Abstract
We aim to investigate whether the phenomenon of “sudden drop” in blood pressure (BP) within the first 2 hours is associated with vessel recanalization.
We retrospectively examined clinical and imaging data from a consecutive series of patients with stroke with large vessel occlusion treated with intravenous thrombolysis (IVT). BP was monitored every 15 minutes during the first 2 hours, then every 30 minutes for 6 hours, and then every hour for 16 hours.
We observed the phenomenon of “sudden drop” in systolic BP (≥20 mm Hg) in 82 (50.9%) patients in the first 2 hours and vessel recanalization in 87 (54.0%) patients 24 hours after treatment. This phenomenon was independently associated with recanalization (odds ratio 2.100; 95% confidence interval: 1.085–4.062; P = 0.028) after adjusting for the history of atrial fibrillation, coronary heart disease, and hypertension.
The phenomenon of “sudden drop” in systolic BP with 20 mm Hg or greater between 2 continuous measurements within the first 2 hours is associated with recanalization after IVT in patients with large vessel occlusion, especially for middle cerebral artery occlusion.
INTRODUCTION
Approximately 80% of patients with ischemic stroke have an elevated blood pressure (BP) when measured within 24 to 48 hours of onset, which starts to fall within hours of onset and then normalize over the first weeks after stroke.1 BP elevation may herald arterial occlusion in acute ischemic stroke. The course of BP after thrombolysis has been reported to be related to the severity of neurological deficits,2,3 hemorrhagic transformation,4 and diffusion-weighted imaging (DWI) lesion growth.5
Intravenous thrombolysis (IVT) is currently the most promising treatment for acute ischemic stroke.6 The goal of recombinant tissue plasminogen activator (rtPA) is to achieve recanalization and restore blood flow to the ischemic brain. Continuous transcranial Doppler (TCD) ultrasonography has demonstrated that the majority of rtPA-induced recanalizations occur during the first hour after treatment, while recanalization rarely presented among patients with persistent occlusion at 2 hours after IVT.7 Most recently, studies indicated that decreases in systolic but not diastolic BP might be a clinical sign of recanalization after thrombolysis.8,9 However, previous studies only evaluated the declines of BP from baseline to 12 or 24 hours after thrombolysis, which, to some extent, dampens the clinical significance of this phenomenon in the early stage. We thus aim to investigate whether the phenomenon of “sudden drop” in BP presented within the first 2 hours is associated with recanalization. To verify or reject this hypothesis, we monitored BP every 15 minutes for 2 hours from the start of rtPA therapy and investigated the relationship between “sudden drop” in BP and arterial recanalization.
SUBJECTS AND METHODS
Study Subjects
We retrospectively reviewed our database for acute ischemic stroke patients who were admitted to our stroke care unit and received thrombolytic therapy between June 2009 and May 2014. We included patients who had a diagnosis of acute ischemic stroke confirmed by computed tomography (CT) or DWI; received intravenous rtPA; underwent magnetic resonance angiography (MRA) or CT angiography (CTA) before rtPA infusion; underwent follow-up MRA or CTA 24 hours after rtPA infusion; and had large vessel occlusion demonstrated on baseline noninvasive angiography, including middle cerebral artery (MCA) (M1 and M2), internal carotid artery (ICA), anterior cerebral artery (ACA), posterior cerebral artery (PCA), or basilar artery (BA). We excluded patients who were treated with combined endovascular and rtPA therapy, as the potential effect on BP; received antihypertensive agents (BP>185/110 mm Hg) before or during rtPA infusion; whose image quality was poor due to motion artifacts; and whose medical record was not available to abstract BP measurements of every 15 minutes for 2 hours from the start of rtPA therapy.
Demographics characteristics, stroke severity measure by National Institutes of Health Stroke Scale (NIHSS) score,10 risk factors (atrial fibrillation, hypertension, coronary heart disease, smoking, and diabetes mellitus), medication history, time interval from stroke onset to rtPA infusion, serum glucose, platelet and international normalized ratio level on admission, systolic and diastolic BP every 15 minutes for 2 hours from the start of rtPA therapy, admission and follow-up imaging scans data, and modified Rankin Scale (mRS) score after 3 months were documented.11
Ethics Statement
The protocols of MRI-guided IVT had been approved by the Human Ethics Committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine. All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained for all patients.
