Abstract
The aim of this study was to compare the long-term safety and efficacy of laparoscopic surgery and laparotomy for high-risk endometrial cancer (EC).
A retrospective analysis based on our decade of clinical data of patients with high-risk EC who were comprehensively surgically staged by laparotomy or laparoscopy was performed. The surgical outcomes were compared between different approaches using propensity score matching (PSM).
Eighty-one pairs of patients from the initial 220 enrolled ones were matched by PSM. The mean operative time is similar between laparotomy and laparoscopy groups (258 minutes vs. 253 minutes). The laparoscopy cohort has less blood loss (107 mL vs.414 mL, P < 0.01), shorter hospital stay (14.7 days vs. 17.7 days, P = 0.02) and significant fewer intraoperative complications (6.2% vs. 25.9%, P < 0.01). The pelvic lymph nodes dissected by laparoscopy (16.4) were significant less than that dissected by laparotomy (21.9). The 5- and 10-year survival rate for laparotomy were 89.2% and 75.8% compared with 85.3% and 85.3% for the laparoscopy. There was no significant difference in overall survival (P = 0.97).
Laparoscopy is as effective as laparotomy in the long term and can be safely carried out in patients with high-risk EC for surgery treatment.
INTRODUCTION
Endometrial cancer (EC) is one of the most common gynecologic malignancies, occupying the sixth place in malignant tumor throughout the world with an increasing trend.1 The initial treatment of EC is comprehensive staging surgery, which consists of total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy and peritoneal cytology.2 Laparotomy is the traditional approach, but laparoscopic surgery is widely proposed recent years since it was first reported using for the treatment of EC in 1992 by Childers and Surwit.3 In lots of retrospective and prospective studies, laparoscopy has been showed with many advantages compared to laparotomy, such as smaller incision, less pain, and faster recovery.4–16 It seems that laparoscopic surgery for EC also has similar recurrence rate and survival outcome with laparotomy procedure.17–19 However, most of these studies have focused on patients with early stage or low-risk disease and few specifically on patients with advanced stage or high risk. What's more, data of long-term survival outcomes about laparoscopic surgery remain limited. Willis et al20 have published an article about laparoscopic hysterectomy in a high-risk series of patients with EC, showed that laparoscopy provide all the benefits inherent in minimal access surgery with no compromise in terms of outcomes, but there was no laparotomy data as control. Therefore, to compare the long-term safety and efficacy between laparoscopic surgery and laparotomy in patients with high-risk EC, we conducted a retrospective analysis based on our decade of clinical data using propensity score matching (PSM) in this research.
MATERIALS AND METHODS
A total of about 400 medical case notes of patients with histologically confirmed EC who has been received surgical treatment in Beijing Chao-Yang Hospital affiliated Capital Medical University from 2000 to April 2014 were reviewed. We selected subjects according to the following criteria: high-risk (grade ≥2, histologic nonendometrioid, myometrial invasion ≥1/2, positive pelvic or para-aortic nodes or stages II–IV) patients who had undergone comprehensive surgical staging in the form of peritoneal cytology, total hysterectomy, bilateral salpingo-oophorectomy, pelvic lymphadenectomy and para-aortic lymphadenectomy or sampling, +/− cytoreductive surgery by the same experienced surgical team via laparotomy (total abdominal hysterectomy, TAH) or laparoscopy (total laparoscopic hysterectomy, TLH). Para-aortic lymphadenectomy was performed when para-aortic lymph nodes sampled positive and nonendometrioid carcinomas. Patients who were enrolled (excepted staged I aG2 with histologic endometrioid) received postoperative adjuvant therapy including 3 to 6 cycles of chemotherapy (paclitaxel, 175 mg/m2 + carboplatin, area under the concentration–time curve [AUC] 5 or adriamycin, 45 mg/m2 + cisplatin, 50 mg/m2 + paclitaxel, 160 mg/m2) and vaginal cuff brachytherapy or pelvic irradiation (45 Gy over 5 weeks). Patients had antibiotic prophylaxis (cephalosporins or quinolones) half an hour before operation and venous thrombosis prophylaxis after operation by using molecular weight enoxaparin (0.4 mL every day for 4–5 days) and lower extremity sequential compression device or graduated compression stocking. Patients associated with severe cardiopulmonary disease (myocardial infarction, heart failure, or cerebral infarction), other malignancies or a bulky uterus ≥12 weeks of gestation were excluded. Because our data source was case notes and the missing data were few, we deleted the individuals with missing data.
