Abstract
The association between membranous nephropathy (MN) and immunological disorder-related liver disease has not been extensively investigated, and the specific features of this uncommon association, if any, remain to be determined.
We retrospectively identified 10 patients with this association. We aimed to describe the clinical, biological, and pathological characteristics of these patients and their therapeutic management. The possible involvement of the phospholipase A2 receptor (PLA2R) in these apparent secondary forms of MN was assessed by immunohistochemistry with renal and liver biopsy specimens.
The mean delay between MN and liver disease diagnoses was 3.9 years and the interval between the diagnosis of the glomerular and liver diseases was <1.5 years in 5 patients. MN was associated with a broad spectrum of liver diseases including primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), and primary sclerosing cholangitis (PSC). AIH whether isolated (n = 3) or associated with PBC (n = 2) or PSC (n = 2) was the most frequent autoimmune liver disease. Circulating PLA2R antibodies were detected in 4 out of 9 patients but the test was performed under specific immunosuppressive treatment in 3 out of 9 patients. Seven of the 9 patients with available renal tissue specimens displayed enhanced expression of PLA2R in glomeruli whereas PLA2R was not expressed in liver parenchyma from these patients or in normal liver tissue. The study of immunoglobulin (Ig) subclasses of deposits in glomeruli revealed that the most frequent pattern was the coexistence of IgG1 and IgG4 immune deposits with IgG4 predominating.
Detection of PLA2R antibodies in glomeruli but not in liver parenchyma is a common finding in patients with MN associated with autoimmune liver disease, suggesting that these autoantibodies are not exclusively detected in idiopathic MN.
INTRODUCTION
Membranous nephropathy (MN) is considered as a primary glomerular disease, also referred to as “idiopathic,” in 80% of cases, whereas it occurs in association with various clinical conditions including systemic autoimmune disease, chronic infection, malignancies, or drug exposure in about 20% of cases (secondary MN).1–3 Idiopathic MN is currently considered as an autoimmune disease whereas secondary forms may be related to the accumulation exogenous antigens (viral, tumor) without a clear evidence for a direct pathogenic role.4 Numerous studies have investigated the antigens involved in human MN and have led to major advances in understanding the pathogenesis of adult MN.2 M-type phospholipase A2 receptor (PLA2R) was the first protein identified as a target antigen in human idiopathic MN.5 PLA2R antibodies were initially found in the serum of 70% idiopathic, but not in secondary forms of MN.5 Subsequent studies demonstrated that circulating PLA2R antibodies are exclusively detected in cases of idiopathic MN, but not in healthy individuals, in patients with other glomerular diseases, or in patients with autoimmune disorders. These autoantibodies may therefore be a useful biomarker for the diagnosis of MN and monitoring of idiopathic MN treatment.1,2,6 In some patients, circulating PLA2R antibodies are not found whereas PLA2R antigens are present in their glomerular immune deposits.7,8 Circulating PLA2R antibodies seem to be rarely detected in patients with an apparent secondary form of MN occurring in patients with neoplasia, viral hepatitis B infection, systemic lupus erythematous (SLE), sarcoidosis, or graft-versus-host disease.9–11 The systemic diseases most frequently associated with MN are SLE, immunoglobulin (Ig)G4-related immunological disorders, and sarcoidosis.12–14 Nevertheless, some case reports suggest a strong association between MN and liver diseases associated with immune dysfunction, such as primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), and primary sclerosing cholangitis (PSC), pointing out a possible pathophysiological link.15–25 We therefore assessed the significance of this association, by reviewing clinical, histological, biological, and therapeutic data for 10 patients with a diagnosis of MN occurring in the context of PBC, PSC, and/or AIH. We also studied by immunohistochemistry whether the PLA2R antigen could be expressed in kidney and liver biopsy specimens in these patients.
METHODS
This multicenter retrospective survey, approved by our local Ethics Committee, was conducted in 13 French health departments. A questionnaire to identify patients with MN, occurring in the context of PBC or PSC or AIH, was sent to all French hepatology and nephrology centers. Ten adult patients (>18 years) were retrospectively identified. Demographic, clinical, biological, and histological data were assessed for each patient at the time of MN diagnosis.
MN Diagnosis
All patients included in this study underwent a renal biopsy for a nephrotic syndrome (NS) or a significant proteinuria (albumin excretion rate of >0.3 g/d). Immunofluorescence (IF) on renal biopsies was performed using monoclonal antibodies specific for IgG1, IgG2, IgG3, and IgG4 (Sigma-Aldrich : Saint Louis, MO, USA; 1 in 30 dilution). IF staining intensity was graded on a scale of 0 to 3+ by one observer (A.M.). In one case with interstitial nephritis associated with glomerular lesions, IgG4 labeling was performed by immunohistochemistry (monoclonal antibody against human IgG4, binding site; 1:200 dilution). Reduced kidney function was defined as a permanent (at least 3 months) decrease of estimated glomerular filtration rate (eGFR) to <60 mL/min/1.73 m2 according to the modification of diet in renal disease formula.26 Acute renal failure was defined according to Acute Kidney Injury Network (AKIN) criteria.27 Complete remission was defined as proteinuria of <0.3 g/d at the 6-month follow-up; patients with proteinuria between 0.3 and 3 g/d and those with at least a 50% reduction in proteinuria, with an albumin level >30 g/L, were considered to be in partial remission.
