Abstract
Multifocal or multiple osteonecrosis (ON), defined by the involvement of 3 or more anatomic sites, is unusual, being observed in only 3%–10% of patients diagnosed with ON. We report the clinical characteristics of a cohort of 29 patients with multifocal ON from a single center and evaluate the prevalence of associated prothrombotic abnormalities in 26 of these patients. We conducted a retrospective study of all patients diagnosed with multifocal ON evaluated in our institution during the last 20 years. We recorded clinical manifestations and underlying diagnoses. A wide thrombophilic profile was performed, including antithrombin, protein C, protein S, lupus anticoagulant, anticardiolipin antibodies, activated protein C resistance, factor V Leiden, mutation G-20210-A of the prothrombin gene, and factor VIII. Coagulation test results were compared with those in a healthy control group and a group of patients with history of lower-extremity deep venous thrombosis.
The mean age of the patients was 49.2 ± 15 years (range, 28–81 yr). The mean number of ON localizations per patient was 5.2 ± 2.3 (range, 3–11). Hips were the most commonly affected joint (82%), followed by knees (58%), shoulders (37%), and ankles (13%). Most patients had an underlying disease process, and 12 of 25 (48%) patients had coagulation test abnormalities. The most common alterations were high factor VIII levels and antiphospholipid antibody (aPL) positivity in 24% and 20% of cases, respectively. These abnormalities were more prevalent in patients with multifocal ON compared with patients in the control groups.
Sixty-one percent of patients had a history of corticosteroid treatment. Patients with coagulation abnormalities had a higher number of ON localizations per patient (6.5 ± 2.7 vs. 3.88 ± 0.8; p = 0.002) and a higher prevalence of atypical ON localizations (25% vs. 0%; p = 0.05).
In conclusion, in the present cohort of patients with multifocal ON, 48% of the patients had at least 1 prothrombotic factor, especially high levels of factor VIII and aPL. These findings have major implications for the diagnosis and treatment of multifocal ON and clearly indicate the need to perform a thrombophilic profile in these patients.
INTRODUCTION
Osteonecrosis (ON), also known as avascular necrosis of bone, is the result of an interruption in blood circulation. This leads to a disparity between the oxygen requirements of the bone cell and the ability of the local circulation to supply the need, leading to bone and bone marrow cell death, which may finally result in mechanical failure and joint destruction.
A variety of traumatic and nontraumatic factors have been implicated in the pathogenesis of ON. Corticosteroid use and excessive alcohol intake are the main nontraumatic factors related, accounting for more than 90% of cases.4 Other processes, such as decompression disease (Caisson disease), human immunodeficiency virus (HIV) infection, radiation therapy, inheritable COL2A1 gene mutations, Gaucher disease, or sickle cell hemoglobinopathies, among others, have been associated with the development of ON.26 In relation to the intrinsic nature of this process, several prothrombotic conditions have been evaluated and described in patients with ON, including antiphospholipid antibodies (aPL),25 factor V Leiden,19 protein C deficiency,9,22 protein S deficiency,9 hyperhomocysteinemia,5 methylenetetrahydrofolate reductase (MTHFR) mutations,6 elevated factor VIII levels,21 and elevated plasminogen activator inhibitor,17 among others.24 Indeed, histologic findings in nontraumatic ON usually reveal thrombosis of terminal arteries in subchondral bone, which, due to the few collaterals of these arteries, can trigger progressive involvement of venules, veins, and arterioles.4,24
Multifocal or multiple ON, defined by the involvement of 3 or more anatomic sites, is unusual, being observed in only 3%–11% of patients diagnosed with ON.18,34 This process can affect any skeletal bone, with particular involvement in the femur, tibia, and talus, and has been associated mainly with a previous history of high-dose corticosteroid therapy, alcohol consumption, and systemic disorders such as systemic lupus erythematosus (SLE), solid organ transplantation, and hematologic diseases. Nevertheless, a few studies have indicated that this process may also be associated with hypercoagulation disorders or a hypofibrinolysis state, further suggesting the convenience of evaluating the presence of prothrombotic abnormalities in these patients.17,24,27,34
Therefore, we conducted the present study to analyze the clinical characteristics of and the prevalence of prothrombotic abnormalities in patients with multifocal ON and to review the literature related to this subject.
METHODS
Patients
We performed a retrospective study including all patients with a diagnosis of multifocal ON evaluated in our department over the 20-year period from 1990 to 2010. Clinical data were obtained from a detailed review of the medical records, with special reference to history of risk factors for ON, corticosteroid treatment, and associated clinical conditions. Clinical manifestations, location, and evolution were recorded in all patients.
Radiologic (including plain X-ray, computed tomography [CT] scan, and/or magnetic resonance imaging [MRI]) and scintigraphic results were recorded in all patients. Diagnosis of ON was established by compatible radiographic study, and diagnosis of multifocal ON was established by involvement of 3 or more anatomic sites.
Thrombophilic Profile
All patients with multifocal ON attended in our department from 1990 to 2010 were evaluated with a standardized protocol for analyzing procoagulant conditions through an extensive thrombophilic profile.
The thrombophilic profile included the following: prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma fibrinogen, functional antithrombin, functional protein C, free protein S, total protein S, plasminogen, lupus anticoagulant (LA), anticardiolipin antibodies (aCL), activated protein C resistance, factor V Leiden, mutation G-20210-A of the prothrombin gene, and coagulation factor VIII.
PT and aPTT were determined in an automated BCS XP analyzer (Siemens, Marburg, Germany) using standard reagents (Thromborel and Actin FS, Siemens). Fibrinogen was measured by the Clauss technique. Protein C activity was quantified by a colorimetric assay (Chromogenix IL, Milano, Italy). Free and total protein S were quantified by enzyme-linked immunosorbent assay (ELISA) (Stago, Asnières, France). Antithrombin and plasminogen activity were measured using chromogenic assays (Siemens). Platelet function testing was done by transmission light aggregometry in platelet-rich plasma using adenosine diphosphate (ADP) and epinephrine as agonists. The diagnosis of “sticky platelet syndrome” was established by evidence of hyperaggregability of platelets in platelet-rich plasma with ADP, epinephrine, or both measured as preservations of the response to the referred agonists at very low concentrations.36 Factor V Leiden and prothrombin gene G20210A mutations were determined by real-time polymerase chain reaction (PCR) (Roche, Mannheim, Germany). Coagulation factor VIII was determined using a chromogenic assay (Chromogenix). The normal range of factor VIII in our laboratory is 65–135 IU/dL. Lupus anticoagulant was detected following the guidelines of the International Society on Thrombosis and Haemostasis: the diluted Russell viper venom test (dVVT) and diluted aPTT were employed for the screening, and dilution (1:1) in normal plasma tests and dVVT after the addition of an excess of phospholipids were used for confirmation. Anticardiolipin antibodies were measured by a standardized ELISA according to the international standards of IgM (MPL) and IgG phospholipid units (GPL) (Cheshire Diagnostics, Chester, UK).
All patients with positive LA and/or aCL were confirmed by at least 2 determinations separated by 12 weeks according to international recommendations at that time.40 Factor VIII was considered to be elevated in cases with levels above the 98th percentile of levels determined in blood donors (>200 IU/dL).52
For comparisons in the thrombophilic profile, a control group of 200 healthy individuals and a group of 100 patients with first lower-extremity deep venous thrombosis (DVT) were selected. These patients and the healthy blood donors were consecutively recruited in our institution in a separate case-control study on thrombophilia parameters in DVT.
Statistical Analysis
All data are expressed as mean ± SD. The Student t test and the nonparametric Kruskal-Wallis test were used to compare differences for continuous variables. Differences between proportions were assessed with the chi-square test, and the Fisher exact test was used when appropriate. A p value <0.05 was considered statistically significant.
With a 2-sided α of 0.05 and a statistical power of 90%, we estimated that a sample of 200 healthy controls was needed to detect a difference of 20% in proportions for coagulation abnormalities among ON patients and controls.
