Supplemental Digital Content is available in the text
Abstract
This article compares the clinical characteristics and prognosis of young patients in different age groups with liver cancer (LC).
In this retrospective study, we searched the Surveillance, Epidemiology, and End Results population-based database and identified 2641 patients who had been diagnosed with LC between 1988 and 2005. These patients were categorized into 2 different age ranges: Group 1 (≤35 years) and Group 2 (36–45 years). Five-year cancer-specific survival (CSS) data were obtained. Kaplan–Meier methods and multivariable Cox regression models were used to analyze the long-term survival outcomes and risk factors.
There were significant differences between the age groups for stage and tumor size (P < 0.001). The 5-year liver CSS rate was 20.4% and 14.5%, respectively (P < 0.001). Univariate and multivariate analysis also confirmed the difference (P < 0.001). Further analysis showed that this significant difference existed in localized, regional, and distant-stage patients.
Young patients with LC of age 18 to 45 years are inherently heterogeneous. Patients aged ≤35 years have better CSS than those aged 36 to 45 years, despite exhibiting unfavorable clinicopathological characteristics.
INTRODUCTION
Liver cancer (LC) is the sixth most common cancer worldwide and the third leading cause of cancer-related death globally.1–3 People with cirrhosis or hepatitis B or C have an increased risk of LC, especially in male patients.4,5 Trends in liver and intrahepatic bile duct cancer have shown significant increases over the past few years.6 An estimated 30,640 adults in the United States were diagnosed with primary LC in 2003.7 However, it also uncharacteristically affects young people who do not have these related risk factors. Although LC remains an affliction of elderly people, there has been a shift toward relatively younger individuals.8 Therefore, LC in young people is a topical issue in oncology. LC in young patients usually presents at an advanced stage with a poor pathological grading, which tends to have a poorer prognosis compared to elderly patients.9,10 However, other studies have demonstrated that even though young patients have unfavorable pathological features, they have a better, at least no worse, long-term survival than elderly patients.11,12 As young age has wider subgroups with potential heterogeneous, different subgroups may cause different prognosis.
In this study, we searched the Surveillance, Epidemiology, and End Results (SEER) population-based database for young patients with LC and divided them into 2 subgroups: aged ≤35 years and 36 to 45 years. The purpose of this population-based study was to analyze the clinicopathological characteristics and cancer-specific survival (CSS) of these subgroups of young patients with LC in the SEER population-based database.
METHODS
Patients
The SEER Cancer Statistics Review (http://seer.cancer.gov/data/citation.html), a report on the most recent cancer incidence, mortality, survival, prevalence, and lifetime risk statistics, is published annually by the Data Analysis and Interpretation Branch of the National Cancer Institute (NCI; Bethesda, MD). The current SEER database consists of 17 population-based cancer registries that represent ∼26% of the population in the United States. The SEER data contain no identifiers and are publicly available for studies of cancer-based epidemiology and survival analysis. The National Cancer Institute's SEER∗Stat software (Version 8.1.5) (www.seer.cancer.gov/seerstat) was used to identify patients whose pathological diagnosis as LC based on International Classification of Diseases for Oncology topography codes (C22.0 and C22.1) between 1988 and 2005, for liver and intrahepatic bile duct cancers, respectively. Morphology codes for LC were expanded to include the following histologies: 8170, 8171, 8172, 8173, 8174, 8175, 8160, and 8180 (ie, not otherwise specified, fibrolamellar, scirrhous, spindle cell variant, clear cell type, pleomorphic-type hepatocellular carcinoma [HCC], cholangiocarcinoma, and combined HCC and cholangiocarcinoma). Only patients who underwent surgical treatment at age 18 to 45 years were included in the current study. Patients diagnosed after 2006 were excluded to ensure an adequate follow-up time. Patients were excluded if they had no evaluation on follow-up. Liver cancer-specific survival (LCSS) was assessed depending on age, sex, race, histological type, stage, tumor grade, and tumor size. Adjuvant chemotherapy was not evaluated because the SEER registry does not include such information. The primary endpoint of the study was LCSS, which was calculated from the date of diagnosis to the date of cancer-specific death. Deaths attributed to the cancer of interest were treated as events and deaths from other causes were treated as censored observations.
