD relapse, and isolated CNS infection subgroups is shown in Figure 1.
FIGURE 1.
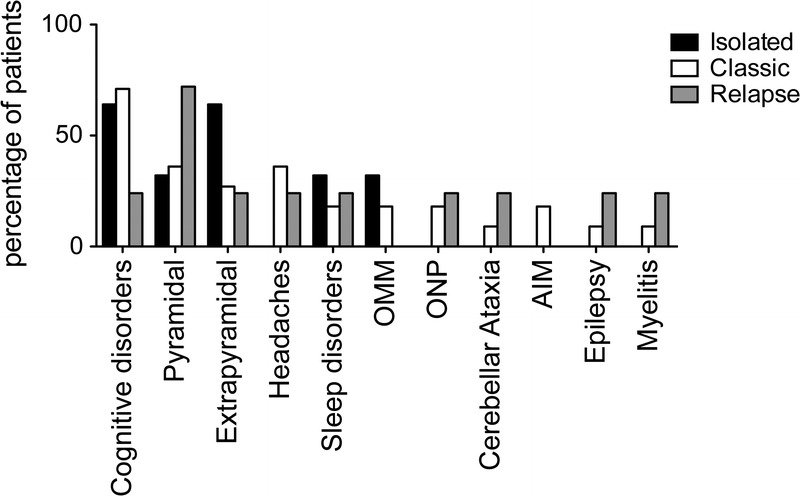
CNS symptoms in neurologic WD subgroups. The bars represent the percentage of patients with isolated CNS disease (black bar), classic WD (white bar), and CNS relapse (gray bar) displaying any of the neurologic symptoms listed in abscissa. Abbreviations: AIM = abnormal involuntary movement, OMM = oculomasticatory myorhythmia, ONP = oculomotor nerve palsy.
Brain Magnetic Resonance Imaging
Brain MRI data were available for 17 patients. The MRI was normal in 3 cases despite neurologic symptoms including cognitive defects, extrapyramidal symptoms, oculomotor nerve palsy, or abnormal movements (Case 2).
In 6 patients, MRI revealed a unique focal lesion involving the temporal lobe, fronto-temporal lobe, pituitary gland, or brainstem with high T2 signal intensity, which was most evident on fluid-attenuated inversion recovery (FLAIR) sequences. Contrast enhancement on T1-weighted sequences was observed in 3 patients. In 3 cases, the focal lesion was described as a pseudotumoral mass.
In 4 cases, multifocal T2-weighted high signal lesions were observed in the brainstem, temporal lobe, and periventricular white matter. Two patients presented with periventricular diffuse leukopathy, and 3 patients had diffuse cortical atrophy. Isolated major dilatation of ventricles was observed in 1 case. In 2 patients, MRI revealed pachymeningitis.
The spinal cord was involved in 2 cases with FLAIR and T2-weighted high signal lesions in the white matter. It is noteworthy that 2 patients had ischemic sequelae in the cerebellum at diagnosis.
The distribution of brain MRI lesions in the classic WD, WD relapse, and isolated CNS infection subgroups is shown in Table 2. Examples of brain MRI patterns are shown in Figure 2.
TABLE 2.
Distinct Patterns of Brain MRI Lesions According to the Phenotype of Neurologic Involvement in WD
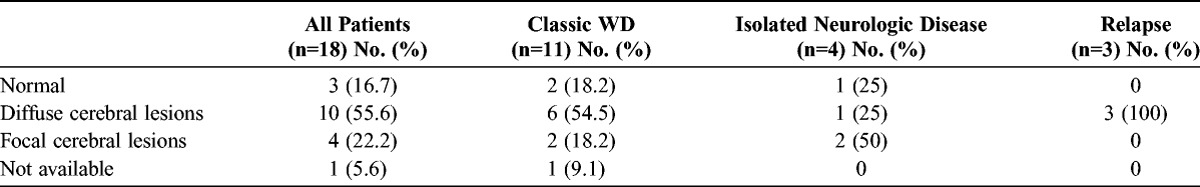
FIGURE 2.