Literature Review
Additionally, we carried out a computer-assisted (MEDLINE, National Library of Medicine, Bethesda, MD) literature search to locate all reports of multiple ON and prothrombotic abnormalities published in English since 1961 when initial reports indicated a possible association.
RESULTS
General Characteristics
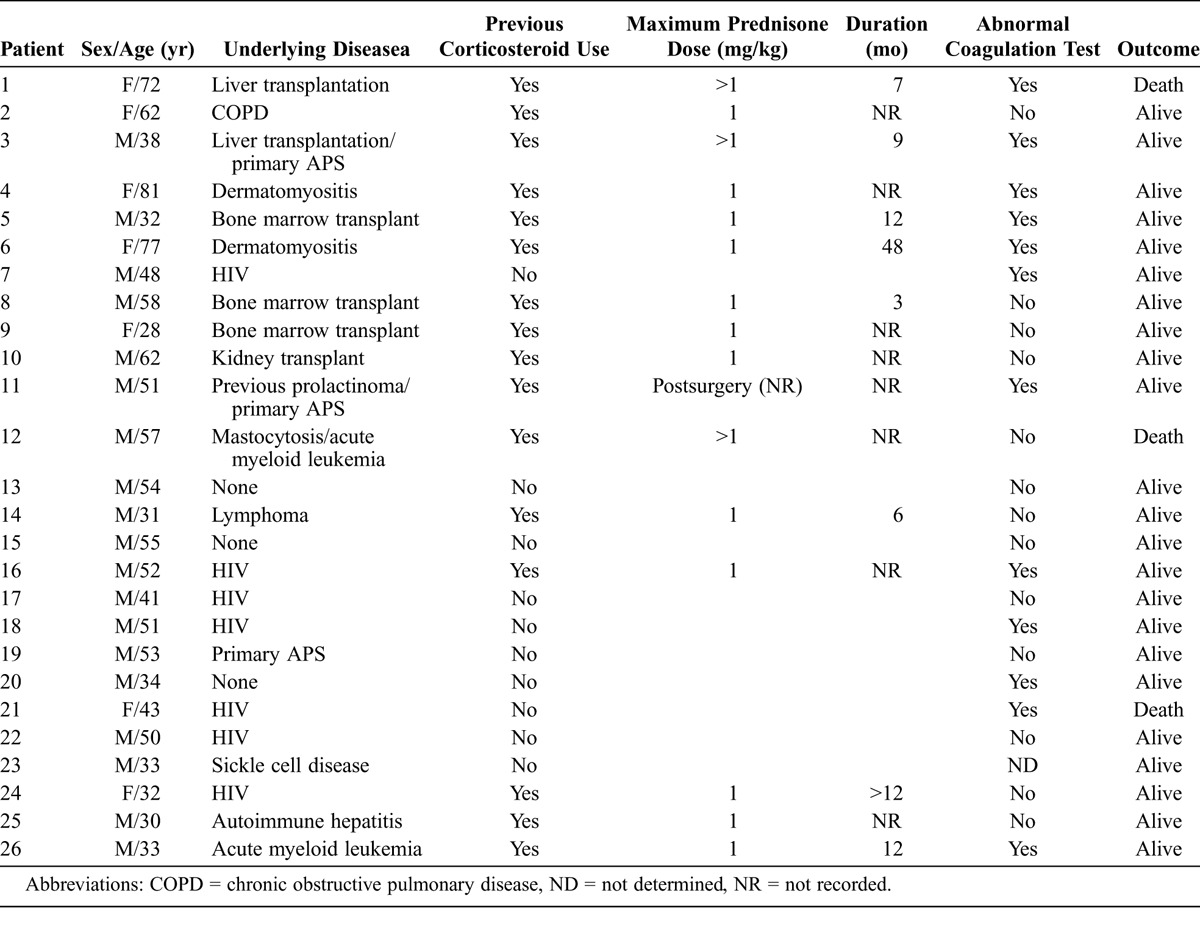
Of the 29 patients with multifocal ON included in the study, the thrombophilic profile was performed in 25 patients. In 4 patients coagulation profile tests were not undertaken: 2 patients were not available for study, 1 patient died, and in 1 case (a patient with sickle cell disease) the tests were not performed because the cause of ON was attributed to the underlying disease. The mean current age was 49.2 ± 15 years (range, 28–81 yr), and 69% of the patients were men. The general clinical characteristics of the patients included are shown in Table 1. Sixty-one percent of the patients had a previous history of high or maintained doses of corticosteroids. The main underlying disorders were HIV in 7 (24%) patients; lymphoproliferative disorders in 6 (21%), 3 of whom had undergone a previous bone marrow transplant; orthotopic liver transplantation in 4 (14%) patients; primary antiphospholipid syndrome (APS) in 3 (10%); and dermatomyositis in 2 (7%) patients. Other conditions were found in 1 case each including renal transplant, sickle cell disease, chronic obstructive pulmonary disease, and autoimmune hepatitis. One patient had a previous history of spontaneous hemorrhage, and 4 patients had no underlying disease (idiopathic ON). In 2 patients 2 comorbidities potentially related to the development of ON were detected: prolactinoma plus APS in 1 patient and mastocytosis plus acute myeloid leukemia in the other.
TABLE 1.
General Characteristics of 26 Patients With Multifocal Osteonecrosis
Coagulation Abnormalities
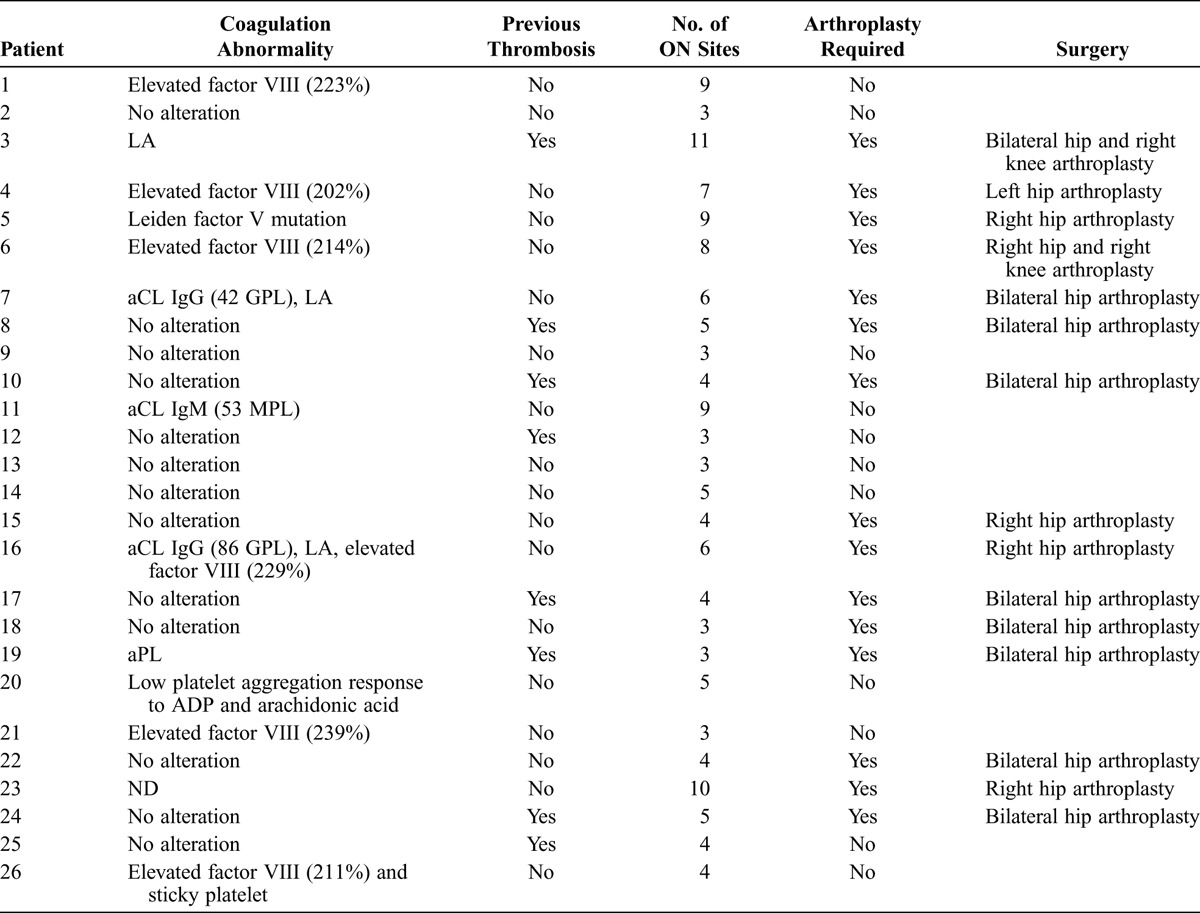
Coagulation abnormalities and clinical outcomes are summarized in Table 2. Briefly, 12 (48%) of 25 patients showed abnormalities on coagulation tests: 11 (44%) patients had 1 alteration, and 2 (8%) had 2 alterations. The most common alterations were high factor VIII levels (>200) in 6 (24%) patients and aPL positivity (LA and/or aCL) in 5 (20%) patients. Interestingly, 8 (27.6%) patients had a previous history of DVT, but only 1 had abnormal coagulation tests (1 with APS). Sixty-one percent of patients with coagulation abnormalities had a previous history of corticosteroid therapy, and 4 of 7 patients with HIV also had coagulation test abnormalities. All HIV patients were under high active antiretroviral therapy, including protease inhibitors.
