Supplemental Digital Content is available in the text
Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with risk factors for cardiovascular disease. The cardioankle vascular index (CAVI), a new measure of arterial stiffness, was recently developed and is independent of blood pressure. We investigated whether NAFLD is associated with arterial stiffness as measured using the CAVI in an apparently healthy population.
A total of 2954 subjects without any known liver diseases were enrolled. NAFLD was diagnosed via typical ultrasonography. The clinical characteristics examined included age, sex, body mass index (BMI), waist circumference (WC), and the levels of aspartate aminotransferase, alanine aminotransferase, total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol triglycerides, and glucose. Arterial stiffness was defined using an age- and sex-specific threshold of the upper quartile of the CAVI.
NAFLD was found in 1249 (42.3%) of the analyzed subjects. Using an age-, sex-, and BMI-adjusted model, NAFLD was associated with a 42% increase in the risk for arterial stiffness (highest quartile of the CAVI). The risk for arterial stiffness increased according to the severity of NAFLD (adjusted odds ratio [95% confidence interval], 1.27 [1.02 − 1.57] vs 1.78 [1.37 − 2.31], mild vs moderate-to-severe, respectively). When adjusted for other risk factors, including BMI, WC, smoking status, diabetes, and hypertension, these relationships remained statistically significant.
Patients with NAFLD are at a high risk for arterial stiffness regardless of classical risk factors. The presence of cardiometabolic risk factors may attenuate the prediction of arterial stiffness by means of NAFLD presence. Thus, physicians should carefully assess subjects with NAFLD for atherosclerosis and associated comorbidities.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease with an estimated prevalence of 20% to 30% in the West and 16% to 33% in Korea.1–3 Because the development of NAFLD has been linked to insulin resistance and metabolic syndrome,4–6 NAFLD is closely associated with obesity, dyslipidemia, type II diabetes, and cardiovascular disease.7–9 Evidences suggest that the severity of NAFLD is associated with the extent of increased cardiovascular risk, independent of conventional risk factors.10,11
Arterial stiffness has been established as a surrogate marker for the prognosis of cardiovascular disease.12 Arterial stiffness is a strong predictor of future cardiovascular events and all-cause mortality, and is among the earliest detectable manifestations of adverse structural and functional changes to blood vessel walls. Increased arterial stiffness is found in patients with cardiometabolic risk factors including hypertension13 and metabolic syndrome.14 The association between arterial stiffness and NAFLD has been reported.15–17 Many methods such as pulse wave velocity (PWV), the augmentation index, and the β-stiffness index have been designed to assess arterial stiffness.18 However, most of these approaches have the drawback of affecting blood pressure during measurement. Additionally, β-stiffness is limited in that it is applicable to only a local segment of the artery.
The cardioankle vascular index (CAVI) is a new index representing the stiffness of entire arterial segments from the aorta to the ankle; it is independent of the blood pressure at the time of the measurement.19 The CAVI is highly reproducible and easy to measure.20,21 The CAVI has been demonstrated as a superior index of arterial stiffness compared with previously established parameters, such as brachial-ankle PWV, and displays good correlations with left ventricular diastolic indices and lipid profiles in patients with angina pectoris.22 Associations between CAVI and coronary atherosclerosis,23 cardiac function,24,25 hypertension,26 and stroke27 have been shown. However, no data regarding the association between CAVI and NAFLD have been reported. In this study, we aimed to evaluate the association between NAFLD and arterial stiffness using the CAVI in the apparently healthy general population.
PATIENTS AND METHODS
Study Population
A cross-sectional study was conducted to evaluate the association between NAFLD and the CAVI. The participants who underwent abdominal ultrasonography and the CAVI on the same day at the Seoul National University Hospital's Gangnam Healthcare Center in Seoul, Korea, for routine health checkups from 2010 to 2013 were recruited. Most of the study population voluntarily paid for their health checkups, whereas others were supported by their company. Patients with previous peripheral artery disease, an ankle–brachial index <0.9, or a history of clinically significant valvular heart disease were excluded from CAVI analysis.
Of a total of 119 subjects who were positive for hepatitis B virus, 36 subjects who were positive for hepatitis C virus and 448 subjects with a history of alcohol consumption (>30 g/d for males and >20 g/d for females) or had a history of other types of hepatitis were excluded. Finally, 2954 subjects were enrolled in this study. Ethical approval for this study was obtained from the institutional review board of the Seoul National University Hospital with an informed consent waiver prior to the study.