This study was based on public data from the SEER database, and we obtained permission to access the research data files with the reference number 11928-Nov2013. It did not include interaction with human subjects or use personal identifying information. The study did not require informed consent and was approved by the Review Board of Nanjing Medical University, Nanjing, China.
Statistical Analysis
Years of diagnosis, sex, age, race, primary site, pathological grading, histological type, stage, tumor size, survival time, and LCSS were extracted from the SEER database. Young patients with LC were divided into 2 age groups: Group 1 (≤35 years) and Group 2 (36–45 years). The primary endpoint of this study was LCSS, which was calculated from the date of diagnosis to the date of cancer-specific death. Deaths attributed to the LC of interest were treated as events and deaths from other causes were treated as censored observations. Statistical association of age with clinicopathological parameters was analyzed by χ2 test. Survival curves were generated using Kaplan–Meier estimates, and differences between the curves were analyzed by log-rank test. Multivariable Cox regression models were constructed for analysis of risk factors for survival outcomes. All of the statistical analyses were done using SPSS for Windows version 17 (Chicago, IL). Results were considered statistically significant when a 2-tailed test achieved P < 0.05.
RESULTS
Patient Characteristics
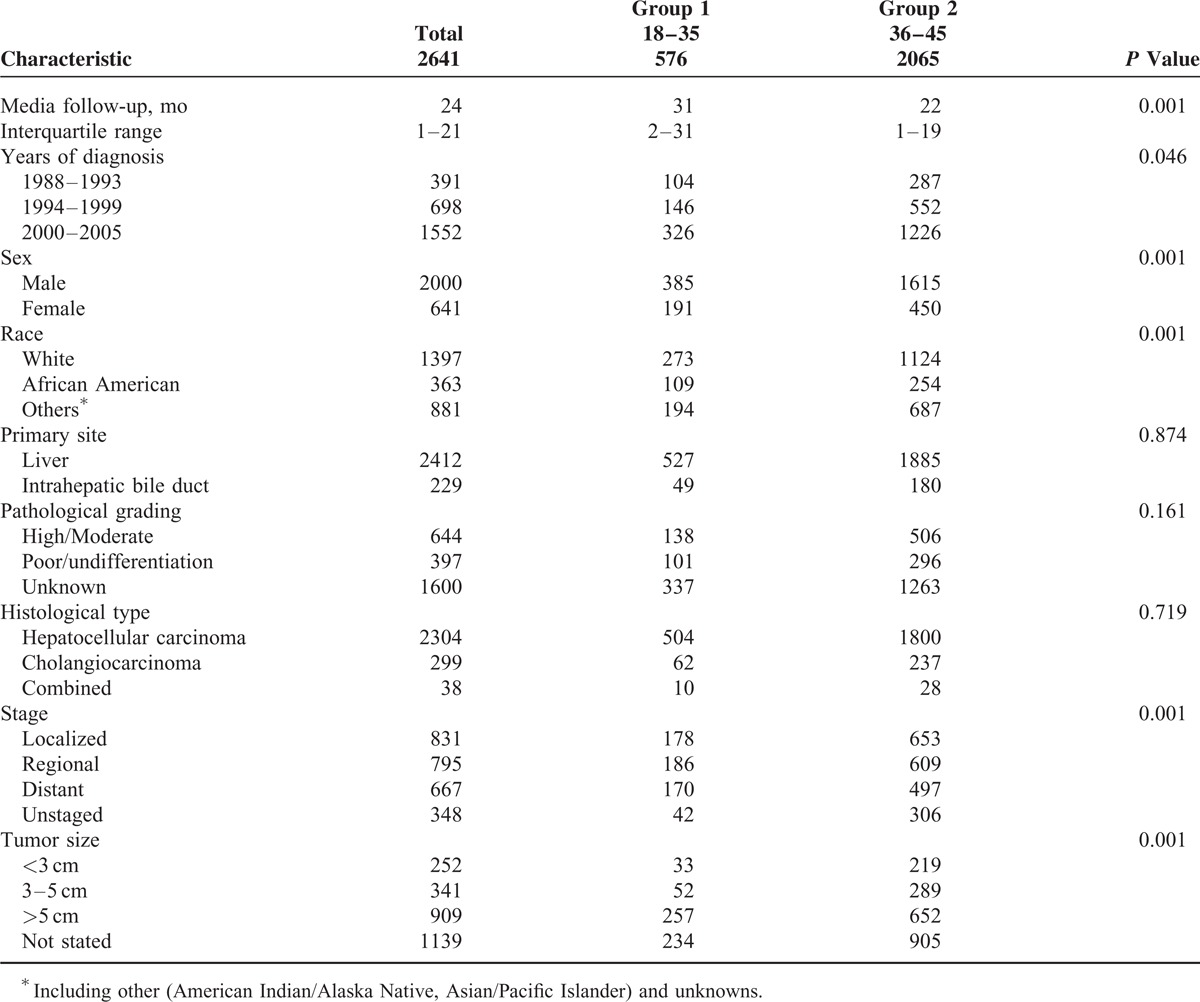