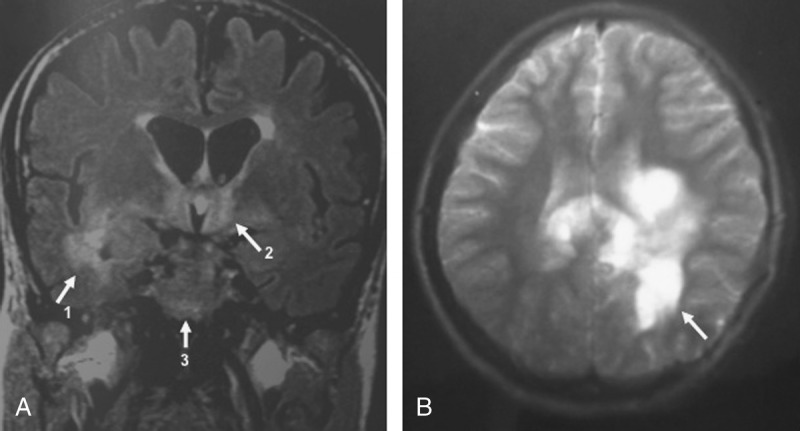
Brain MRI in WD with neurologic involvement. A, Flair-weighted MRI showed diffuse hyperintensities involving medial temporal lobes (arrow 1), hypothalamic regions (arrow 2), and a focal lesion of the left cerebral peduncle (arrow 3). B, T2-weighted MRI showed large pseudotumoral and nodular hyperintense lesions involving corpus callosum and periventricular white matter (arrow) with less mass effect than should be expected.
Diagnosis
The average time between the first symptom and diagnosis of WD was 2 years (range, 1 mo to 11 yr). The WD diagnosis was confirmed by PCR of T. whipplei DNA in CSF (n = 12), brain biopsy showing PAS-positive cells (n = 4), and positive PCR and/or PAS-positive cells in duodenal biopsies (n = 2). No patient had primary immunodeficiency.
Cerebrospinal Fluid
A lumbar puncture was performed in 16 patients. In 6 cases, CSF analysis showed lymphocytic meningitis with 5–335 cells/mm3, which was associated with high protein levels in 3 cases. The PCR analysis for T. whipplei was positive in 4 cases.
In 10 patients, the white cell count in CSF was normal, whereas the protein level was high in 4 cases. Despite a normal cytologic analysis, the CSF was PCR positive for T. whipplei in all tested cases (n = 7) (Case 2).
Overall, the CSF cytology was normal in 62% of patients (10/16), but T. whipplei DNA was found in 92% (11/12) of those tested.
Duodenal Biopsy
Gastro-duodenoscopy was performed in 17 patients. Duodenal tissue analysis provided a WD diagnosis in 14 patients. Duodenal histology showed PAS-positive cells associated with a PCR+ result for T. whipplei in 7 cases. The PCR assay was positive with negative PAS staining in 7 cases. No patients had positive PAS staining with a negative PCR analysis. Interestingly, PCR analysis for T. whipplei and PAS staining of duodenal biopsies were positive in 4 (23%) and 3 (17%) patients, respectively, with no digestive symptoms.
Cerebral Biopsy
A cerebral biopsy was performed in 4 patients. In 3 cases, there was a brain pseudotumor, and in 1 case, there were unexplained multifocal lesions. PAS-positive cells were observed in all cases. Electron microscopy was performed on brain specimens, with a positive diagnosis in 1 case (full data not available). T. whipplei PCR was not performed.
Of these 4 patients, 2 had isolated CNS disease.