Clinical and Laboratory Assessments
Each subject completed a questionnaire on past medical history and lifestyle. Current smokers were defined as having smoked at least 1 cigarette per day during the previous year. Former smokers were defined as prior regular cigarette smokers.28 All subjects received an anthropometric assessment and the laboratory and radiologic tests on the same day. Body weight and height were measured using a digital scale, and body mass index (BMI) was calculated as the weight (kilogram) divided by the height (meter) squared. Waist circumference (WC) was measured at the midpoint between the lower costal margin and the anterior superior iliac crest by a well-trained individual using a tape measure. Systolic and diastolic blood pressures were measured twice, and the average values were recorded. Hypertension was defined as treatment with an antihypertensive drug, a systolic blood pressure >140 mm Hg, or a diastolic blood pressure >90 mm Hg.
Blood samples were collected before 10:00 am after a 12-hour overnight fast. All laboratory tests were performed using standard laboratory methods. Laboratory tests included alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, triglycerides, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, fasting glucose, hepatitis B surface antigen, and hepatitis C virus antibody levels. Diabetes mellitus was defined as either a fasting serum glucose level ≥126 mg/dL or the use of blood glucose-lowering agents.
Assessment of NAFLD
NAFLD was defined as the presence of fatty liver disease as determined via ultrasonography in the absence of the following: a positive serologic marker for hepatitis B surface antigen or hepatitis C virus serological marker, excessive alcohol intake (>30 g/d for males and >20 g/d for females), medications known to produce fatty liver disease, and other specific hepatic disease. Ultrasonographic examination of the liver was performed by experienced radiologists blinded to the patients’ clinical characteristics. The diagnosis of fatty liver was performed via ultrasonography (Acuson, Sequoia 512; Siemens, Mountain View, CA) using previously described standardized categories as follows29: normal, normal echogenicity; mild, slight diffuse increase in bright homogenous echoes in the liver parenchyma, with normal visualization of the diaphragm and the portal and hepatic vein borders, and normal hepatorenal contrast if echogenic; moderate, diffuse increase in bright echoes in the liver parenchyma, with slightly impaired visualization of the peripheral portal and hepatic vein borders; severe, marked increase in bright echoes at a shallow depth, with deep attenuation and impaired visualization of the diaphragm and marked vascular blurring.
The CAVI Measurement
The CAVI was measured using a VaSera VS-1000 (Fukuda Denshi Co Ltd, Tokyo, Japan) according to previous descriptions.20,30 Briefly, the brachial pulse pressure was measured with an automated cuff oscillometer on seated individuals following a 5-minute rest. The average value of 2 measurements was obtained to determine the systolic and diastolic pressures and pulse pressure. Next, the cuffs were applied to ankles and both upper arms with the individuals in a resting lying position. After 10 minutes of rest, the measurement was performed. A phonocardiogram used for the detection of heart sounds was placed over the right sternum between the second intercostal spaces, and electrocardiogram electrodes were applied on both wrists. The PWV was calculated as the vascular length (L) by the time (T) required for the pulse wave to propagate from the aortic valve to the ankle. Because the initiation of blood release from the aortic valve is difficult to identify based on the opening sound of the valve, T is difficult to determine; thus, T value was defined as summing the interval between the initiation of the brachial pulse waveform and the initiation of the ankle pulse waveform, and the interval between the closing sound of the aortic valve and the notch of the brachial pulse waveform. Measurements were performed by a well-trained staff member. The CAVI was determined using the following equation: CAVI = a([2p/ΔP] × ln [Ps/Pd] × PWW2) + b
where Ps and Pd are the systolic and diastolic blood pressures, respectively, ΔP is Ps–Pd, ρ is the blood density, and a and b are constants. The mean values of the left and right CAVI were used. Given the lack of data regarding an appropriate reference of “arterial stiffness,” we selected the age- (10-year interval) and sex-specific highest quartile of the CAVI as the arterial stiffness group.