Imaging Acquisition
MRI was performed on a 3.0 T system (Signa Excite HD; General Electric Medical System, Milwaukee, WI) equipped with an 8-channel phased-array head coil, including DWI sequence (TR = 4000 ms; TE = 69.3 ms; b-value = 1000 s/mm2; slice thickness = 5.0 mm; interslice gap = 1.0 mm) and time-of-flight (TOF)-MRA (TR = 20 ms; TE = 3.2 ms; flip angle = 15°; slice thickness = 1.4 mm, 3 slabs).
The multimodal CT was performed on a 64-slice CT scanner (SOMATOM Definition Flash; Siemens Healthcare Sector, Forchheim, Germany), including unenhanced CT (contiguous 5 mm axial slices) and volumetric perfusion CT (VPCT) (slice thickness 1.5 mm, collimation 32 × 1.2 mm). VPCT consisted of 26 consecutive spiral acquisitions of the brain. CTA was performed after VPCT with acquisition from the base of the skull to the top of the lateral ventricles.
BP Measurements and Management
BP was measured with the traditional Riva Rocci method using an arm cuff and a sphygmomanometer. According to published guidelines,12 antihypertensive agents were administered when BP exceeded 185/110 mm Hg before rtPA therapy, and 180/105 mm Hg during and after rtPA therapy.
For this study we collected BP recordings at admission and every 15 minutes for 2 hours from the start of rtPA therapy, that is 9 values of BP with 8 time intervals for each patient. A “sudden drop” in BP was defined as decline in systolic BP of 20 mm Hg or greater between 2 continuous BP measurements, while a cutoff value of 15, 25, and 30 mm Hg was also tested, respectively. In strict definition, it was not a “sudden drop” in BP if systolic BP increased 20 mm Hg or greater first, and then decreased 20 mm Hg or greater. In addition, BP decline from admission to 6/12/18/24 hours after IVT was calculated.
Evaluation of Outcome
We used the Arterial Occlusive Lesion (AOL) scale to define recanalization or no recanalization based simply on the presence (grades 2 or 3) or absence (grades 0 or 1) of any downstream flow, according to a recent consensus statement of recommendations on cerebral angiographic revascularization grading standards.13 Hemorrhagic transformation was classified by hemorrhagic infarction (HI), parenchymal hemorrhage (PH), and symptomatic intracranial hemorrhage (sICH) as defined in European Cooperative Acute Stroke Study (ECASS) II trial.14 sICH was defined as hemorrhagic transformation with an increase of >4 points of NIHSS score or leading to death. Outcome at 3 months was assessed by using mRS score. Good clinical outcome was defined as mRS score 0 to 2, and poor clinical outcome (including death within the first 90 days as the worst outcome) was defined as mRS score 3 to 6.
Statistical Analysis
The patients were dichotomized according to vessel recanalization. Fisher exact test was used to compare the dichotomous variables between groups, while independent samples or paired-sample 2-tailed t test or Mann–Whitney U test was used for the continuous variables, as appropriate. Variables with a P value of <0.1 in univariate analyses were included in the binary logistic regression model to select independent associated variables for recanalization, hemorrhagic transformation, and poor clinical outcome, respectively. Medical history of atrial fibrillation, coronary heart diseases, and hypertension was forced into the model, as the potential effect on BP. Receiver operating characteristics curve analysis was used to determine predictive value. All analyses were performed blinded to participant identifying information. The odds ratio (OR) and 95% confidence interval (CI) were obtained. Statistical significance was set at a P value of <0.05. All statistical analysis was performed with SPSS package (14.0 for Windows).
RESULTS
A total of 215 acute ischemic stroke patients with large vessel occlusion who received rtPA had baseline and follow-up angiography during the study period. Of these, 54 patients were excluded for the following reasons: 9 were treated with combined endovascular and rtPA therapy, 6 imaging quality was poor for analysis, 11 without continuous BP records, and 28 received antihypertensive agents. Thus, 161 remaining patients were included for the final analysis. Demographic and laboratory data were not different between included and excluded subjects. Of the included patients, 52 (32.3%) were women, with a median age of 69 years (mean 68.81 ± 11.49 years, range 43–94 years) and an acute large vessel occlusion: 26 (16.1%) ICA, 77 (47.8%) M1, 39 (24.4%) M2, 2 (1.2%) ACA, 5 (3.1%) PCA, and 12 (7.5%) BA. Mean time from onset to rtPA infusion was 232.2 ± 94.8 minutes. Baseline NIHSS score was 12.71 ± 6.07.