Treatment
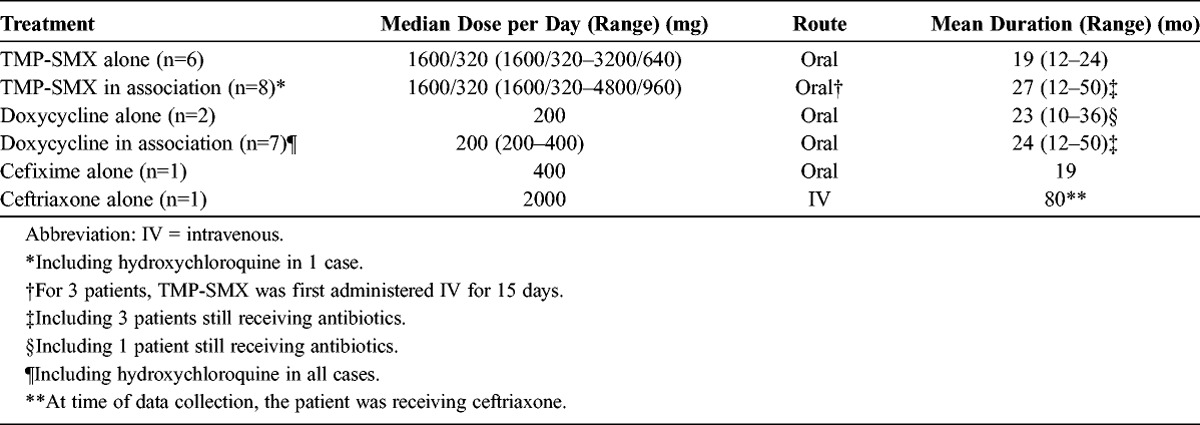
All patients were treated with antimicrobial therapy. The average treatment duration was 2 years (range, 1–7 yr). Ten patients received monotherapy with TMP-SMX (n = 6), doxycycline (n = 2), or third-generation cephalosporins (n = 2). Eight patients were treated with a combination of the following antibiotics: TMP-SMX and tetracycline (n = 6); TMP-SMX and rifampicin (n = 1); or TMP-SMX, tetracycline, and third-generation cephalosporins (n = 1). In 5 patients, oral treatments followed a 2-3 week combination of parenteral antibiotics with beta-lactams (amoxicillin 4 g 3 times per day, ceftriaxone 2 g twice or once daily) and aminoglycosides (streptomycin 1 g per day, amikacin 15 mg/kg once a day). Eight patients received hydroxychloroquine with the following: doxycycline (n = 1); TMP-SMX and doxycycline (n = 5); TMP-SMX, doxycycline, and cefixime (n = 1); or cefixime (n = 1). Three patients were treated with steroids. Dose, route, and duration of treatment are described in Table 3.
TABLE 3.
Antimicrobial Therapy Received by Patients
Outcome
At the time of data collection, the median follow-up duration was 6 years (range, 1–19 yr). Only 5 patients were still receiving antibiotics. Thirteen of the 18 patients (72%) had stopped treatment for an average time of 4 years (range, 0.7–14 yr). Of the 7 patients treated by hydroxychloroquine and doxycycline, 1 relapsed. Of the 11 other patients, 3 relapsed, and 2 died.
Clinical Outcome
At the time of data collection, clinical improvement had been observed in 14 patients (78%). Five patients were in complete remission. Nine patients displayed mild to moderate neurologic sequelae such as central motor symptoms, abnormal movements, oculomotor palsy, and cognitive disorders.
Notably, 5 of these 14 patients had transient clinical worsening during treatment, without evidence of ongoing infection (T. whipplei PCR-negative CSF). It occurred in the first 2 months of antimicrobial treatment in 3 cases. Immune reconstitution syndrome (n = 1), resistance to TMP-SMX (n = 1), or TMP-SMX-related aseptic meningitis (n = 1) were suspected. TMP-SMX was switched for cefixime or doxycycline in 2 cases and improvement was eventually observed. Two patients had new neurologic symptoms (paraplegia, hemiplegia, sensory deficit) after 2 and 4 years of doxycycline alone and doxycycline with TMP-SMX, respectively. Clinical improvement was observed in both cases after corticosteroid treatment without antimicrobial therapy modification.
Two of the 14 patients with prolonged clinical improvement had WD relapses after the discontinuation of antimicrobial treatment including TMP-SMX; these relapses were neurologic, with choreiform movements (n = 1), or digestive, with proved pulmonary involvement (n = 1). In both cases, the clinical symptoms eventually improved under treatment with third-generation cephalosporins or TMP-SMX alone.
After a transient improvement, 4 patients displayed persistent clinical worsening under antimicrobial therapy including severe frontal lobe syndrome associated with refractory epilepsy (n = 2), persistent hemiplegia caused by a stroke (n = 1), or nosocomial pneumopathy (n = 1). Two patients were receiving TMP-SMX alone; 1 patient, third-generation cephalosporins alone; and 1, TMP-SMX with rifampicin. Two of these patients died because of diffuse encephalitis (n = 1) and pulmonary infection (n = 1).
Follow-Up Brain MRI
A follow-up brain MRI was available for 13 patients. In 7 patients, the MRI lesions normalized (n = 1), improved (n = 3), or stabilized (n = 3). For 3 patients, new lesions appeared despite clinical improvement; there were new high T2-weighted signals in cerebellum and lenticular nuclei (n = 1) and ischemic strokes (n = 2). In 3 cases, the MRI outcome paralleled clinical alterations with worsening of cerebellar and frontal atrophy (n = 1), expanding cystic lesions (n = 1), and ischemic stroke (n = 1).
