Abstract
The aim of the present article was to evaluate the association of N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) with contrast-induced nephropathy (CIN) and long-term outcomes in patients with chronic kidney disease (CKD) and relative preserved left ventricular function (LVF) undergoing percutaneous coronary intervention (PCI).
We prospectively enrolled 1203 consecutive patients with CKD and preserved LVF undergoing elective PCI. The primary end point was the development of CIN, defined as an absolute increase in serum creatinine (SCr) ≥0.5 mg/dL, from baseline within 48 to 72 hours after contrast medium exposure.
CIN incidence varied from 2.2% to 5.2%. Univariate logistic analysis showed that lg-NT-pro-BNP was significantly associated with CIN (odds ratio [OR] = 3.93, 95% confidence interval [CI], 2.22–6.97, P < 0.001). Furthermore, lg-NT-pro-BNP remained a significant predictor of CIN (OR = 3.30, 95% CI, 1.57–6.93, P = 0.002), even after adjusting for potential confounding risk factors. These results were confirmed by using other CIN criteria, which were defined as elevations of the SCr by 25% or 0.5 and 0.3 mg/dL from the baseline. The best cutoff value of lg-NT-pro-BNP for detecting CIN was 2.73 pg/mL (537 pg/mL) with 73.1% sensitivity and 70.0% specificity according to the receiver operating characteristic (ROC) analysis (C statistic = 0.754, 95% CI, 0.67–0.84, P < 0.001). In addition, NT-pro-BNP ≥537 pg/mL (2.73 pg/mL, lg-NT-pro-BNP) was associated with an increased risk of all-cause mortality and composite end points during 2.5 years of follow-up.
NT-pro-BNP ≥537 pg/mL is independently associated with an increased risk of CIN with different definitions and poor clinical outcomes in patients with CKD and relative preserved LVF undergoing PCI.
INTRODUCTION
Contrast-induced nephropathy (CIN) is a significant and well-known complication in patients undergoing percutaneous coronary intervention (PCI).1 Development of CIN after PCI is associated with poor clinical outcomes including increased rates of end-stage renal failure, and short- and long-term mortality.2 Except for patients with clinical heart failure (HF) or low left ventricular ejection fraction (LVEF <40%),3 CIN also occurs more frequently in patients with chronic kidney disease (CKD) and relative preserved left ventricular function (LVF).4,5 Currently, few agents have proven to be effective for treating CIN; therefore, identifying the patients at high risks of CIN before procedure is urgent, especially in patients with CKD and preserved LVF, as this population receives less attention compared with patients with clinical HF.
N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) is synthesized and secreted from the cardiac ventricular myocardium, in response to increased volume or pressure overload, or myocardial ischemia.6–8 This marker is closely linked to poor hemodynamics, and is triggered by proinflammatory cytokines and activation of the renin–angiotensin–aldosterone system (RAAS), and sympathetic nervous system,9 all of which play an important role in the development of CIN.10 In addition, plasma B-type natriuretic peptide (BNP) is useful for predicting future cardiovascular events in patients with CKD in the general population.11 Furthermore, NT-pro-BNP is strongly associated with prevalent left ventricular (LV) systolic dysfunction in CKD patients without clinical HF.12
Consequently, NT-pro-BNP may serve as a cardiac and renal biomarker that is potentially associated with the pathological processes that lead to CIN. However, little information is available regarding the predictive value of NT-pro-BNP for CIN and long-term outcomes in patients with CKD and relative preserved LVF undergoing PCI. We aimed to investigate whether NT-pro-BNP is a risk factor for CIN and long-term outcomes in these patients.
MATERIALS AND METHODS
Ethics Statement
This study protocol was approved by the Guangdong General Hospital ethics committee and the study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before the procedure.
Patient and Study Design
We prospectively enrolled 1203 consecutive patients with CKD and preserved LVF undergoing PCI at Guangdong Cardiovascular Institute, Guangdong General Hospital, between March 2010 and July 2012. Inclusion criteria included an estimated glomerular filtration rate (eGFR) ≤90 mL/min/1.73 m2, LVEF ≥40%, and heart function classified as New York Heart Association (NYHA) I/II class at clinical assessment. Patients were excluded if they had a history of known chronic HF (NYHA >II), LVEF <40%, acute coronary syndrome (ACS) requiring primary or rescue PCI, cardiogenic shock, pregnancy, contrast medium (CM) allergy, CM exposure within the previous 7 days, or treatment with nephroprotective drugs or nephrotoxic drugs. We also excluded patients with renal transplantation, dialysis, severe hepatic insufficiency, or chronic respiratory problems.
Laboratory Investigations
Serum creatinine (SCr) was measured at admission and within 48 to 72 hours after CM exposure. NT-pro-BNP was measured using a chemoluminescent immunoassay kit (Roche Diagnostics, Grenzach Wyhlen, Germany) at admission (prior to PCI). We also measured blood urea nitrogen, high-sensitivity C-reactive protein (hs-CRP), creatine kinase MB, fasting glucose, electrolytes, fasting lipid profiles, albumin, and other standard clinical parameters on the morning of the day before the procedure. LVF was evaluated in all patients using echocardiography within 24 hours before the procedure. We evaluated eGFR using the 4-variable Modification of Diet in Renal Disease equation for Chinese patients.13
PCI and Medications
PCI was performed by experienced interventional cardiologists according to standard clinical practice using standard technique. We used nonionic, low-osmolar CM in all patients (either Iopamiron or Ultravist, both 370 mg I/mL). In addition, 0.9% normal saline at a rate of 1 mL/kg/h was administered intravenously approximately 12 hours before and 12 to 24 hours after exposure to CM. The use of antiplatelet agents (aspirin/clopidogrel), β-adrenergic blocking agents, statins, angiotensin-converting enzyme inhibitors, and inotropic drugs was at the discretion of cardiologists according to clinical protocols based on interventional guidelines.
Clinical Follow-Up for Outcomes
All patients included in the study were subject to follow-up for ≥1 year. Follow-up events were carefully monitored and recorded by trained nurses using either outpatient clinical visits or telephone contact with the patients or their relatives after discharge.