Statistical Analysis
Comparisons of continuous variables between the 2 groups were performed using Student t test, and categorical variables were compared using a χ2 test or Fisher exact test. Analysis of variance and analysis of covariance (ANCOVA) were used to compare dependent variables. Logistic regression analysis was used to analyze the association between NAFLD and arterial stiffness while controlling for potential confounders. All statistical analyses were performed using SPSS 19.0 software (SPSS Inc, Chicago, IL). P values <0.05 were considered to be statistically significant.
RESULTS
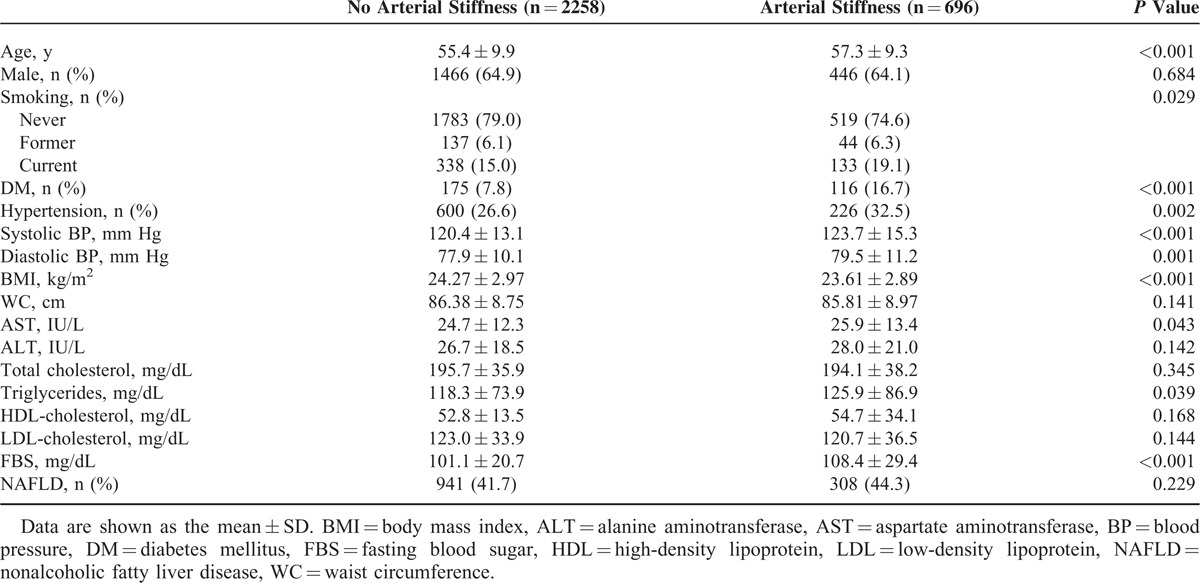
A total of 2954 subjects (mean age 55.5 ± 9.6, male 64.7%) were analyzed. The characteristics of the study subjects are shown in Table 1. Older age, currently smoking, increased prevalence of diabetes mellitus, hypertension, higher blood pressure, and levels of triglycerides and fasting glucose were found in the arterial stiffness group (highest age- and sex-specific quartile of the CAVI) compared with the nonarterial stiffness group. However, BMI was lower in the arterial stiffness group than in the nonarterial stiffness group. There were no differences in the rates of NAFLD depending on the presence of arterial stiffness.
TABLE 1.
