Abstract
We performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the protective effects of rosuvastatin on contrast-induced acute kidney injury (CI-AKI) and major adverse cardiovascular events (MACEs) in patients undergoing cardiac catherization.
PubMed, MEDLINE, Web of Science, EMBASE, ClinicalTrials.gov, and the Cochrane Central RCTs were searched for RCTs from inception to May 2015, to compare rosuvastatin for preventing CI-AKI with placebo treatment in patients undergoing cardiac catherization.
Five RCTs with a total of 4045 patients involving 2020 patients pretreated with rosuvastatin and 2025 control patients were identified and analyzed. Patients treated with rosuvastatin had a 51% lower risk of CI-AKI compared with the control group based on a fixed-effect model (OR = 0.49, 95% CI = 0.37–0.66, P < 0.001), and showed a trend toward a reduced risk of MACEs (OR = 0.62, 95% CI = 0.36–1.07, P = 0.08). A subgroup analysis showed that studies with Jadad score ≥3 showed a significant reduction of CI-AKI (OR = 0.53, 95% CI, 0.38–0.73, P < 0.001). However, the risk of CI-AKI did not significantly differ in the studies with Jadad score <3 (OR = 0.54, 95% CI, 0.13–2.24, P = 0.40). In addition, the rosuvastatin treatment showed no effect for preventing CI-AKI in patients with chronic kidney disease (CKD) undergoing elective cardiac catherization (I2 = 0%, OR = 0.81, 95% CI = 0.41–1.61, P = 0.55).
This updated meta-analysis demonstrated that preprocedural rosuvastatin treatment could significantly reduce the incidence of CI-AKI, with a trend toward a reduced risk of MACEs in patients undergoing cardiac catheterization. However, rosuvastatin treatment did not seem to be effective for preventing CI-AKI in CKD patients undergoing elective cardiac catheterization.
INTRODUCTION
Contrast-induced acute kidney injury (CI-AKI) remains one of the most important clinical complications of cardiac catheterization, associated with both short- and long-term renal and cardiovascular adverse outcomes.1 An increasing number of studies have been performed to determine new or timely effective strategies for preventing CI-AKI, other than periprocedural hydration and limiting the volume of contrast medium (CM).
Increasing evidence has demonstrated that statins can reduce the risk of CI-AKI by means of pleiotropic effects on factors contributing to the progression of CI-AKI, such as improving endothelial function, maintaining nitric oxide production, and reducing oxidative stress.2 Previous studies focused on beneficial effects of classic statins such as atorvastatin on CI-AKI.3 However, pleiotropic effects of different statins (such as rosuvastatin and atorvastatin) were not the same. The differences might be associated with lipophilicity, antiinflammatory effects, low-density lipoprotein cholesterol lowering potency, renoprotection, and the effects on myocardial function.4 Recent randomized clinical trials (RCTs)5–7 were performed to demonstrate the effect of rosuvastatin on CI-AKI. However, results of these studies were conflicting, and few meta-analyses have been performed to explore this issue. Therefore, we performed a meta-analysis of RCTs, to evaluate the effects of rosuvastatin on CI-AKI prevention.
METHODS
Ethics Statement
As this study is a meta-analysis, ethical approval was not required.
Search Strategies
All studies relevant to the effect of rosuvastatin on CI-AKI in patients undergoing cardiac catheterization were identified by searching PubMed (1998 through February 10, 2015), MEDLINE, EMBASE (1998 through February 10, 2015), ClinicalTrials.gov, and the Cochrane Central Register of Controlled Trials databases. According to optimal search strategies, we used terms such as rosuvastatin AND (contrast OR contrast media OR contrast medium OR contrast agent) AND (nephropathy OR kidney injury OR renal injury OR renal failure) AND (coronary angiography or percutaneous interventions or cardiac catheterization). Additionally, a manual search was conducted on the scientific sessions of the European Society of Congress, the American Heart Association, and the American College of Cardiology from 1998 to 2015. We included unpublished studies which only had abstracts online, and published studies in English.
Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: RCTs investigating the effect of rosuvastatin on CI-AKI; compared with a placebo, the treatment groups received rosuvastatin before CM exposure at any dose, for any length of time; studies directly comparing 2 different doses of rosuvastatin; and studies that provided oral or intravenous N-acetylcysteine (NAC) preparations were only included if both arms received NAC. Exclusion criteria for studies were as follows: nonrandomized trials; unclear CI-AKI definitions not in accordance with the current guideline; duplicate publications; and abstracts that did not contain complete results.
Study Selection and End Points
Two independent reviewers (YY and YXW) screened the selected studies based on their titles and abstracts. The full text of an article was reviewed carefully for inclusion if the topic was unclear after screening its title and abstract. Any disagreements between the investigators were resolved through by a third reviewer (YZH).
The primary endpoint was the incidence of CI-AKI, defined as an absolute increase in serum creatinine (SCr) ≥0.5 mg/dL or an increase ≥25% from baseline within 72 hours after CM exposure.8 The secondary endpoints were major adverse cardiovascular events (MACEs), including all-cause mortality, the requirement of renal replacement therapy, nonfatal myocardial infarction, or ischemic cerebrovascular accidents.
Data Extraction
Two independent reviewers (YY and YXW) extracted data from the identified studies using a standard data extraction form. The following data was extracted: study characteristics, patient characteristics, inclusion and exclusion criteria, treatment protocol, the type and dose of CM, and definitions and incidences of CI-AKI.
Quality Assessment
The identified studies were assessed for methodological quality by 2 independent reviewers using the Jadad scoring system.9 The quality assessment was judged according to concealment of treatment allocation, the similarity of the study groups at baseline, eligibility criteria, any blinding procedure used, reporting on those lost to follow-up, and intention to treat analysis.10 Disagreements were resolved by a third reviewer (YZH).
Statistical Analysis
The effect of statins on CI-AKI development was given as an odds ratio (OR) with the 95% confidence interval (CI). The Q statistic was calculated and heterogeneity was quantified using the I2 statistic. We regarded I2 ≤ 25%, 25–50%, and >50% as low, moderate, and high heterogeneity, respectively.11 An I2 > 50% indicates at least moderate statistical heterogeneity. When a pooled analysis resulted in significant heterogeneity, the random effects model was used. Otherwise, a fixed effect model was used. We also performed subgroup analyses based on patients with chronic kidney disease (CKD), studies with a Jadad risk score ≥3 or not, to identify potential differences in treatment across the trials. Publication bias was evaluated using a funnel plot. Egger's regression asymmetry test was also performed to explore potential publication bias. All statistical analyses were performed using Review Manager Version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and STATA version 11 (Stata Corp, College Station, TX). All tests were 2-tailed and P < 0.05 was considered significant.
RESULTS
Characteristics of the Included Studies
The flow chart of the search strategy is provided in Figure 1. After excluding 33 duplicates and 11 clearly irrelevant papers, 20 articles remained. The clearly irrelevant papers included nonrandomized studies, and no target outcomes or intervention procedures were described after carefully reading the titles and abstracts. After systematical assessment of the 20 potentially relevant articles, 5 RCTs were included in the analysis.5–7,12,13
FIGURE 1.
