Abstract
The elevated platelet-to-lymphocyte ratio (PLR), determined using an easy blood test based on platelet and lymphocyte counts, is reported to be a predictor of poor survival in patients with several cancers. The prognostic role of preoperative PLR in patients with intrahepatic cholangiocarcinoma (ICC) has, until now, been rarely investigated. The purpose of our study was to evaluate the prognostic significance of PLR in a large cohort of ICC patients after hepatic resection.
We obtained data from 322 consecutive nonmetastatic ICC patients who underwent hepatectomy without preoperative therapy between 2005 and 2011. Clinicopathological parameters, including PLR, were evaluated. Overall survival (OS) and recurrence-free survival (RFS) were assessed using the Kaplan–Meier method. Using multivariate Cox regression models, the independent prognostic value of preoperative PLR was determined.
Our results showed that PLR represents an independent adverse prognostic factor for OS and RFS in ICC patients using univariate and multivariate analyses. The optimal PLR cutoff value was 123 using receiver operating curve analyses. The 5-year OS and RFS rates after hepatectomy were 30.3% and 28.9% for the group with PLR 123 greater, compared with 46.2% and 39.4% for the group with PLR less than 123 (P = 0.0058 and 0.0153, respectively). In addition, high PLR values were associated with tumor size (P = 0.020).
Our results suggest that preoperative PLR might represent a novel independent prognostic factor for OS and RFS in ICC patients with hepatic resection.
INTRODUCTION
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumor after hepatocellular carcinoma (HCC), and accounts for 10% to 15% of all primary liver malignancies.1–3 Worldwide data accumulated over long periods of time have shown marked increases in ICC incidence and mortality.3,4 Long-term survival of patients with unresectable ICC is dismal, with less than 5% to 10% of the patients alive 5 years after diagnosis.5 The only potentially curative treatment option for patients with resectable disease is surgery. Unfortunately, even after curative-intent surgery, the clinical outcomes of patients undergoing liver resection are disappointing, with 5-year survival rates of 20% to 35%.6–10 Therefore, it is critical to accurately predict which patients have high risk of recurrence and to develop novel anticancer strategies. Although, several clinicopathological factors, including tumor size,11 intrahepatic satellite lesions,11 lymph node metastasis,12 vascular invasion,13 and resection margin involvement,11 have been identified as predictors for poor survival following resection of ICC.
Inflammation has emerged as the seventh hallmark of cancer.14 Over the last decade, it has been established that cancer-related inflammation is involved in many aspects of malignancy, and particularly enhances tumor cell survival, proliferation, and metastasis.15,16 Recently, cumulating evidence suggests that increased systemic inflammation might represent an independent adverse prognostic factor in different types of cancer.15,17,18 Among these inflammatory biomarkers, the preoperative platelet-to-lymphocyte ratio (PLR), an easy blood test based on platelet and lymphocyte counts, is associated with poor prognosis in patients with various cancers, including ovarian cancer,19 colorectal cancer,20 and breast cancer.21 Recent data have suggested that an elevated preoperative PLR is associated with early recurrence of HCC and worse survival after hepatectomy,22 as well as the need for liver transplantation for HCC.23
We hypothesized that inflammation is associated with ICC prognosis and that PLR values may be good indicators of the inflammatory process. Therefore, in this study, we evaluated the effect of preoperative PLR values on OS and RFS in 322 ICC patients in order to clarify the prognostic value of preoperative PLR for ICC patients who underwent hepatic resection.
PATIENTS AND METHODS
Patient Selection and Follow-Up Strategy
A total of 322 patients with histologically confirmed ICC were included in this study. All patients underwent surgical resection with curative intent between 2005 and 2011 in our department (Liver Cancer Institute, Zhongshan Hospital of Fudan University, Shanghai, China). A preoperative blood cell counts were obtained within 3 days prior to surgery. The Zhongshan Hospital Ethics Committee approved this study, and informed consent was obtained from each patient according to institutional review board protocols. Patients who underwent preoperative therapy, such as transarterial chemoembolization, radiofrequency ablation, or percutaneous ethanol injection, were excluded from this study.
The patient follow-up and postoperative treatment were administrated as described previously according to our established guidelines.24,25 Briefly, all patients were followed up monthly with screens for recurrence using tumor markers such as CA19-9, as well as liver ultrasonography. Every 6 months, computerized tomography scanning, magnetic resonance imaging (MRI), or bone scans were selected as needed. If recurrence was suspected, additional examinations, such as hepatic angiography, were performed. While ICC recurrence was being confirmed, a second hepatectomy, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, or external radiotherapy was administered according to the number, size, and site of the recurrent tumor.26 Time to recurrence (TTR) was defined as the interval between the date of surgery and the first recurrence, or from the date of surgery to the date of last follow-up patients without recurrence. OS was defined as the interval between surgery and death, or the interval between surgery and the last observation for surviving patients. Data were censored at the last follow-up for living patients.