TABLE 2.
Coagulation Abnormalities and Clinical Features
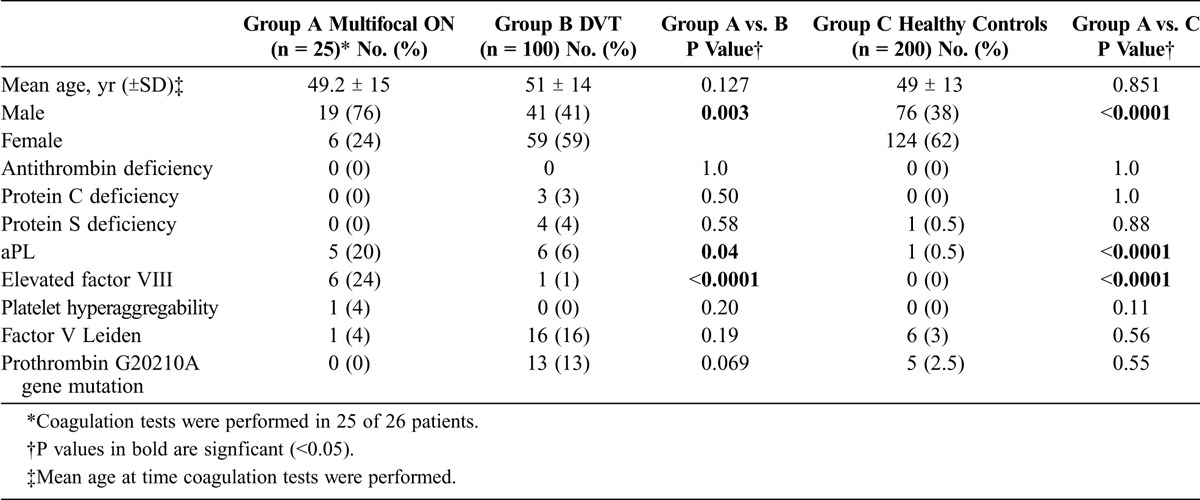
As shown in Table 3, patients with multifocal ON had a statistically significant higher prevalence of aPL and elevated factor VIII compared with patients with DVT and healthy controls. Conversely, patients with DVT tended to have a higher prevalence of Factor V Leiden and prothrombin G20210A gene mutation than patients with multifocal ON.
TABLE 3.
Coagulation Abnormalities in Patients With Multifocal ON, Patients With DVT, and Healthy Controls
Clinical Features and Outcome
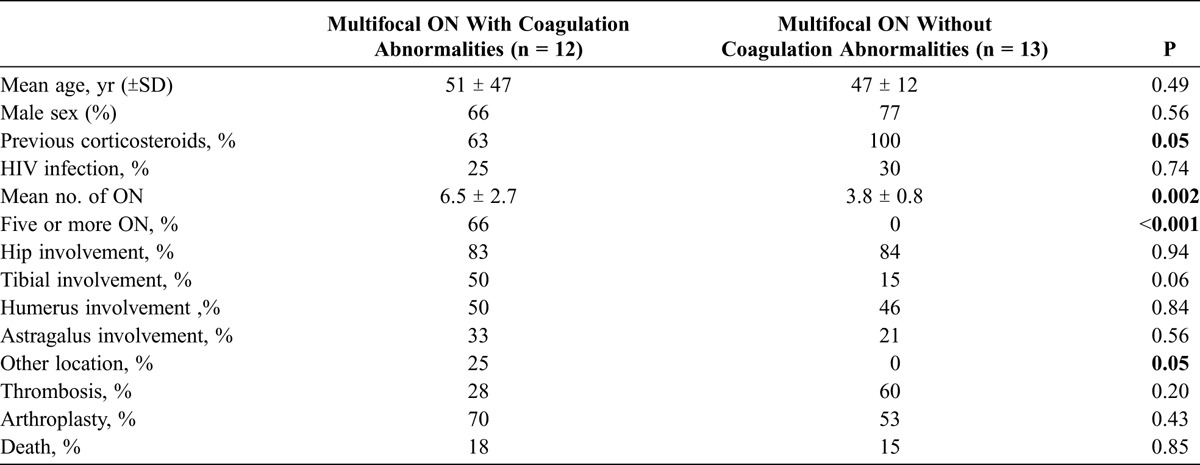
The mean number of bones affected with ON per patient was 5.2 ± 2.3 (range, 3–11). Patients with coagulation abnormalities had a higher number of ON-affected bones (6.5 ± 2.7 vs. 3.8 ± 0.8; p = 0.002). Conversely, patients without coagulation abnormalities had a more frequent history of corticosteroid use (100% vs. 63%; p = 0.05). Patients with coagulation abnormalities had a higher prevalence of atypical localizations of ON (25% vs. 0%; p = 0.05), and additionally had a trend for more tibial involvement (50% vs. 15%; p = 0.06), compared with patients without coagulation abnormalities. There were no significant differences in terms of age, sex, other ON localizations, previous thrombosis, comorbidities, or arthroplasty requirement among patients with or without coagulation abnormalities (Table 4).
TABLE 4.
Patients With Multifocal ON, With or Without Coagulation Abnormalities
Four of the 5 patients with associated aPL received anticoagulation therapy (all with APS), and the remaining patient received antiaggregation therapy. In spite of anticoagulation treatment within the therapeutic international normalized ratio (INR) range, 2 patients developed a new episode of ON (Table 5). The remaining patients with other associated coagulation abnormalities were treated with low-dose aspirin, and no further ON episodes were observed.
TABLE 5.
Follow-Up of Patients With Antiphospholipid Antibodies (aPL)
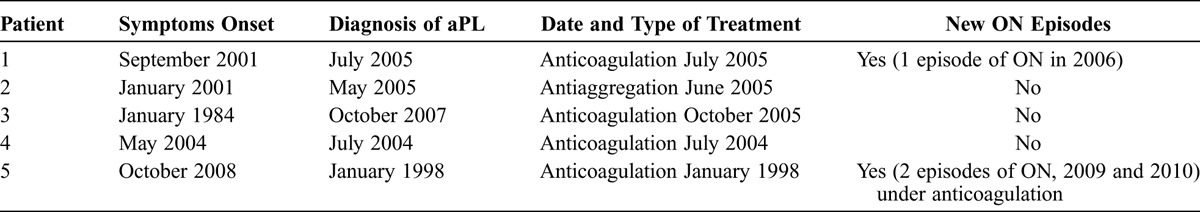
Hips were the joint most commonly affected (82%; bilateral in 72.4% of cases), followed by the knees (58%; bilateral in 48%; Figure 1), shoulders (37%; bilateral in 24%), and ankles (13%; bilateral in 3.4%; Figure 2). Other common locations affected were the talus (17%; bilateral in 7%; Figure 3), and the calcaneus (10%). Vertebral involvement was uncommon, observed in only 1 case.