The primary end point was the development of CIN, defined as an absolute increase in SCr ≥0.5 mg/dL from baseline within 48 to 72 hours after CM exposure (CIN0.5).14 Additional end points included other CIN criteria, which were defined as elevations of the SCr by 25% or 0.5 mg/dL (CIN0.5 or 25%) and 0.3 mg/dL (CIN0.3) from the baseline,15 all-cause mortality, and composite end points, including all-cause mortality, renal replacement therapy, nonfatal myocardial infarction, acute HF, target vessel revascularization, or cerebrovascular accident during in-hospital as well as follow-up.
Statistical Analysis
SPSS software (version 16.0; SPSS Inc, Chicago, IL) was used for all analyses. The continuous variables are described as the mean ± standard deviation or median. The categorical variables are expressed as absolute values (percentages). Comparisons of difference between groups were performed using Student t test or Wilcoxon rank sum test for continuous variables and χ2 or Fisher exact test for categorical variables. Because of the skewed distribution of admission NT-pro-BNP and hs-CRP concentrations, logarithmic transformation was performed. Multivariate logistic regression analysis was performed to determine if NT-pro-BNP is an independent predictor of CIN with different definitions after adjustment for significant clinical variables associated with CIN. The adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated. Receiver operating characteristic (ROC) curve analysis was conducted to determine the best cutoff value of NT-pro-BNP for detecting composite end points and CIN especially for CIN0.5, which is more sensitive because it more selectively recognizes those patients with a higher risk of mortality and morbidity.14,16 Differences in the area under the curve (AUC) between NT-pro-BNP and Mehran risk score were compared using MedCalc statistical software (MedCalc Software bvba, version 12.7.10, Ostend, Belgium). Cox proportional hazards regression was performed to evaluate independent predictors of major clinical adverse events (MACEs) by adjusting for factors that were significantly associated with clinical outcomes (including traditional risk factors, LVEF, and NT-pro-BNP). Survival analysis was performed using the Kaplan–Meier method and survival differences between patients with lg-NT-pro-BNP levels ≥2.73 or <2.73 pg/mL were compared using the log-rank test. All statistical tests were 2-tailed and statistical significance was accepted if P < 0.05.
RESULTS
Baseline Clinical Characteristics and In-Hospital Clinical Events
A total of 1203 consecutive patients with CKD and preserved LVF were analyzed (mean age 65.2 ± 10.3 years; mean lg-NT-pro-BNP 2.3 ± 0.7 pg/mL; mean eGFR 69.3 ± 15.4 mL/min/1.73 m2 and mean Mehran score 4.5 ± 3.5). Overall, CIN was observed in 63 patients using CIN0.5 or 25% (5.2%), 26 (4.8%) using CIN0.3, and 25 (2.2%) using CIN0.5. Compared with patients with 60 < eGFR ≤ 90 mL/min/1.73 m2, the incidence of CIN was more commonly observed in patients with eGFR ≤60 mL/min/1.73 m2 (CIN0.5 or 25%: 8.5% vs 4.1%, CIN0.3: 12.0% vs 2.3%; CIN0.5: 5.4% vs 1.0%, all P < 0.05).