CSF Follow-Up
CSF follow-up was available for 15 patients. In 8 cases, a lumbar puncture was performed because of clinical worsening despite antibiotic treatment, with only 2 cases eventually diagnosed as neurologic relapse. The CSF analysis showed meningitis in 63% (5/8) of cases. The PCR for T. whipplei was negative in all tested cases. Notably, the CSF T. whipplei PCR was not performed in the 2 cases diagnosed as neurologic relapse. The relapse was proven with cerebral biopsy in 1 case and with duodenal biopsy in the other case.
CSF analysis was also systematically performed in patients with clinical improvement. Overall, 25 lumbar punctures were performed between 1 and 72 months after the WD diagnosis. Although the PCR was negative in all cases, meningitis was observed in 24% (6/25) of patient CSF (5–15 cells/mm3).
DISCUSSION
Whipple disease is a bacterial, relapsing, chronic systemic disease with frequent CNS involvement that follows 3 distinct patterns: classic WD with neurologic involvement, isolated WD neurologic involvement, and neurologic relapse of previously treated WD.12 Neurologic involvement is the third major manifestation of the disease (10%–43% of patients).7,23 WD rarely presents with isolated neurologic involvement (4%–8%).7,16,17,23,26 We report 18 cases of WD with CNS involvement, including 4 cases (22%) with isolated neurologic disease, with a median follow-up of 6 years.
The median age at onset was 47 years. Compared with series of patients with systemic WD, women were more frequently affected in the current study (44% of patients in the current study were women, compared with 27% of all classic WD patients).7,23
In a previous report,7 the average delay between first symptoms and diagnosis was 72, 48, and 30 months for joint, digestive, and neurologic symptoms, respectively. In the current study, the average time to WD diagnosis was 24 months. It is noteworthy that the delay was only 2 months in patients with pseudotumoral brain lesions.
The neurologic symptoms were various, often complex, and intermingled, underscoring the wide brain tissue distribution of WD lesions. Cognitive changes, affecting 61% of patients, were always associated with other CNS symptoms. Other features included (in decreasing order of frequency): pyramidal and extrapyramidal signs, supranuclear ophthalmoplegia, headache and seizures, as reported elsewhere.17,24 Notably, 39% of patients had abnormal movements, suggesting the specific involvement of the basal ganglia, which is consistent with extrapyramidal symptoms. Sleep disorders, mostly hypersomnia, were also observed (17% of patients), and may be related to the hypothalamic involvement. Two patients had spinal cord involvement. A 2011 study11 showed that progressive dementia and ataxia were significantly more frequent in patients with isolated T. whipplei encephalitis than in patients with classic WD with neurologic involvement. In the current study, the patterns of neurologic features did not differ between the subgroups of patients with untreated classic WD with CNS involvement, WD with isolated CNS involvement, and CNS relapse.
Among the 18 patients with neurologic symptoms, 14 also had non-neurologic disease manifestations including diarrhea (64%) and joint (57%), eye (14%), or lung involvement (14%). In these 14 patients, duodenal biopsies exhibited PAS-positive macrophages and/or a positive result from T. whipplei PCR. It is noteworthy that duodenal biopsies sometimes provided a diagnostic clue without obvious clinical intestinal tract involvement. The lack of significant weight loss, a common manifestation of classic WD, observed in the 4 subjects with isolated CNS disease was consistent with the absence of digestive involvement. No patient had weight gain, as reported in several cases of isolated neurologic WD.11 Brain MRI for diagnosis of CNS WD is more sensitive than a CT scan.11 In the current study, brain MRI revealed a unique focal lesion (35%) or multifocal lesions (23%) with high signals for T2 and FLAIR sequences. In 4 cases, MRI disclosed a focal tumorlike brain lesion, which is a rare presentation of CNS WD.11,26,30 As previously reported, lesions were present in the medial temporal lobe, midbrain, hypothalamus, and thalamus, with transient minimal enhancement and no restricted diffusion.3,26 Periventricular diffuse leukopathy, diffuse cortical atrophy, and pachymeningitis were observed in 6%, 18%, and 12% of patients, respectively, in the current study. The spinal cord was involved in 2 cases. Notably, 4 patients in the current retrospective study had ischemic sequelae on MRI at diagnosis or during follow-up. Ischemic lesions in CNS WD were rarely reported9,27,28 and could be caused by emboli migrating from T. whipplei endocarditis.2,26 Alternatively, arterial or arteriolar fibrosis adjacent to chronic inflammation or a true vasculitic process could provoke ischemic lesions in WD.28 Notably, the MRI was normal despite CNS symptoms in 17% of the patients in the current study.