Mean systolic BP at admission was 147.98 ± 20.21 mm Hg, and mean diastolic BP was 83.01 ± 15.07 mm Hg. Systolic BP was elevated (>140 mm Hg) in 103 patients, and diastolic BP was elevated (>90 mm Hg) in 51 patients. Systolic BP or diastolic BP or both were increased in 111 patients (68.9%). There was no association between BP at admission or 6/12/18/24 hours after IVT and severity of stroke measured with the NIHSS (P > 0.05 for all variables).
The AOL scale was evaluated in 129 patients on TOF-MRA and 32 on reconstructed CTA. Follow-up angiography 24 hours after rtPA infusion revealed recanalization in 87 (54.0%) patients and no recanalization in 74 (46.0%) patients. Table 1 shows the characteristics of patients with and without recanalization for comparison. Patients with recanalization had more hemorrhagic transformation, less poor clinical outcome. Baseline diastolic BP was marginally associated with recanalization. Only “sudden drop” in systolic BP with 20 mm Hg or greater was independently associated with recanalization after adjusting for medical history of atrial fibrillation, coronary heart disease, and hypertension (Table 2). Eighty-two (50.9%) patients presented the phenomenon of “sudden drop” in BP (≥20 mm Hg), among whom 65 (79.3%) patients had the elevated baseline BP. The predictive value of “sudden drop” in BP (≥20 mm Hg) for recanalization was shown in Table 3. This phenomenon predicted recanalization better in the patients with ICA or MCA occlusion, and the predictive ability increased by using the strict definition.
TABLE 1.
Characteristics Between Patients With and Without Recanalization
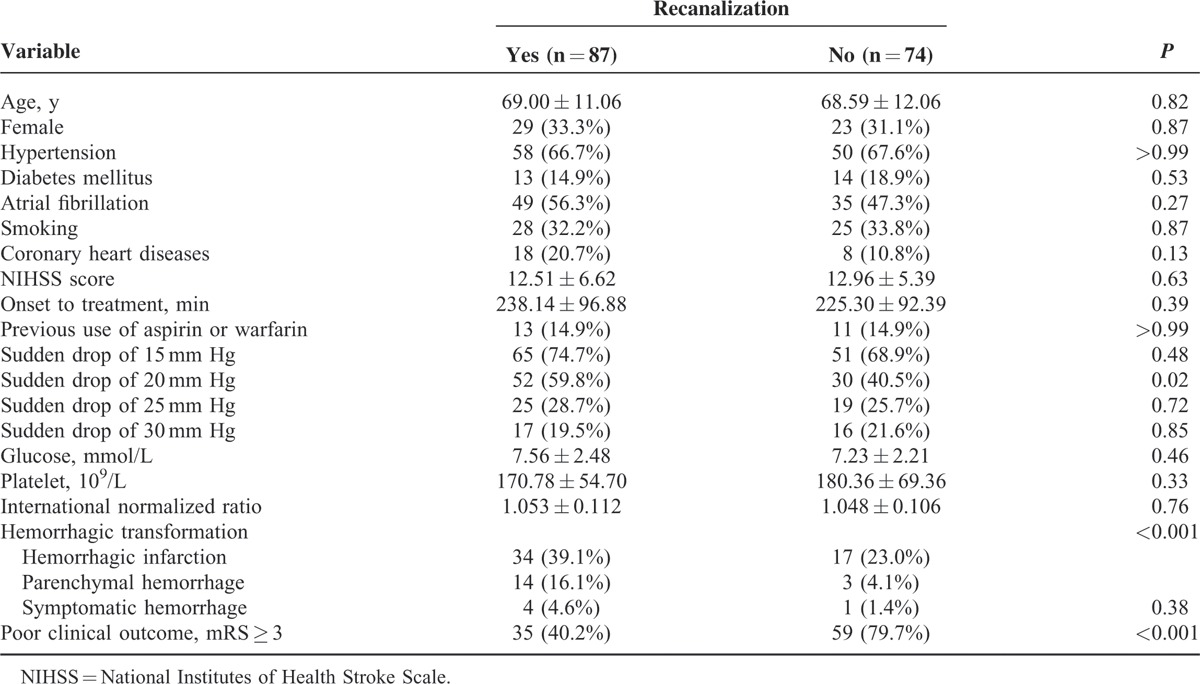
TABLE 2.