There were 2641 cases of LC diagnosed between 1988 and 2005 in the SEER database. At first, as shown in Table S1, http://links.lww.com/MD/A233, these young patients were divided into 4 age groups: 294 patients in Group I (≤30 years), 282 in Group II (31–35 years), 610 in Group III (36–40 years), and 1455 in Group IV (41–45 years). However, no differences in LCSS were observed between Groups I and II, or Groups III and IV (Figure 1A). Thus, Groups I and II were merged into Group 1 (≤35 years) and Groups III and IV were merged into Group 2 (36–45 years) to increase patient numbers. There were 2000 (75.7%) male and 641 (24.3%) female patients, which was consistent with epidemiological results in which men have higher LC rates than women. The median age was 39 years. The median follow-up period was 24 months. Patient demographics and pathological features are summarized in Table 1.
FIGURE 1.
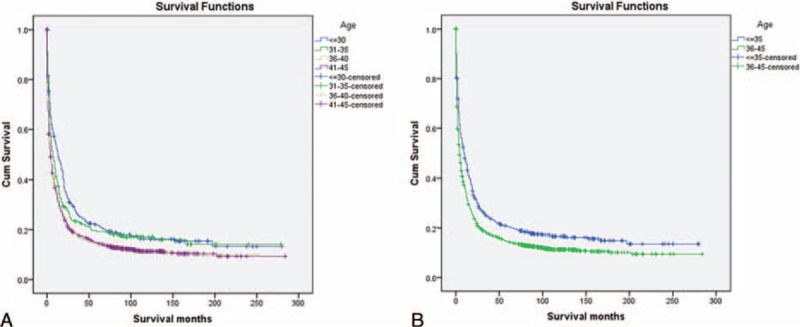
Survival curves in patients with liver cancer according to different age groups. A: Group I versus Group II, χ2 = 2.243, P = 0.134; Group I versus Group III, χ2 = 17.304, P < 0.001; Group I versus Group IV, χ2 = 22.360, P < 0.001; Group II versus Group III, χ2 = 5.474, P = 0.019; Group II versus Group IV, χ2=8.469, P = 0.004; Group III versus Group IV, χ2 = 0.252, P = 0.616. (B) Group 1 versus Group 2, χ2 = 26.990, P < 0.001.
TABLE 1.
Characteristics of Patients From SEER Database by Age
Clinicopathological Differences Between the Groups
As illustrated in Table 1, there were significant differences between the 2 groups, including years of diagnosis (more frequent in 2000–2005, P = 0.046), sex (more frequent in women, P < 0.001), race (less frequent in whites, P < 0.001), stage (less localized, P < 0.001), and tumor size (<3 cm, P < 0.001). With regard to primary site (P = 0.874), pathological grading (P = 0.161), and histological type (P = 0.719), no significant differences were found. With respect to the median follow-up, there was a significant difference between the 2 groups (P < 0.001).