Diagnosis of Liver Diseases Related to Autoimmune and/or Immunological Disorders
Three patterns of liver diseases including PBC, PSC, and AIH previously described as being significantly associated with MN were considered in this study. We excluded patients with MN in a context of viral hepatitis B or C infection. Definitive diagnosis of PBC required 2 of the 3 following criteria: biochemical evidence of chronic cholestasis with increased alkaline phosphatase activity, presence of antimitochondrial antibodies (AMAs), and liver biopsy demonstrating compatible pathological lesions (nonsuppurative cholangitis and interlobular bile duct injury).28,29 A diagnosis of PSC was made in patients with biochemical markers of cholestasis not otherwise explained, when cholangiography and/or liver biopsy showed typical findings (multiple bile duct strictures and/or obliterative fibrosing cholangitis) and when causes of secondary sclerosing cholangitis were excluded.30 The diagnosis of AIH required unexplained elevated serum transaminase levels associated with the presence of ≥1 relevant autoantibodies (smooth muscle actin, antinuclear antibody [ANA], liver–kidney microsomal antibodies, soluble liver/pancreas antibodies) and histological interface hepatitis. A score ≥6 points by the simplified scoring system for AIH was required for this diagnosis.31 Specific treatments for liver diseases including ursodeoxycholic acid and/or immunosuppressive agents were recorded for all patients. Biochemical remission of liver disease was defined according to standard guidelines.32,33
Determining Serum PLA2R Antibodies and PLA2R Immunostaining in Renal and Liver Biopsies
Serum was tested for PLA2R antibodies by IF assay using cells transiently transfected with full-length complementary DNA encoding a PLA2R1 isoform.2 Expression of PLA2R in tissue biopsies was tested by immunohistochemistry with a rabbit polyclonal anti-PLA2R1 antibody (Sigma-Aldrich, product number HPA012657). Liver biopsies were fixed in formaldehyde, whereas renal biopsies were fixed in formol acetic alcohol/Duboscq Brazil and embedded in paraffin. Positive controls consisted of idiopathic MN with circulating PLA2R antibodies. Protocolar renal graft biopsies exhibiting negative IF (Igs and complement) were used as negative controls. Renal sections were stained for PLA2R1 at a dilution of 1:400 and incubated in DAB enhancer (Bond DAB enhancer; Leica : Newcastle, United Kingdom). Formaldehyde-fixed liver biopsies from patients with PBC, PSC, or AIH associated with MN (patients 3, 4, 5, 6, 7, 8, 10) or without known glomerular disease (controls) were stained for PLA2R1 at a dilution of 1:1000. To investigate basal expression of PLA2R in normal liver tissue, liver biopsies from patients who underwent liver function assessment during pretherapeutic workup for liver tumors were used as controls.
RESULTS
Clinical and Biological Data for Patients With MN and Liver Diseases Related to Autoimmune and/or Immunological Disorders
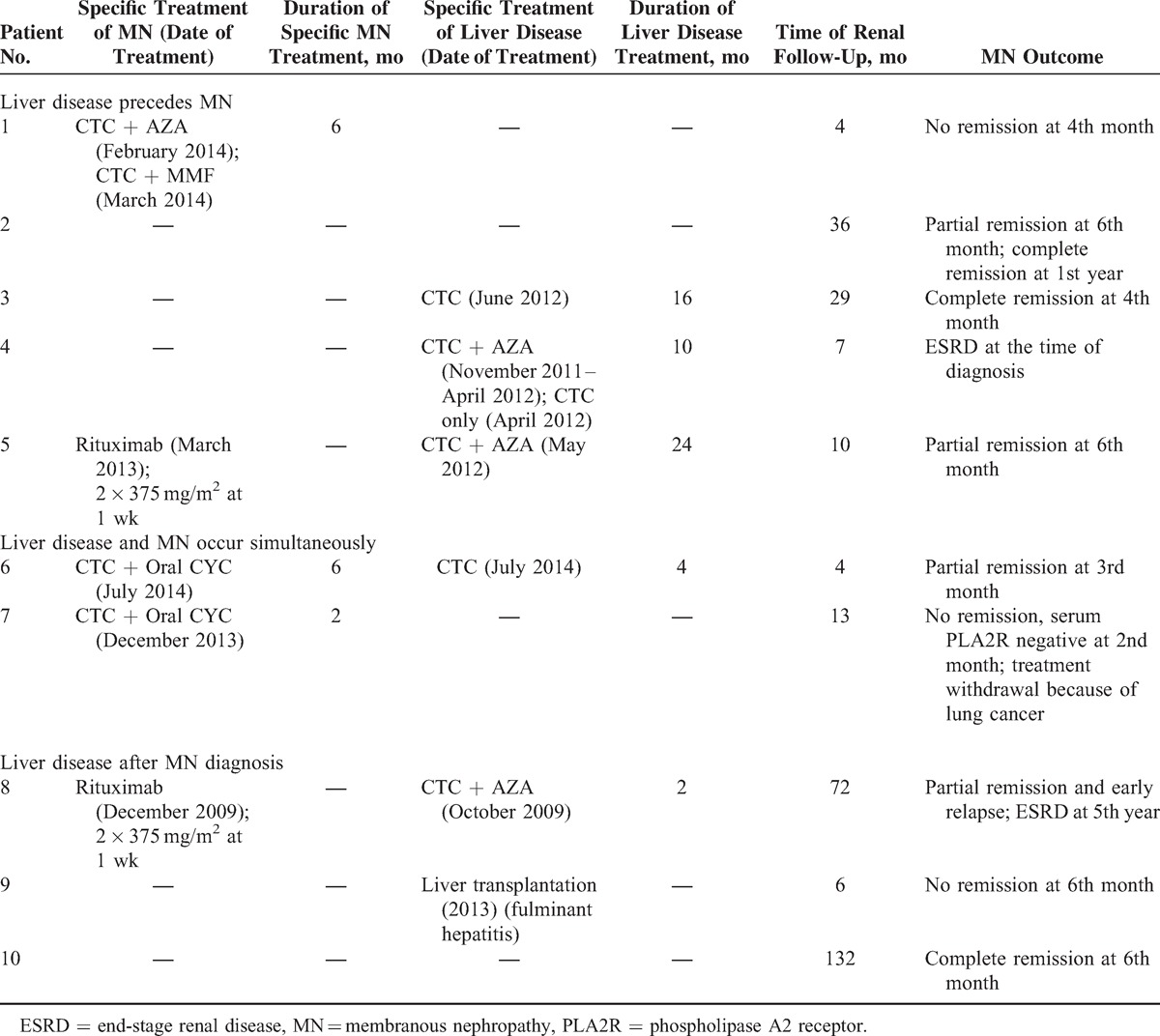