Multivariate Logistic Regression Analysis of Predictors for Recanalization
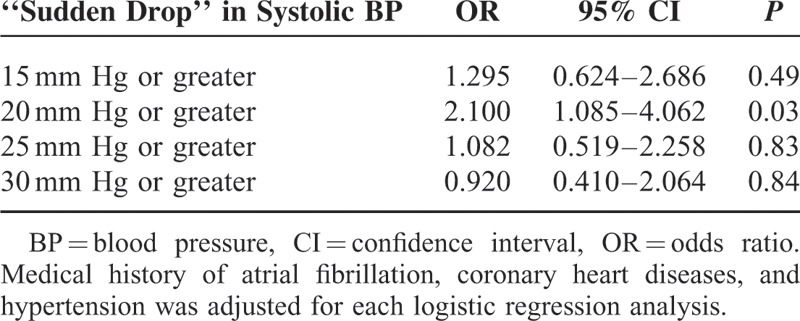
TABLE 3.
Predictive Value of “Sudden Drop” in BP (≥20 mm Hg) for Recanalization
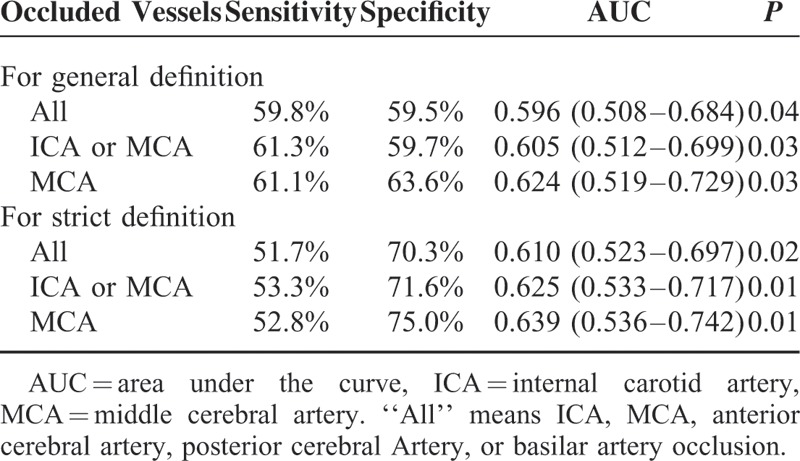
For general definition, 15 (18.3%) patients had 2 “sudden drop” in BP with 20 mm Hg or greater and 2 patients had 3 episodes, while 65 (79.3%) only had 1 “sudden drop” in BP. The distribution of this phenomenon during the first 2 hours from the start of rtPA therapy was shown in Figure 1. The predictive ability increased, when only one episode of “sudden drop” was used to predict recanalization (area under the curve [AUC] = 0.611, P = 0.015, for All; AUC = 0.626, P = 0.010, for ICA or MCA; AUC = 0.666,Pp = 0.003, for MCA).
FIGURE 1.
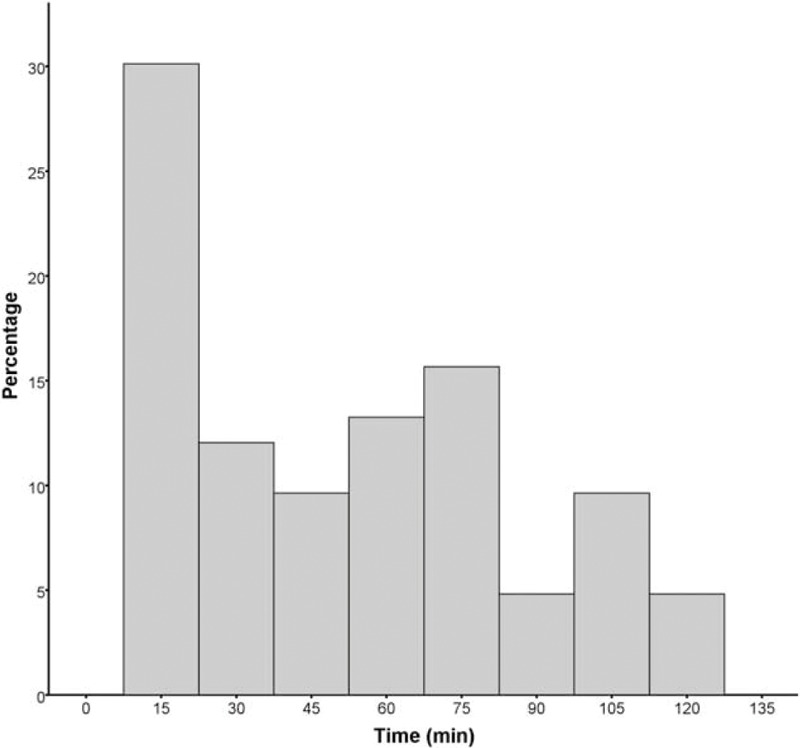
Distribution of the first episode of “sudden drop.” Timing of the first episode of “sudden drop” in systolic blood pressure during the first 2 hours after intravenous thrombolysis.