In our study, CSF cytology was normal in more than 50% of cases at diagnosis. This frequency is approximately consistent with previous reports.17,24 Hence, normal CSF microscopic analysis cannot rule out neurologic involvement in WD. Although previously reported in half of the cases, PAS-positive cells were not present in CSF in the current study.17 These 2 observations highlight the importance of the PCR assay for confirming CNS involvement in patients with WD. CSF was PCR positive in 92% (12/13) of current cases with data available, including cases with no meningitis. Moreover, T. whipplei DNA has also been detected in CSF in classic WD with no obvious clinical neurologic involvement.31 However, in some cases documented by brain biopsy, CSF is PCR negative.31 As observed in the current study, CSF analysis may indicate persistent or de novo mild meningitis in patients with improved CNS disease without further evidence of an ongoing infection. In such cases, meningitis could be related to the so-called immune reconstitution syndrome occurring in the early months of treatment.21 Hence, PCR assay on CSF—and T. whipplei cultivation when feasible—should be obtained in all WD patients at diagnosis or when a relapse is suspected.
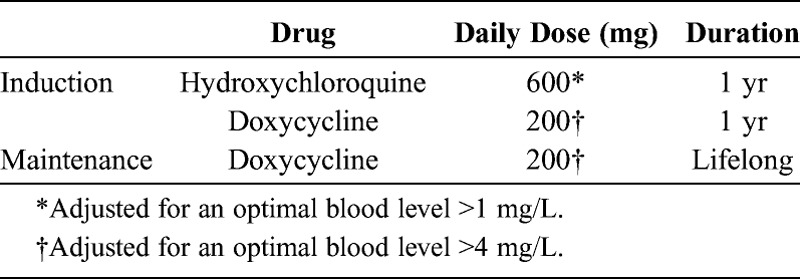
The current recommended treatment—with agents that cross the blood-brain barrier—includes a 2-week course of parenteral meropenem or ceftriaxone followed by 1 year of oral TMP-SMX.15 However, trimethoprim and ceftriaxone may be ineffective in vitro against T. whipplei.4 Moreover, secondary acquired resistance to sulfamethoxazole has been reported,14,22 which explains why CNS involvement occurred despite prolonged TMP-SMX treatment in 3 of our patients. In contrast, our data suggest that the combination of doxycycline and hydroxychloroquine is more efficient (Table 4).
TABLE 4.
Suggested Optimal Treatment of WD With CNS Involvement
With previous reports of 46%-59% improvement in WD with CNS involvement, the prognosis of WD with neurologic complications is poor.17,22 The severity of the disease and the poor response to treatment explain why CNS involvement was a common cause of death in patients with WD.29 In contrast, the current study shows improvement in 14 patients (78%), including 5 cases of complete remission with a median follow-up of 6 years. Moreover, only 2 patients (11%) died during the long-term follow-up, as compared to a reported death rate as high as 27%.29 The discrepancies with previous series may be explained by earlier diagnosis with PCR and better use of antibiotics, including hydroxychloroquine, in the current series.12,18
In conclusion, the current retrospective study reveals some key information regarding neurologic involvement in WD. We found that the brain MRI may be normal and the PCR assay of CSF may be positive when the CSF cytology is normal (that is, no meningitis). The PAS staining and PCR assay may be positive in duodenal tissue in patients with WD and no digestive symptoms. CNS involvement may occur during TMP-SMX treatment. Transient clinical worsening, which is attributed to immune reconstitution syndrome, may occur in the early months of treatment. The late prognosis may be better than previously reported due to earlier diagnosis and better use of antimicrobial therapy, including the combination of hydroxychloroquine and doxycycline.