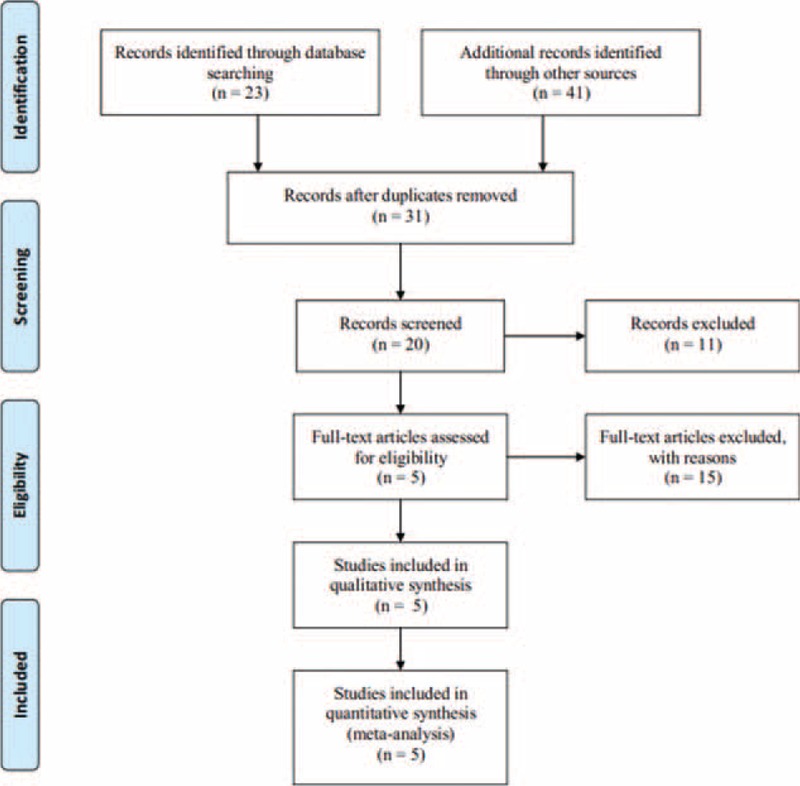
Flow diagram showing the exclusion and inclusion of trials in this meta-analysis.
A total of 4045 patients who underwent cardiac catheterization (coronary angiography/percutaneous coronary interventions) were included, 2020 in the rosuvastatin group and 2025 in the control group. The mean age of patients included in the studies ranged from 52 ± 11 to 68 ± 9 years. The mean baseline SCr level ranged from 0.81 ± 0.2 to 1.4 ± 0.5 mg/dL. The mean CM volume ranged from 72.2 to 150 mL. Four of the 5 trials evaluated the effect of statins on CI-AKI in patients with an estimated glomerular filtration rate (eGFR) ≤60 mL/minute/1.73 m2 or a creatinine clearance ≤60 mL/minute (Table 1).
TABLE 1.
Baseline Characteristic of Patients and Interventions of Included Studies
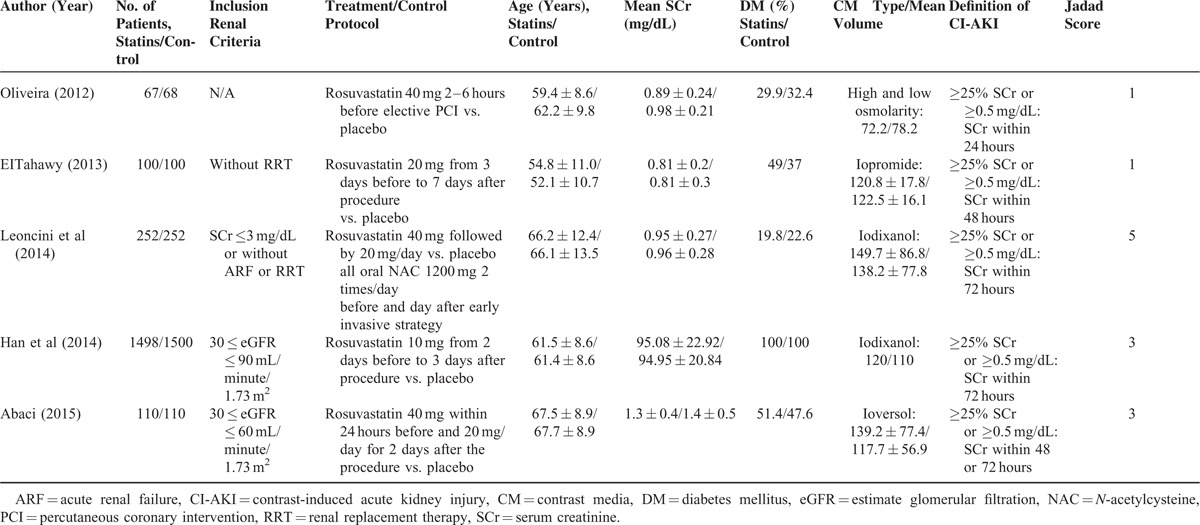
The criteria used to define CI-AKI were similar among the individual studies. Three trials used the definition of an absolute increase in the SCr of >0.5 mg/dL (44.2 mmol/L) or a relative increase of >25% from baseline to within 72 hours after CM exposure, and 1 trial employed the same SCr changes within 24 hours, and 1 trial within 48 hours.