Comparison of Baseline Characteristics Between Subjects With and Without Arterial Stiffness
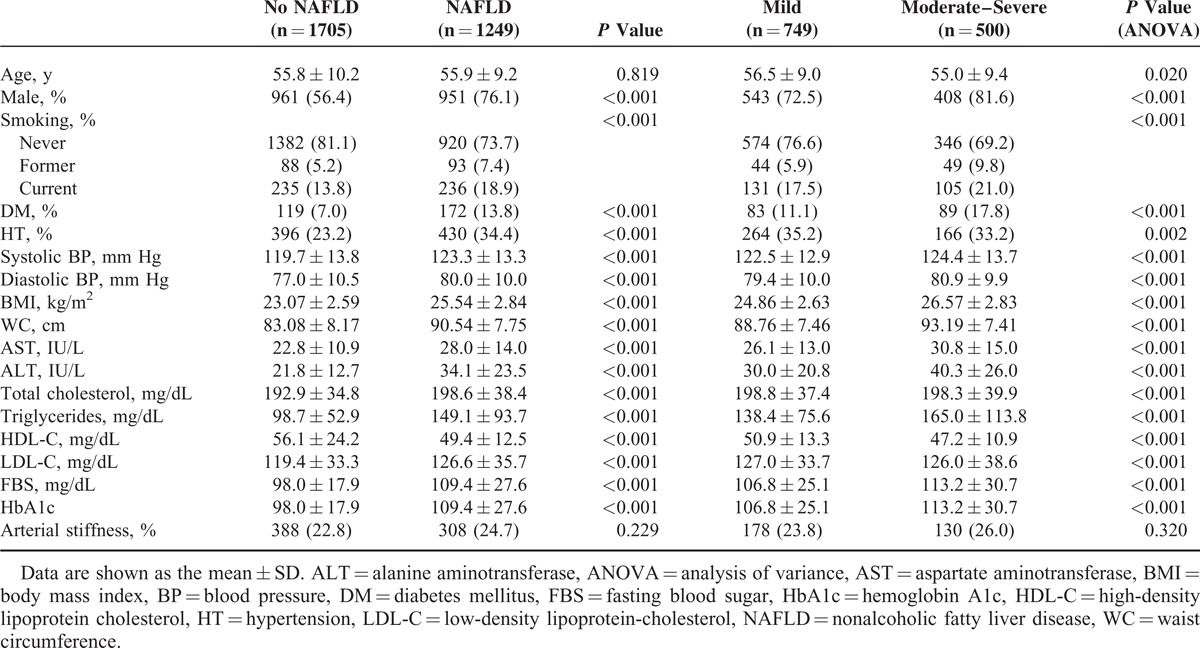
Of the 2954 subjects, 1249 (42.3%) had ultrasonographically diagnosed NAFLD. Table 2 compares the subjects with and without NAFLD. The individuals with NAFLD had a higher prevalence of diabetes mellitus and hypertension, higher blood pressure, BMI, WC, and serum levels of AST, ALT, total cholesterol, triglycerides, LDL-cholesterol, fasting glucose, hemoglobin A1c (HbA1c) and lower levels of HDL-cholesterol than those without NAFLD. Individuals with moderate-to-severe NAFLD had a higher prevalence of diabetes mellitus and hypertension, higher blood pressure, BMI, WC, and serum levels of AST, ALT, total cholesterol, triglycerides, LDL-cholesterol, fasting glucose, and HbA1c, and lower levels of HDL-cholesterol than those with mild NAFLD.
TABLE 2.
Comparison of Baseline Characteristics Between Subjects With and Without NAFLD
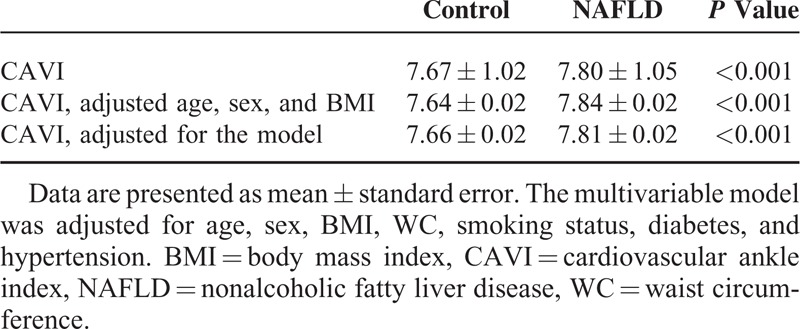
The mean values of the CAVI for the subjects with and without NAFLD are shown in Table 3 and Supplementary Figure 1, http://links.lww.com/MD/A231. The mean value of the CAVI was significantly higher in the individuals with NAFLD than in those without NAFLD (7.80 ± 1.05 vs 7.67 ± 1.02, respectively, P < 0.001), and this difference remained significant after adjusting for multiple metabolic variables such as age, sex, BMI, WC, smoking status, diabetes, and hypertension (7.81 ± 0.02 vs 7.66 ± 0.02, respectively, P < 0.001).
TABLE 3.