Ten patients (6 men and 4 women) diagnosed with MN between 1993 and 2014, occurring in a context of liver disease associated with immune disorders, were identified retrospectively. Their clinical and biological data at the time of MN diagnosis are summarized in Table 1. They were aged from 28 to 77 years (mean age 56.4 years) at the time of the MN diagnosis. All patients underwent a liver biopsy to definitively confirm the diagnosis of PBC or PSC and/or AIH. Liver disease included isolated PSC for 2 patients (patients 3 and 10), isolated AIH for 3 patients (patients 2, 5, and 9), and isolated PBC for 1 patient (patient 1). Two patients had both PBC and AIH (patients 4 and 7) and 2 had both PSC and AIH (patients 6 and 8) (Table 1). AMAs were positive in 1 patient with PBC (patient 1) and in 1 patient with PBC associated with AIH (patient 4). Six patients (1 patient with isolated PBC and 5 with AIH diagnosis) had ANA, but none of them fulfilled the SLE diagnosis criteria of the American College of Rheumatology.34 The mean delay between the diagnosis of the glomerular and liver diseases was 3.9 years (range 0–17 years). The interval between the onset of liver and glomerular diseases was <1.5 year in 5 cases (patients 4, 5, 6, 7, 8). Both clinical conditions were diagnosed concomitantly in 2 patients (patients 6 and 7). The diagnosis of the hepatic disease preceded that of MN in 5 patients (patients 1 to 5), 2 of these 5 patients were treated for their liver disease with steroids (patient 4) or with steroids and azathioprine (patient 5) when renal biopsy was performed whereas other patients did not receive steroids or immunosuppressive agents at the time of renal biopsy. At the time of hepatic disease, the liver tests showed isolated hepatic cytolysis in 2 cases (patients 2 and 5), isolated cholestasis in 2 cases (patients 1 and 10), and both cytolysis and cholestasis in 6 cases (patients 3, 4, 6, 7, 8, and 9). While presenting with MN, all except 1 patient (patient 1) had nephrotic range proteinuria, with a mean value of 5.5 g/d (range 1.6–11 g/d). The mean serum albumin concentration was 24.1 g/L (range 9.6–34 g/L) and the mean eGFR was 75.5 mL/min/1.73 m2 (range 14–132 mL/min/1.73 m2). Three patients displayed reduced kidney function (patients 4, 6, 8). None of the patients displayed acute kidney injury according to AKIN criteria. The results of tests for serum anti-PLA2R antibodies were available for 9 patients (not available for patient 10). The test was positive for 4 patients (patients 5, 6, 7, 9); of the 5 patients negative for serum anti-PLA2R antibodies, 3 were under steroid and/or immunosuppressive therapy at the time of PLA2R antibody determination (patient 1 received steroids and mycophenolate mofetil for MN treatment, patient 4 was treated exclusively with steroids for AIH, and patient 8 was given steroids and azathioprine for AIH and rituximab for MN treatment) and 2 were not receiving treatment (patients 2 and 3).
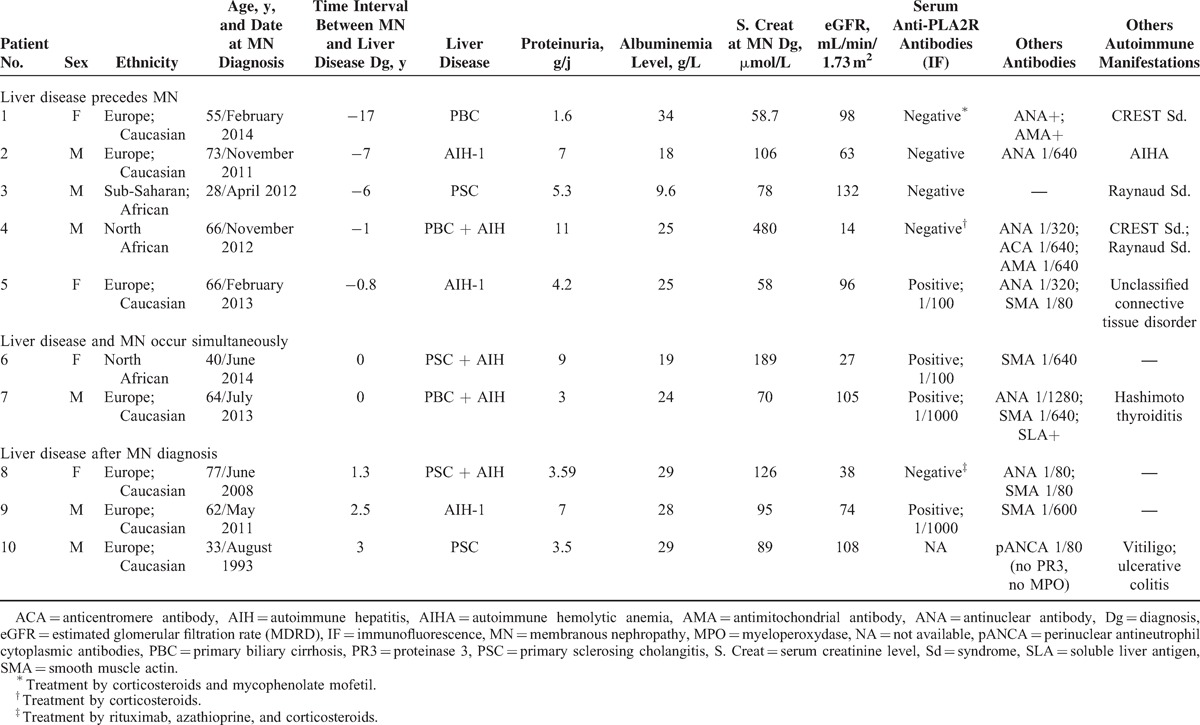
TABLE 1.