Assessment of the Study Quality and Publication Bias
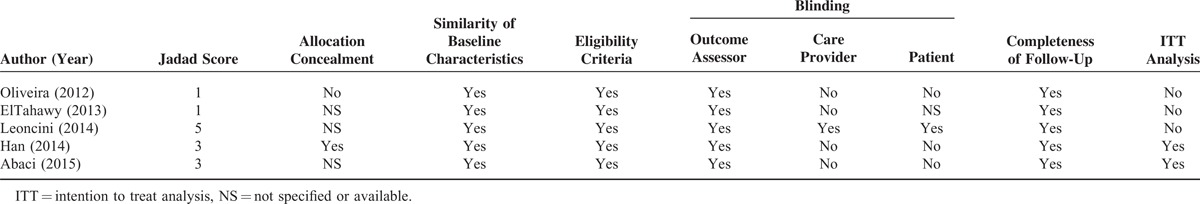
All of the studies included patients with similar baseline characteristics, and provided details about the eligibility criteria and the completeness of the follow-up. The intention to treat analysis was provided for 2 researches. Allocation concealment and blinding were both used in only 1 study and the quality characteristics of the studies are shown in Table 2.
TABLE 2.
Quality of Included Randomized Controlled Trials
Three of the studies were deemed to have a high risk of bias, while the remaining 2 had a low risk. The details of the funnel plot are shown in Figure 2. The funnel plot was relatively symmetrical, and the result of Egger's test demonstrated no obvious publication bias among the included trials for CI-AKI (P = 0.231) or MACE (P = 0.324).
FIGURE 2.
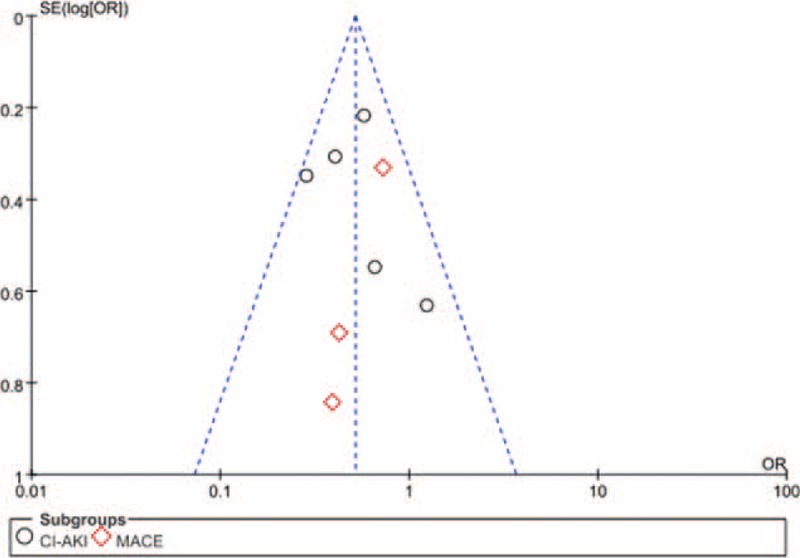
Funnel plot with 95% confidence interval (CI) for the subjective assessment of bias among the included studies.
Study Outcomes
All of the studies provided information on the CI-AKI incidence. Patients receiving rosuvastatin had a 51% lower risk of CI-AKI compared with controls based on a fixed-effect model (OR = 0.49, 95% CI = 0.37–0.66, P < 0.001) (Figure 3A). No significant heterogeneity existed across studies (I2 = 30%, P = 0.22).
FIGURE 3.