Patients with CIN were older, more likely complicated with hypoalbuminemia, and displayed relatively lower eGFR and LVEF, compared with patients without CIN. Increased NT-pro-BNP and hs-CRP levels, as well as increased Mehran CIN risk scores, were more prevalent in patients with CIN than in patients without CIN. However, the prevalence of hypertension, diabetes, anemia, smoking, and CM volume did not significantly differ between groups (Table 1).
TABLE 1.
Baseline Clinical Features in Patients With and Without CIN
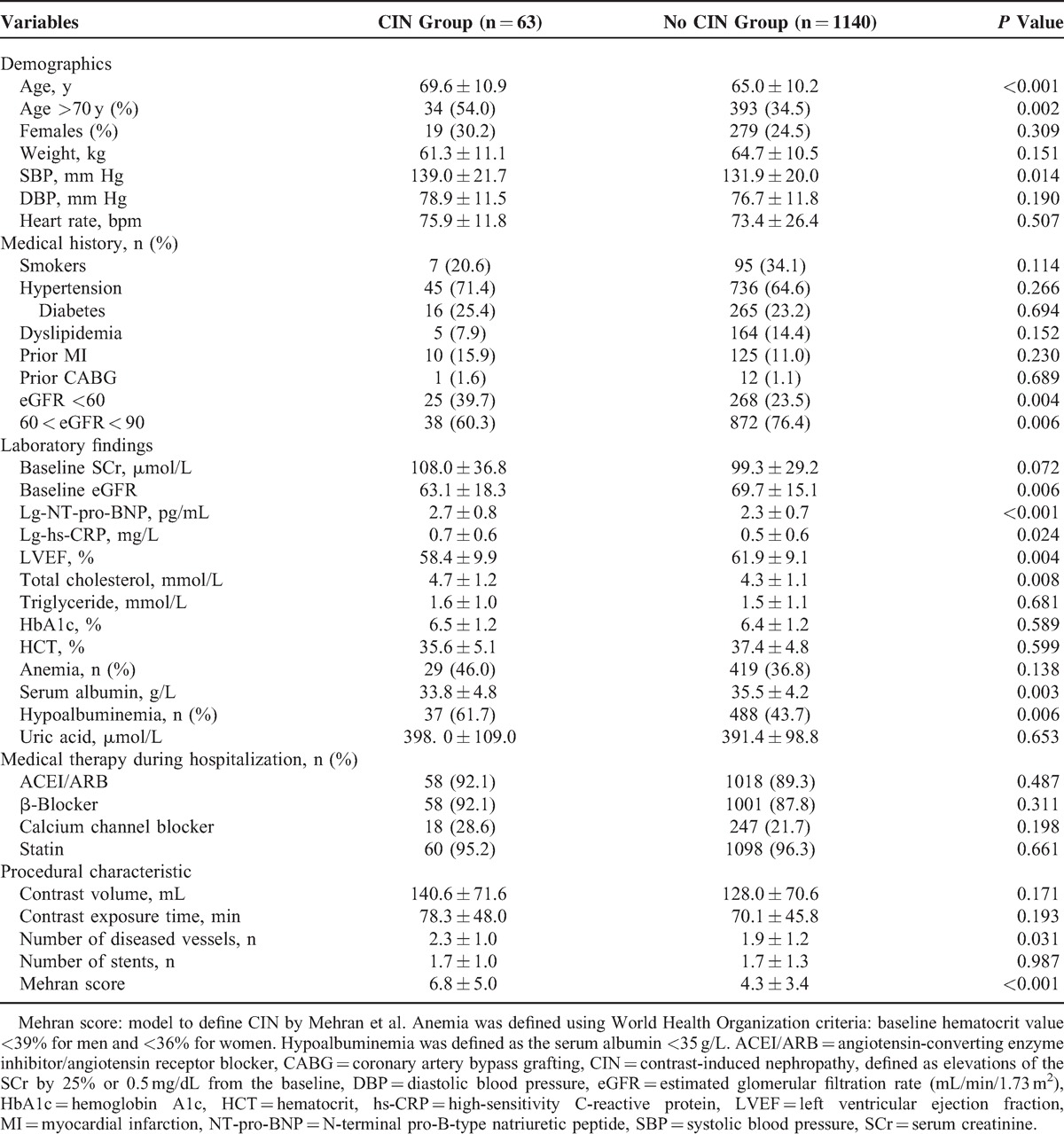
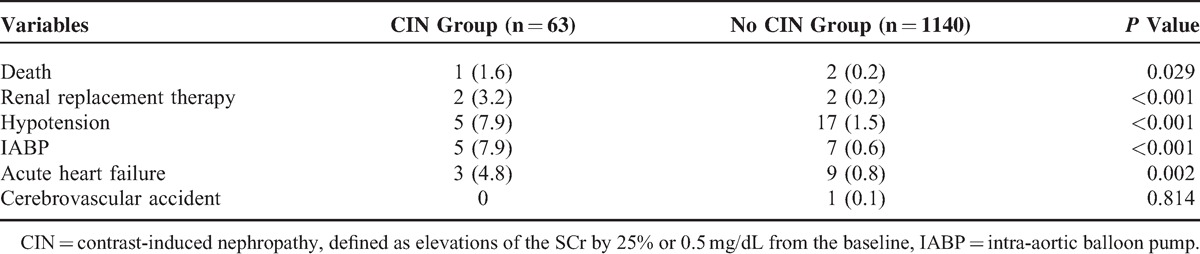
Compared with patients without CIN, patients with CIN exhibited a significantly higher rate of in-hospital mortality (1.6% vs 0.2%, P = 0.029), and other in-hospital complications, such as requirement for renal replacement therapy (3.2% vs 0.2%, P < 0.001), intra-aortic balloon pump (7.9% vs 0.6%, P < 0.001), and acute HF (4.8% vs 0.8%, P = 0.002) (Table 2).
TABLE 2.
In-Hospital Events in Patients With and Without CIN
Role of NT-Pro-BNP in CIN
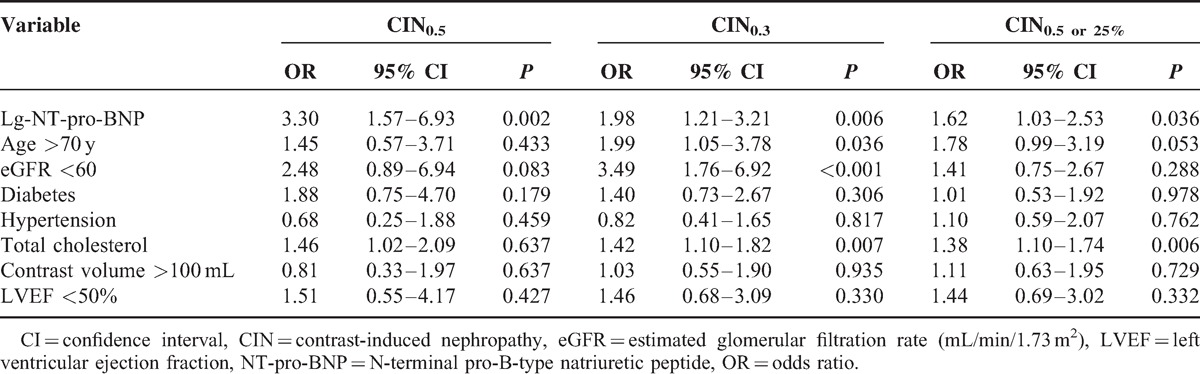
Using univariate logistic regression analysis, lg-NT-pro-BNP was significantly associated with CIN (CIN0.5: OR = 3.93, 95% CI, 2.22–6.97, P < 0.001; CIN0.5 or 25%: OR = 2.09, 95% CI, 1.46–2.98, P < 0.001; CIN0.3: OR = 2.93, 95% CI, 2.00–4.29, P < 0.001). Additional significant variables included older age, eGFR <60 mL/min/1.73 m2, LVEF <50%, and total cholesterol. Multivariate logistic regression analysis showed that lg-NT-pro-BNP remained a strong significant predictor of CIN (CIN0.5: OR = 3.30, 95% CI, 1.57–6.93, P = 0.002; CIN0.5 or 25%: OR = 1.62, 95% CI, 1.03–2.53, P = 0.036; CIN0.3: OR = 1.98, 95% CI, 1.21–3.21, P = 0.006), even after adjusting for potential confounding risk factors. Age >70 years and eGFR <60 mL/min/1.73 m2 were also independent predictor of CIN0.3 in this population (OR = 1.99, 95% CI, 1.05–3.78, P = 0.036; OR = 3.49, 95% CI, 1.76–6.92, respectively, P < 0.001) (Table 3).
TABLE 3.
Multivariate Logistic Analysis Associating CIN Risk Indicators
ROC analysis revealed that the AUC of the predictive performance of CIN0.5, based on the previous risk score system suggested by Mehran et al,17 was 0.790 (95% CI, 0.69–0.89, P < 0.001), whereas the AUC (0.754, 95% CI, 0.67–0.84, P < 0.001) for NT-pro-BNP levels was similar to the Mehran risk score (P = 0.508). Similar results were demonstrated for other definitions of CIN (Table 4). Moreover, NT-pro-BNP and the Mehran risk score for composite end point display similar good predictive value (AUC: 0.716, 95% CI, 0.64–0.80, P < 0.001; 0.754, 95% CI, 0.66–0.85, P < 0.001, respectively, P = 0.359). In addition, the best cutoff lg-NT-pro-BNP value for detecting CIN0.5 was 2.73 pg/mL with 73.1% sensitivity and 70.0% specificity (Figure 2).