Demographic, Clinical, and Laboratory Characteristics of the 10 Patients With MN Associated With Liver Diseases Related to Immunological Disorders
Kidney and Liver Biopsy Findings
The nature of Ig and complement subepithelial deposits is shown in Table 2. C1q deposits were weakly positive for 2 patients (patients 2 and 4) with positive ANA but without diagnostic criteria for SLE. One patient displayed acute interstitial nephritis associated with MN diagnosis but immunohistochemistry for IgG4 antibody was negative, excluding the diagnosis of IgG4-related disease (data not shown). The most frequent IF pattern observed in 4 patients (patients 2, 6, 8, 9) was the coexistence of IgG1 and IgG4 immune deposits with predominance of IgG4 (Figure 1). In 2 patients, IgG4 was the predominant glomerular Ig subclass in association with IgG1 and IgG3 deposits in 1 case (patient 3) and exclusively associated with IgG3 deposits in the other (patient 7). In 1 patient (patient 4), IgG1 was the only Ig subclass found in glomerular deposits. Consistent with a previous study,35 we found a very weak basal expression of PLA2R in normal human kidney (Figure 2A). On the contrary, PLA2R expression, with a fine granular pattern, was clearly induced in glomeruli from patients with idiopathic MN (Figure 2B). A similar distribution was found in 7 of the 9 patients (77.8%) in whom renal biopsy was available (patients 1 and 3 were negative for PLA2R immunostaining and renal tissue specimen was not available for patient 2) (Figure 2C and D). We next analyzed PLA2R expression in liver parenchyma. In normal liver tissue, PLA2R was undetectable (Figure 3A). Then, hepatic biopsies from autoimmune liver diseases with (n = 7) and without MN (n = 7) were studied (Figure 3B and C). Regardless of the nature of liver diseases (including AIH, PBC, and PSC) and the presence or absence of MN, we did not detect significant PLA2R staining in liver biopsies (Table 2). PLA2R was not expressed in physiological or pathological conditions in intrahepatic bile duct, hepatocytes, portal areas, or central venules. The only positive signals observed were nonspecific staining in lipofuscin and pigmented macrophages (Figure 3D).
TABLE 2.
Renal and Liver Biopsy Findings of the 10 Patients With MN Associated With Liver Diseases Related to Immunological Disorders
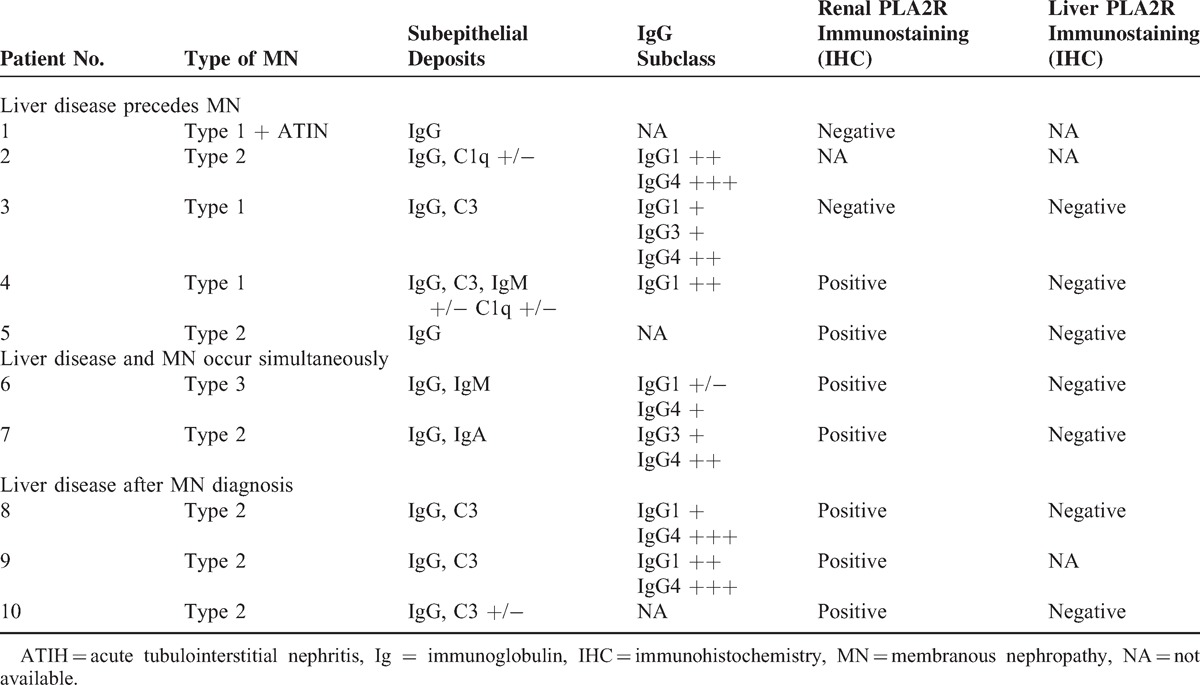
FIGURE 1.