The collected data of patients included age, body mass index (BMI), the scope of operation, operating time, blood loss, intraoperative and postoperative complications, first flatus time, postoperative hospital stay, surgical stage, histologic type and grade, number of lymph nodes dissection, and clinical outcome. The staging of patients was done according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 staging classification.21 Intraoperative complications included injuries to bladder, ureter, intestine or large vessels, bleeding requiring blood transfusion, conversion from laparoscopy to laparotomy and transferred to intensive care unit (ICU) for any unintended reasons. Postoperative complications included fever (>38°C) after 24 hours postoperatively, wound infection or dehiscence, deep-vein thrombosis (DVT), and pulmonary embolism (PE) within 30 days after operation. Recurrences were classified by the site that was first confirmed. The overall survival (OS) was defined as the period from the date of surgery to death or the date of last contact for living people. Follow-up is completed by telephone, E-mail, or outpatient service. All procedures were approved by the Ethics Committee for human experiments of the Capital Medical University.
Because parts of the baseline characteristics were statistical different between patients received laparoscopic surgery and laparotomy, PSM was used to control the imbalance. Eight covariates entered in the propensity model, including age, BMI, the scope of operation, myometrial invasion, pathologic type and grade, lymph node metastasis, and surgical stage. Propensity scores were calculated using a nonparsimonious multivariable logistic regression model to estimate the conditional probability of a patient receiving a surgery approach.22 Then a 1:1 match between the laparoscopy and laparotomy groups was performed by using the nearest available neighbor matching.23
After matching, data of patient baseline characteristics, operation outcome and incidence of intraoperative and postoperative complication were compared using t test, Chi-square test, or Fisher exact test. Survival data were estimated using Kaplan–Meier curves and compared between groups by log-rank statistics. The 5- and 10-year survival rate and OS were reported. All of the statistical analysis was completed by SPSS 19.0 (SPSS version 19.0; SPSS, Inc., Chicago, IL). Statistical significance was defined as P < 0.05.