Statistical Analysis
The PLR was calculated as the absolute platelet count (measured as ×109 L−1) divided by the absolute lymphocyte count (measured as ×109 L−1). Data are expressed as the mean ± standard deviation. The optimal cutoff value for the PLR was determined using time-dependent receiver operating characteristic curve.27 Qualitative variables were compared using χ2 or Fisher exact tests. Survival rates, including OS and RFS, were calculated using the Kaplan–Meier method and evaluated using the log-rank tests. Multivariate analyses of survival were performed using the Cox proportional hazards model. All statistical analyses were performed using the Statistical Package for Social Sciences version 16.0 (SPSS Inc, Chicago, IL). P values less than 0.05 were considered statistically significant.
RESULTS
Clinical and Pathologic Characteristics
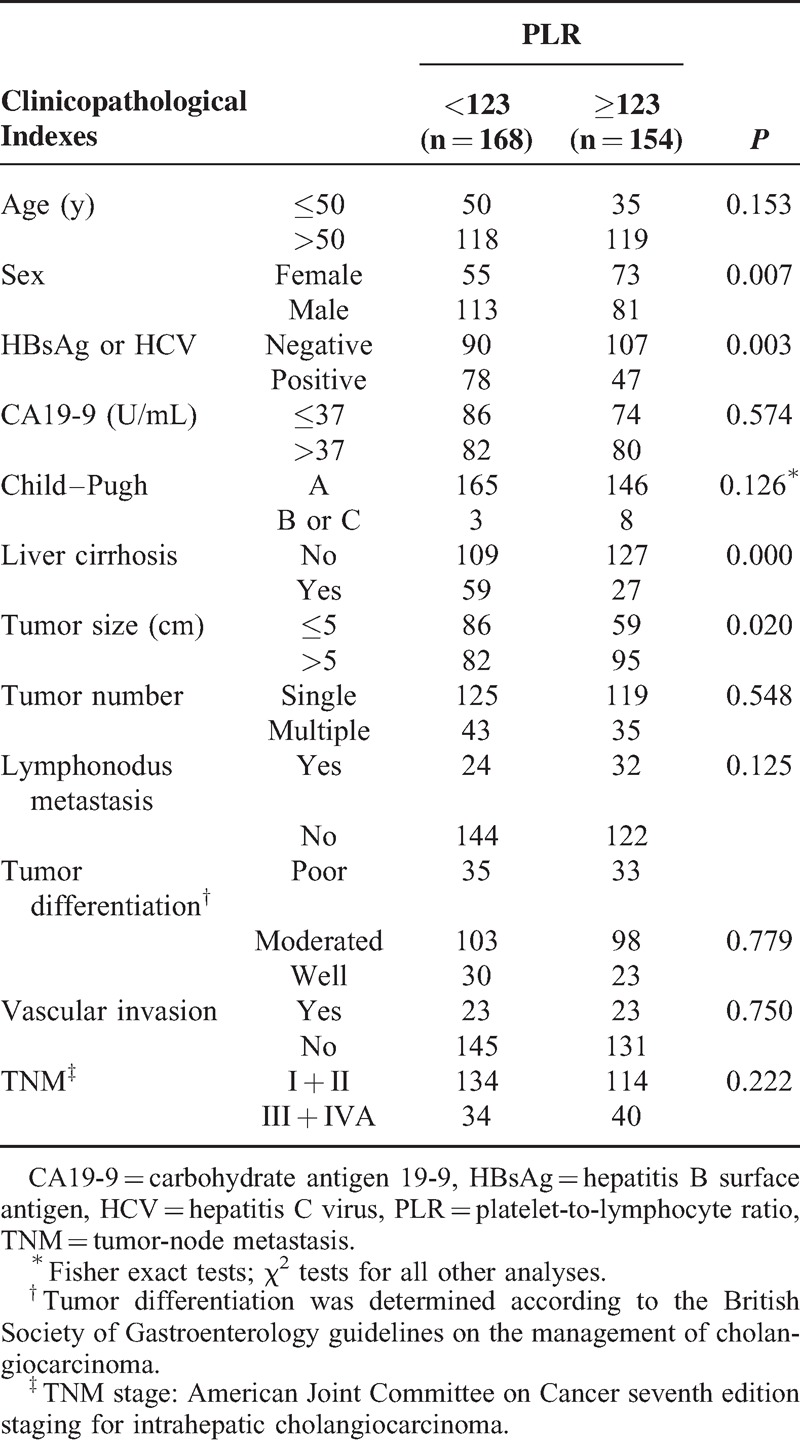
The clinicopathological features of the 322 patients included in the study are listed in Table 1. The median age at the time of diagnosis was 57.8 ± 11.2 years. Mean platelet and lymphocyte counts were 185 ± 63.4 and 1.54 ± 0.6, respectively. The mean PLR was 139.7 ± 84.2. Using time-dependent receiver operating characteristic curves, we determined a cutoff PLR value of 123 for postoperative prognosis. Patients were divided into 2 groups: the low (<123) PLR group (n = 168) and high (≥123) PLR group (n = 154). When we compared the clinical and pathological data of the low and high PLR groups, we observed high PLR values significantly correlated with sex, hepatitis B surface antigen (HBsAg), liver cirrhosis, and tumor size (all P < 0.05). However, high PLR values did not correlate with age, CA19-9, Child–Pugh score, tumor number, lymph node involvement, tumor differentiation, vascular invasion, and high TNM tumor stage (Table 1).