CAVI Between Control and NAFLD
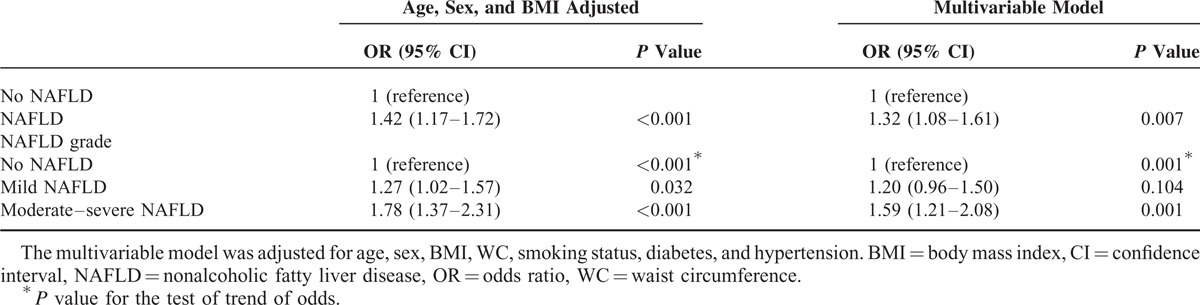
Next, we analyzed the association between NAFLD and the presence of arterial stiffness (highest quartile of the CAVI). The associations between NAFLD and arterial stiffness, as measured using the CAVI, appeared to be robust to the influences of age, sex, and BMI. Based on an age-, sex-, and BMI-adjusted model, NAFLD was associated with a 42% increase in the risk for arterial stiffness. The risk for arterial stiffness increased according to the severity of NAFLD (adjusted odds ratio [OR] [95% confidence interval, CI], 1.27 [1.02 − 1.57] vs 1.78 [1.37 − 2.31], mild vs moderate-to-severe NAFLD, respectively, as shown in Table 4 and Supplementary Table 1, http://links.lww.com/MD/A231). This effect of NAFLD was attenuated, but remained significant based on multivariable analyses in which other well-identified risk factors for arterial stiffness were considered. When adjusted for age, sex, BMI, WC, smoking status, diabetes, and hypertension, NAFLD was associated with a 32% increase in the risk for arterial stiffness compared with the control. The risk for arterial stiffness increased according to the severity of NAFLD (adjusted OR [95% CI], 1.20 [0.96 − 1.50] vs 1.59 [1.21 − 2.08], mild vs moderate to severe NAFLD, respectively).
TABLE 4.
Univariable and Multivariable Binary and Ordinal Analyses of the Risk for Arterial Stiffness in Subjects With Versus Without NAFLD
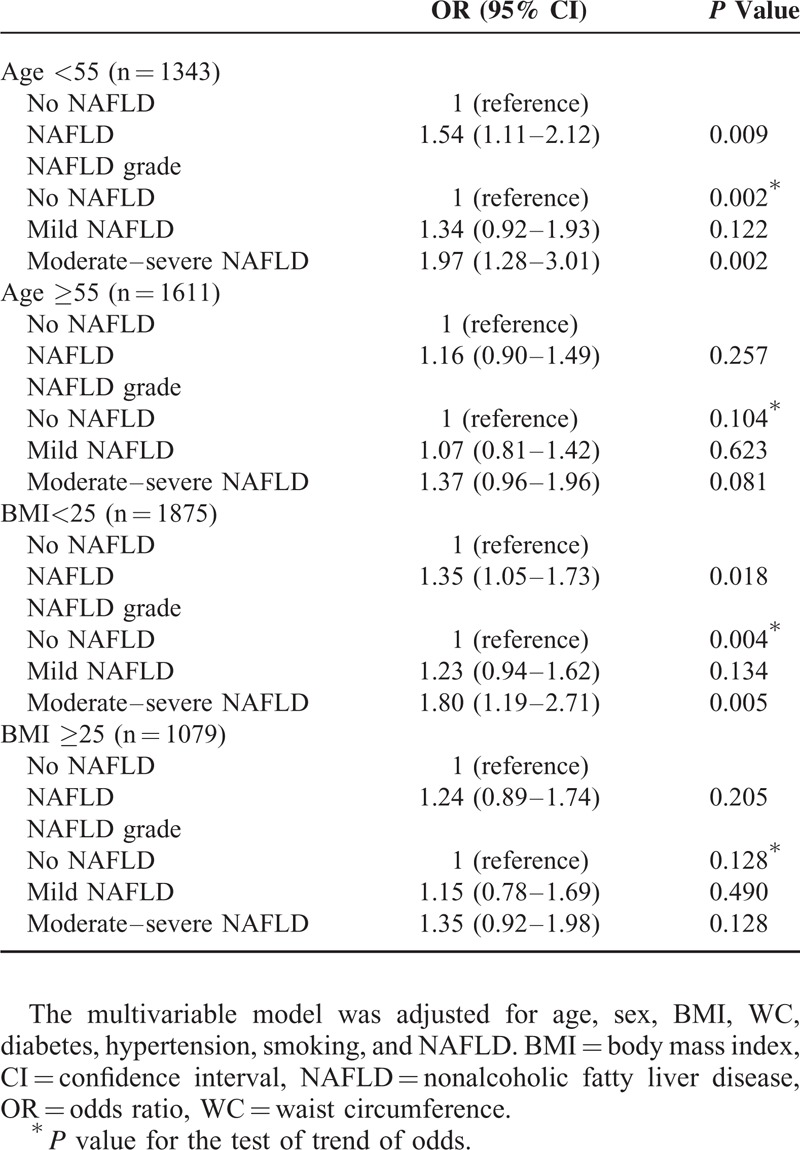
We also analyzed the association between NAFLD and arterial stiffness according to age and obesity (Table 5). Multivariate analyses showed an independent (OR 1.54, 95% CI 1.11–2.12) and dose-dependent relationship (moderate–severe NAFLD: OR 1.97, 95% CI 1.28–3.01, P for trend = 0.002) between NAFLD and arterial stiffness in the younger group (age < 55). In contrast, subjects over 55 years showed an insignificant association with the presence of NAFLD and the degree of NAFLD. Likewise, the presence and degree of NAFLD was associated with arterial stiffness in a dose-dependent manner, especially in the nonobese group (OR 1.35, 95% CI 1.05–1.73; moderate–severe NAFLD: OR 1.80, 95% CI 1.19–2.71, P for trend = 0.004).
TABLE 5.