RESULTS
Baseline Characteristics
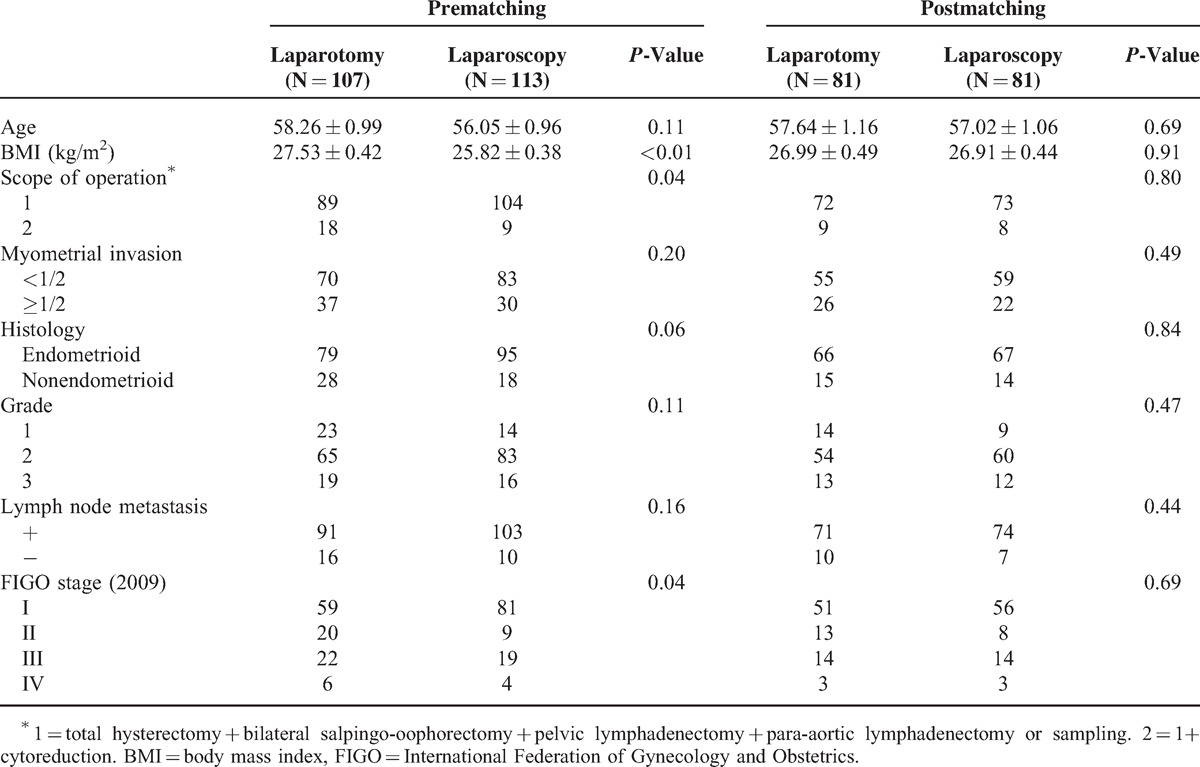
Three hundred eighty-two case notes were reviewed including 255 patients with high-risk EC. Among these 225 patients, 31 individuals with severe cardiopulmonary disease, other malignancies or uterus ≥12 weeks of gestation were excluded; 4 individuals with missing surgical or pathological data were deleted. Finally, a total of 220 patients with high-risk EC met the study criteria, 107 were treated by laparotomy and 113 by laparoscopy. Table 1 provides details of patient characteristics across the laparotomy and laparoscopy group pre- and postpropensity score matching. Before matching, significant differences were not found in age, myometrial invasion, pathological type, and grade and lymph node metastasis between 2 groups but in BMI, scope of operation, and FIGO stage which could be the confounding factors. After matching, 81 pairs of patients were matched successfully. In the matched cohorts, there was no significant difference in baseline patient characteristics between 2 groups.
TABLE 1.
Baseline Patient Characteristics Pre- and Postpropensity Score Matching
Operation Outcome
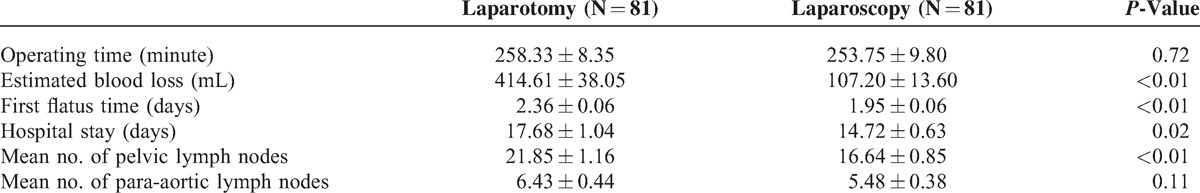
The mean operative time was similar between 2 groups, 253 minutes in laparoscopy group and 258 minutes in laparotomy group. The mean blood loss were significant less in laparoscopy group (107 mL) than laparotomy group (414 mL) (P < 0.01). The laparoscopy cohort also has significant shorter first flatus time (2.0 days vs. 2.4 days, P < 0.01) and postoperative hospital stay (14.7 days vs. 17.7 days, P = 0.02) than laparotomy which meant that patients received laparoscopic surgery had more rapid recovery than that received laparotomy. The mean numbers of pelvic lymph nodes dissected by laparoscopy were 16.6 compared 21.9 by laparotomy with significant difference (P < 0.01). However, the mean numbers of para-aortic lymph nodes were comparable between 2 groups (P = 0.11) (Table 2).