TABLE 1.
Correlation Between PLR and Clinicopathological Characteristics in ICC (n = 322)
Association of the High PLR Values With Poor Survival
In the 322 ICC patients undergoing hepatic resection, the median survival time was 33.8 ± 3.6 months and median RFS was 18.0 ± 3.1 months. We found that an elevated PLR was associated with worse OS (PLR < 123 vs. PLR ≥ 123, median OS 40.5 ± 5.3 vs. 20.7 ± 3.2 months). The patients with liver cirrhosis are always present with hypersplenism, which may accelerate platelet turnover and reduce platelet production. We divided the low PLR and high PLR patients into 4 subgroups: PLR low, PLR low + liver cirrhosis, PLR high, and PLR high + liver cirrhosis. When compared with the OS and RFS rates in 4 groups, there were no significant difference between PLR low + liver cirrhosis and PLR high + liver cirrhosis groups, as shown in the Figures 1 and 2. We found that the 1-, 3-, and 5-year OS rates were 82.5%, 55.6%, and 46.2%, respectively, in the low PLR group, which were significantly higher compared with the high PLR group (70.0%, 42.7%, and 30.3%, respectively, P = 0.0058, Figure 3). In addition, elevated PLR values significantly correlated with ICC recurrence following hepatic resection. High preoperative PLR was also associated with worse RFS (PLR < 123 vs. PLR ≥ 123, median RFS 24.0 ± 5.6 vs. 12.3 ± 2.2 months). The 1-, 3-, and 5-year RFS rates were significantly lower in the high PLR group (50.9%, 32.6%, and 28.9%, respectively) compared with the low PLR group (66.1%, 49.4%, and 39.4%, respectively, P = 0.0153, Figure 4). These data suggest that high PLR may be a marker of early ICC recurrence after hepatic resection.
FIGURE 1.
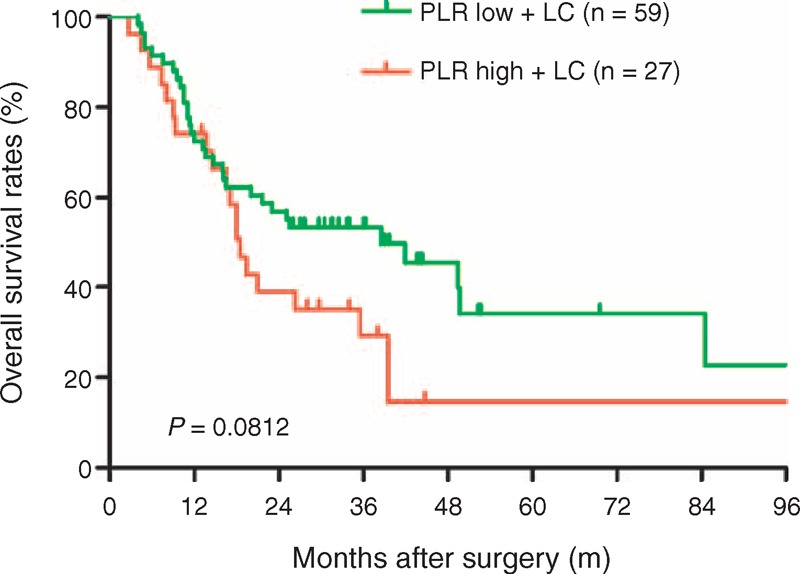
Comparison of overall survival rates in the PLR low + liver cirrhosis and PLR high + liver cirrhosis groups. The overall survival rate was no significant difference between PLR high + liver cirrhosis and PLR low + liver cirrhosis groups (P = 0.0812). LC = liver cirrhosis, PLR = platelet-to-lymphocyte ratio.