TABLE 4.
AUC of NT-Pro-BNP and Mehran Risk Score for CIN
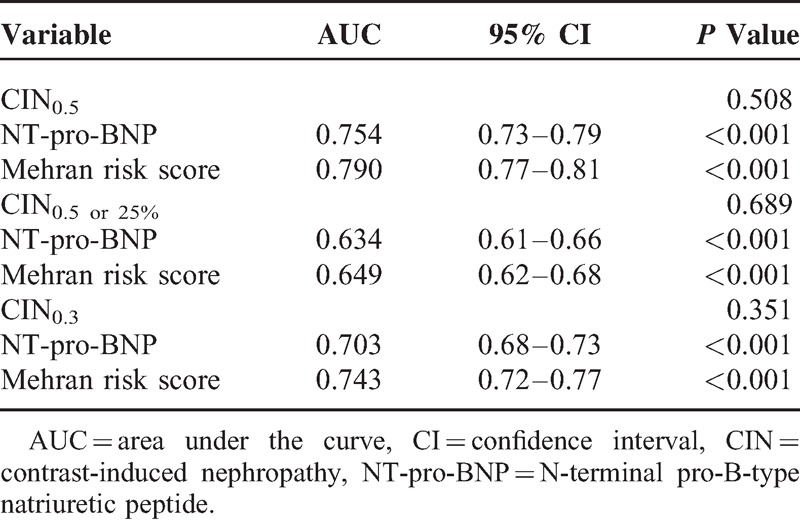
FIGURE 2.
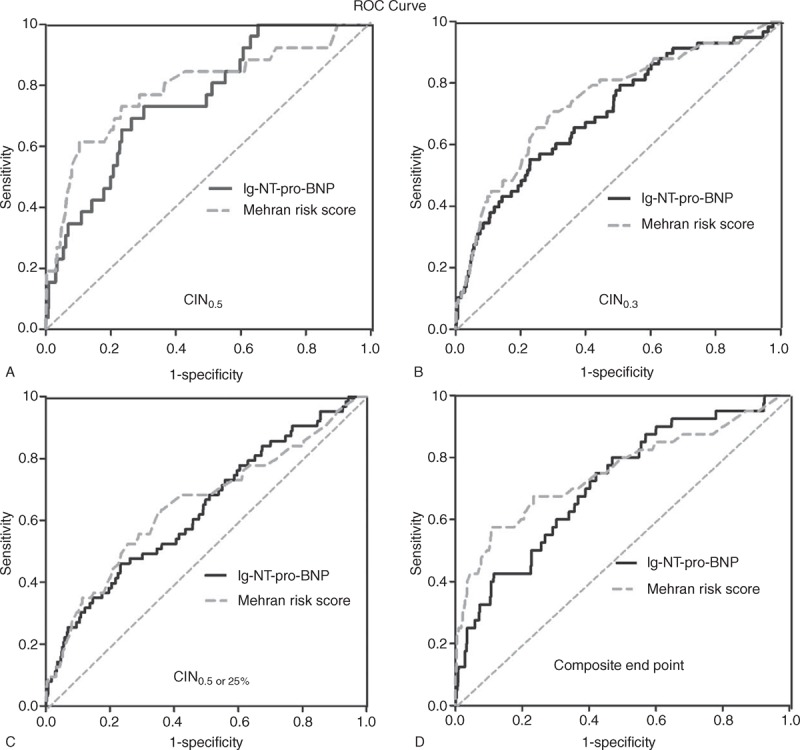
The ROC curve for NT-pro-BNP and Mehran risk score in order to predict (A) CIN0.5, (B) CIN0.3, or (C) CIN0.5 or 25% as well as (D) composite end point. CIN = contrast-induced nephropathy, NT-pro-BNP = N-terminal pro-B-type natriuretic peptide, ROC = receiver operating characteristic.
Compared with patients with low-NT-pro-BNP (<2.73 pg/mL), patients with high-NT-pro-BNP (≥2.73 pg/mL) displayed significantly higher rates of CIN0.5 (5.0% vs 0.8%, P < 0.001), CIN0.5 or 25% (8.2% vs 3.9%, P < 0.001), CIN0.3 (9.0% vs 2.9%, P < 0.001), and composite end points (6.3% vs 1.9%, P < 0.001) (Figure 1).
FIGURE 1.
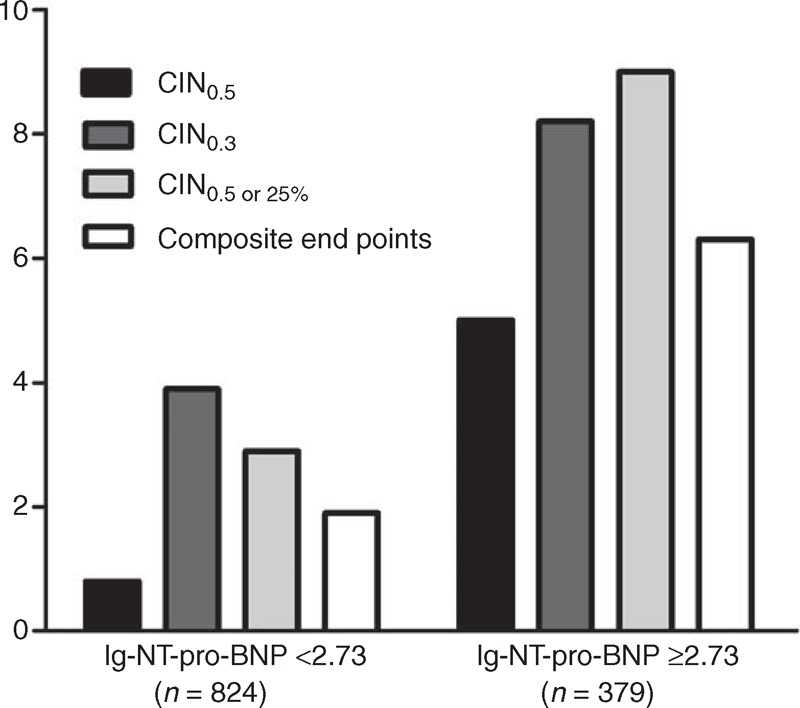
The prevalence of CIN or in hospital composite end points in patients with lg-NT-pro-BNP levels ≥2.73 or <2.73 pg/mL. CIN = contrast-induced nephropathy, NT-pro-BNP = N-terminal pro-B-type natriuretic peptide.