TABLE 2.
Comparison of Operation Outcome
Intraoperative and Postoperative Complication
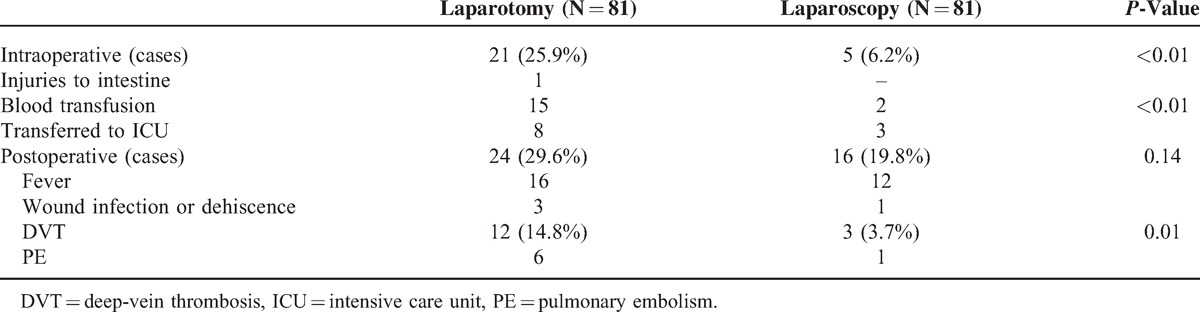
Intraoperative complications were significant different between the 2 groups (6.2% for laparoscopy and 25.9% for laparotomy, P < 0.01). None of the laparoscopic cases was converted into open procedures. The patients received transfusion during surgery in laparotomy group was statistically more than that in laparoscopy (P < 0.01). For the incidence of transferred to ICU on account of poor cardiopulmonary resuscitation, it was also statistically higher in patients treated by laparotomy than by laparoscopy. Moreover, no injury to bladder, ureter, or large vessels occurred in both 2 groups, only 1 patient in laparotomy group had the intestine injury because of heavy pelvic adhesion that had been received intestinal repair.
There was no significant difference in postoperative complications between the 2 groups (19.8% for laparoscopy and 29.6% for laparotomy, P = 0.14). DVT occurred more often in laparotomy patients than laparoscopy patients, 14.8% versus 3.7% (P = 0.01). Six patients developed PE in laparotomy group compared 1 in laparoscopy with no statistically difference (Table 3).
TABLE 3.
Comparison of Intraoperative and Postoperative Complications
Clinical Outcome
Clinical outcome of 2 groups was shown in Table 4. The median follow-up was 45 months (rang 5–176). During the follow-up time, there were 16 (9.9%) recurrences which were similar between the 2 treatment cohorts, 7 (8.6%) were observed in laparotomy group at peritoneal, liver, lung, vaginal, and abdominal incision versus 9 (11.1%) in laparoscopy group at peritoneal, liver, lung, and vaginal. No port-site metastases were observed in laparoscopy cohort. Twenty patients died, 11 in the laparotomy group and 9 in the laparoscopy group. The Kaplan–Meier estimate for survival rate in laparotomy and laparoscopy at 5 years was 89.2%, 85.3% and at 10 years was 75.8%, 85.3%, respectively. No statistically significant difference was found between the 2 groups when OS was compared (P = 0.97) (Figure 1).
TABLE 4.
Comparison of Clinical Outcome
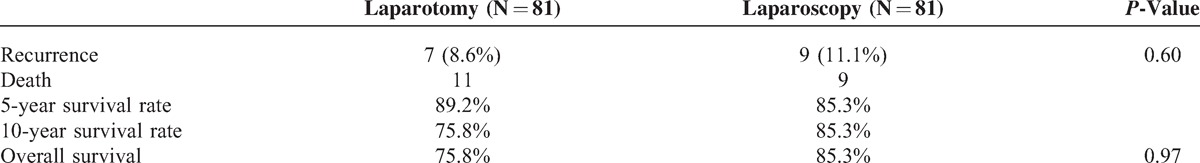
FIGURE 1.