FIGURE 1.
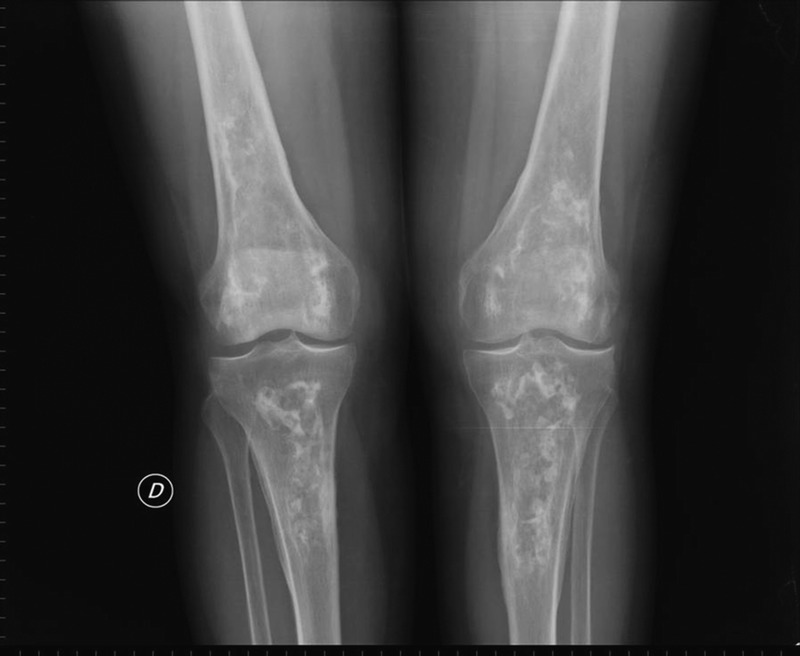
Bilateral knee X-rays showing extensive bilateral bone infarcts in the distal femur and proximal tibia. (“D” indicates the right side.)
FIGURE 2.
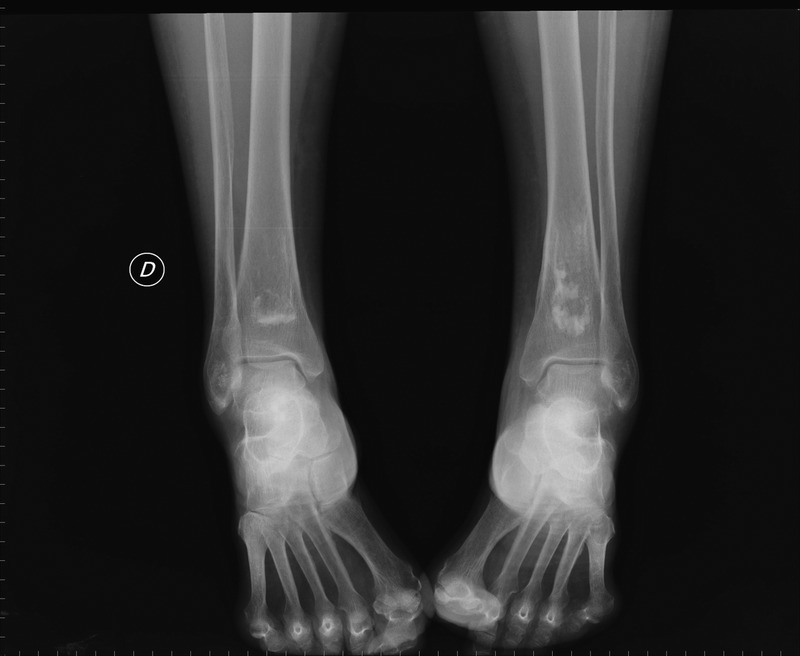
Bilateral ankle X-rays showing bilateral bone infarcts in the distal tibia and fibula. (“D” indicates the right side.)
FIGURE 3.
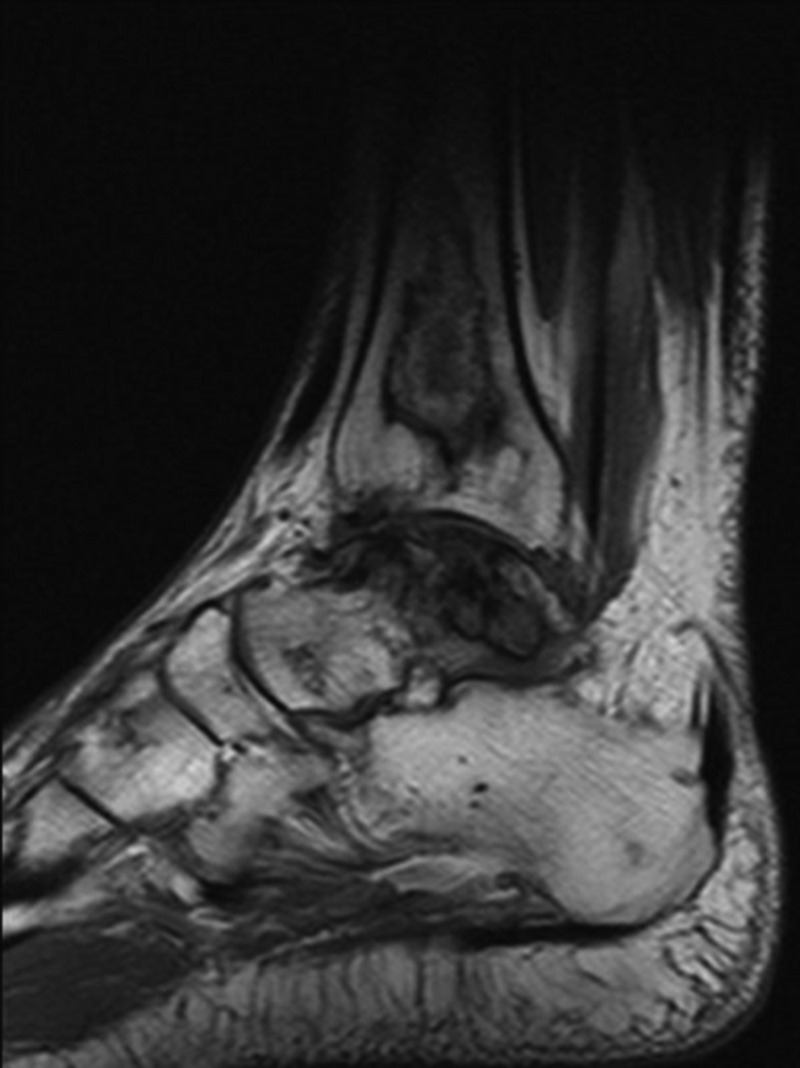
Sagittal MRI demonstrating serpentine areas of bone infarcts in the distal tibia and talus and ON of cuneiform bone.
Sixteen of 26 (61.5%) patients followed underwent joint replacement surgery: 6 patients had single joint replacement, 9 patients had bilateral hip replacement, and 1 patient required 3 arthroplasties. Fifteen of 16 patients treated with arthroplasty had positive X-rays and signs of degenerative changes with Ficat stage IV; the remaining patient had Ficat stage III.
At the study’s end, 25 (86%) patients remained alive and 4 (14%) had died due to complications related to the underlying process (not related to ON or joint replacement surgery).
DISCUSSION
The present study shows a high prevalence of coagulation disorders in patients with multifocal ON, even in patients with other associated clinical conditions such as corticosteroid therapy and HIV infection. Literature regarding multifocal ON is limited. Clinical data from the largest cohorts published to date (including the present study) are summarized in Table 6.