Association Between NT-Pro-BNP, CIN, and Composite End Point
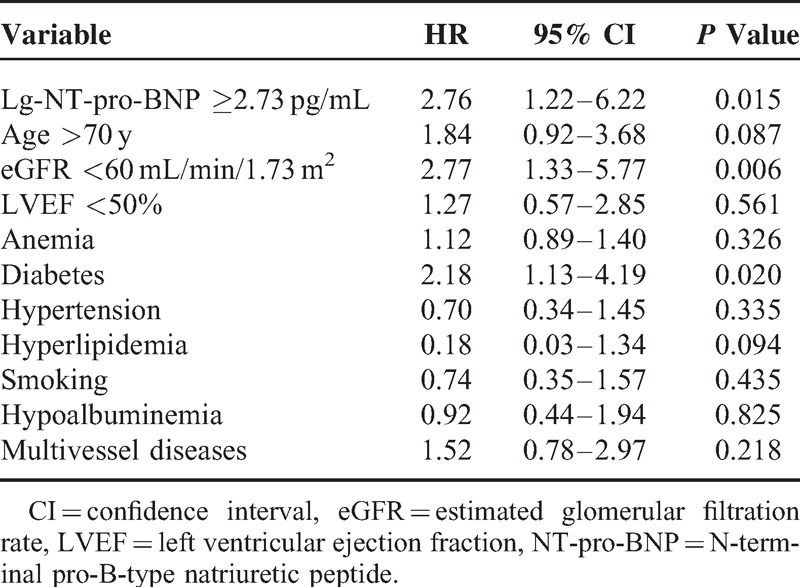
Clinical outcome during a mean of 26 months of follow-up was available for 1063 patients (88.4%). Composite end points occurred in 56 patients, including 42 patient deaths, 6 cases of acute HF, 4 cases of nonfatal myocardial infarction, and 4 cases of ischemic stroke. To identify the independent predictors of future outcomes, multivariate Cox regression analysis was performed with factors including age, anemia, diabetes, hypertension, hyperlipidemia, smoking, hypoalbuminemia, eGFR, multivessel diseases, and lg-NT-pro-BNP. Lg-NT-pro-BNP ≥2.73 pg/mL remained a significant predictor of all-cause mortality (hazard ratio = 2.76, 95% CI, 1.22–6.22, P = 0.015) (Table 5).
TABLE 5.
Multivariate Cox Analysis: Independent Predictors of All-Cause Mortality
Based on the Kaplan–Meier analysis, clinical outcomes, including all-cause mortality and composite end points, were significantly worse in patients with lg-NT-pro-BNP ≥2.73 pg/mL compared with those in patients with lg-NT-pro-BNP <2.73 pg/mL (both P < 0.001) (Figure 3). For patients with 60 < eGFR ≤ 90 mL/min/1.73 m2, outcome was significantly worse in patients with lg-NT-pro-BNP ≥2.73 pg/mL compared with that in patients with lg-NT-pro-BNP <2.73 pg/mL (cumulative rate of mortality 3.3% vs 1.2%, P = 0.029; Figure 4). For patients with eGFR ≤60 mL/min/1.73 m2, Kaplan–Meier analysis also indicated a significantly worse prognosis in subjects with lg-NT-pro-BNP ≥2.73 pg/mL compared with that in patients with lg-NT-pro-BNP <2.73 pg/mL (cumulative rate of mortality 13.6% vs 3.4%, P = 0.029; Figure 4).
FIGURE 3.
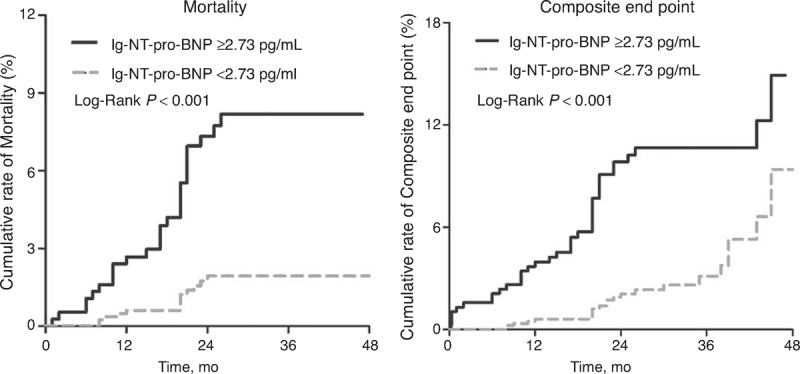
Cumulative rate of all-cause mortality or composite end points in patients with lg-NT-pro-BNP levels ≥2.73 or <2.73 pg/mL. NT-pro-BNP = N-terminal pro-B-type natriuretic peptide.
FIGURE 4.
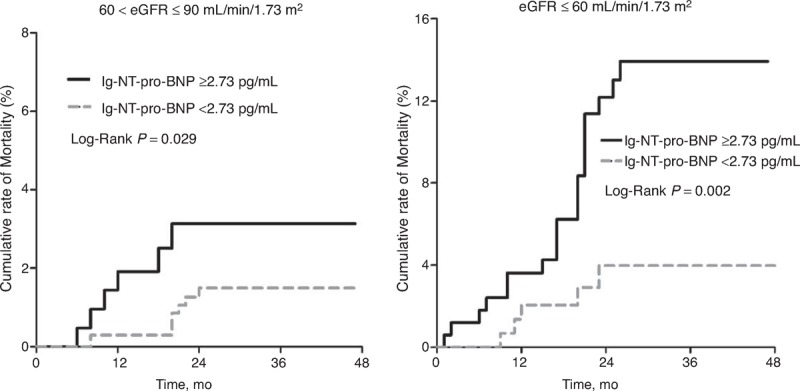
Cumulative rate of all-cause mortality between patients with lg-NT-pro-BNP levels ≥2.73 or <2.73 pg/mL, and among subgroups with eGFR ≤60 mL/min/1.73 m2 or 60 < eGFR ≤ 90 mL/min/1.73 m2. eGFR = estimated glomerular filtration rate, NT-pro-BNP = N-terminal pro-B-type natriuretic peptide.