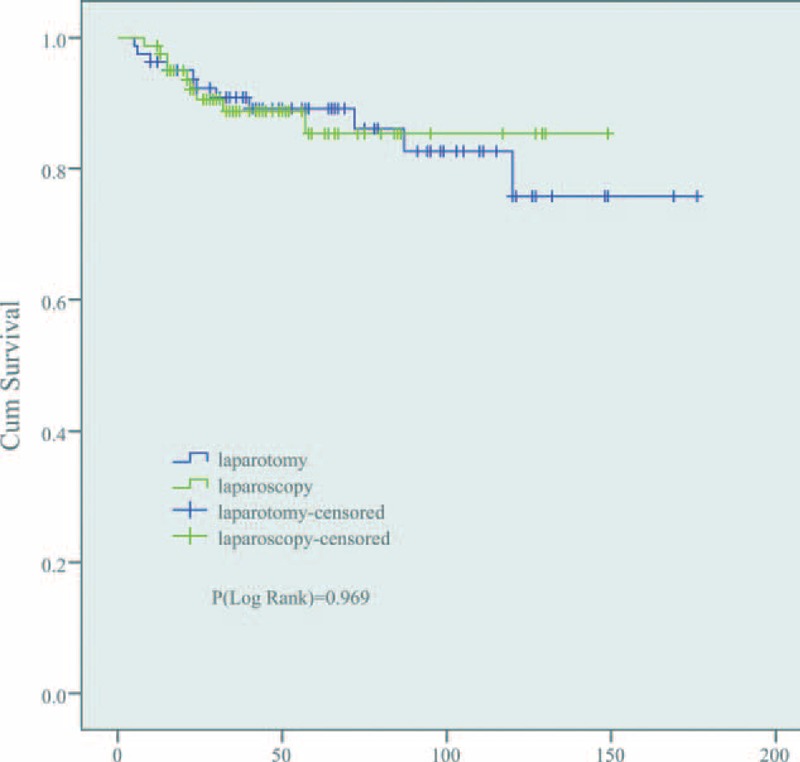
Overall survival. It showed that no significant difference between laparotomy and laparoscopy cohorts (75.8% vs. 85.3%, P = 0.97).
DISCUSSION
For accurately estimating the effect of intervention, randomized controlled trial (RCT) is considered the optimal approach but often limited by ethic and other problems. PSM can eliminate the imbalance between groups and reduce the effects of confounding to achieve a random effect in observational studies.24 In the present study, the patient baseline characteristics of laparotomy and laparoscopy group were adequately balanced after matching, and then, the surgery outcomes achieved comparability. This retrospective study demonstrated that laparoscopic technique is effective and even safer compared with laparotomy for patients with high-risk EC.
In 2009, Gynecologic Oncology Group (GOG) compared laparoscopy and laparotomy for comprehensive surgical staging of EC in a randomized multicenter trial which involved 2616 patients and reported that laparoscopic surgery resulted in fewer complications and shorter hospital stay but longer operation time.14 In another large randomized trial published in 2010, laparoscopic surgery was associated with a shorter hospital stay and less amount of blood loss, but no beneficial effect was observed in terms of major complications and operation time.10 However, our data showed evidence of less blood loss, lower proportion of major complications and shorter hospital stay with laparoscopy versus laparotomy and a similar operation time. Operation time is much related to the experience of the surgical team and learning curve. As the improvement of surgical technique, it is a trend that laparoscopic operation time becomes shorter.25 Moreover, our study indicated that the first flatus time was shorter in laparoscopy group than in laparotomy. Patients treated with laparoscopic surgery had smaller incision and less pain compared to those treated with laparotomy. Laparoscopy can also bring out an earlier resumption of bowel function and shorter hospital stays. We found that the postoperative hospital stays in our data were longer than that reported in most of the previously studies and just similar to Kong's study in Korea.8 Kong reported a mean hospitalization of 16.4 days for laparoscopy and 23.3 days for laparotomy. But in GOG study, the median hospital stays was 3 days for laparoscopy and 4 days for laparotomy.14 Patients in China are accustomed to a longer hospitalization, which is mainly decided by the social factors. In Chinese traditional opinion, patients are willing to discharge from hospital only when they have complete recovery (after removing drainage tube and urinary catheter or taking out stitches), what's more, they need not worry about the medical expense because of the coverage of insurance. So, some patients even have been in hospital until the end of the first chemotherapy.
