Abstract
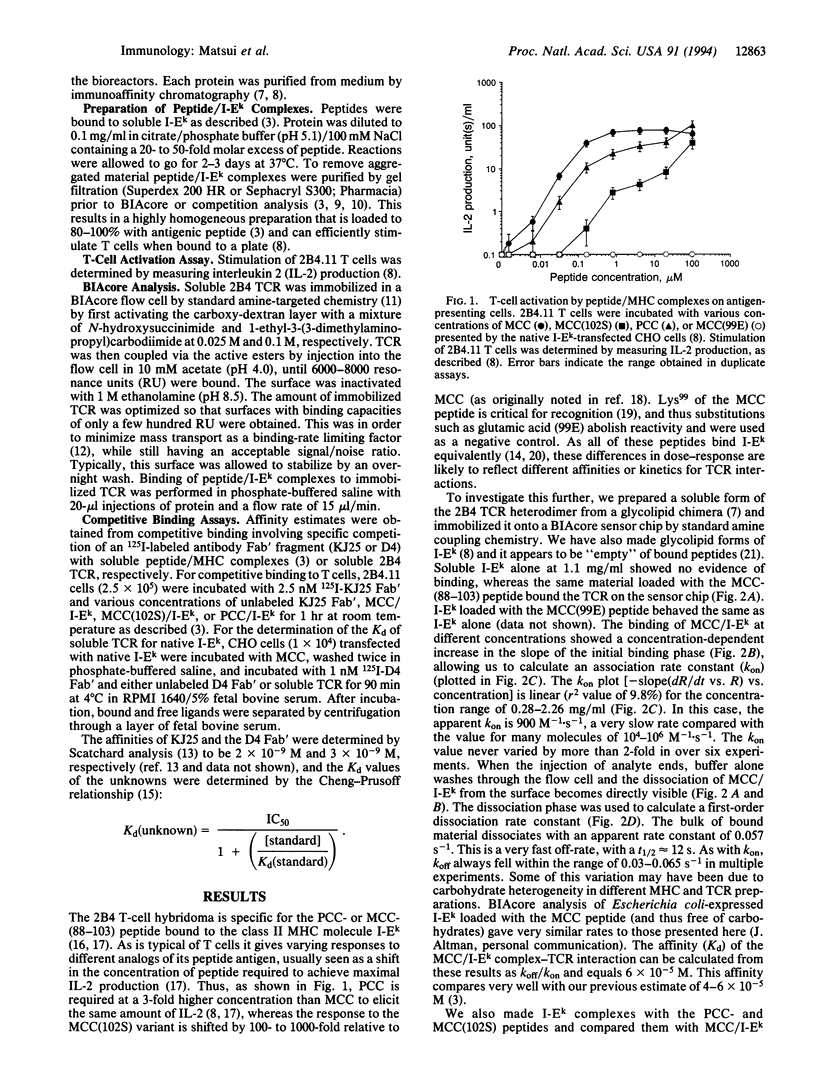
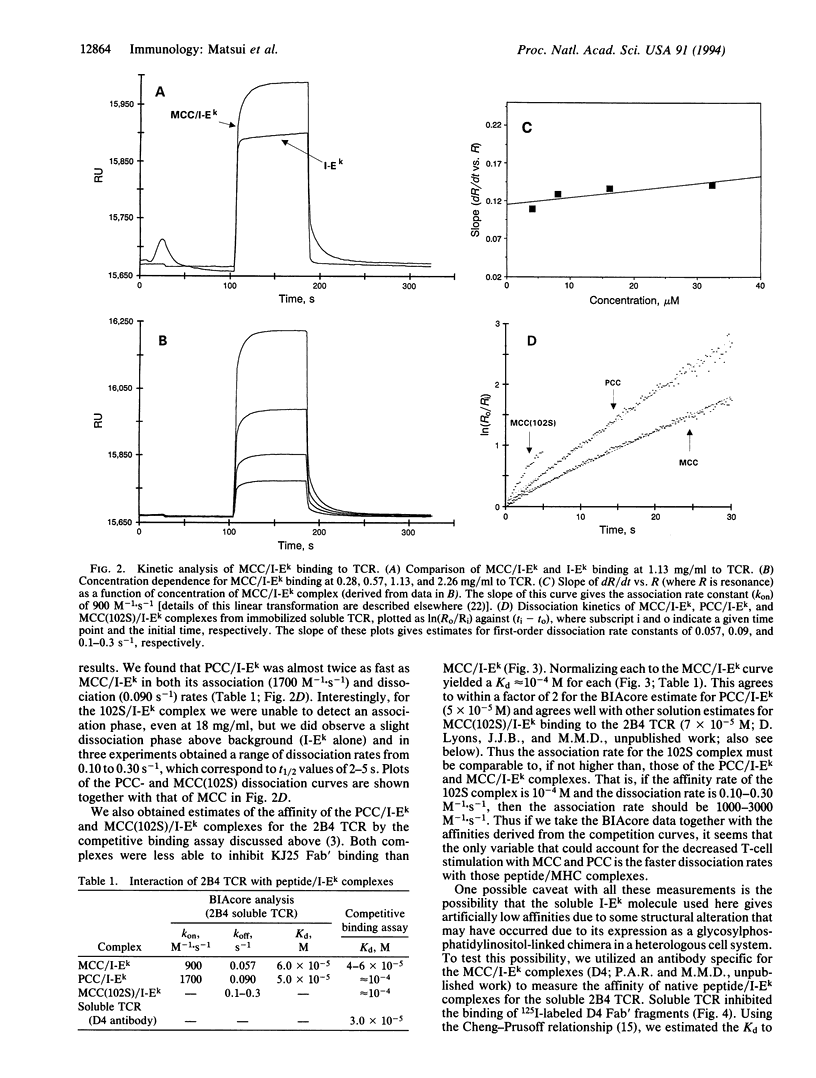
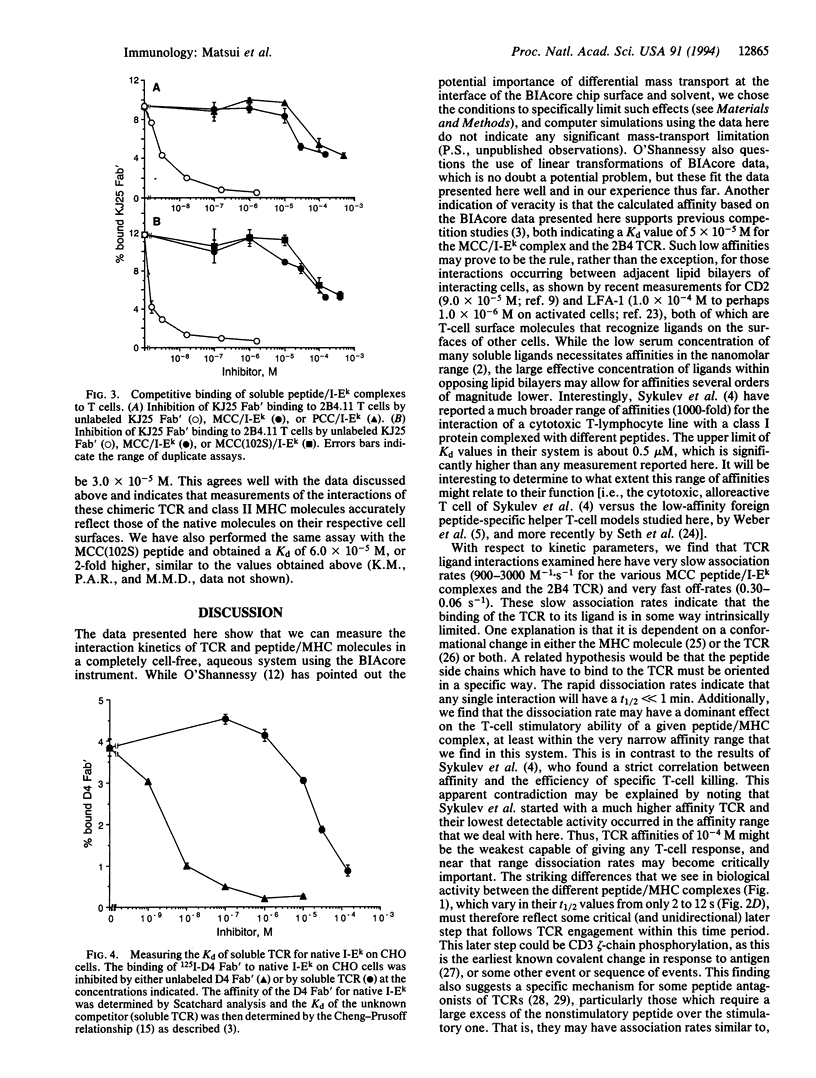
Recognition by T-cell antigen receptors (TCRs) of processed peptides bound to major histocompatibility complex (MHC) molecules is required for the initiation of most T-lymphocyte responses. Despite the availability of soluble forms of TCRs and MHC heterodimers, this interaction has proven difficult to study directly due to the very low affinity. We report here on the kinetics of TCR binding to peptide/MHC complexes in a cell-free system using surface plasmon resonance. The apparent association rates for the interactions of related peptide/MHC complexes to one such TCR are relatively slow (900-3000 M-1.s-1) and dissociation rates are very fast (0.3-0.06 s-1) with t1/2 of 2-12 s at 25 degrees C. The calculated affinity of the engineered soluble molecules compares well with previously reported competition data for native TCRs or competition data reported here for native peptide/MHC complexes, indicating that these soluble heterodimers bind in the same manner as the original molecules expressed on cells. We also find that the peptide variants which give weaker T-cell stimulatory responses have similar affinities but distinctly faster dissociation rates compared with the original peptide (when loaded onto the MHC molecule) and that this later property may be responsible for their lower activity. This has implications for both downstream signaling events and models of TCR-peptide antagonists.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhayani H., Paterson Y. Analysis of peptide binding patterns in different major histocompatibility complex/T cell receptor complexes using pigeon cytochrome c-specific T cell hybridomas. Evidence that a single peptide binds major histocompatibility complex in different conformations. J Exp Med. 1989 Nov 1;170(5):1609–1625. doi: 10.1084/jem.170.