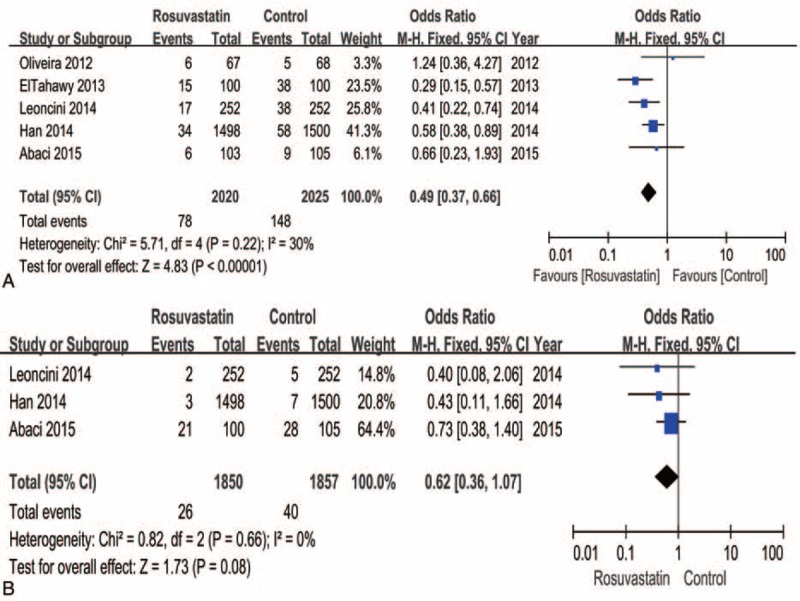
Forest plot of the odds ratio (OR) and 95% confidence interval (CI) for contrast-induced acute kidney injury (CI-AKI) (A) and major adverse cardiovascular events (MACEs) (B) among patients assigned to rosuvastatin versus placebo therapy.
The incidence of MACEs was only reported in 3 trials, but the trend indicated that rosuvastatin treatment reduced MACEs compared to controls (I2 = 0%, OR = 0.62, 95% CI = 0.36–1.07, P = 0.08) (Figure 3B).
Subgroup Analysis
We performed a subgroup analysis according to whether the Jadad score was ≥3 or not. The result indicated that studies with Jadad score ≥3 had significantly reduced CI-AKI (OR = 0.53, 95% CI, 0.38–0.73, P < 0.001). However, the risk of CI-AKI did not significantly differ in the studies with Jadad score <3 (OR = 0.54, 95% CI, 0.13–2.24, P = 0.40) (Figure 4).
FIGURE 4.
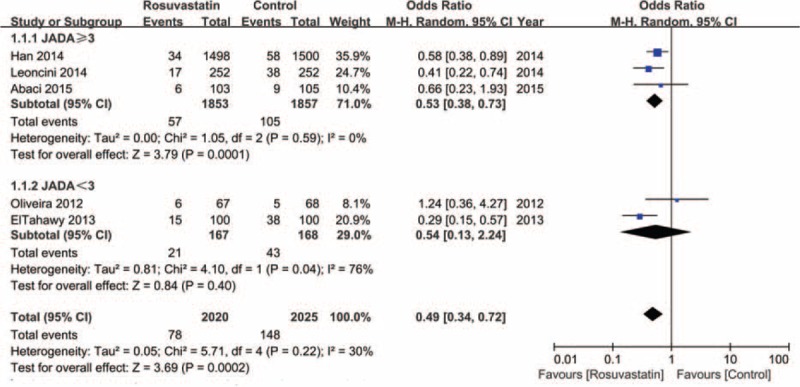
Subgroup analysis of the forest plot of odds ratio (OR) and 95% confidence interval (CI) for contrast-induced acute kidney injury (CI-AKI) among patients assigned to rosuvastatin versus placebo therapy according to the Jadad score.
Analysis of the subgroup of patients with CKD indicated that rosuvastatin treatment was beneficial for preventing CI-AKI compared to controls (I2 = 8%, OR = 0.57, 95% CI = 0.34–0.96, P = 0.04) (Figure 5A). However, when including CKD patients undergoing elective cardiac catheterization, rosuvastatin treatment showed no effect on preventing CI-AKI (I2 = 0%, OR = 0.81, 95% CI = 0.41–1.61, P = 0.55) (Figure 5B).
FIGURE 5.