In the present study, intraoperative complications were significantly lower in the laparoscopy group and no injuries to bladder, ureter, or large vessels occurred. The incidence of major complications can be highly reduced when the procedure was performed by experience surgeon.10 However, it seems that laparoscopy had no benefit in the incidence of postoperative complications. But we found DVT occurred significantly often in patients treated by laparotomy than those treated by laparoscopy. In GOG study, there was no significant difference in incidence of venous thromboembolism (VTE) and PE between different approaches.14 In contrast, Fader's study showed that the laparoscopy cohort had significantly fewer episodes of VTE.5 Lots of studies indicate that the combination of underlying malignancy and pelvic surgery is the risk for developing a VTE.26,27 In a Japanese large research about risk factors for VTE, the age (≥50 or ≥55 years), a diagnosis of cancer, operating time (≥4 hours), blood loss (≥1000 mL), and blood transfusion (≥2000 mL) were reported significantly related to postoperative VTE.28 It may account for the difference of DVT incidence between the 2 groups that laparotomy had longer operating time and more blood loss in our study. What's more, Trendelenburg position during laparoscopic surgery may play a role in preventing VTE.
Most of the previous studies demonstrate that the pelvic or para-aotic lymph nodes count removed by laparoscopy approach were similar to those dissected by laparotomy.8,29–32 Laparoscopy has been proven to be even more efficient in lymphadenectomy because of its clearer vision. However, Obermair and Kalogiannidis reported lower number of lymph node dissections in the laparoscopy group.6,11 In the present study, the mean number of dissected para-aotic lymph nodes was comparable between the 2 groups, but the pelvic lymph nodes were significant fewer in laparoscopy cohort than laparotomy. Actually, it remains controversially that whether lymphadenectomy has prognostic value for EC. Two large RCTs reported by Benedett and Kitchener showed no evidence that lymphadenectomy could decrease the risk of recurrence or mortality rate in patients with early EC.33,34 Conversely, another retrospective research gave a result that lymphadenectomy improved the survival of patients with high-risk EC and stage I B to stage IV.35 Moreover, an article published in LANCET in 2010 reported that para-aotic had a significantly benefit in terms of OS for patients with EC at mediate or high risk.36 It seems that the difference of pelvic lymph nodes count between the laparoscopy and laparotomy groups had not influenced the OS in our study. We got a similar survival outcome between the 2 study cohorts.
Lots of previous studies have proven that laparoscopy surgery was similar to laparotomy in short-term survival.9,12,15,17 A meta-analysis reported in 2012 based on 8 RCTs showed that there was no significant difference in disease-free survival, cancer-related survival, or OS.31 However, long-term survival data were still not much. Cho et al4 reported a similar 5-year OS and progression-free survival between laparoscopy and laparotomy groups based on 10-year experience. In 2012, the GOG published the survival data of their large RCT in 2009 that the 5-year OS was almost identical in both laparoscopy and laparotomy cohorts at 89.8%.19 Between 2000 and 2010, we conducted a randomized trial and reported that the 5-year OS rates were 91% of laparotomy and 96% of laparoscopy with no significantly difference.37 In another retrospective study focus on high-grade EC in 2012, progression-free and OS were not significantly different between the surgical cohorts at 44-month median follow-up.5 In the present study, parts of patients were follow-up more than 10 years. With a median follow-up time of 45 months, the 5- and 10-year survival rate for the laparotomy were 89.2% and 75.8% compared with 85.3% and 85.3% for the laparoscopy. There was no significant difference in OS between 2 cohorts and also the recurrence rate.