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr M., Slanetz A. E., Boyd L. F., Jelonek M. T., Khilko S., al-Ramadi B. K., Kim Y. S., Maher S. E., Bothwell A. L., Margulies D. H. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994 Aug 12;265(5174):946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Chien Y. Topology and affinity of T-cell receptor mediated recognition of peptide-MHC complexes. Curr Opin Immunol. 1993 Feb;5(1):45–49. doi: 10.1016/0952-7915(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Davis M. M. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- De Magistris M. T., Alexander J., Coggeshall M., Altman A., Gaeta F. C., Grey H. M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992 Feb 21;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Chen C., Fraga E., French C. A., Singh B., Schwartz R. H. Functionally distinct agretopic and epitopic sites. Analysis of the dominant T cell determinant of moth and pigeon cytochromes c with the use of synthetic peptide antigens. J Immunol. 1987 Sep 1;139(5):1578–1588. [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Johnsson B., Löfås S., Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991 Nov 1;198(2):268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Michaelsson A., Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J Immunol Methods. 1991 Dec 15;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- Lollo B. A., Chan K. W., Hanson E. M., Moy V. T., Brian A. A. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J Biol Chem. 1993 Oct 15;268(29):21693–21700. [PubMed] [Google Scholar]
- Malmqvist M. Biospecific interaction analysis using biosensor technology. Nature. 1993 Jan 14;361(6408):186–187. doi: 10.1038/361186a0. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- O'Shannessy D. J. Determination of kinetic rate and equilibrium binding constants for macromolecular interactions: a critique of the surface plasmon resonance literature. Curr Opin Biotechnol. 1994 Feb;5(1):65–71. doi: 10.1016/s0958-1669(05)80072-2. [DOI] [PubMed] [Google Scholar]