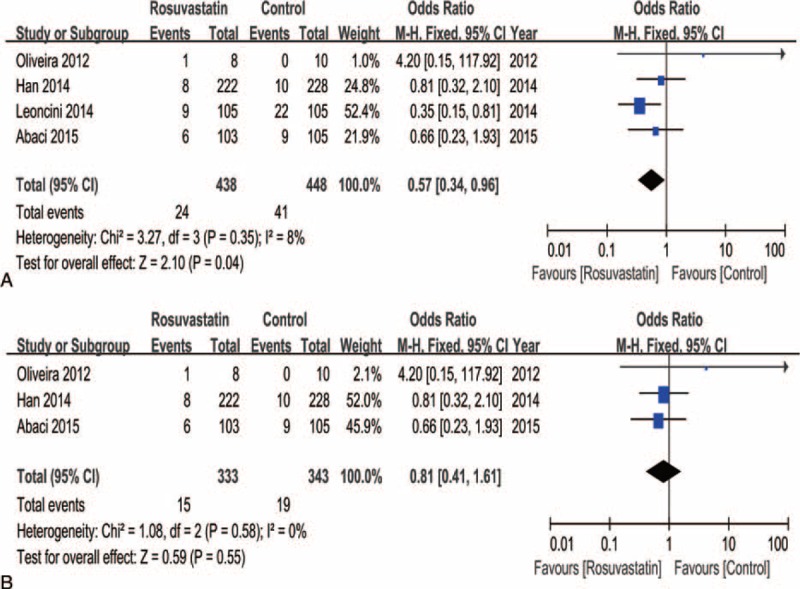
Subgroup analysis of the forest plot of odds ratio (OR) and 95% confidence interval (CI) for contrast-induced acute kidney injury (CI-AKI) among patients assigned to rosuvastatin versus placebo therapy with patients grouped based on chronic kidney disease (A) or treatment with elective cardiac catheterization (B).
DISCUSSION
The present meta-analysis demonstrated that pretreatment with rosuvastatin significantly decreased the incidence of CI-AKI in patients undergoing cardiac catheterization. Rosuvastatin treatment showed a nonsignificant trend toward decreasing the incidence of MACE. However, rosuvastatin therapy did not show a preventive effect on CI-AKI in CKD patients after elective cardiac catheterization.
CI-AKI, as a common serious complication after the use of CM, is the third most common cause of hospital-acquired renal insufficiency. The reported incidence of CI-AKI ranges from <5% in low-risk patients to 50% in high-risk patients, such as patients with diabetes mellitus or CKD.14 CI-AKI development has been associated with increased in-hospital and long-term morbidity and mortality, prolonged hospitalization, and long-term renal impairment.1 However, other than adequate periprocedure hydration and limiting the amount of CM used, few strategies have been proven effective for preventing CI-AKI. Strategies for reducing the CI-AKI risk have been a highly topical subject for years.
Recently, increasing evidence has demonstrated that statins could reduce the CI-AKI risk, through having beneficial effects on endothelial function, maintaining nitric oxide production, and reducing oxidative stress.15,16 Although meta-analyses evaluated the effect of statins on preventing CI-AKI, they mainly focused on beneficial effects of classic statins-like atorvastatin on CI-AKI.3 However, pleiotropic effects of different statins (such as rosuvastatin and atorvastatin) were not the same. The differences might be related to lipophilicity, antiinflammatory effects, low-density lipoprotein cholesterol lowering potency, renoprotection, and the effects on myocardial function.4 A large number of studies have been performed to evaluate the effect of rosuvastatin on CI-AKI, and the results remains conflicting. However, few meta-analyses that explore this issue have been done. To our knowledge, the present meta-analysis is the first to evaluate the role of rosuvastatin on CI-AKI development, and demonstrate that rosuvastatin significantly decreases CI-AKI risk in patients undergoing cardiac catheterization.
Rosuvastatin is a hydrophilic statin with acute pleiotropic effects; and it is different from a lipophilic statin such as atorvastatin. It has a longer plasma half-life and stronger antiinflammatory effects than atorvastatin.17 A recent experimental study performed by Ferreira et al demonstrated that rosuvastatin performed better than atorvastatin or simvastatin through attenuating both inflammation and oxidative stress parameters.18 In addition, short-term treatment with rosuvastatin may also improve eGFR, independent of lipid fraction changes, supporting the hypothesis that statins might have pleiotropic mechanisms of action that include renal benefits.19 Furthermore, rosuvastatin is a particularly potent HMG-CoA reductase inhibitor, reducing protein reabsorption in the proximal tubules.20 Reduced protein trafficking in the proximal tubules may result in reduced inflammation, endothelial dysfunction, and tubulointerstitial fibrosis, which are important contributors to CI-AKI development. Rosuvastatin was demonstrated to increase apolipoprotein A-I levels at all doses more than atorvastatin in a recent meta-analysis.21 Apolipoprotein A-I was proven to have antiinflammatory and antioxidant properties,22 which was involved in CI-AKI development.