FIGURE 2.
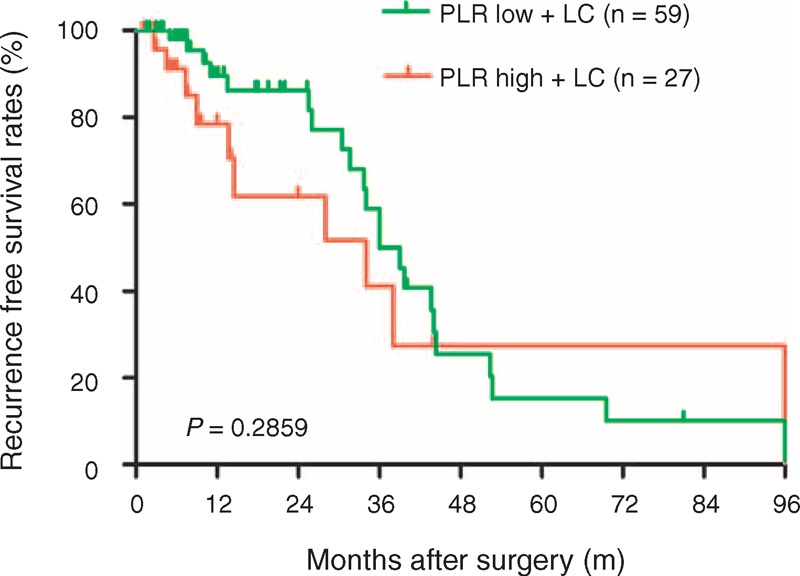
Comparison of recurrence-free survival rates in the PLR low + liver cirrhosis and PLR high + liver cirrhosis groups. The recurrence-free survival rate was no significant difference between PLR high + liver cirrhosis and PLR low + liver cirrhosis groups (P = 0.2859). LC = liver cirrhosis, PLR = platelet-to-lymphocyte ratio.
FIGURE 3.
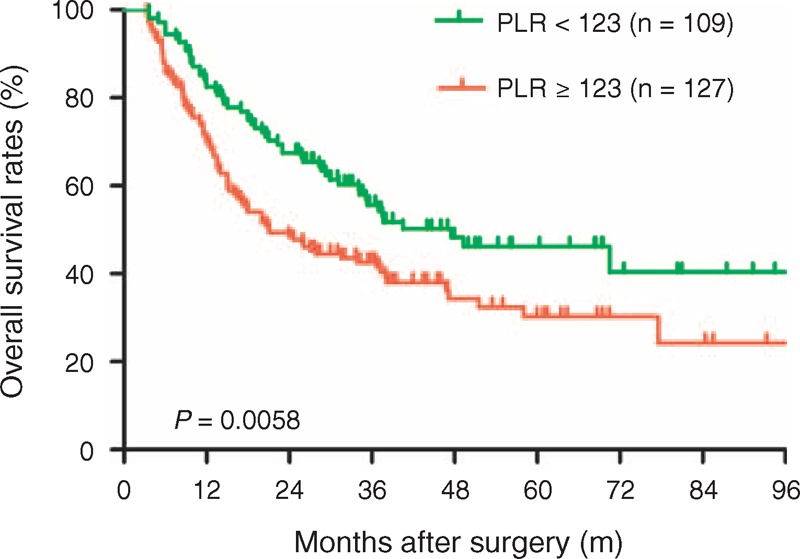
Comparison of overall survival rates in the low (<123) and high (≥123) PLR groups. The 1-, 3-, and 5-year overall survival rates were 82.5%, 55.6%, and 46.2%, respectively, in the low (<123) PLR group, which were significantly higher compared with the high (≥123) PLR group (70.0%, 42.7%, and 30.3%, respectively, P = 0.0058). PLR = platelet-to-lymphocyte ratio.
FIGURE 4.