Both CIN definitions were related to all-cause mortality evaluations at follow-up. Patients who developed CIN0.5 displayed a higher rate of all-cause mortality compared with those who did not (cumulative rate of mortality 19.2% vs 3.2%, P < 0.001) or those who developed CIN0.5 or 25% and CIN0.3 (Figure 5).
FIGURE 5.
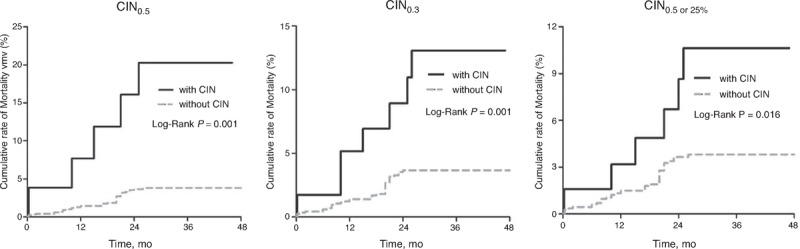
Cumulative rate of all-cause mortality between patients with CIN and without CIN, and among subgroups with CIN0.5, CIN0.3, or CIN0.5 or 25%. CIN = contrast-induced nephropathy.
DISCUSSION
The present study indicates that measurement of NT-pro-BNP at admission is associated with the development of various definitions of CIN, and poor in-hospital and long-term outcomes in patients with CKD and relative preserved LVF undergoing PCI. Moreover, NT-pro-BNP ≥537 pg/mL is a significantly good predictor of CIN and composite end points during follow-up.
Patients with CKD but without clinical HF will often be considered as patients at low risk of CIN and be ignored to some degree in clinical practice; for example, the appropriate use of fluids for these patients without clinical HF might be overlooked. However, such patients are also at high risk of CIN and poor outcomes after PCI. Koo et al4 indicated that CIN occurred in 7.8% of patients with LVEF ≥40% and mean of eGFR 74.5 ± 20.5 mL/min/1.73 m2. In addition, Pyxaras et al5 demonstrated that the CIN incidence was up to 15% in patients with LVEF ≥40% and CKD, which was defined as an eGFR ≤60 mL/min/1.73 m2. Therefore, there is a need to identify asymptomatic or minimally symptomatic patients (NYHA I/II class) with preserved LVEF and CKD who are at risk for CIN.
NT-pro-BNP is a well-established predictor of short- and long-term outcomes (regardless of LV systolic function) in numerous clinical conditions, including stable coronary artery disease,18 ACS,19,20 and essential hypertension.21 Recently, NT-pro-BNP was demonstrated to be a valuable biomarker for assessing prognosis in patients with CKD (with or without HF) and even the general population.11,12,22,23 Our findings supplement these observations by demonstrating that NT-pro-BNP, especially NT-pro-BNP ≥537 pg/mL, retains independent predictive value for CIN in patients with CKD and preserved LVF, even after adjusting for potential confounding factors. The NT-pro-BNP predictive accuracy for CIN as defined by various definitions in our study (CIN0.5: AUC = 0.754, 95% CI = 0.73–0.79, P < 0.001) was similar to that reported in recent study about investigating NT-pro-BNP for early prediction of acute kidney injury (AKI) in community-acquired pneumonia.24 Furthermore, the predictive value of NT-pro-BNP for CIN is similar to the classic Mehran CIN score (P = 0.508). However, Mehran risk score includes many procedural factors, such as the type and volume of CM, and are not convenient in clinical practice and could not be used to evaluate the risk of CIN before PCI. NT-pro-BNP, as a simple and sensitive marker, is readily obtainable before the procedure, and may be important and useful for clinicians to rapidly evaluate individual patient risk for developing CIN before PCI.
In 2 previous studies on patients presenting with ST-segment elevation myocardial infarction (STEMI), the authors reported that BNP measurements at admission enhanced the identification of patients at risk for developing CIN after PCI.25,26 In another study of patients undergoing elective cardiac surgery, preoperative BNP marginally improved classification for risk of AKI.27 More recently, Moltrasio et al analyzed the relationship between BNP and risk of AKI defined by Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE) classification in patients with ACS. They found that BNP was associated with AKI as well as its severity in patients with ACS.28 However, these studies reported BNP instead of NT-pro-BNP levels. NT-pro-BNP levels may have analytical advantages over BNP, given the enhanced stability due to a longer half-life, which allows for increased accumulation and potentially greater sensitivity for detecting more subtle structural and functional changes.29 In addition, they did not report the effects of BNP on long-term outcomes (except 6-month mortality),25 but our study provided additional information regarding the effects of NT-pro-BNP on long-term mortality in patients with CKD and preserved LVF. Moreover, previous studies almost included STEMI or non-STEMI patients. In fact, SCr changes in these patients were mainly the result of acute hemodynamic impairment rather than a direct effect of CIN.30 However, we focus our main investigation of NT-pro-BNP and CIN in patients with relative preserved LVF to limit the confounding effect of patients with renal impairment secondary to hypotension possibly associated with reduced LVF. Accordingly, the underlying comorbidities and exposure to CM may play a major role in the development of CIN in our study.
Although the pathophysiological mechanism of high NT-pro-BNP and CIN remains ill-defined and poorly understood, multiple important mechanisms may be involved. Previous studies reported that anemia is an independent predictor for elevated NT-pro-BNP via downregulation of NT-pro-BNP clearance receptors adapted to hypoxia caused by anemia. Our study also demonstrated that anemia and low serum hemoglobin are more prevalent in patients with high NT-pro-BNP. Reduced serum hemoglobin can decrease the oxygen-carrying capacity of blood.31,32 CM could increase the oxygen affinity of hemoglobin and decrease oxygen delivery to the renal tissues, especially in patients with anemia, resulting in renal medullary hypoxia and CIN aggravation. Therefore, anemia is associated with CIN and may influence the relationship between NT-pro-BNP and CIN. However, the relationship should be interpreted with caution, because we found that patients with anemia had relatively lower LVEF than patients without anemia (60.9 ± 9.3 vs 62.2 ± 9.1, P = 0.027). Even if it shows small differences, it might also influence the incidence of CIN.