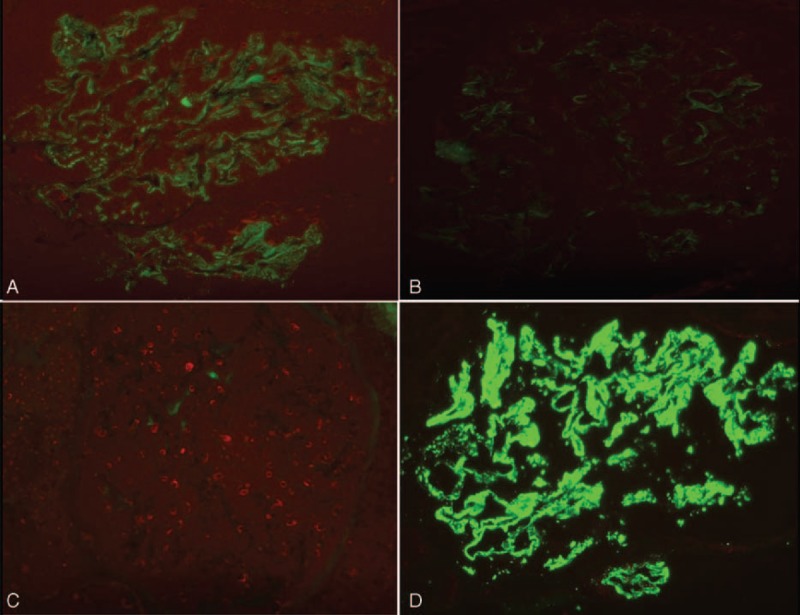
Immunofluorescence staining for IgG subclasses in patient 8 with PSC and AIH (original magnification ×400). Granular staining for (A) IgG1 and (D) IgG4 in the subepithelial portion along the glomerular capillary wall with predominance of IgG4. Immunofluorescence staining for (B) IgG2 and (C) IgG3 was negative. AIH = autoimmune hepatitis, IgG = immunoglobulin G, PSC = primary sclerosing cholangitis.
FIGURE 2.
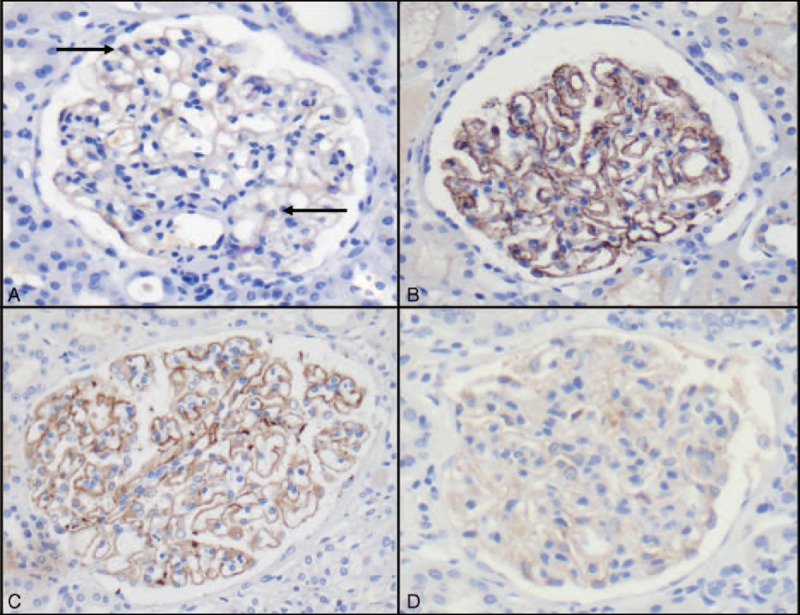
PLA2R immunohistochemistry analysis (original magnification ×400). (A) Normal kidney showed a weak PLA2R signal in podocyte cytoplasm (arrow). (B) Granular staining along glomerular basement membrane in 1 patient with idiopathic MN and who was positive for circulating PLA2R antibodies (positive control). (C) Parietal and granular staining for PLA2R in 1 patient with MN associated with liver overlap syndrome (patient 8). (D) No significant PLA2 staining was detected in podocytes (enhancement without parietal staining) from patient 1. PLA2R = phospholipase A2 receptor.
FIGURE 3.
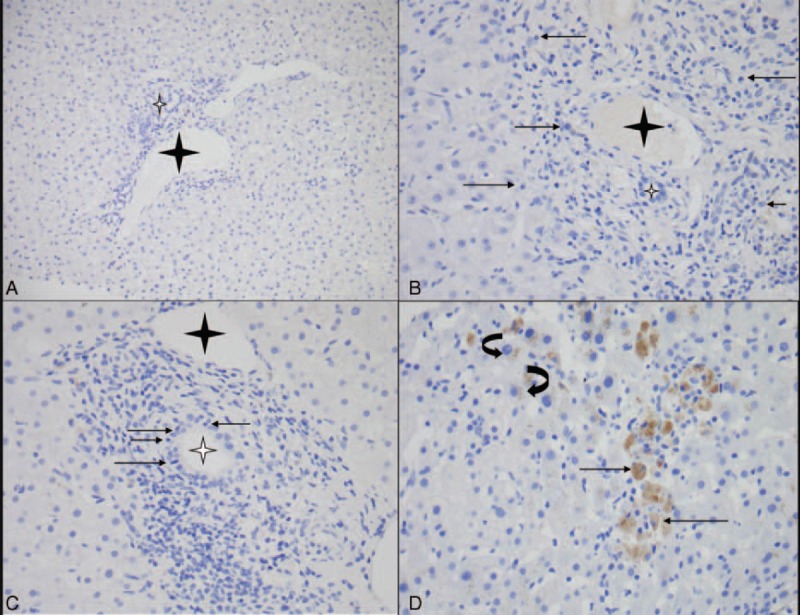
PLA2R immunostaining in normal liver and cases of autoimmune liver diseases (with or without MN). (A) Absence of PLA2R expression in hepatocytes and portal area in normal liver tissue specimen (original magnification ×20). (B) PLA2R immunostaining gave negative results in AIH (control) with portal infiltrate rich in plasmocytes (arrow) with interface hepatitis (original magnification ×40) and (C) in PBC (control) with portal inflammation and bile duct damage (arrow: intraepithelial lymphocyte in bile duct) (original magnification ×40). A similar pattern (no significant PLA2R signal) was observed in patients with autoimmune and/or immunological-related liver disease associated with MN (Table 3). (D) Nonspecific cytoplasmic staining in macrophages (arrow) and hepatocytes (lipofuschines: curved arrow) in patient 5 (original magnification ×40). The interlobular bile duct and portal vein are indicated with white and black stars, respectively. PLA2R = phospholipase A2 receptor.