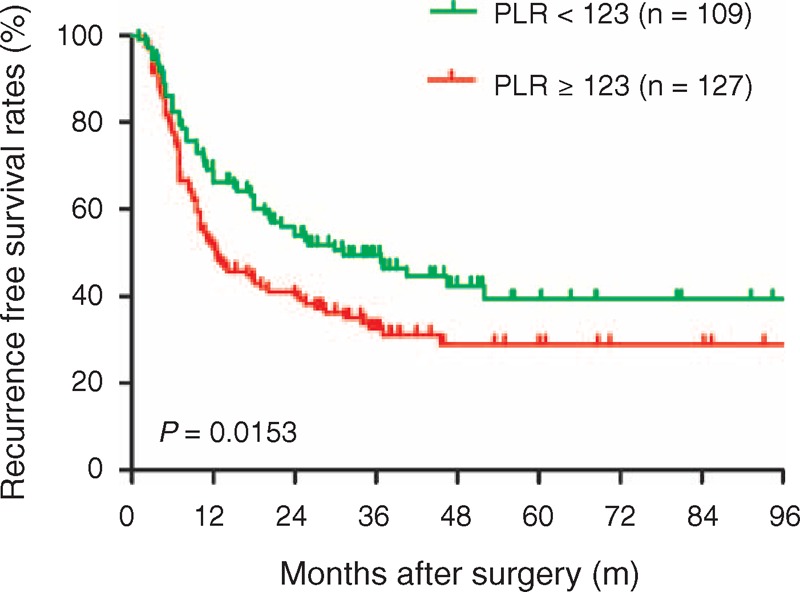
Comparison of recurrence-free survival rates in the low (<123) and high (≥123) PLR groups. The recurrence-free survival rate was significantly higher in the low PLR group than in the high PLR group (P = 0.0153). PLR = platelet-to-lymphocyte ratio.
PLR as an Independent Prognostic Factor
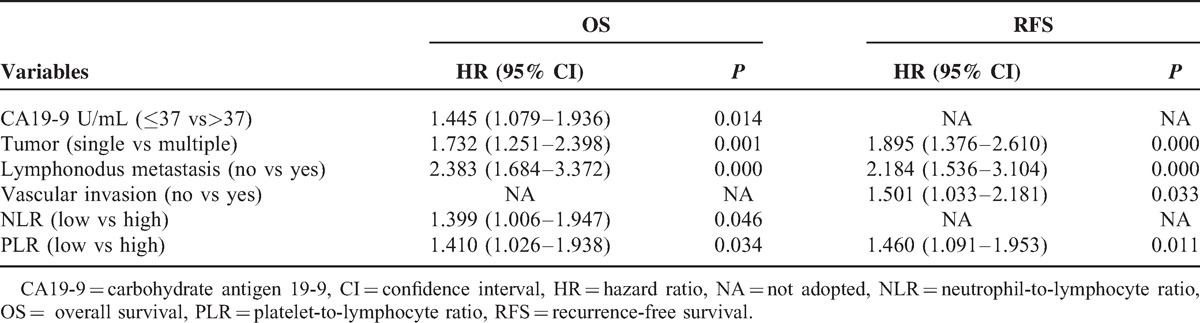
Univariate analyses revealed high PLR values significantly impacted ICC patient survival (hazard ratio, HR 1.594, 95% CI 1.194-2.128, P = 0.002, Table 2). In multivariable analyses that included age, sex, hepatitis history, cirrhosis, CA19-9, Child–Pugh score, tumor differentiation, lymph node involvement, vascular invasion, TNM stage, neutrophil-to-lymphocyte ratio (NLR), and PLR, we observed that PLR values ≥123 were independent prognostic factors of poor outcome in ICC patients after liver section (HR 1.410, 95% CI 1.026–1.938, P = 0.034, Table 3). In addition, CA19-9, number of tumor, NLR, and lymph node metastasis were independently associated with OS. Furthermore, we found that elevated preoperative PLR was significantly associated with ICC recurrence (HR 1.404, 95% CI 1.056–1.866, P = 0.019, Table 2). Multivariable analyses showed high PLR values were independent prognostic factors for poor RFS (HR 1.460, 95% CI 1.091–1.953, P = 0.011, Table 3). These results suggest that ICC patients with high PLR values should be closely followed for ICC recurrence.
TABLE 2.