TABLE 6.
Patients With Multifocal ON, Previous and Present Studies
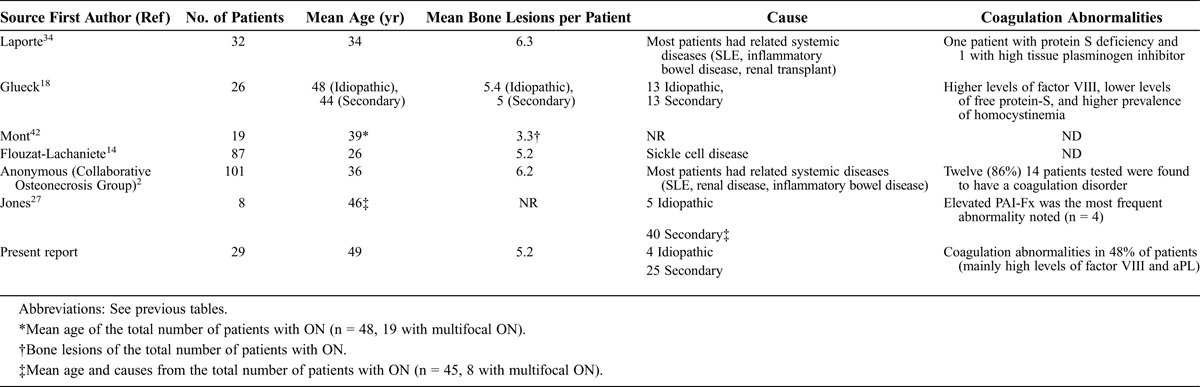
Similar to previous studies, in the present series femoral head, humeral head, and distal femur were the ON locations most frequently affected. Nevertheless, multifocal ON may involve any bone location, including metacarpal50 and tarsal bones44 and ribs.62 Since several patients with ON may follow an asymptomatic course, high clinical suspicion is important. Bone scan is considered the least expensive and most cost-efficient screening method for the initial assessment of suspected multifocal ON,55 with a sensitivity and specificity of about 80% for the diagnosis of ON.54,55 However, MRI is the most sensitive method for diagnosis.42
LaPorte et al34 described one of the largest series of multifocal ON to date. The authors retrospectively reviewed a series of 1056 patients with ON, with only 32 (3%) having multifocal ON. The main disorders associated with multifocal ON were SLE (13 patients), inflammatory bowel disease (5 patients), neoplasia (4 patients), and renal transplantation (3 patients). Most patients were women (75%), with a mean age of 34 years. All patients had involvement of bilateral femoral heads, and 30 also had bilateral involvement of the knees. Other affected areas were the shoulders (78%), ankles (20%), elbows (9%), carpus (6%) and calcaneus (3%). The most common clinical presentation was polyarthralgia, including the hip syndrome, although 8 patients presented with isolated knee pain. Patients in the current study frequently reported previous corticosteroid therapy and showed similar bone involvement, with more than 60% requiring surgical joint replacement.
In 1999, the Collaborative Osteonecrosis Group2 reported the results of a multicenter study that included 101 patients with multifocal ON collected in 21 centers during 16 years. In this cohort, hips were involved in all patients, and there was a bilateral predominance at all sites. Additionally, 89% of knees were affected, followed by the shoulders and ankles in 73% and 35% of cases, respectively. It is noteworthy that 12 (86%) of 14 patients tested for thrombophilia or hypofibrinolysis were found to have a coagulation disorder, including familial protein S deficiency, high tissue plasminogen activator inhibitor, and Factor V Leiden deficiency.
More recently, a French group14 evaluated the clinical course of 200 patients with sickle cell disease over a period of 15 years with 87 patients having multifocal ON, affecting 455 sites. The most commonly affected areas were the proximal femur, followed by proximal humerus, distal femur, proximal tibia, distal tibia, and talus. The very high incidence of ON in this French series was probably related to the routine use of MRI and the particular characteristics of the cohort (sickle cell disease patients). Such a high percentage is not usually seen in other populations and clinical conditions. Indeed, 1 of the patients in the present series had sickle disease, with 8 vertebrae affected by ON; this was the only case of the series with this type of involvement.
Although the pathogenesis of nontraumatic ON is not well understood, several factors have been proposed, such as genetic factors, coagulation abnormalities including inherited and acquired thrombophilias, and hypofibrinolysis or even endothelial factors, among others, suggesting a multifactorial process.3 Indeed, a 2010 case-control study29 including 1450 patients with ON of the femoral head and 7250 controls demonstrated that patients with ON of the femoral head had an increased risk for the development of coronary heart disease during the first 3 years after the diagnosis of ON (HR, 1.43; 95% CI, 1.10–1.86). This increased risk was higher in males and in patients younger than 65 years of age. These data suggest that coagulation abnormalities and endothelial dysfunction involve not only bone tissue circulation but also other cardiovascular areas. On the other hand, 2 different studies20,30 have demonstrated the association between polymorphisms of the endothelial nitric oxide synthetase gene with the development of idiopathic ON, probably related to a reduction in nitric oxide production.
The evaluation of diverse prothrombotic factors in patients with idiopathic ON has shown discrepant results. In a case-control study that evaluated several prothrombotic factors including aCL, anti-β2 glycoprotein I antibodies, S protein, antithrombin, mutation of factor V Leiden, G-20210-A mutation in prothrombin gene, lipoprotein (a) (Lp[a]), and mutation A223 V MTHFR, Mehsen and colleagues37 did not find an increased frequency in any of these factors in patients with ON. On the other hand, Zalavras and colleagues63 reported a high prevalence of the factor V Leiden mutation in a series of 74 patients with ON (23 idiopathic and 49 secondary ON). The prevalence of the factor V Leiden mutation was 18% in ON patients compared to 4.6% in the control group, with 21% prevalence in the idiopathic ON subgroup. Nevertheless, in the current study, we found only 1 case (4%) with the factor V Leiden mutation, similar to the expected prevalence of this mutation in the general Spanish population15 and in our healthy control group. Conversely, this was the most frequent coagulation abnormality found in the present DVT group, being observed in 16% of the patients.
Glueck et al21 compared measures of thrombophilia and hypofibrinolysis in 133 patients with idiopathic (n = 71) and secondary hip ON (n = 62) with measures in healthy control subjects. Hypofibrinolysis studies included plasminogen activator inhibitor (PAI-1) and Lp(a). The authors reported higher levels of factor VIII (>150%) and Lp(a) in patients with idiopathic ON, whereas in patients with secondary ON, high factor VIII, factor V Leiden heterozygosity, and resistance to activated protein C, were more frequently observed. Jones et al27 also indicated a relationship between procoagulant factors and ON in a series of 45 patients with large joint ON: 82.2% of these patients had at least 1 procoagulant factor, compared with 30% of controls. The presence of high levels of aCL IgG and/or low levels of resistance to activated protein C was the most common alteration. Cenni and coworkers7 evaluated several thrombotic and fibrinolytic factors in a 2011 case-control study including 18 patients with idiopathic ON, 18 patients with corticosteroid-associated ON, and 44 healthy controls; they observed significantly higher plasminogen activity in patients with idiopathic ON, a finding that was not observed in patients with corticosteroid-associated ON. The frequency of inherited thrombophilia (factor V Leiden, prothrombin gene mutation and genotypes 4G/5G and 4G/4G of the PAI-1) was similar to that of the controls.