Treatment and Outcome of MN Associated With Autoimmune or Inflammatory Liver Disease
All patients received a renin–angiotensin–aldosterone system blockade treatment at the time of MN diagnosis. When liver disease was diagnosed, ursodeoxycholic acid therapy was administered to all except 1 patient (patient 9). Steroids, either alone or in association with other immunosuppressive agents (azathioprine in 3 cases), were started for AIH patients (patients 4, 5, 6, and 8) (Table 3). One patient with AIH associated with fulminant hepatitis (patient 9) and another (patient 2) did not receive steroid therapy at the time of AIH diagnosis. Three patients (patients 3, 4, 10) developed progressive cirrhosis, requiring liver transplantation in 2 cases (patients 3 and 10). One patient (patient 9) underwent liver transplantation quickly because of fulminant hepatitis related to AIH. Spontaneous complete remission of NS was observed in 3 cases (patients 2, 3, 10) whereas 1 untreated patient (patient 9) had persistent nephrotic proteinuria at the end of the follow-up (6 months). Five patients received specific treatment for MN (patients 1, 5, 6, 7, 8) (Table 3). In 1 case (patient 7), immunosuppressive therapy was stopped because of the emergence of lung cancer; 3 patients went into partial remission under immunosuppressive therapy. Among the patients with MN occurring after the diagnosis of liver disease (patients 1 to 5), 2 were considered to be in remission of liver disease when the NS was diagnosed (patients 1 and 2). In 2 cases (patients 6 and 8), there was simultaneous remission of MN and liver disease under specific treatment, but there was early relapse of MN in patient 8. After a mean follow-up of 31 months (range from 4 to 132 months), 2 patients required intermittent hemodialysis (patients 4 and 8).
TABLE 3.
Treatment and Outcome of the 10 Patients With MN Associated With Liver Diseases Related to Immunological Disorders
DISCUSSION
One of the first objectives of the clinical management of a patient with recently diagnosed MN is to determine if this glomerular disease should be considered to be idiopathic or secondary to an underlying disease. Indeed, this distinction is of value for the therapeutic management of MN. MN can be associated with a broad spectrum of clinical conditions including chronic infections, cancer, drug exposure, and autoimmune diseases.3 Case reports have described the occurrence of MN in the context of liver diseases related to immunological disorders suggesting a potential relationship between MN and these conditions. However, there have been no extensive studies of the clinical, biological, pathological data and therapeutic management of these patients.
One of the aims of this study was to identify features specific to this association and thereby help elucidate the pathophysiology of these diseases. The recent discovery of PLA2R as a major target antigen of idiopathic MN led us to investigate whether PLA2R antibodies are involved in the pathogenesis of MN associated with immunological disorder-related liver disease. Four out of 9 patients had circulating serum PLA2R antibodies. In 3 of the cases scoring seronegative, the test was performed while the patient was under steroid and/or immunosuppressive treatments that significantly reduce the level of PLA2R antibodies.36,37 We demonstrated a significant PLA2R immunostaining in 7 patients (7 out of 9, 77.8%). As reported by Debiec and Ronco,7 this suggests that the absence of detectable circulating antibodies in the serum does not preclude positive immunostaining for PLA2R in renal biopsy specimens. Only 2 patients were negative for both circulating PLA2R antibodies and PLA2R staining on renal biopsy. This is consistent with MN occurring in a context of autoimmune liver disease being a fortuitous association rather than a related disorder. However, it is also possible that PLA2R detection in this context is not specific, and that the true autoantigen target remains to be discovered. Recent studies yielded contradictory results concerning the prevalence of PLA2R antibodies in possible secondary MN. Hoxha et al35 showed that secondary MN is characterized by the absence of PLA2R immunostaining in glomerular deposits. By contrast, in the study by Larsen et al,10 positive glomerular staining for PLA2R was a frequent finding among patients with secondary MN (64% for hepatitis C-related MN, 75% for sarcoidosis-related MN, and 25% for neoplasia-related MN). In our study, the high frequency of patients with PLA2R antigen in glomerular deposits led us to test for PLA2R antigen in the liver: it may be a target for both glomerular and liver injury. PLA2R was not expressed in normal liver tissue and our immunohistochemistry study of liver biopsy specimens from patients with PBC, AIH, and PSC associated or not with MN did not reveal any specific induction of PLA2R in liver parenchyma.
Regarding neoplasia-related MN, light microscopy examination is generally not sufficient to distinguish primary from secondary forms of MN, but the presence of >8 inflammatory cells infiltrating the glomeruli seems to strongly increase the likelihood of malignancy.38 Our pathological study did not reveal light microscopy-specific features concerning MN glomerular lesions in patients with liver disease related to immunological disorders. The IgG subclass distribution in glomerular deposits seems to be helpful for differentiating primary from secondary MN. IgG4 is predominantly found in patients with idiopathic MN39 whereas IgG1 and IgG2 seem to be preferentially found in malignancy-associated MN40 and IgG1 and IgG3 in lupus-related MN.12,39 The absence of IgG4 may be associated with the subsequent risk of developing secondary malignancy.41 In all except 1 of our patients, IgG4 was the major component of the glomerular immune deposits. This and the high frequency of PLA2R deposits in glomeruli in these patients raise the question of whether MN in cases of liver disease related to autoimmune or immunological disorders is coincidental and unrelated. Nevertheless, in the study of Larsen et al,10 all cases of secondary MN that were PLA2R positive showed IgG4-predominant deposits.