Univariate Analyses of Factors in Relation to Overall and Recurrence-Free Survival, Using the Cox Proportional Hazards Model (n = 322)
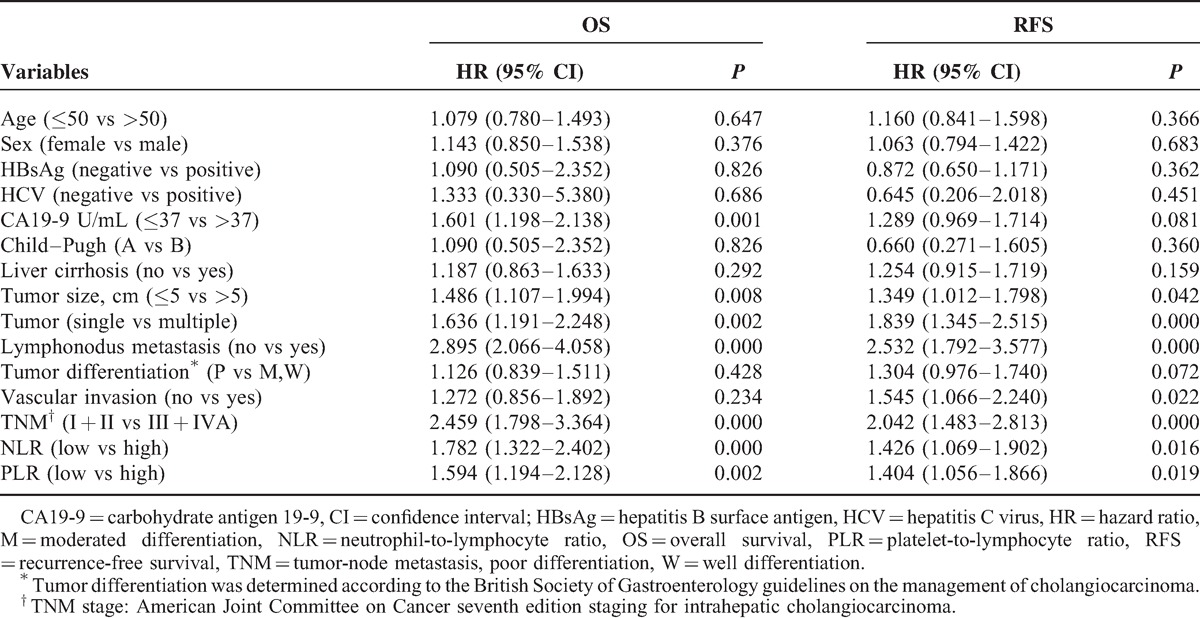
TABLE 3.
Multivariate Analyses of Factors in Relation to Overall and Recurrence-Free Survival, Using the Cox Proportional Hazards Model (n = 322)
DISCUSSION
Tumor progression and metastasization is the result of dynamic interactions between tumor cells themselves and with components of the tumor inflammatory environment.28 The inflammatory environment includes inflammatory mediators that support angiogenesis as well as inflammatory cells.15,18 The preoperative systemic inflammation response (e.g., C-reactive protein, NLR, and PLR) has been shown to independently predict cancer-specific survival in patients undergoing curative resection of several solid tumors.21,23,29,30 Mano et al31 have recently demonstrated that a NLR ≥2.81 was an independent predictor of recurrence and poor overall survival in patients with HCC. Similarly, only 1 relatively small-scale study of 27 patients has found that the preoperative NLR ≥5 was a prognostic indicator of survival after hepatic resection for ICC.32 These findings may provide new insights regarding the influence of peripheral blood cells (such as neutrophils, lymphocytes, and platelets) on tumor pathogenesis and progression.