In addition, both the activation of RAAS and sympathetic nervous system involved in the development of CIN are known to stimulate NT-pro-BNP. The increased activity of the RAAS in patients with CKD and even in the very early, clinically asymptomatic stages of chronic HF may influence NT-pro-BNP concentrations, because RAAS increases the level of circulating vasoconstrictive neurohormones (including catecholamines and angiotensin II), and elevates systemic vascular resistance. NT-pro-BNP can be induced by these neurohormones in cardiac myocytes and counteract water and sodium retention caused by activated RAAS. Thus, high NT-pro-BNP concentration reflects the high degree of activated RAAS, and may be responsible for systemic vasodilation and renal hypoperfusion, which in turn potentiates CIN.
Furthermore, NT-pro-BNP is found to be increased in a model of systemic inflammation in healthy men with normal HF.33 Proinflammatory cytokines, such as interleukin-1 or 6, and Tumor necrosis factor-a, induce NT-pro-BNP secretion from cultured myocytes in vitro.9,34 Moreover, Lazzeri et al reported that NT-pro-BNP is correlated with CRP-determined inflammation. High hs-CRP is also concomitant with high NT-pro-BNP levels in patients, which is consistent with the result of present study.35 Our recent study showed that hs-CRP, as a marker of systemic inflammation, is strongly associated with CIN.36,37 Thus, NT-pro-BNP levels appear to reflect the extent of systemic inflammation, which is an important contributor to CIN pathogenesis.
Neutrophil gelatinase-associated lipocain (NGAL), a marker of acute renotubular damage, is a powerful predictor of early CIN.38 A recent study reported that patients with high levels of NT-pro-BNP also display high NGAL levels and are more prone to develop AKI.39 It can be speculated that high NT-pro-BNP levels may be associated with CIN through these mechanisms.
In addition, we demonstrated that patients with CIN displayed worse long-term outcomes. Patients who developed CIN0.5 had a higher risk of hard clinical end points, which is consistent with recent studies.14,16 The results of our multivariate analysis demonstrated that elevated NT-pro-BNP is strongly associated with higher prevalence of all-cause mortality, even after adjustment for possible confounding factors, in patients with CKD and preserved LVF. In addition, this might be the first study to confirm the best cutoff value of NT-pro-BNP to estimate the risk of CIN with different definitions and all-cause mortality. Therefore, careful risk stratification by using NT-pro-BNP levels could identify patients who would benefit the most from specific treatment strategies and make it possible to avoid overtreating low-risk patients with limited available resources.
STUDY LIMITATIONS
There are several limitations to this study. First, this is a prospective, observational study that was conducted in a single center. Validation of the NT-pro BNP cutoff to predict CIN is required in other cohorts or at other centers. Second, our study population was limited to CKD patients with preserved LVF; therefore, the conclusions cannot be extended to patients without CKD or with CKD and decreased LVF. Third, due to variations in time measurement, we may have missed peak SCr values that occurred after the procedure. In addition, we did not measure cystatin C, which is the more sensitive biomarker that increases faster than SCr after CIN. Thus, the true incidence of CIN may have been underestimated. Fourth, SCr levels were not systematically measured during the follow-up period.
CONCLUSIONS
Our results demonstrate that NT-pro-BNP, as a simple and sensitive marker, is readily obtainable before the procedure, and displays a good predictive power for various definitions of CIN beyond traditional risk factors and poor clinical outcomes in CKD patients with preserved LVF undergoing PCI. Patients with NT-pro-BNP ≥537 pg/mL have a significantly higher probability of developing CIN or death during the follow-up. Future studies are warranted to determine whether treatment strategies guided by NT-pro-BNP levels could reduce CIN and MACE in this patient population treated with PCI.
Acknowledgments
We appreciate the efforts of our statistical consultant, Prof Ping-Yan Chen, MS, and Chong-Yang Duan, MD, of the Department of Biostatistics, Southern Medical University, China.
Footnotes
Abbreviations: CIN = contrast-induced nephropathy, CKD = chronic kidney disease, CM = contrast medium, eGFR = estimated glomerular filtration rate, HF = heart failure, hs-CRP = high-sensitivity C-reactive protein, LVEF = left ventricular ejection fraction, LVF = left ventricular function, NT-pro-BNP = N-terminal pro-B-type natriuretic peptide, OR = odds ratio, PCI = percutaneous coronary intervention, ROC = receiver operating characteristic, SCr = serum creatinine.
Y-hL and YL contributed equally to this work.