In the current study, the most frequent prothrombotic abnormality was the increase in factor VIII levels, which was found in about one-quarter of patients. Conversely, no individual from the control group showed increased values of this factor, and only 1 patient with DVT had increased values. High levels of factor VIII have been recognized as an independent risk factor for DVT.52 Indeed, patients with factor VIII levels >150 IU/dL had an almost 5-fold higher risk of thrombosis than those with normal levels (<100 IU/dL).32 In addition, elevated factor VIII was shown to constitute a dose-dependent risk factor for DVT, with increases of 10% in the risk for first DVT for each 10 IU/dL increment in plasma factor VIII.33 High factor VIII concentrations represent a risk factor for thrombosis similar to the deficiencies of inhibitors such as proteins C and S and activated protein C resistance.59 The Austrian Study on Recurrent Venous Thromboembolism33 demonstrated that the risk of recurrent thrombosis is 7-fold higher among patients with factor VIII levels exceeding the 90th percentile (>234 IU/dL), and this factor also constitutes a risk factor for the development of thrombosis in non-Western populations, as occurs in portal vein thrombosis and DVT in Indian31 and Japanese48 populations, respectively.
The role of factor VIII as a procoagulant factor has been debated by some authors interpreting its function as an acute phase reactant, with controversy as to whether factor VIII represents a congenital prothrombotic predisposition, an acquired prothrombotic tendency, or merely a secondary reactive phenomenon.51 However, very elevated levels of factor VIII over a relatively long time are suggestive of a factor VIII elevation independent of inflammation.35 In addition, there is consistent evidence that the elevation of factor VIII may not be an acute post-thrombotic phenomenon. Several studies have demonstrated that this factor remains elevated several months and even years after the thrombotic event.28,32,33,47 Moreover, a 2011 prospective study in patients with DVT57 demonstrated that factor VIII levels are partially influenced by the acute phase reaction, but remain elevated during follow-up, despite treatment with oral anticoagulants. Other studies have also demonstrated that the elevation of factor VIII is independent from elevations of other acute phase reactants, such as C-reactive protein or fibrinogen.46
In the current study, aPL positivity was the second most common prothrombotic alteration. It was more frequently observed in patients with multifocal ON (20% presenting with aPL), compared with 6% of patients with DVT and none of the control patients. Additionally, 3 of our patients fulfilled criteria for primary APS.40 Although the frequency of ON in primary APS seems to be low, it is probably an underestimated clinical complication. Of the different reported cohorts of patients with primary APS, to our knowledge only 2 have reported associated cases of ON: Asherson et al3 described 2 (3%) cases out of 70 patients, and in the “Euro-phospholipid” cohort,8 2.4% of the 1000 patients had associated ON, with none of the other series of primary APS13,16,23,38,60 describing other cases of ON. The association between primary APS and ON is derived from isolated case reports25 and, especially, from a single controlled study of 75 APS patients.56 This study prospectively evaluated the prevalence of ON in asymptomatic APS patients by MRI of the femoral heads. The study included 30 patients with primary APS, 19 patients with SLE who were not previously treated with corticosteroids (10 negative for aCL and 9 with aCL but not APS), and 30 healthy subjects. It is noteworthy that asymptomatic ON was revealed in 20% of the patients with primary APS, whereas none of the SLE patients or control patients had positive MRI findings for ON, thereby suggesting that ON represents a common feature of primary APS that can be detected by MRI in its early stages.
APS patients may also present with ON at atypical sites, such as the vertebral body or the lunate bone (Kienböck disease),1 occasionally being the only clinical manifestation of APS. Additionally, multifocal ON has been described in association with catastrophic APS.11 To date, 5 cases with ON of a total of 282 patients collected from the catastrophic APS Registry have been described (http://infmed.fcrb.es/es/web/caps).
The risk of thrombosis in aPL-positive carriers is difficult to estimate accurately because of the multifactorial nature of thrombosis. Currently, it is well known that patients with triple positivity for aPL (LA, aCL, and anti-β2-glycoprotein I) are considered to have a higher risk for thrombosis.49 In fact, these patients may develop recurrent thrombotic episodes, despite the use of oral anticoagulants.
The effectiveness of aspirin in aPL-positive carriers without previous thrombosis is not fully supported by the current literature: in a randomized, double-blind, placebo-controlled trial, low-dose aspirin (81 mg daily) seemed to be no better than placebo in preventing first thrombotic episodes in asymptomatic, persistently aPL-positive patients.12 Nonetheless, based on its low cost and toxicity, prophylactic treatment with aspirin seems to be a easonable option in asymptomatic patients persistently positive for aPL.58
Based on current recommendations for the treatment of APS patients, at present there is no clear indication for anticoagulation in patients with ON as the only thrombotic manifestation of the disease.58 The best approach might be antiaggregation (low dose of aspirin) with strict control of other thrombotic risk factors. In patients with multifocal ON or with the onset of new ON in other territories, oral anticoagulation could be a good, albeit not yet proven, option.25 Four APS patients in the current series received anticoagulation therapy, and 2 of them had additional ON episodes. One patient with associated aCL had antiaggregation treatment and did not have any new ON episodes.
Seven of 29 patients in the current series had HIV infection. These patients are at a substantially increased risk for the development of ON, occurring not infrequently in the form of multifocal ON and early in the course of the disease. In HIV patients, the main risk factors for the development of ON include the HIV infection per se; corticosteroid use; antiretroviral therapy, especially protease inhibitors; and aPL.45 In a recent case-control study, a CD4 cell count <60 cells/μL and corticosteroid use were the main factors associated with the increased risk for ON. No specific thrombophilia tests were significantly associated with ON.10 Nevertheless, in an earlier study, Miller et al39 observed MRI findings consistent with ON of the femoral head in 4.4% of a group of 339 HIV-infected patients, with 93% of these patients having aCL (half with antibody levels >23 IgG phospholipid units). In a subsequent study by the same group,43 239 of 339 original participants underwent a second MRI screening, with a mean follow-up period of 23 months after the initial MRI (range, 17–31 mo). The incidence of hip ON was 0.65 cases per 100 patient-years, showing a 100-fold elevated risk of ON in HIV-infected patients compared with the general population.41 Other series have also confirmed the presence of prothrombotic abnormalities in HIV individuals, especially the presence of aCL observed in 77% and anti-protein S antibodies in 28% of the patients, respectively.53 Four of the 7 HIV-infected patients in the current series had prothrombotic abnormalities; elevated factor VIII and the presence of aCL were the most frequent.
Finally, a paradoxic case in the current study involved a 34-year-old man with a previous history of spontaneous hemorrhage (subdural hematoma) with low platelet aggregation tests, including acid arachidonic and adenosine diphosphate, and no other known risk factors related to the development of ON. To our knowledge, low platelet aggregation has not previously been related to ON. Conversely, as expected, animal studies have demonstrated that the use of antiplatelet drugs, such as clopidogrel, prevented the development of corticosteroid-induced ON.61 The pathogenesis of ON in this particular case remains unclear.
The current study has certain strengths and limitations. It could be argued that we have included a relatively low number of patients. Nevertheless, taking the low prevalence of this process into account, this study describes one of the largest cohorts of multifocal ON from a single center to date. Although the specific role of the observed coagulation abnormalities in the development of ON is not known, considering the previous history of corticosteroid use in most of the patients, it seems logical to assume these abnormalities are a contributory factor. Indeed, patients with associated coagulation abnormalities also had a higher number of ON-affected bones.
In conclusion, in the current study most patients with multifocal ON had secondary conditions that may be associated with the development of ON, such as steroid treatment or HIV infection. However, more than half of these patients also had prothrombotic-associated factors, especially high levels of factor VIII and aPL, which can contribute to the development of multifocal ON. The current findings may have major implications for the diagnosis and treatment of this condition, and clearly indicate that a thrombophilic profile must be performed in these patients.