Although the patients enrolled were not assigned to different approaches randomly which was a limitation of our study, we performed a PSM and successfully to control a certain confounding factors yet. When randomized trial was limited by the objective conditions, PSM might be a feasible alternative to reduce selection bias. In conclusion, it seems that laparoscopic surgery is as effective as laparotomy in the long term and can be safely carried out in patients with high-risk EC for surgery treatment. Furthermore, other long-term follow-up data on high-risk EC of randomized trials is still needed to confirm these results.
Footnotes
Abbreviations: AUC = area under the concentration–time curve, BMI = body mass index, DVT = deep-vein thrombosis, EC = endometrial cancer, FIGO = International Federation of Gynecology and Obstetrics, GOG = Gynecologic Oncology Group, ICU = intensive care unit, OS = overall survival, PE = pulmonary embolism, PSM = propensity score matching, RCT = randomized controlled trial, TAH = total abdominal hysterectomy, TLH = total laparoscopic hysterectomy, VET = venous thromboembolism.
This study was supported in part by the Chinese High-tech R&D (863) Program.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists (ACOG). ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol 2005; 106:413–425. [DOI] [PubMed] [Google Scholar]
- 3.Childers JM, Surwit EA. Combined laparoscopic and vaginal surgery for the management of two cases of stage I endometrial cancer. Gynecol Oncol 1992; 45:46–51. [DOI] [PubMed] [Google Scholar]
- 4.Cho YH, Kim DY, Kim JH, et al. Laparoscopic management of early uterine cancer: 10-year experience in Asan Medical Center. Gynecol Oncol 2007; 106:585–590. [DOI] [PubMed] [Google Scholar]
- 5.Fader AN, Seamon LG, Escobar PF, et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: a multi-site study performed at high volume cancer centers. Gynecol Oncol 2012; 126:180–185. [DOI] [PubMed] [Google Scholar]
- 6.Kalogiannidis I, Lambrechts S, Amant F, et al. Laparoscopy-assisted vaginal hysterectomy compared with abdominal hysterectomy in clinical stage I endometrial cancer: safety, recurrence, and long-term outcome. Am J Obstet Gynecol 2007; 196:248.e1–248.e8. [DOI] [PubMed] [Google Scholar]
- 7.Kluivers KB, Ten Cate FA, Bongers MY, et al. Total laparoscopic hysterectomy versus total abdominal hysterectomy with bilateral salpingo-oophorectomy for endometrial carcinoma: a randomised controlled trial with 5-year follow-up. Gynecol Surg 2011; 8:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong TW, Lee KM, Cheong JY, et al. Comparison of laparoscopic versus conventional open surgical staging procedure for endometrial cancer. J Gynecol Oncol 2010; 21:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: a prospective randomized study. Gynecol Oncol 2009; 112:126–133. [DOI] [PubMed] [Google Scholar]
- 10.Marian JEM, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer a randomised trial. Lancet Oncol 2010; 11:763–771. [DOI] [PubMed] [Google Scholar]
- 11.Obermair A, Manolitsas TP, Leung Y, et al. Total laparoscopic hysterectomy for endometrial cancer: patterns of recurrence and survival. Gynecol Oncol 2004; 92:789–793. [DOI] [PubMed] [Google Scholar]
- 12.Tozzi R, Malur S, Koehler C, et al. Laparoscopy versus laparotomy in endometrial cancer: first analysis of survival of a randomized prospective study. J Minim Invasive Gynecol 2005; 12:130–136. [DOI] [PubMed] [Google Scholar]
- 13.Vaisbuch E, Goldchmit C, Ofer D, et al. Laparoscopic hysterectomy versus total abdominal hysterectomy: a comparative study. Eur J Obstet Gynecol Reprod Biol 2006; 126:234–238. [DOI] [PubMed] [Google Scholar]