Previous case reports suggested that PBC was the immunological liver disease most frequently associated with MN.15–22,24 We report here that MN did not occur only in patients with PBC but can also affect patients with PSC and AIH. As these liver disorders do not share common pathophysiological mechanisms, the existence of a direct molecular link between MN and these liver diseases remains uncertain. Nevertheless, the close temporal relationship found in 5 patients (the interval between the diagnoses of glomerular and liver disease being <1.5 years) strongly suggests that MN and liver involvement may be linked in some cases.
PBC and AIH, like MN, are currently considered to be 2 genuine autoimmune diseases characterized by the presence of ≥1 circulating autoantibodies associated with specific histological lesions in the liver.42,43 By contrast, autoantibodies are not key diagnostic factors for PSC although this liver disease seems to be triggered by several immunological mechanisms.44 Note that the definitive diagnosis of liver disease included both AIH and PBC or AIH and PSC in 4 cases (2 cases for each association), and 7 patients exhibited MN in association with AIH suggesting that specific antibodies might be involved in these patients. Genetic susceptibility has been demonstrated as promoting the risk of developing MN, particularly in the context of human leukocyte antigen (HLA) system polymorphism.2 Findings of a genome-wide association study (GWAS) of idiopathic MN patients with European or Asian ancestries revealed a significant association with HLA-DQA1 and PLA2R1 loci.45,46 GWAS analyses in a context of autoimmune liver diseases have led to similar observations.43,47,48 Altogether, these various findings highly suggest that HLA polymorphisms may be involved in the pathogenesis of MN in a context of autoimmune liver disorders. Unfortunately, the retrospective nature of our study did not allow us to test this hypothesis.
The optimal therapeutic management of secondary MN is to treat the underlying disorder implicated in the etiology of MN.3 Our retrospective study did not demonstrate a direct relationship in terms of remission between glomerular and liver diseases. Among the 5 patients with a close temporal relationship (interval of <1.5 years) between MN and liver disease diagnoses, specific immunosuppressive therapy used to treat MN (rituximab administration in 2 cases and steroids in association with cyclophosphamide in 2 cases) was associated with partial remission of MN simultaneously with complete remission of liver disease in only 2 cases. The 2 other patients displayed partial remission of their glomerular disease without concomitant remission of liver disease. The mean delay between MN and liver disorder was greater in other patients, such that this retrospective study cannot draw firm conclusions about any potential correlation between immunological activity of MN and liver disease based on therapy responsiveness.
CONCLUSION
We report here a unique series of 10 patients with MN in a context of autoimmune and/or immunological-related liver disease. Given the limited number of patients reported in this cohort study, no firm conclusion can be drawn on pathophysiological mechanisms linking MN to liver disease. We found that isolated AIH or AIH with concomitant PBC or PSC liver injury were the most frequent immunological liver disorder associated with MN. It is plausible that there is a common molecular link, involving genetic susceptibility, between MN and immunological liver disease; however, the small number of patients exhibiting this association, as well as the results of PLA2R immunostaining and the distribution of IgG subclasses in renal biopsy specimens did not provide evidence that AIH, PBC, and PSC are among the underlying conditions promoting MN occurrence. Whether MN and immunological-related liver diseases share a common causal mechanism remains to be determined.
Acknowledgments
The authors would like to thank Professor Elie Serge Zafrani (Department of Pathology, Henri Mondor Hospital), Professor Dominique Wendum (Department of Pathology, Saint Antoine Hospital), Professor Marianne Ziol (Department of Pathology, Jean Verdier Hospital), Dr Laurence Choudat (Department of Pathology, Bichat Hospital), Dr Francine Walker (Department of Pathology, Bichat Hospital), Dr Julien Calderaro (Department of Pathology, Henri Mondor Hospital), and Dr Nouria Bey Boumezrag (Department of Pathology, Saint Antoine Hospital) for providing liver biopsies from patients and controls.
Footnotes
Abbreviations: ACR = American College of Rheumatology, AIH = autoimmune hepatitis, AKI = acute kidney injury, AKIN = Acute Kidney Injury Network, AMAs = antimitochondrial antibodies, ANA = antinuclear antibody, GFR = glomerular filtration rate, GWAS = genome-wide association study, HLA = human leukocyte antigen, IF = immunofluorescence, Ig = immunoglobulin, LKM = liver-kidney microsomal antibodies, MN = membranous nephropathy, NS = nephrotic syndrome, PBC = primary biliary cirrhosis, PLA2R = phospholipase A2 receptor, PSC = primary sclerosing cholangitis, SLE = systemic lupus erythematous, SMA = smooth muscle actin.