The prognostic role of preoperative PLR in patients with ICC has, until now, been rarely investigated. We found an elevated PLR might be a significant prognostic factor in ICC patients, potentially allowing accurate predicted survival after surgical treatment. PLR values ≥123 showed the greatest correlation with early recurrence and worse OS. The reason for elevated PLR among patients with malignancies who have poor prognoses is not clearly defined. High PLR values may reflect relatively depleted lymphocytes, which impairs the host immune response to malignancy. We also found the lymphocyte number was associated with survival outcomes, consistent with the previous studies.29,33 Lymphocytes participate in tumor immunosurveillance and inhibit tumor cell proliferation as well as metastasis.34 An elevated lymphocyte count is also associated with prolonged OS in patients with multiple myeloma.35 Several studies have demonstrated that patients with weaker lymphocytic infiltrates at hepatic tumor margins have worse prognoses.36,37 In our previous study, we showed that high intratumoral-activated CD8-positive cytotoxic cells were an independent prognostic factor for both improved disease-free survival and OS. However, the cause of relationship between intratumoral infiltration of immune cells and peripheral blood cells that constitute the systemic inflammatory response remains unclear. 21
Numerous studies have suggested that systemic inflammation is associated with the release of inflammatory mediators (e.g. interleukin [IL]-1, IL-3, and IL-6) that are released in different types of cancer and stimulate the proliferation of megakaryocytes, the platelet progenitor cells.38 The elevated peripheral blood platelet counts might reflect the tumor-induced systemic inflammatory response.21 Platelet aggregation and degranulation, along with the consequent release of platelet-derived proangiogenic mediators, platelet-derived growth factor, vascular endothelial growth factor, and angiopoetin-1, have been suggested as important determinants of tumor growth, and possibly angiogenesis.39–41 Natural killer (NK) cells provide the most effective antitumor cell activity of all circulating immune cells.42 However, their mode of action requires direct contact with the tumor cell.43 Tumor cell–induced platelet aggregation results in “platelet coating” of the tumor cells which protects them from NK cells. In addition, circulating tumor cells of cancer patients display coexpression of platelet markers. The resulting “phenotype of false pretenses” disrupts recognition of tumor cell missing self, thereby impairing cytotoxicity and IFN-γ production by NK cells.44 There is strong evidence to support the concept that platelets can limit the ability of NK cells to lyse tumor cells in vitro and in vivo, an observation that is reversed after depletion of these platelets.45,46 Recent data show that the prometastatic effects of platelets are in large part mediated via activation of the TGF-β signaling pathway, and that abrogating either TGF-β signaling in tumor cells or platelet-derived TGF-β is sufficient to inhibit metastasis and the epithelial-mesenchymal transition of tumor cells.47 These results indicate platelets may contribute to accelerated tumor metastasis and progression in cancers. Therefore, the mechanisms underlying the interactions of platelet-tumor cell need to be studied in future studies, in an effort to provide individual patients in high-risk situations for cancer cell spreading with therapies.
There is an increasing body of evidence that supports the use of several antiangiogenic and anti-inflammatory agents to improve survival and decrease recurrence rates in hepatic cancer and other malignancies. Antiplatelet drugs are widely used to prevent cardiovascular disease, and in these studies, have additionally been found to reduce the rates of overall cancer deaths.48 In prospective studies, daily use of aspirin significantly reduced the incidence of colorectal adenomas,49 breast cancer,50 and lung cancer.51 In addition, Sitia et al.52 have recently demonstrated that antiplatelet drugs, such as aspirin and clopidogrel, effectively prevented or delayed HCC and improved survival in a mouse model of chronic immune-mediated hepatitis B. In a previous study, our results demonstrated that aspirin minimized the prometastasis effect of sorafenib by upregulating the tumor suppressor HTATIP2.53 Therefore, we suggest that antiplatelet drugs may constitute a simple, well-tolerated, and inexpensive additional approach for the prevention or delay of ICC recurrences.
To our knowledge, this is the first study to use preoperative PLR as a biomarker for ICC patients undergoing hepatic resection to reflect the systemic inflammatory response. Our findings indicate that an elevated PLR might be a poor prognostic factor in ICC patients after hepatic resection. Patients with elevated PLR values might be considered candidates for additional, more aggressive treatment approaches as well as more stringent follow-up schedules. However, there still exist several drawbacks such as the elevated PLR was not predictor for ICC patients with liver cirrhosis in this study. The combined use of the other preoperative systemic inflammation response such as C-reactive protein may be able to accurately predict the prognosis of ICC patients with liver cirrhosis.
CONCLUSION
In conclusion, PLR has been proposed as an accessible measurable inflammatory biomarker. Our results demonstrate that the preoperative PLR value is a novel independent indicator that is predictive of poor prognosis and early recurrence in patients with ICC after hepatic resection. Preoperative prediction of recurrence and outcome using preoperative PLR has potentially valuable implications with regard to guiding both pre- and postoperative therapies to improve outcomes. The optimal PLR cutoff level should be clarified and our findings should be independently validated.
Footnotes
Abbreviations: CA19-9 = carbohydrate antigen 19-9, CI = confidence interval, HBsAg = hepatitis B surface antigen, HCV = hepatitis C virus, HR = hazard ratio, ICC = Intrahepatic cholangiocarcinoma, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, OS = overall survival, PLR = platelet-to-lymphocyte ratio.
QC, ZD, and DY contributed equally to this work.