ACKNOWLEDGMENTS
The authors are grateful to Drs. G. Besson (Department of Neurology, Grenoble University Hospital, France), B. Brochet (Department of Neurology, Bordeaux University Hospital, France), J. M. Brisseau (Department of Geriatric Medicine, Nantes University Hospital, France), H. Charlanne (Department of Neurology, Lille University Hospital, France), C. Confavreux (Department of Neurology, Lyon University Hospital, France), S. Vukusic (Department of Neurology, Lyon University Hospital, France), S. Démeret (Department of Neurology, University Paris-6, France), J. M. Gérard (Department of Neurology, Ambroise Paré Hospital, Mons, Belgium.), C. Lebrun-Frenay (Department of Neurology, Nice University Hospital, France), D. Maltête (Department of Neurology, Rouen University Hospital, France), J. Schoenen (Department of Neurology, University of Liège, Belgium), and E. Vidal (Department of Internal Medicine, Limoges University Hospital, France) for their participation in the study and their assistance in collecting clinical data.
Abbreviations
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CT
computed tomography
- FLAIR
fluid-attenuated inversion recovery
- MRI
magnetic resonance imaging
- PAS
periodic acid–Schiff
- PCR
polymerase chain reaction
- TMP-SMX
trimethoprim-sulfamethoxazole
- WD
Whipple disease
Footnotes
*Both authors contributed equally to this work.
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1. Agard C, Brisseau JM, Grossi O, Pattier S, Espitia-Thibault A, Le Goff B, Audrain M, Ponge T, Hamidou M. Two cases of atypical Whipple’s disease associated with cytoplasmic ANCA of undefined specificity. Scand J Rheumatol. 2012; 41: 246– 248. [DOI] [PubMed] [Google Scholar]
- 2. Besnard S, Cady A, Flecher S, Fily F, Revest M, Arvieux C, Donnio PY, Michelet C, Tattevin P. Should we systematically perform central nervous system imaging in patients with Whipple’s endocarditis? Am J Med. 2010; 123: 962,e961–964. [DOI] [PubMed] [Google Scholar]
- 3. Black DF, Aksamit AJ, Morris JM. MR imaging of central nervous system Whipple disease: a 15-year review. AJNR Am J Neuroradiol. 2010; 31: 1493– 1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulos A, Rolain JM, Raoult D. Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob Agents Chemother. 2004; 48: 747– 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dobbins WO., III The diagnosis of Whipple’s disease. N Engl J Med. 1995; 332: 390– 392. [DOI] [PubMed] [Google Scholar]
- 6. Drancourt M, Raoult D, Lepidi H, Fenollar F, Birg ML, Bodaghi B, Hoang PL, Lelievre JD. Culture of Tropheryma whippelii from the vitreous fluid of a patient presenting with unilateral uveitis. Ann Intern Med. 2003; 139: 1046– 1047. [DOI] [PubMed] [Google Scholar]
- 7. Durand DV, Lecomte C, Cathebras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Societe Nationale Francaise de Medecine Interne. Medicine (Baltimore). 1997; 76: 170– 184. [DOI] [PubMed] [Google Scholar]
- 8. El Helou J, Saliba G, Kolev I, Pierrot-Deseilligny C. Neuro-Whipple confirmed five years after a presumptive diagnosis of a primitive CNS vasculitis. J Neurol. 2008; 255: 925– 926. [DOI] [PubMed] [Google Scholar]
- 9. Famularo G, Minisola G, De Simone C. A patient with cerebral Whipple’s disease and a stroke-like syndrome. Scand J Gastroenterol. 2005; 40: 607– 609. [DOI] [PubMed] [Google Scholar]
- 10. Fenollar F, Laouira S, Lepidi H, Rolain JM, Raoult D. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: usefulness of saliva and stool specimens for first-line screening. Clin Infect Dis. 2008; 47: 659– 667. [DOI] [PubMed] [Google Scholar]
- 11. Fenollar F, Nicoli F, Paquet C, Lepidi H, Cozzone P, Antoine JC, Pouget J, Raoult D. Progressive dementia associated with ataxia or obesity in patients with Tropheryma whipplei encephalitis. BMC Infect Dis. 2011; 11: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenollar F, Puechal X, Raoult D. Whipple’s disease. N Engl J Med. 2007; 356: 55– 66. [DOI] [PubMed] [Google Scholar]