MD and AM contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol 2012; 8:203–221. [DOI] [PubMed] [Google Scholar]
- 2.Debiec H, Ronco P. Immunopathogenesis of membranous nephropathy: an update. Semin Immunopathol 2014; 36:381–397. [DOI] [PubMed] [Google Scholar]
- 3.Glassock RJ. The pathogenesis of membranous nephropathy: evolution and revolution. Curr Opin Nephrol Hypertens 2012; 21:235–242. [DOI] [PubMed] [Google Scholar]
- 4.Glassock RJ. Human idiopathic membranous nephropathy: a mystery solved? N Engl J Med 2009; 361:81–83. [DOI] [PubMed] [Google Scholar]
- 5.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck LH, Jr, Salant DJ. Membranous nephropathy: from models to man. J Clin Invest 2014; 124:2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011; 364:689–690. [DOI] [PubMed] [Google Scholar]
- 8.Svobodova B, Honsova E, Ronco P, et al. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 2013; 28:1839–1844. [DOI] [PubMed] [Google Scholar]
- 9.Qin W, Beck LH, Jr, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 2011; 22:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Messias NC, Silva FG, et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 2013; 26:709–715. [DOI] [PubMed] [Google Scholar]
- 11.Byrne-Dugan CJ, Collins AB, Lam AQ, et al. Membranous nephropathy as a manifestation of graft-versus-host disease: association with HLA antigen typing, phospholipase A2 receptor, and C4d. Am J Kidney Dis 2014; 64:987–993. [DOI] [PubMed] [Google Scholar]
- 12.Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis 1994; 23:358–364. [DOI] [PubMed] [Google Scholar]
- 13.Alexander MP, Larsen CP, Gibson IW, et al. Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 2012; 83:455–462. [DOI] [PubMed] [Google Scholar]
- 14.Stehlé T, Joly D, Vanhille P, et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: a multicenter study. Orphanet J Rare Dis 2013; 8:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bindi D, Johanet C, Gilson B, et al. Membranous glomerulonephritis associated with primary biliary cirrhosis: a pathogenic role of anti-M2 antibodies? Gastroenterol Clin Biol 1993; 17:142–143. [PubMed] [Google Scholar]
- 16.Carella G, Marra L, Bevilacqua E. A case of membranous glomerulonephritis in the course of primary biliary cirrhosis. Am J Gastroenterol 1989; 84:579–580. [PubMed] [Google Scholar]
- 17.Efe C, Ozaslan E, Purnak T, et al. Membranous glomerulonephritis associated with autoimmune hepatitis and primary biliary cirrhosis overlap syndrome: a very rare condition. Eur J Gastroenterol Hepatol 2010; 22:1149–1150. [DOI] [PubMed] [Google Scholar]
- 18.Goto T, Komatsu M, Fujii T, et al. Primary biliary cirrhosis associated with membranous glomerulonephritis. Intern Med 1999; 38:22–26. [DOI] [PubMed] [Google Scholar]
- 19.Rai GS, Hamlyn AN, Dahl MG, et al. Primary biliary cirrhosis, cutaneous capillaritis, and IgM-associated membranous glomerulonephritis. Br Med J 1977; 1:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reitsma DJ, Gratama S, Vroom TM. Clinical remission of membranous glomerulonephritis in primary biliary cirrhosis with cutaneous vasculitis. Br Med J (Clin Res Ed) 1984; 288:27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamaki Y, Hayashi M, Wakino S, et al. A case of membranous nephropathy with primary biliary cirrhosis and cyclosporine-induced remission. Intern Med 2011; 50:233–238. [DOI] [PubMed] [Google Scholar]
- 22.Singhal PC, Scharschmidt LA. Membranous nephropathy associated with primary biliary cirrhosis and bullous pemphigoid. Ann Allergy 1985; 55:484–485. [PubMed] [Google Scholar]
- 23.Verresen L, Waer M, Verberckmoes R, et al. Primary sclerosing cholangitis associated with membranous nephropathy. Ann Intern Med 1988; 108:909–910. [DOI] [PubMed] [Google Scholar]
- 24.Vlassopoulos D, Divari E, Savva S, et al. Membranous glomerulonephritis associated with primary biliary cirrhosis. Nephrol Dial Transplant 1998; 13:459–461. [DOI] [PubMed] [Google Scholar]
- 25.Warling O, Bovy C, Coïmbra C, et al. Overlap syndrome consisting of PSC-AIH with concomitant presence of a membranous glomerulonephritis and ulcerative colitis. World J Gastroenterol 2014; 20:4811–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 27.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindor KD, Gershwin ME, Poupon R, et al. American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 2009; 50:291–308. [DOI] [PubMed] [Google Scholar]
- 29.Selmi C, Bowlus CL, Gershwin ME, et al. Primary biliary cirrhosis. Lancet 2011; 377:1600–1609. [DOI] [PubMed] [Google Scholar]
- 30.EASL. Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009; 51:237–267. [DOI] [PubMed] [Google Scholar]
- 31.Hennes EM, Zeniya M, Czaja AJ, et al. International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008; 48:169–176. [DOI] [PubMed] [Google Scholar]
- 32.Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008; 48:871–877. [DOI] [PubMed] [Google Scholar]
- 33.Manns MP, Czaja AJ, Gorham JD, et al. American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51:2193–2213. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 35.Hoxha E, Kneissler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 2012; 82:797–804. [DOI] [PubMed] [Google Scholar]
- 36.Bech AP, Hofstra JM, Brenchley PE, et al. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2014; 9:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck LH, Jr, Fervenza FC, Beck DM, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 2011; 22:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefaucheur C, Stengel B, Nochy D, et al. GN-PROGRESS Study Group. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int 2006; 70:1510–1517. [DOI] [PubMed] [Google Scholar]
- 39.Kuroki A, Shibata T, Honda H, et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern Med 2002; 41:936–942. [DOI] [PubMed] [Google Scholar]
- 40.Ohtani H, Wakui H, Komatsuda A, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 2004; 19:574–579. [DOI] [PubMed] [Google Scholar]
- 41.Qu Z, Liu G, Li J, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant 2012; 27:1931–1937. [DOI] [PubMed] [Google Scholar]
- 42.Oertelt S, Rieger R, Selmi C, et al. A sensitive bead assay for antimitochondrial antibodies: chipping away at AMA-negative primary biliary cirrhosis. Hepatology 2007; 45:659–665. [DOI] [PubMed] [Google Scholar]
- 43.Mackay IR. A 50-year experience with autoimmune hepatitis: and where are we now? J Gastroenterol 2011; 46 suppl 1:17–28. [DOI] [PubMed] [Google Scholar]
- 44.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet 2013; 382:1587–1599. [DOI] [PubMed] [Google Scholar]
- 45.Stanescu HC, Arcos-Burgos M, Medlar A, et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 2011; 364:616–626. [DOI] [PubMed] [Google Scholar]
- 46.Lv J, Hou W, Zhou X, et al. Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol 2013; 24:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Invernizzi P, Lu Y, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet 2010; 42:658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 2010; 138:1102–1111. [DOI] [PubMed] [Google Scholar]