Several recent studies have demonstrated that rosuvastatin therapy can reduce the risk of CI-AKI after CM exposure, consistent with the present meta-analysis result. Previous studies have indicated that rosuvastatin could exert a beneficial renoprotective effect even in patients with CKD.23 Patients with CKD had a significant higher mean C reactive protein concentration,24 which contributed to the development of CI-AKI,25 therefore rosuvastatin may be effective in such patients. However, when the study by Leoncini et al was excluded from our analysis, rosuvastatin was not effective at preventing CI-AKI in CKD patients undergoing elective cardiac catheterization, in accordance with studies by Han et al,5 Abaci et al,7 and Yun et al.26 The 2014 European Society of Cardiology's revascularization guidelines27 recommended as a Class IIa indication that patients with moderate to severe CKD be treated with statins (rosuvastatin or atorvastatin) before CM exposure for protection against CI-AKI. Leoncini et al6 and the meta-analysis by Liu et al3 were cited for this recommendation. However, Leoncini et al evaluated patients with acute coronary syndrome (ACS), who had inflammation in various organs, and the majority of the patients had an eGFR ≥60 mL/minute/1.73 m2. Their research demonstrated that rosuvastatin appears to exert more effective kidney protection in ACS subjects with higher baseline high sensitivity C reactive protein levels compared with those with lower levels.28 The majority of the patients in the meta-analysis by Liu et al also had an eGFR ≥60 mL/minute/1.73 m2. A preventive effect of statins on CI-AKI in patients with an eGFR <60 mL/minute/1.73 m2 was not demonstrated. Therefore, the present meta-analysis, together with previous studies,5,9,11 suggests that rosuvastatin might not be effective at preventing CI-AKI in stable CKD patients without evident inflammation who are referred for elective cardiac catheterization.
LIMITATIONS
This study had several limitations. First, patients in the rosuvastatin group were treated with different dose regimens for varied periods of time in the different studies, which might influence the conclusions about the efficacy of rosuvastatin in preventing CI-AKI. Second, publication bias is always a potential limitation since neutral or negative studies might not be published in a peer reviewed journal, whereas positive studies are more likely to be published. Third, even though SCr was used to define CI-AKI, limitations exist in its utility as a surrogate marker of renal function. The studies included in the present meta-analysis only used SCr to define CI-AKI, not cystatin C, which is a potentially more sensitive biomarker. Despite these limitations, the present review provides the most comprehensive analysis of the benefits of rosuvastatin for prevention of CI-AKI to date.