Impact of Age on LC Survival Outcomes
Univariate log-rank test showed that the overall 5-year LCSS was 20.4% in Group 1 and 14.5% in Group 2 (P < 0.001) (Figure 1B). Moreover, male sex, early year of diagnosis (1988–1993), African–American race, poor/undifferentiation grade, advanced stage, and larger tumor size were regarded as significant risk factors for poor prognosis, by univariate analysis (P < 0.001) (Table 2). Multivariate analysis was also performed by Cox regression model. We identified the following 7 factors as independent prognostic factors (Table 3): year of diagnosis, sex, age, race, pathological grading, stage, and tumor size. Patients aged ≤35 years experienced a significantly lower liver cancer-specific mortality (LCSM) compared with the patients aged 36 to 45 years (hazard ratio [HR] 1.436, 95% confidence interval [CI] 1.293–1.596, P < 0.001; patients aged 18–35 years as the reference). Patients with tumors <3 cm also experienced a significantly lower LCSM compared with tumors 3 to 5 cm (HR 1.516, 95% CI 1.241–1.852, P < 0.001; <3 cm as the reference) and >5 cm (HR 1.939, 95% CI 1.622–2.317, P < 0.001; <3 cm as the reference).
TABLE 2.
Univariate Survival Analyses of Patients With LC According to Various Clinicopathological Variables
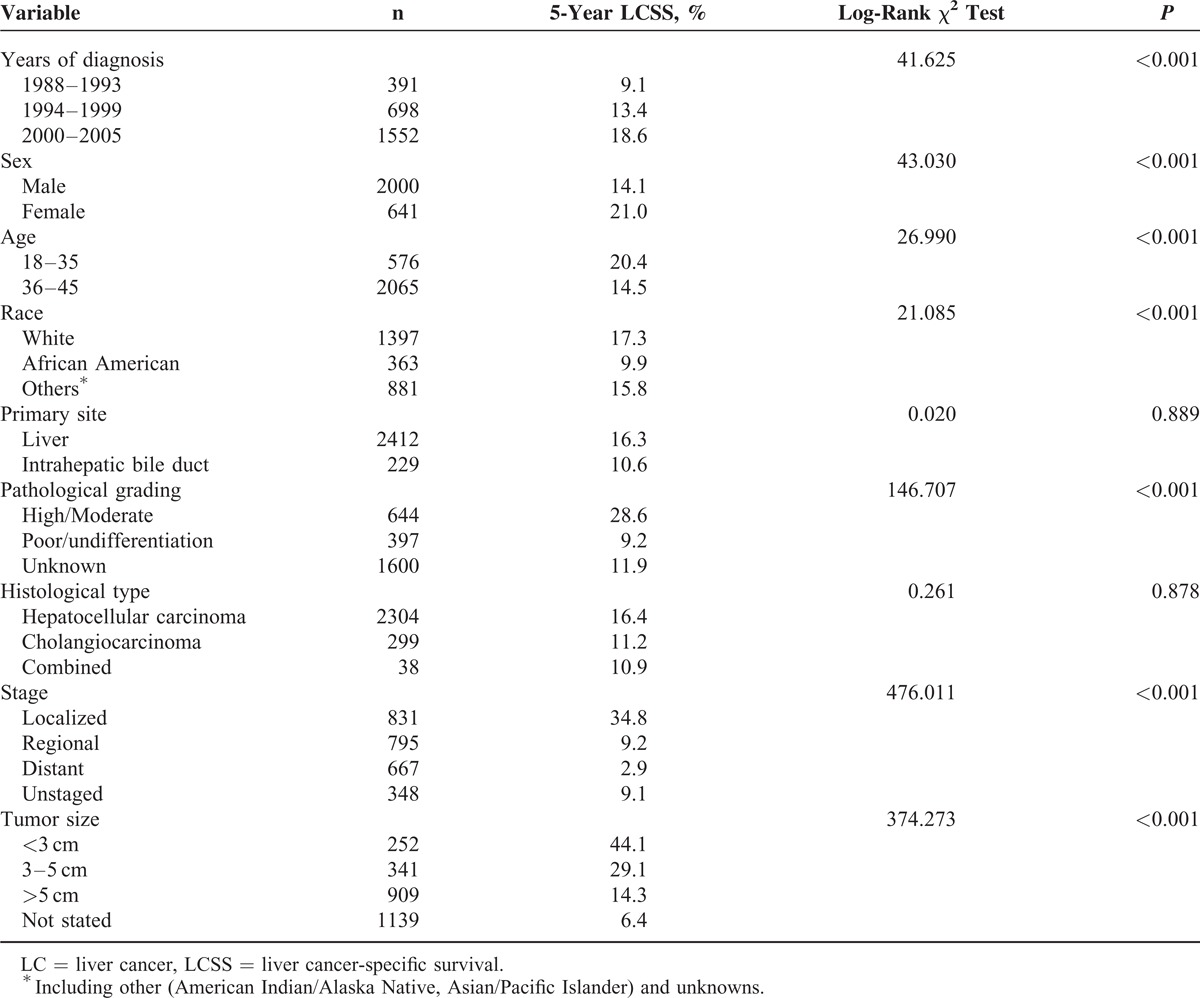
TABLE 3.
Multivariate Cox Model Analyses of Prognostic Factors of LC
Stratified Analysis of Age on Cancer Survival According to Stage
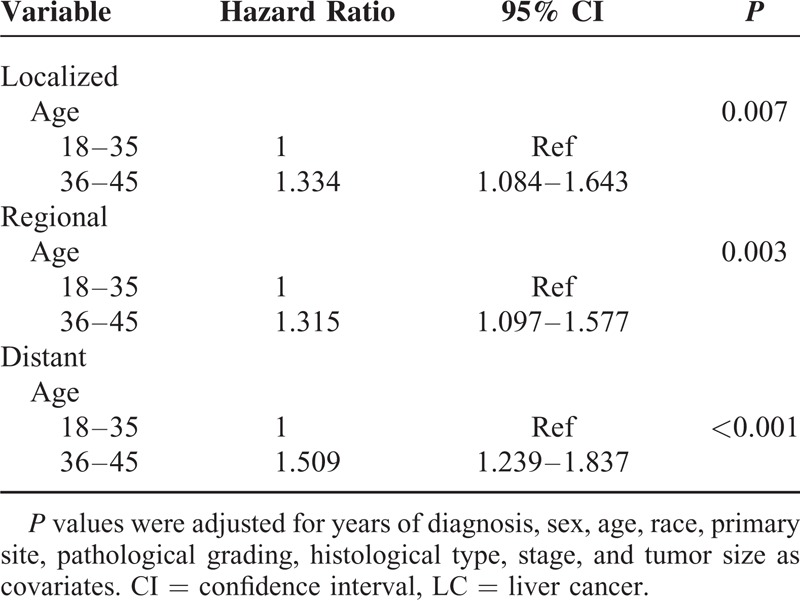
We analyzed whether age was associated with 5-year LCSS at different stages. Univariate analysis showed that patients aged ≤35 years had a better 5-year LCSS than those aged 36 to 45 years with localized (P = 0.040), regional (P = 0.002), and distant-stage (P < 0.001) LC (Table 4). Multivariate Cox regression analyses were performed at different stages and the results showed that age was validated as an independent predictor of survival for localized stage (Group 2, HR 1.334, 95% CI 1.084–1.643), regional stage (Group 2, HR 1.315, 95% CI 1.097–1.577), and distant stage (Group 2, HR 1.509, 95% CI 1.239–1.837) (Table 5).
TABLE 4.