- 14.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 2009; 27:5331–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zullo F, Palomba S, Falbo A, et al. Laparoscopic surgery vs laparotomy for early stage endometrial cancer: long-term data of a randomized controlled trial. Am J Obstet Gynecol 2009; 200:296.e1–296.e9. [DOI] [PubMed] [Google Scholar]
- 16.Hahn HS, Kim HJ, Yoon SG, et al. Laparoscopy-assisted vaginal versus abdominal hysterectomy in endometrial cancer. Int J Gynecol Cancer 2010; 20:102–109. [DOI] [PubMed] [Google Scholar]
- 17.Ju W, Myung SK, Kim Y, et al. Korean Meta-Analysis Study G. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a meta-analysis. Int J Gynecol Cancer 2009; 19:400–406. [DOI] [PubMed] [Google Scholar]
- 18.Palomba S, Falbo A, Mocciaro R, et al. Laparoscopic treatment for endometrial cancer: a meta-analysis of randomized controlled trials (RCTs). Gynecol Oncol 2009; 112:415–421. [DOI] [PubMed] [Google Scholar]
- 19.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 2012; 30:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willis SF, Barton D, Ind TE. Laparoscopic hysterectomy with or without pelvic lymphadenectomy or sampling in a high-risk series of patients with endometrial cancer. Int Semin Surg Oncol 2006; 3:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009; 105:103–104. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127 (8 Pt 2):757–763. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009; 51:171–184. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soliman HO, Elsebaie HI, Gad ZS, et al. Laparoscopic hysterectomy in the treatment of endometrial cancer: NCI experience. J Egypt Natl Cancer Inst 2011; 23:101–104. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell GL, Clarke-Pearson D. Pulmonary embolism after major abdominal surgery in gynecologic oncology. Obstet Gynecol 2006; 108:209.author reply 209–211. [DOI] [PubMed] [Google Scholar]
- 27.Schmeler KM, Wilson GL, Cain K, et al. Venous thromboembolism (VTE) rates following the implementation of extended duration prophylaxis for patients undergoing surgery for gynecologic malignancies. Gynecol Oncol 2013; 128:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki N, Yoshioka N, Ohara T, et al. Risk factors for perioperative venous thromboembolism: a retrospective study in Japanese women with gynecologic diseases. Thromb J 2010; 8:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorlu CG, Simsek T, Ari ES, et al. Laparoscopy or laparotomy for the management of endometrial cancer. J Soc Laparoendoscopic Surg 2005; 9:442–446. [PMC free article] [PubMed] [Google Scholar]
- 30.He H, Zeng D, Ou H, Tang Y, Li J, Zhong H. Laparoscopic treatment of endometrial cancer: systematic review. J Minim Invasive Gynecol 2013; 20:413–423. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Cui J, Jia L, et al. Comparison of laparoscopy and laparotomy for endometrial cancer. Int J Gynaecol Obstet 2012; 116:185–191. [DOI] [PubMed] [Google Scholar]
- 32.Zullo F, Falbo A, Palomba S. Safety of laparoscopy vs laparotomy in the surgical staging of endometrial cancer: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol 2012; 207:94–100. [DOI] [PubMed] [Google Scholar]
- 33.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008; 100:1707–1716. [DOI] [PubMed] [Google Scholar]
- 34.Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009; 373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CY, Ho CM, Chen YL, et al. Impact of lymphadenectomy in uterine endometrioid carcinoma. Eur J Surg Oncol 2013; 39:350–357. [DOI] [PubMed] [Google Scholar]
- 36.Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 2010; 375:1165–1172. [DOI] [PubMed] [Google Scholar]
- 37.Lu Q, Liu H, Liu C, et al. Comparison of laparoscopy and laparotomy for management of endometrial carcinoma: a prospective randomized study with 11-year experience. J Cancer Res Clin Oncol 2013; 139:1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]