Abbreviations
- aCL
anticardiolipin antibodies
- aPL
antiphospholipidantibodies
- APS
antiphospholipid syndrome
- aPTT
activated partial thromboplastin time
- DVT
deep venous thrombosis
- ELISA
enzyme-linked immunosorbent assay
- HIV
human immunodeficiency virus
- LA
lupus anticoagulant
- Lp(a)
lipoprotein (a)
- MRI
magnetic resonance imaging
- MTHFR
methylenetetrahydrofolate reductase
- ON
osteonecrosis
- PAI-1
plasminogen activator inhibitor
- PT
prothrombin time
- SLE
systemic lupus erythematosus
Footnotes
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1. Alijotas J, Argemi M, Barquinero J. Kienbock’s disease and antiphospholipid antibodies. Clin Exp Rheumatol. 1990; 8: 297– 298. [PubMed] [Google Scholar]
- 2.Anonymous. Symptomatic multifocal osteonecrosis. A multicenter study. Collaborative Osteonecrosis Group. Clin Orthop Relat Res. 1999; 369: 312– 326. [PubMed] [Google Scholar]
- 3. Asherson RA, Khamashta MA, Ordi-Ros J, Derksen RH, Machin SJ, Barquinero J, Outt HH, Harris EN, Vilardell-Torres M, Hughes GR. The “primary” antiphospholipid syndrome: major clinical and serological features. Medicine (Baltimore). 1989; 68: 366– 374. [PubMed] [Google Scholar]
- 4. Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002; 32: 94– 124. [PubMed] [Google Scholar]
- 5. Blanche P, Si-Larbi AG, Jouve P. Femoral head necrosis and hyperhomocysteinemia. J Rheumatol. 2001; 28: 1469. [PubMed] [Google Scholar]
- 6. Celik A, Tekis D, Saglam F, Tunali S, Kabakci N, Ozaksoy D, Manisali M, Ozcan MA, Meral M, Gulay H, Camsari T. Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant Proc. 2006; 38: 512– 516. [DOI] [PubMed] [Google Scholar]
- 7. Cenni E, Fotia C, Rustemi E, Yuasa K, Caltavuturo G, Giunti A, Baldini N. Idiopathic and secondary osteonecrosis of the femoral head show different thrombophilic changes and normal or higher levels of platelet growth factors. Acta Orthop. 2011; 82: 42– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Boffa MC, Hughes GR, Ingelmo M. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002; 46: 1019– 1027. [DOI] [PubMed] [Google Scholar]
- 9. Chotanaphuti T, Heebthamai D, Chuwong M, Kanchanaroek K. The prevalence of thrombophilia in idiopathic osteonecrosis of the hip. J Med Assoc Thai. 2009; 92 (Suppl 6): S141– 146. [PubMed] [Google Scholar]
- 10. de Larranaga G, Bottaro E, Martinuzzo M, Figueroa R, Iglesias Varela ML, Peres Wingeyer S, Forastiero R, Adamczuk Y, Corti M, Puga L, Benetucci J. Thrombophilia in human immunodeficiency virus-infected patients with osteonecrosis: is there a real connection? The first case-control study. Clin Appl Thromb Hemost. 2009; 15: 340– 347. [DOI] [PubMed] [Google Scholar]
- 11. Egan RM, Munn RK. Catastrophic antiphospholipid antibody syndrome presenting with multiple thromboses and sites of avascular necrosis. J Rheumatol. 1994; 21: 2376– 2379. [PubMed] [Google Scholar]
- 12. Erkan D, Harrison MJ, Levy R, Peterson M, Petri M, Sammaritano L, Unalp-Arida A, Vilela V, Yazici Y, Lockshin MD. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum. 2007; 56: 2382– 2391. [DOI] [PubMed] [Google Scholar]
- 13. Erkan D, Yazici Y, Sobel R, Lockshin MD. Primary antiphospholipid syndrome: functional outcome after 10 years. J Rheumatol. 2000; 27: 2817– 2821. [PubMed] [Google Scholar]
- 14. Flouzat-Lachaniete CH, Roussignol X, Poignard A, Mukasa MM, Manicom O, Hernigou P. Multifocal joint osteonecrosis in sickle cell disease. Open Orthop J. 2009; 3: 32– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Gala JM, Alvarez V, Pinto CR, Soto I, Urgelles MF, Menendez MJ, Carracedo C, Lopez-Larrea C, Coto E. Factor V Leiden (R506Q) and risk of venous thromboembolism: a case-control study based on the Spanish population. Clin Genet. 1997; 52: 206– 210. [DOI] [PubMed] [Google Scholar]
- 16. Gattorno M, Falcini F, Ravelli A, Zulian F, Buoncompagni A, Martini G, Resti M, Picco P, Martini A. Outcome of primary antiphospholipid syndrome in childhood. Lupus. 2003; 12: 449– 453. [DOI] [PubMed] [Google Scholar]
- 17. Glueck CJ, Fontaine RN, Gruppo R, Stroop D, Sieve-Smith L, Tracy T, Wang P. The plasminogen activator inhibitor-1 gene, hypofibrinolysis, and osteonecrosis. Clin Orthop Relat Res. 1999; 366: 133– 146. [DOI] [PubMed] [Google Scholar]
- 18. Glueck CJ, Freiberg RA, Boppana S, Wang P. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am. 2008; 90: 2220– 2229. [DOI] [PubMed] [Google Scholar]
- 19. Glueck CJ, Freiberg RA, Boriel G, Khan Z, Brar A, Padda J, Wang P. The role of the factor V Leiden mutation in osteonecrosis of the hip. Clin Appl Thromb Hemost. 2013; 19: 499– 503. [DOI] [PubMed] [Google Scholar]
- 20. Glueck CJ, Freiberg RA, Oghene J, Fontaine RN, Wang P. Association between the T-786C eNOS polymorphism and idiopathic osteonecrosis of the head of the femur. J Bone Joint Surg Am. 2007; 89: 2460– 2468. [DOI] [PubMed] [Google Scholar]
- 21. Glueck CJ, Freiberg RA, Wang P. Heritable thrombophilia-hypofibrinolysis and osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008; 466: 1034– 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glueck CJ, McMahon RE, Bouquot JE, Tracy T, Sieve-Smith L, Wang P. A preliminary pilot study of treatment of thrombophilia and hypofibrinolysis and amelioration of the pain of osteonecrosis of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85: 64– 73. [DOI] [PubMed] [Google Scholar]
- 23. Gomez-Puerta JA, Martin H, Amigo MC, Aguirre MA, Camps MT, Cuadrado MJ, Hughes GR, Khamashta MA. Long-term follow-up in 128 patients with primary antiphospholipid syndrome: do they develop lupus? Medicine (Baltimore). 2005; 84: 225– 230. [DOI] [PubMed] [Google Scholar]
- 24. Gomez-Puerta JA, Peris P, Guanabens N. Osteonecrosis multiple. Patogenesis, caracteristicas clinicas y tratamiento. Semin Fund Esp Reumatol. 2007; 8: 185– 192. [Google Scholar]
- 25. Gomez-Puerta JA, Pons-Estel GJ. Skeletal involvement in antiphospholipid syndrome. Curr Rheumatol Rev. 2010; 6: 25– 31. [Google Scholar]
- 26.Jones L. Osteonecrosis (avascular necrosis of bone). UpToDate (www.uptodate.com); 2012.