This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81270286). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Moreover, the work was not funded by any industry sponsors.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Seeliger E, Sendeski M, Rihal CS, et al. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012; 33:2007–2015. [DOI] [PubMed] [Google Scholar]
- 2.Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI Registry. JACC Cardiovasc Interv 2014; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenstock JL, Gilles E, Geller AB, et al. Impact of heart failure on the incidence of contrast-induced nephropathy in patients with chronic kidney disease. Int Urol Nephrol 2010; 42:1049–1054. [DOI] [PubMed] [Google Scholar]
- 4.Koo HM, Doh FM, Ko KI, et al. Diastolic dysfunction is associated with an increased risk of contrast-induced nephropathy: a retrospective cohort study. BMC Nephrol 2013; 14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyxaras SA, Sinagra G, Mangiacapra F, et al. Contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention without acute left ventricular ejection fraction impairment. Am J Cardiol 2013; 111:684–688. [DOI] [PubMed] [Google Scholar]
- 6.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994; 90:195–203. [DOI] [PubMed] [Google Scholar]
- 7.Nadir MA, Witham MD, Szwejkowski BR, et al. Meta-analysis of B-type natriuretic peptide's ability to identify stress induced myocardial ischemia. Am J Cardiol 2011; 107:662–667. [DOI] [PubMed] [Google Scholar]
- 8.Xia WJ, Huang YY, Chen YL, et al. Acute myocardial ischemia directly modulates the expression of brain natriuretic peptide at the transcriptional and translational levels via inflammatory cytokines. Eur J Pharmacol 2011; 670:7–12. [DOI] [PubMed] [Google Scholar]
- 9.Weinfeld MS, Chertow GM, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J 1999; 138 (2 Pt 1):285–290. [DOI] [PubMed] [Google Scholar]
- 10.Wong PC, Li Z, Guo J, et al. Pathophysiology of contrast-induced nephropathy. Int J Cardiol 2012; 158:186–192. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma M, Nakamura M, Tanaka F, et al. Plasma B-type natriuretic peptide level and cardiovascular events in chronic kidney disease in a community-based population. Circ J 2010; 74:792–797. [DOI] [PubMed] [Google Scholar]
- 12.Mishra RK, Li Y, Ricardo AC, et al. Association of N-terminal pro-B-type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]). Am J Cardiol 2013; 111:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 14.Slocum NK, Grossman PM, Moscucci M, et al. The changing definition of contrast-induced nephropathy and its clinical implications: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J 2012; 163:829–834. [DOI] [PubMed] [Google Scholar]
- 15.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011; 21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 16.Budano C, Levis M, D’Amico M, et al. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am Heart J 2011; 161:963–971. [DOI] [PubMed] [Google Scholar]
- 17.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004; 44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 18.Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 2005; 352:666–675. [DOI] [PubMed] [Google Scholar]
- 19.Casals G, Azzalini L, Tomas C, et al. Admission B-type natriuretic peptide retains prognostic value in patients with acute coronary syndrome and preserved left ventricular ejection fraction. Int J Cardiol 2012; 158:459–460. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Alvarez A, Regueiro A, Hernandez J, et al. Additional value of B-type natriuretic peptide on discrimination of patients at risk for mortality after a non-ST-segment elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2014; 3:132–140. [DOI] [PubMed] [Google Scholar]
- 21.Paget V, Legedz L, Gaudebout N, et al. N-terminal pro-brain natriuretic peptide: a powerful predictor of mortality in hypertension. Hypertension 2011; 57:702–709. [DOI] [PubMed] [Google Scholar]
- 22.Bruch C, Fischer C, Sindermann J, et al. Comparison of the prognostic usefulness of N-terminal pro-brain natriuretic peptide in patients with heart failure with versus without chronic kidney disease. Am J Cardiol 2008; 102:469–474. [DOI] [PubMed] [Google Scholar]
- 23.Spanaus KS, Kronenberg F, Ritz E, et al. B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem 2007; 53:1264–1272. [DOI] [PubMed] [Google Scholar]
- 24.Nowak A, Breidthardt T, Dejung S, et al. Natriuretic peptides for early prediction of acute kidney injury in community-acquired pneumonia. Clin Chim Acta 2013; 419:67–72. [DOI] [PubMed] [Google Scholar]
- 25.Akgul O, Uyarel H, Pusuroglu H, et al. High BNP level as risk factor for acute kidney injury and predictor of all-cause mortality in STEMI patients. Herz 2014; 39:507–514. [DOI] [PubMed] [Google Scholar]
- 26.Jarai R, Dangas G, Huber K, et al. B-type natriuretic peptide and risk of contrast-induced acute kidney injury in acute ST-segment-elevation myocardial infarction: a substudy from the HORIZONS-AMI trial. Circ Cardiovasc Interv 2012; 5:813–820. [DOI] [PubMed] [Google Scholar]
- 27.Patel UD, Garg AX, Krumholz HM, et al. Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery. Circulation 2012; 125:1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moltrasio M, Cabiati A, Milazzo V, et al. B-type natriuretic peptide and risk of acute kidney injury in patients hospitalized with acute coronary syndromes. Crit Care Med 2014; 42:619–624. [DOI] [PubMed] [Google Scholar]
- 29.van Kimmenade RR, Januzzi JJ, Bakker JA, et al. Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol 2009; 53:884–890. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg A, Hammerman H, Petcherski S, et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J 2005; 150:330–337. [DOI] [PubMed] [Google Scholar]
- 31.Metivier F, Marchais SJ, Guerin AP, et al. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant 2000; 15 (suppl 3):14–18. [DOI] [PubMed] [Google Scholar]
- 32.Fukuta H, Ohte N, Mukai S, et al. Anemia is an independent predictor for elevated plasma levels of natriuretic peptides in patients undergoing cardiac catheterization for coronary artery disease. Circ J 2008; 72:212–217. [DOI] [PubMed] [Google Scholar]
- 33.Vila G, Resl M, Stelzeneder D, et al. Plasma NT-proBNP increases in response to LPS administration in healthy men. J Appl Physiol (1985) 2008; 105:1741–1745. [DOI] [PubMed] [Google Scholar]
- 34.Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol 2004; 36:505–513. [DOI] [PubMed] [Google Scholar]
- 35.Lazzeri C, Valente S, Attana P, et al. The prognostic role of chronic obstructive pulmonary disease in ST-elevation myocardial infarction after primary angioplasty. Eur J Prev Cardiol 2013; 20:392–398. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Tan N, Zhou YL, et al. High-sensitivity C-reactive protein predicts contrast-induced nephropathy after primary percutaneous coronary intervention. J Nephrol 2012; 25:332–340. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH, Liu Y, Tan N, et al. Predictive value of GRACE risk scores for contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction before undergoing primary percutaneous coronary intervention. Int Urol Nephrol 2014; 46:417–426. [DOI] [PubMed] [Google Scholar]
- 38.Liebetrau C, Gaede L, Doerr O, et al. Neutrophil gelatinase-associated lipocalin (NGAL) for the early detection of contrast-induced nephropathy after percutaneous coronary intervention. Scand J Clin Lab Invest 2014; 74:81–88. [DOI] [PubMed] [Google Scholar]
- 39.Breidthardt T, Christ-Crain M, Stolz D, et al. A combined cardiorenal assessment for the prediction of acute kidney injury in lower respiratory tract infections. Am J Med 2012; 125:168–175. [DOI] [PubMed] [Google Scholar]