- 13. Fenollar F, Raoult D. Molecular techniques in Whipple’s disease. Expert Rev Mol Diagn. 2001; 1: 299– 309. [DOI] [PubMed] [Google Scholar]
- 14. Fenollar F, Rolain JM, Alric L, Papo T, Chauveheid MP, van de Beek D, Raoult D. Resistance to trimethoprim/sulfamethoxazole and Tropheryma whipplei. Int J Antimicrob Agents. 2009; 34: 255– 259. [DOI] [PubMed] [Google Scholar]
- 15. Feurle GE, Junga NS, Marth T. Efficacy of ceftriaxone or meropenem as initial therapies in Whipple’s disease. Gastroenterology. 2010; 138: 478– 486. [DOI] [PubMed] [Google Scholar]
- 16. Fleming JL, Wiesner RH, Shorter RG. Whipple’s disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988; 63: 539– 551. [DOI] [PubMed] [Google Scholar]
- 17. Gerard A, Sarrot-Reynauld F, Liozon E, Cathebras P, Besson G, Robin C, Vighetto A, Mosnier JF, Durieu I, Vital Durand D, Rousset H. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002; 81: 443– 457. [DOI] [PubMed] [Google Scholar]
- 18. Ghigo E, Capo C, Aurouze M, Tung CH, Gorvel JP, Raoult D, Mege JL. Survival of Tropheryma whipplei, the agent of Whipple’s disease, requires phagosome acidification. Infect Immun. 2002; 70: 1501– 1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrera C, Myressiotis S, Schoenen J, Ansseau M. [Clinical case of the month. Whipple’s disease: inaugural psychiatric symptoms]. Rev Med Liege. 2001; 56: 676– 680. [PubMed] [Google Scholar]
- 20. La Scola B, Fenollar F, Fournier PE, Altwegg M, Mallet MN, Raoult D. Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int J Syst Evol Microbiol. 2001; 51: 1471– 1479. [DOI] [PubMed] [Google Scholar]
- 21. Lagier JC, Fenollar F, Lepidi H, Liozon E, Raoult D. Successful treatment of immune reconstitution inflammatory syndrome in Whipple’s disease using thalidomide. J Infect. 2010; 60: 79– 82. [DOI] [PubMed] [Google Scholar]
- 22. Lagier JC, Fenollar F, Lepidi H, Raoult D. Failure and relapse after treatment with trimethoprim/sulfamethoxazole in classic Whipple’s disease. J Antimicrob Chemother. 2010; 65: 2005– 2012. [DOI] [PubMed] [Google Scholar]
- 23. Lagier JC, Lepidi H, Raoult D, Fenollar F. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore). 2010; 89: 337– 345. [DOI] [PubMed] [Google Scholar]
- 24. Louis ED, Lynch T, Kaufmann P, Fahn S, Odel J. Diagnostic guidelines in central nervous system Whipple’s disease. Ann Neurol. 1996; 40: 561– 568. [DOI] [PubMed] [Google Scholar]
- 25. Misbah SA, Ozols B, Franks A, Mapstone M. Whipple’s disease without malabsorption: new atypical features. QJM. 1997; 90: 765– 772. [DOI] [PubMed] [Google Scholar]
- 26. Mohamed W, Neil E, Kupsky WJ, Juhasz C, Mittal S, Santhakumar S. Isolated intracranial Whipple’s disease—report of a rare case and review of the literature. J Neurol Sci. 2011; 308: 1– 8. [DOI] [PubMed] [Google Scholar]
- 27. Naegeli B, Bannwart F, Bertel O. An uncommon cause of recurrent strokes: Tropheryma whippelii endocarditis. Stroke. 2000; 31: 2002– 2003. [DOI] [PubMed] [Google Scholar]
- 28. Peters G, du Plessis DG, Humphrey PR. Cerebral Whipple’s disease with a stroke-like presentation and cerebrovascular pathology. J Neurol Neurosurg Psychiatry. 2002; 73: 336– 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schnider PJ, Reisinger EC, Gerschlager W, Muller C, Berger T, Krejs GJ, Auff E. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996; 8: 899– 903. [PubMed] [Google Scholar]
- 30. Vital Durand D, Gerard A, Rousset H. [Neurological manifestations of Whipple disease]. Rev Neurol (Paris). 2002; 158: 988– 992. [PubMed] [Google Scholar]
- 31. von Herbay A, Ditton HJ, Schuhmacher F, Maiwald M. Whipple’s disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997; 113: 434– 441. [DOI] [PubMed] [Google Scholar]