- 27. Jones LC, Mont MA, Le TB, Petri M, Hungerford DS, Wang P, Glueck CJ. Procoagulants and osteonecrosis. J Rheumatol. 2003; 30: 783– 791. [PubMed] [Google Scholar]
- 28. Kamphuisen PW, Houwing-Duistermaat JJ, van Houwelingen HC, Eikenboom JC, Bertina RM, Rosendaal FR. Familial clustering of factor VIII and von Willebrand factor levels. Thromb Haemost. 1998; 79: 323– 327. [PubMed] [Google Scholar]
- 29. Kang JH, Lin HC. Increased risk for coronary heart disease after avascular necrosis of femoral head: a 3-year follow-up study. Am Heart J. 2010; 159: 803– 808 e1. [DOI] [PubMed] [Google Scholar]
- 30. Koo KH, Lee JS, Lee YJ, Kim KJ, Yoo JJ, Kim HJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res. 2006; 24: 1722– 1728. [DOI] [PubMed] [Google Scholar]
- 31. Koshy A, Jeyakumari M. High FVIII level is associated with idiopathic portal vein thrombosis in South India. Am J Med. 2007; 120: 552 e9– 11. [DOI] [PubMed] [Google Scholar]
- 32. Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995; 345: 152– 155. [DOI] [PubMed] [Google Scholar]
- 33. Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K, Eichinger S. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000; 343: 457– 462. [DOI] [PubMed] [Google Scholar]
- 34. LaPorte DM, Mont MA, Mohan V, Jones LC, Hungerford DS. Multifocal osteonecrosis. J Rheumatol. 1998; 25: 1968– 1974. [PubMed] [Google Scholar]
- 35. Livesey JA, Manning RA, Meek JH, Jackson JE, Kulinskaya E, Laffan MA, Shovlin CL. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. 2012; 67: 328– 333. [DOI] [PubMed] [Google Scholar]
- 36. Mammen EF. Sticky platelet syndrome. Semin Thromb Hemost. 1999; 25: 361– 365. [DOI] [PubMed] [Google Scholar]
- 37. Mehsen N, Barnetche T, Redonnet-Vernhet I, Guerin V, Bentaberry F, Gonnet-Gracia C, Schaeverbeke T. Coagulopathies frequency in aseptic osteonecrosis patients. Joint Bone Spine. 2009; 76: 166– 169. [DOI] [PubMed] [Google Scholar]
- 38. Mejia-Romero R, Garcia-Carrasco M, Galarza-Maldonado C, Santos P, Mendoza-Pinto C, Escarcega RO, Salinas-Saldivar S, Soto-Vega E, Lopez-Colombo A, Cervera R. Primary antiphospholipid syndrome in Latin American mestizo patients: clinical and immunologic characteristics and comparison with European patients. Clin Rheumatol. 2008; 27: 891– 897. [DOI] [PubMed] [Google Scholar]
- 39. Miller KD, Masur H, Jones EC, Joe GO, Rick ME, Kelly GG, Mican JM, Liu S, Gerber LH, Blackwelder WC, Falloon J, Davey RT, Polis MA, Walker RE, Lane HC, Kovacs JA. High prevalence of osteonecrosis of the femoral head in HIV-infected adults. Ann Intern Med. 2002; 137: 17– 25. [DOI] [PubMed] [Google Scholar]
- 40. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PG DEG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4: 295– 306. [DOI] [PubMed] [Google Scholar]
- 41. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995; 77: 459– 474. [DOI] [PubMed] [Google Scholar]
- 42. Mont MA, Ulrich SD, Seyler TM, Smith JM, Marker DR, McGrath MS, Hungerford DS, Jones LC. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008; 35: 1629– 1634. [PubMed] [Google Scholar]
- 43. Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, Kovacs JA. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007; 44: 739– 748. [DOI] [PubMed] [Google Scholar]
- 44. Mundo J, Peris P, Monegal A, Navasa M, Cervera R, Guaniabens N. Multifocal avascular necrosis after liver transplantation: an unusual presentation of the antiphospholipid syndrome. Lupus. 2006; 15: 304– 307. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen BY, Reveille JD. Rheumatic manifestations associated with HIV in the highly active antiretroviral therapy era. Curr Opin Rheumatol. 2009; 21: 404– 410. [DOI] [PubMed] [Google Scholar]
- 46. O’Donnell J, Mumford AD, Manning RA, Laffan M. Elevation of FVIII:C in venous thromboembolism is persistent and independent of the acute phase response. Thromb Haemost. 2000; 83: 10– 13. [PubMed] [Google Scholar]
- 47. O’Donnell J, Tuddenham EG, Manning R, Kemball-Cook G, Johnson D, Laffan M. High prevalence of elevated factor VIII levels in patients referred for thrombophilia screening: role of increased synthesis and relationship to the acute phase reaction. Thromb Haemost. 1997; 77: 825– 828. [PubMed] [Google Scholar]
- 48. Ota S, Yamada N, Ogihara Y, Tsuji A, Ishikura K, Nakamura M, Wada H, Ito M. High plasma level of factor VIII: an important risk factor for venous thromboembolism. Circ J. 2011; 75: 1472– 1475. [DOI] [PubMed] [Google Scholar]
- 49. Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, Testa S, Marongiu F, Bison E, Denas G, Banzato A, Padayattil Jose S, Iliceto S. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010; 8: 237– 242. [DOI] [PubMed] [Google Scholar]
- 50. Robinson AB, Rabinovich CE. Avascular necrosis of the metacarpals in juvenile dermatomyositis. J Clin Rheumatol. 2010; 16: 233– 236. [DOI] [PubMed] [Google Scholar]
- 51. Ryan K, O’Donnell JS. Elevated plasma factor VIII levels in patients with venous thrombosis—constitutional risk factor or secondary epiphenomenon? Thromb Res. 2012; 129: 105– 106. [DOI] [PubMed] [Google Scholar]
- 52. Schambeck CM, Hinney K, Haubitz I, Mansouri Taleghani B, Wahler D, Keller F. Familial clustering of high factor VIII levels in patients with venous thromboembolism. Arterioscler Thromb Vasc Biol. 2001; 21: 289– 292. [DOI] [PubMed] [Google Scholar]
- 53. Sorice M, Griggi T, Arcieri P, Circella A, d’Agostino F, Ranieri M, Modrzewska R, Lenti L, Mariani G. Protein S and HIV infection. The role of anticardiolipin and anti-protein S antibodies. Thromb Res. 1994; 73: 165– 175. [DOI] [PubMed] [Google Scholar]
- 54. Steinberg ME. Early diagnosis of avascular necrosis of the femoral head. Instr Course Lect. 1988; 37: 51– 57. [PubMed] [Google Scholar]
- 55. Stulberg BN, Bauer TW, Belhobek GH, Levine M, Davis A. A diagnostic algorithm for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989; 249: 176– 182. [PubMed] [Google Scholar]
- 56. Tektonidou MG, Malagari K, Vlachoyiannopoulos PG, Kelekis DA, Moutsopoulos HM. Asymptomatic avascular necrosis in patients with primary antiphospholipid syndrome in the absence of corticosteroid use: a prospective study by magnetic resonance imaging. Arthritis Rheum. 2003; 48: 732– 736. [DOI] [PubMed] [Google Scholar]
- 57. Tichelaar V, Mulder A, Kluin-Nelemans H, Meijer K. The acute phase reaction explains only a part of initially elevated factor VIII:C levels: a prospective cohort study in patients with venous thrombosis. Thromb Res. 2012; 129: 183– 186. [DOI] [PubMed] [Google Scholar]
- 58. Tuthill JI, Khamashta MA. Management of antiphospholipid syndrome. J Autoimmun. 2009; 33: 92– 98. [DOI] [PubMed] [Google Scholar]
- 59. van der Meer FJ, Koster T, Vandenbroucke JP, Briet E, Rosendaal FR. The Leiden Thrombophilia Study (LETS). Thromb Haemost. 1997; 78: 631– 635. [PubMed] [Google Scholar]
- 60. Vianna JL, Khamashta MA, Ordi-Ros J, Font J, Cervera R, Lopez-Soto A, Tolosa C, Franz J, Selva A, Ingelmo M, et al. Comparison of the primary and secondary antiphospholipid syndrome: a European multicenter study of 114 patients. Am J Med. 1994; 96: 3– 9. [DOI] [PubMed] [Google Scholar]
- 61. Yamaguchi R, Yamamoto T, Motomura G, Ikemura S, Iwasaki K, Zhao G, Iwamoto Y. Effects of an anti-platelet drug on the prevention of steroid-induced osteonecrosis in rabbits. Rheumatology (Oxford). 2012; 51: 789– 793. [DOI] [PubMed] [Google Scholar]
- 62. Yoo WH. Multiple rib infarcts: a rare form of osteonecrosis in antiphospholipid syndrome. Ann Rheum Dis. 2004; 63: 457– 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Factor V Leiden and prothrombin gene mutations in femoral head osteonecrosis. Thromb Haemost. 2002; 87: 1079– 1080. [PubMed] [Google Scholar]