Multivariate Analysis of the Association Between NAFLD and Risk Factors According to Age and Obesity
DISCUSSION
In this study, we identified a strong relationship between NAFLD and arterial stiffness, a surrogate marker of cardiovascular disease. This association was independent of various well-identified risk factors for arterial stiffness, and canonical risk factors may attenuate the prediction of arterial stiffness by means of the NAFLD presence. Moreover, the risk for arterial stiffness increased with the severity of NAFLD, suggesting an important role for NAFLD in the pathogenesis of arterial stiffness.
Several studies have suggested NAFLD as an independent risk factor of arterial stiffness.31,32 A population-based cohort study of Italian adults showed that arterial stiffness measured using the carotid-femoral PWV was significantly lower in controls than in subjects with NAFLD.31 NAFLD was an independent risk factor of increased PWV in patients with biopsy-proven NAFLD.32 A population-based cohort study of adolescents in Australia showed that NAFLD is only associated with increased arterial stiffness in subjects with adverse metabolic profiles.17 Because subjects with severe fatty liver may have additional risk factors for metabolic syndrome, the findings of our study are in accordance with this previous result. However, it is a novel method of evaluating arterial stiffness, as it is not affected by blood pressure. In addition, our results expand upon the current knowledge by indicating that arterial stiffness increases in accordance with the severity of NAFLD. Consistent with our results, a recent study showed that increased arterial stiffness in patients with NAFLD was related to the histological severity of hepatic fibrosis.33 Although these results were based on histological phenotypes, the previous study was limited by its small sample size. In contrast to our findings, VanWagner et al34 demonstrated that NAFLD was not associated with subclinical atherosclerosis as measured by coronary artery calcification, and Tarantino et al35 also indicated that the severity of hepatic steatosis was not associated with carotid intima media thickness in obese patients. These differences in these results may have been caused by different definitions of NAFLD or heterogeneous study populations.