- Reay P. A., Kantor R. M., Davis M. M. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93-103). J Immunol. 1994 Apr 15;152(8):3946–3957. [PubMed] [Google Scholar]
- Reay P. A., Wettstein D. A., Davis M. M. pH dependence and exchange of high and low responder peptides binding to a class II MHC molecule. EMBO J. 1992 Aug;11(8):2829–2839. doi: 10.1002/j.1460-2075.1992.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo J. M., Janeway C. A., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. J Immunol. 1988 Feb 15;140(4):1081–1088. [PubMed] [Google Scholar]
- Saito H., Maki R. A., Clayton L. K., Tonegawa S. Complete primary structures of the E beta chain and gene of the mouse major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5520–5524. doi: 10.1073/pnas.80.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Seth A., Stern L. J., Ottenhoff T. H., Engel I., Owen M. J., Lamb J. R., Klausner R. D., Wiley D. C. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994 May 26;369(6478):324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Sykulev Y., Brunmark A., Jackson M., Cohen R. J., Peterson P. A., Eisen H. N. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity. 1994 Apr;1(1):15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- Wettstein D. A., Boniface J. J., Reay P. A., Schild H., Davis M. M. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J Exp Med. 1991 Jul 1;174(1):219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P. A., Brown M. H., Davis S. J., Barclay A. N. Affinity and kinetic analysis of the interaction of the cell adhesion molecules rat CD2 and CD48. EMBO J. 1993 Dec 15;12(13):4945–4954. doi: 10.1002/j.1460-2075.1993.tb06188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