This study was supported by National Natural Science Funds of China (No. 81172277; No. 81272724), the National Basic Research Program of China (973 Program) (2011CB504001), Program of Excellent Talents in Shanghai City Health System (No. XBR2011018), and the National Science Foundation for Distinguished Young Scholars of China (81225019).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg 1997; 185:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002; 94:2040–2046. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 4.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013; 145:1215–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004; 24:115–125. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31:1188–1195. [DOI] [PubMed] [Google Scholar]
- 7.Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg 2012; 147:1107–1113. [DOI] [PubMed] [Google Scholar]
- 8.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011; 254:824–829.discussion 830. [DOI] [PubMed] [Google Scholar]
- 9.de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011; 29:3140–3145. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 2011; 22:1644–1652. [DOI] [PubMed] [Google Scholar]
- 11.Uenishi T, Hirohashi K, Kubo S, et al. Clinicopathological factors predicting outcome after resection of mass-forming intrahepatic cholangiocarcinoma. Br J Surg 2001; 88:969–974. [DOI] [PubMed] [Google Scholar]
- 12.Kawarada Y, Yamagiwa K, Das BC. Analysis of the relationships between clinicopathologic factors and survival time in intrahepatic cholangiocarcinoma. Am J Surg 2002; 183:679–685. [DOI] [PubMed] [Google Scholar]
- 13.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001; 193:384–391. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A. Cancer: inflaming metastasis. Nature 2009; 457:36–37. [DOI] [PubMed] [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev 2008; 27:11–18. [DOI] [PubMed] [Google Scholar]
- 17.Proctor MJ, Talwar D, Balmar SM, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer 2010; 103:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 19.Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011; 13:499–503. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012; 17:216–222. [DOI] [PubMed] [Google Scholar]
- 21.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer 2014; 110:2524–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012; 107:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014; 27:32–41. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25:2586–2593. [DOI] [PubMed] [Google Scholar]
- 25.Gu FM, Gao Q, Shi GM, et al. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Annals Surg Oncol 2012; 19:2506–2514. [DOI] [PubMed] [Google Scholar]
- 26.Gao Q, Zhao YJ, Wang XY, et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology 2014; 146:1397–1407. [DOI] [PubMed] [Google Scholar]
- 27.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000; 56:337–344. [DOI] [PubMed] [Google Scholar]
- 28.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 2010; 16:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009; 250:141–151. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 2005; 103:1856–1864. [DOI] [PubMed] [Google Scholar]
- 31.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 2013; 258:301–305. [DOI] [PubMed] [Google Scholar]
- 32.Gomez D, Morris-Stiff G, Toogood GJ, et al. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2008; 97:513–518. [DOI] [PubMed] [Google Scholar]
- 33.Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010; 200:197–203. [DOI] [PubMed] [Google Scholar]
- 34.Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983; 52:126–130. [DOI] [PubMed] [Google Scholar]
- 35.Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol 2008; 141:792–798. [DOI] [PubMed] [Google Scholar]
- 36.Okano K, Maeba T, Moroguchi A, et al. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol 2003; 82:28–33. [DOI] [PubMed] [Google Scholar]
- 37.Unitt E, Marshall A, Gelson W, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol 2006; 45:246–253. [DOI] [PubMed] [Google Scholar]
- 38.Alexandrakis MG, Passam FH, Moschandrea IA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol 2003; 26:135–140. [DOI] [PubMed] [Google Scholar]
- 39.Kepner N, Lipton A. A mitogenic factor for transformed fibroblasts from human platelets. Cancer Res 1981; 41:430–432. [PubMed] [Google Scholar]
- 40.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A 1997; 94:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006; 10:355–362. [DOI] [PubMed] [Google Scholar]
- 42.Vivier E, Ugolini S, Blaise D, et al. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storkus WJ, Dawson JR. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol 1991; 10:393–416. [PubMed] [Google Scholar]
- 44.Placke T, Orgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 2012; 72:440–448. [DOI] [PubMed] [Google Scholar]
- 45.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005; 105:178–185. [DOI] [PubMed] [Google Scholar]
- 46.Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999; 59:1295–1300. [PubMed] [Google Scholar]
- 47.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20:576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012; 379:1602–1612. [DOI] [PubMed] [Google Scholar]
- 49.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 2003; 348:891–899. [DOI] [PubMed] [Google Scholar]
- 50.Gallicchio L, McSorley MA, Newschaffer CJ, et al. Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer 2006; 106:1443–1452. [DOI] [PubMed] [Google Scholar]
- 51.Fontaine E, McShane J, Page R, et al. Aspirin and non-small cell lung cancer resections: effect on long-term survival. Eur J Cardiothorac Surg 2010; 38:21–26. [DOI] [PubMed] [Google Scholar]
- 52.Sitia G, Aiolfi R, Di Lucia P, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A 2012; 109:E2165–E2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu L, Sun HC, Zhang W, et al. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PloS One 2013; 8:e65023. [DOI] [PMC free article] [PubMed] [Google Scholar]