Univariate Analysis of Age on LCSS Based on Different Stages
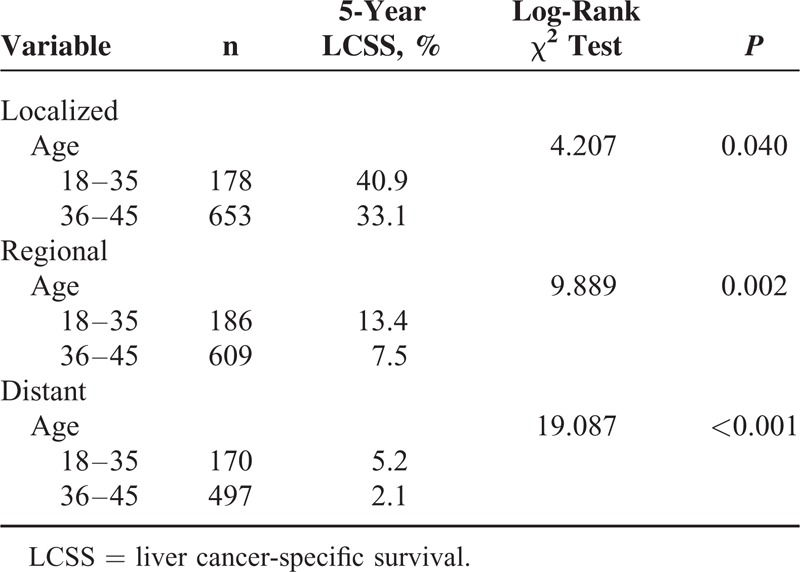
TABLE 5.
Multivariate Cox Model Analyses of Prognostic Factors of LC on Different Stages
DISCUSSION
Although several studies have evaluated the pathological features and survival in young patients with LC,1,13–15 the role of age in the young has not been well characterized. There are still some controversies regarding the prognosis of young patients due to the heterogeneity among these studies. Ni et al9 reported that young patients with LC are considered to have a poorer prognosis compared with elderly patients.10 However, other studies have demonstrated that young patients have better LCSS than elderly patients.11,12 The SEER Program of the NCI also confirms better relative survival rates in young patients.16 These varying results for LC may have been because of the controversy surrounding the definition of young patients with LC. Some studies used 50 years as the cut-off age,15,17 and other studies defined young as 40,18,19 30,20 or even 45 years.21–24 The morbidity of LC is relatively rare and stable until 45 years.25 We defined 45 years as the cut-off for younger age. Moreover, most of the previous studies were based on single-institution experiences or small samples, which makes the conclusions less convincing. Furthermore, young age is consists of wide age ranges, and different subgroups may have different prognosis. Thus, heterogeneity is a characteristic in young patients. Stratified analysis of age groups for CSS and clinicopathological features is meaningful.
In this study, we divided young patients with LC into 4 subgroups: Group I (aged ≤30 years), Group II (31–35 years), Group III (36–40 years), and Group IV (41–45 years). However, there were no significant differences in 5-year LCSS between Groups I and II, as well as Groups III and IV. Thus, Groups I and II were merged into Group 1 (aged ≤35 years) and Groups III and IV were merged into Group 2 (36–45 years) to increase patient numbers. We found fewer patients with localized cancer (21%, 178 of 830) and tumor size <3 cm (13%, 33 of 252) in Group 1. Young patients with LC aged ≤35 years had better long-term survival than patients aged 36–45 years, despite showing advanced tumor stage and poor pathological grading. This confirms our hypothesis that young patients are inherently heterogeneous.
Young patients have more aggressive biological behavior, but it could be compensated by better liver function and faster recovery from surgery, which contribute to longer survival. Univariate and multivariate analysis revealed that young patients with LC (aged ≤35 years) had better 5-year LCSS across several age subgroups.
Our study had some limitations in relation to the data set used. First, the SEER database lacks several important tumor predisposing factors (eg, viral hepatitis, nonalcoholic fatty liver disease, and cirrhosis), cancer therapy (neoadjuvant and adjuvant), as well as α-fetoprotein levels, which could not be adjusted by our analyses. Second, despite our study being based on a large population and multicenter, individual subgroups became small after stratifying by age, yielding limited statistical power. Third, the SEER database only includes patients who have undergone surgical resection for LC; however, these patients cannot represent LC patients who have unresectable tumors. Importantly, because of economic, religious, or poor physical conditions, older patients are more likely to forgo aggressive treatment (chemotherapy and surgery), which may have contributed to poor survival in the older patients. Still, our study was based on a large population and the data was multicenter in origin, was and hence convincing.