In previous studies,36,37 smoking has been reported to have a significant effect on arterial stiffness. However, when we analyzed our data, the effect of smoking was more significant in subjects without NAFLD than those with NAFLD. Another interesting finding in our study was that the association between NAFLD and arterial stiffness was significant in the younger and nonobese group. This finding might suggest that NAFLD may affect the earlier stages of arterial stiffness. Consistent with our results, a recent study in China showed a significant association between NAFLD and arterial stiffness in nonobese, nonhypertensive, and nondiabetic young individuals.38
Although the mechanism by which NAFLD is associated with arterial stiffness is yet to be determined, several plausible explanations have been introduced. Excessive fat accumulation in the liver is closely associated with insulin resistance, which correlates to arterial stiffness.39,40 In addition to hepatic fat accumulation, epicardial adipose tissue, which reflects metabolic risk, displays a significant association with arterial stiffness.30 Moreover, an excess of reactive oxygen species may induce the production of cytokines, such as tumor necrosis factor-α and interleukin-6, leading to lipid peroxidation in hepatocytes and resulting in hepatic inflammation in NAFLD.41,42 Furthermore, NAFLD was associated with increased circulating levels and hepatic expression of molecular mediators of atherosclerosis, such as intracellular adhesion molecule and plasminogen activator inhibitor-1, which may exert a direct effect on arterial stiffness.43
A strength of this study is the first use of the CAVI, a reliable marker of arterial stiffness on a large number of subjects considered to be representative of the general population due to the nature of a health checkup. However, there are several limitations to this study. First, the cross-sectional design of the study made it difficult to evaluate the temporal association between NAFLD and arterial stiffness. Second, we were unable to obtain liver histology, the gold standard for diagnosis of NAFLD. Ultrasonography can lead to false-negative results when fatty infiltration of the liver falls below 30%.44 Additionally, there is inter- and intraobserver diagnostic variability in hepatic ultrasonography. However, it is impossible to perform invasive tests in apparently healthy subjects; therefore, ultrasonography is used as a first-line method to detect NAFLD according to clinical practical guidelines.45,46 Third, this study was performed at a single health screening center in Korea, which may result in selection bias.
In conclusion, our findings show an association between NAFLD and arterial stiffness in NAFLD severity-dependent manner. This finding suggests that canonical risk factors for cardiovascular disease may reduce the prediction of arterial stiffness by means of the NAFLD presence. Thus, physicians should carefully assess subjects with NAFLD for atherosclerosis and its associated comorbidities.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CAVI = cardioankle vascular index, HDL = high-density lipoprotein, LDL = low-density lipoprotein, NAFLD = nonalcoholic fatty liver disease, PWV = pulse wave velocity, WC = waist circumference.
GEC and S-YC were responsible for acquisition of data, drafting of the article, and final approval of the submitted version. DK was responsible for conception and design of the study, analysis and interpretation of data, and final approval of the submitted version. MSK, HEP, MKK, and JYY were responsible for acquisition of data and final approval of the submitted version.
GEC and S-YC contributed equally to this study.
This study was supported by grant no. 34-2014-0240 from the SK Telecom Research Fund.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34:274–285. [DOI] [PubMed] [Google Scholar]
- 2.Choi SY, Kim D, Kim HJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol 2009; 104:1953–1960. [DOI] [PubMed] [Google Scholar]
- 3.Bae JC, Cho YK, Lee WY, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol 2010; 105:2389–2395. [DOI] [PubMed] [Google Scholar]
- 4.Almeda-Valdes P, Cuevas-Ramos D, Aguilar-Salinas CA. Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol 2009; 8 suppl 1:S18–S24. [PubMed] [Google Scholar]
- 5.Chen SH, He F, Zhou HL, et al. Relationship between nonalcoholic fatty liver disease and metabolic syndrome. J Dig Dis 2011; 12:125–130. [DOI] [PubMed] [Google Scholar]
- 6.Khashab MA, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease as a component of the metabolic syndrome. Curr Gastroenterol Rep 2008; 10:73–80. [DOI] [PubMed] [Google Scholar]
- 7.Choi SY, Kim D, Kang JH, et al. Nonalcoholic fatty liver disease as a risk factor of cardiovascular disease: relation of non-alcoholic fatty liver disease to carotid atherosclerosis. Korean J Hepatol 2008; 14:77–88. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 2008; 51:1947–1953. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012; 56:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol 2012; 27:420–428. [DOI] [PubMed] [Google Scholar]
- 11.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010; 51:595–602. [DOI] [PubMed] [Google Scholar]