CONCLUSIONS
In conclusion, our analysis suggests that preprocedural rosuvastatin treatment significantly reduces the risk of CI-AKI. However, rosuvastatin treatment does not seem effective in the prevention of CI-AKI in CKD patients undergoing elective cardiac catheterization.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, CI-AKI = contrast-induced acute kidney injury, CKD = chronic kidney disease, CM = contrast media, MACEs = major adverse cardiovascular events, OR = odds ratio, RCT = randomized clinical trial, SCr = serum creatinine.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI Registry. JACC Cardiovasc Interv 2014; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeliger E, Sendeski M, Rihal CS, et al. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012; 33:2007–2015. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Liu Y, Fu L, et al. Efficacy of short-term high-dose statin in preventing contrast-induced nephropathy: a meta-analysis of seven randomized controlled trials. PLoS One 2012; 7:e34450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Liu YH, Tan N, et al. Comparison of the efficacy of rosuvastatin versus atorvastatin in preventing contrast induced nephropathy in patient with chronic kidney disease undergoing percutaneous coronary intervention. PLoS One 2014; 9:e111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y, Zhu G, Han L, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol 2014; 63:62–70. [DOI] [PubMed] [Google Scholar]
- 6.Leoncini M, Toso A, Maioli M, et al. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J Am Coll Cardiol 2014; 63:71–79. [DOI] [PubMed] [Google Scholar]
- 7.Abaci O, Arat OA, Kocas C, et al. Impact of Rosuvastatin on contrast-induced acute kidney injury in patients at high risk for nephropathy undergoing elective angiography. Am J Cardiol 2015; 115:867–871. [DOI] [PubMed] [Google Scholar]
- 8.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011; 21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998; 51:1235–1241. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira MSD, Martins KBA, Costa JR, Jr, et al. Impact on renal function of rosuvastatin preload prior to elective percutaneous coronary intervention chronic statin users. Revista Brasileira de Cardiologia Invasiva 2012; 20:303–308. [Google Scholar]
- 13.Ahmed Abdul-Aziz ElTahawy, Basem Elsaid Enany, Mohsen Fahmy Role of rosuvastatin pretreatment in prevention of contrast induced nephropathy in patients undergoing coronary angiography. Pak Heart J 2013; 46:250–254. [Google Scholar]
- 14.Aurelio A, Durante A. Contrast-induced nephropathy in percutaneous coronary interventions: pathogenesis, risk factors, outcome, prevention and treatment. Cardiology 2014; 128:62–72. [DOI] [PubMed] [Google Scholar]
- 15.Wassmann S, Faul A, Hennen B, et al. Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition on coronary endothelial function. Circ Res 2003; 93:e98–e103. [DOI] [PubMed] [Google Scholar]
- 16.Ongini E, Impagnatiello F, Bonazzi A, et al. Nitric oxide (NO)-releasing statin derivatives, a class of drugs showing enhanced antiproliferative and antiinflammatory properties. Proc Natl Acad Sci U S A 2004; 101:8497–8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth PP. An update on the benefits and risks of rosuvastatin therapy. Postgrad Med 2014; 126:7–17. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira TS, Lanzetti M, Barroso MV, et al. Oxidative stress and inflammation are differentially affected by atorvastatin, pravastatin, rosuvastatin, and simvastatin on lungs from mice exposed to cigarette smoke. Inflammation 2014; 37:1355–1365. [DOI] [PubMed] [Google Scholar]
- 19.Vidt DG, Harris S, McTaggart F, et al. Effect of short-term rosuvastatin treatment on estimated glomerular filtration rate. Am J Cardiol 2006; 97:1602–1606. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R. Statin induced proteinuria: renal injury or renoprotection? J Am Soc Nephrol 2004; 15:2502–2503. [DOI] [PubMed] [Google Scholar]
- 21.Takagi H, Umemoto T. A meta-analysis of randomized head-to-head trials for effects of rosuvastatin versus atorvastatin on apolipoprotein profiles. Am J Cardiol 2014; 113:292–301. [DOI] [PubMed] [Google Scholar]
- 22.Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med 2004; 255:188–205. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, MacFadyen J, Cressman M, et al. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273. [DOI] [PubMed] [Google Scholar]
- 24.Fox ER, Benjamin EJ, Sarpong DF, et al. The relation of C-reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol 2010; 11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty—contrast-induced nephropathy trial]). Am J Cardiol 2011; 108:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Yun KH, Lim JH, Hwang KB, et al. Effect of high dose rosuvastatin loading before percutaneous coronary intervention on contrast-induced nephropathy. Korean Circ J 2014; 44:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention 2015; 10:1024–1094. [DOI] [PubMed] [Google Scholar]
- 28.Toso A, Leoncini M, Maioli M, et al. Relationship between inflammation and benefits of early high-dose rosuvastatin on contrast-induced nephropathy in patients with acute coronary syndrome: the pathophysiological link in the PRATO-ACS study (Protective Effect of Rosuvastatin and Antiplatelet Therapy on Contrast-Induced Nephropathy and Myocardial Damage in Patients With Acute Coronary Syndrome Undergoing Coronary Intervention). JACC Cardiovasc Interv 2014; 7:1421–1429. [DOI] [PubMed] [Google Scholar]