In conclusion, our analysis reveals that the group of young patients with LC aged ≤45 years is essentially heterogeneous. Patients aged ≤35 years have better LCSS than those aged 36 to 45 years, despite having advanced tumor stage and poor pathological grading.
Footnotes
Abbreviations: CSS = cancer-specific survival, LC = liver cancer, LCSS = liver cancer-specific survival, SEER = Surveillance, Epidemiology, and End-Results.
This work was supported by grants from the National Natural Science Foundation for Distinguished Young Scholars (81225017 to BS), National Basic Research Program of China (2012CB910800 to BS), and National Natural Science Foundation (81302106 to JH; 81201528 to RJ). This work was also supported in part by the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, and the Priority Academic Program of Jiangsu Higher Education Institutions.
B.S. is Yangtze River Scholars Distinguished Professor.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol 2012; 13:817–826. [DOI] [PubMed] [Google Scholar]
- 2.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010; 14:249–258. [PubMed] [Google Scholar]
- 3.Venook AP, Papandreou C, Furuse J, et al. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010; 15 suppl 4:5–13. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45:529–538. [DOI] [PubMed] [Google Scholar]
- 5.Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol 2014; 28:753–770. [DOI] [PubMed] [Google Scholar]
- 6.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011; 103:714–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004; 127 (5 suppl 1):S27–34. [DOI] [PubMed] [Google Scholar]
- 9.Ni YH, Chang MH, Hsu HY, et al. Hepatocellular carcinoma in childhood. Clinical manifestations and prognosis. Cancer 1991; 68:1737–1741. [DOI] [PubMed] [Google Scholar]
- 10.Lee CL, Ko YC. Survival and distribution pattern of childhood liver cancer in Taiwan. Eur J Cancer 1998; 34:2064–2067. [DOI] [PubMed] [Google Scholar]
- 11.Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer 1994; 74:2772–2780. [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka N, Okamoto E, Oriyama T, et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg 1994; 219:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YT, Jen CL, Yang HI, et al. Lifetime risk and sex difference of hepatocellular carcinoma among patients with chronic hepatitis B and C. J Clin Oncol 2011; 29:3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 15.Wong VW, Chan SL, Mo F, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 2010; 28:1660–1665. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. 2007; Bethesda, MD: National Cancer Institute, SEER Program; NIH Pub. No. 076215. [Google Scholar]
- 17.Kim SS, Hwang JC, Lim SG, et al. Effect of virological response to entecavir on the development of hepatocellular carcinoma in hepatitis B viral cirrhotic patients: comparison between compensated and decompensated cirrhosis. Am J Gastroenterol 2014; 109:1223–1233. [DOI] [PubMed] [Google Scholar]
- 18.Sarpel U, Ayo D, Lobach I, et al. Inverse relationship between cirrhosis and massive tumours in hepatocellular carcinoma. HPB (Oxford) 2012; 14:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai FC, Liu CJ, Chen CL, et al. Lower serum viral loads in young patients with hepatitis-B-virus-related hepatocellular carcinoma. J Viral Hepat 2007; 14:153–160. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Choi MS, Lee H, et al. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol 2006; 21:588–594. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B Management and treatment in a large academic medical center. Am J Gastroenterol 2014; 109:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do AL, Wong CR, Nguyen LH, et al. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol 2014; 48:644–649. [DOI] [PubMed] [Google Scholar]
- 23.Furuta T, Kanematsu T, Matsumata T, et al. Clinicopathologic features of hepatocellular carcinoma in young patients. Cancer 1990; 66:2395–2398. [DOI] [PubMed] [Google Scholar]
- 24.Huo TI, Wu JC, Lee PC, et al. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology 1998; 28:231–236. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014; 60:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]