- 12.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006; 113:657–663. [DOI] [PubMed] [Google Scholar]
- 13.Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am J Hypertens 2004; 17:39S–48S. [DOI] [PubMed] [Google Scholar]
- 14.Scuteri A, Cunha PG, Rosei EA, et al. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis 2014; 233:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YJ, Shim JY, Moon BS, et al. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci 2012; 57:196–203. [DOI] [PubMed] [Google Scholar]
- 16.Yu KJ, Zhang MJ, Li Y, et al. Increased whole blood viscosity associated with arterial stiffness in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2014; 29:540–544. [DOI] [PubMed] [Google Scholar]
- 17.Huang RC, Beilin LJ, Ayonrinde O, et al. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology 2013; 58:1306–1314. [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 19.Shirai K, Hiruta N, Song M, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb 2011; 18:924–938. [DOI] [PubMed] [Google Scholar]
- 20.Shirai K, Utino J, Otsuka K, et al. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 21.Kubozono T, Miyata M, Ueyama K, et al. Clinical significance and reproducibility of new arterial distensibility index. Circ J 2007; 71:89–94. [DOI] [PubMed] [Google Scholar]
- 22.Takaki A, Ogawa H, Wakeyama T, et al. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res 2008; 31:1347–1355. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Tomaru T, Yamamura S, et al. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J 2008; 72:598–604. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi T, Doi M, Hirohata S, et al. Cardio-ankle vascular index is independently associated with the severity of coronary atherosclerosis and left ventricular function in patients with ischemic heart disease. J Atheroscler Thromb 2010; 17:249–258. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Ohira M, Iizuka T, et al. Cardio-ankle vascular index relates to left ventricular ejection fraction in patients with heart failure. A retrospective study. Int Heart J 2013; 54:216–221. [DOI] [PubMed] [Google Scholar]
- 26.Okura T, Watanabe S, Kurata M, et al. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res 2007; 30:335–340. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki J, Sakakibara R, Tomaru T, et al. Stroke and cardio-ankle vascular stiffness index. J Stroke Cerebrovasc Dis 2013; 22:171–175. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 29.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750. [DOI] [PubMed] [Google Scholar]
- 30.Park HE, Choi SY, Kim HS, et al. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb 2012; 19:570–576. [DOI] [PubMed] [Google Scholar]
- 31.Salvi P, Ruffini R, Agnoletti D, et al. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio-GOOSE study. J Hypertens 2010; 28:1699–1707. [DOI] [PubMed] [Google Scholar]
- 32.Vlachopoulos C, Manesis E, Baou K, et al. Increased arterial stiffness and impaired endothelial function in nonalcoholic fatty liver disease: a pilot study. Am J Hypertens 2010; 23:1183–1189. [DOI] [PubMed] [Google Scholar]
- 33.Sunbul M, Agirbasli M, Durmus E, et al. Arterial stiffness in patients with non-alcoholic fatty liver disease is related to fibrosis stage and epicardial adipose tissue thickness. Atherosclerosis 2014; 237:490–493. [DOI] [PubMed] [Google Scholar]
- 34.VanWagner LB1, Ning H2, Lewis CE3, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 2014; 235:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarantino G1, Finelli C, Colao A, et al. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma-glutamyl-transferase? J Transl Med 2012; 10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003; 41:183–187. [DOI] [PubMed] [Google Scholar]
- 37.Liang YL, Shiel LM, Teede H, et al. Effects of blood pressure, smoking, and their interaction on carotid artery structure and function. Hypertension 2001; 37:6–11. [DOI] [PubMed] [Google Scholar]
- 38.Yu XY, Zhao Y, Song XX, et al. Association between non-alcoholic fatty liver disease and arterial stiffness in the non-obese, non-hypertensive, and non-diabetic young and middle-aged Chinese population. J Zhejiang Univ Sci B 2014; 15:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang FS, Liu MY, Cheng XL, et al. Insulin resistance correlates with the arterial stiffness before glucose intolerance. Intern Med 2014; 53:189–194. [DOI] [PubMed] [Google Scholar]
- 40.Webb DR, Khunti K, Silverman R, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010; 53:1190–1198. [DOI] [PubMed] [Google Scholar]
- 41.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745–E751. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007; 191:235–240. [DOI] [PubMed] [Google Scholar]
- 43.Sookoian S, Castano GO, Burgueno AL, et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 2010; 209:585–591. [DOI] [PubMed] [Google Scholar]
- 44.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002; 123:1705–1725. [DOI] [PubMed] [Google Scholar]
- 45.The Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol 2013; 19:325–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609. [DOI] [PubMed] [Google Scholar]