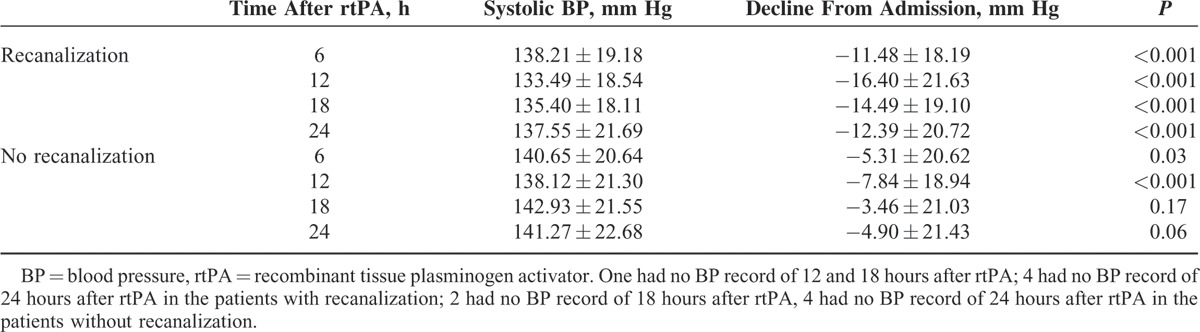
BP of the 161 patients decreased significantly from admission to 12 hours after thrombolysis (148.07 ± 20.24 vs 135.63 ± 19.93, P < 0.001, for systolic BP; 82.98 ± 15.11 vs 75.24 ± 13.04, P < 0.001, for diastolic BP). The absolute systolic BP decline from admission to 6/12/18/24 hours after thrombolysis was larger in patients with recanalization compared with patients without recanalization (Table 4).
TABLE 4.
Mean Systolic BP Course in the Patients With or Without Recanalization
Follow-up scans 24 hours after treatment revealed hemorrhagic transformation in 68 (42.2%; 51 were HI and 17 were PH) patients, and sICH was observed in 5 (3.1%) patients. The phenomenon of “sudden drop” in BP (≥20 mm Hg) was not associated with any hemorrhagic transformation (OR 0.963; 95% CI: 0.500–1.856; P = 0.911) or sICH (OR 1.594; 95% CI: 0.242–9.914; P = 0.644), after adjusting for NIHSS score and age. Moreover, the phenomenon of “sudden drop” in SBP (≥20 mm Hg) could not predict 3 months outcome (OR 1.639; 95% CI: 0.707–3.797; P = 0.249), while age, baseline NIHSS score, and recanalization were independent predictors for clinical outcome.
DISCUSSION
We found that the phenomenon of “sudden drop” in systolic BP with 20 mm Hg or greater between 2 continuous measurements within the first 2 hours was more likely to be associated with recanalization after IVT in patients with large vessel occlusion. This predictive ability was better in the patients with ICA or MCA occlusion. Our finding first underscores the striking association between BP variability within the first 2 hours and artery recanalization after IVT.
Elevated BP is a common phenomenon in the patients with acute ischemic stroke. In a nationally representative large data set, elevated systolic BP was observed in >60% of the patients presenting with stroke to the emergency department.15 Similarly, we found approximately 70% patients had either elevated systolic BP or diastolic BP at baseline. The underlying mechanisms of this BP elevation and dysregulation have not been elucidated to date. One reasonable explanation is that BP elevation may be a natural response of the organism to persistent vessel occlusion in the acute stroke phase, in order to increase the perfusion of salvageable tissue and to minimize the ischemic damage. Therefore, one could posit that the physiological course after recanalization is a pressure decline and more rapid return toward prestroke levels than in those patients with persistent vessel occlusions. Our findings first confirmed the relationship between the phenomenon of “sudden drop” in systolic BP (≥20 mm Hg) and the vessel recanalization. Most (79.3%) of the patients with “sudden drop” in systolic BP (≥20 mm Hg) presented with an elevated baseline systolic BP before treatment. Moreover, we found that most (approximately 80%) of the patients had one “sudden drop” in systolic BP, and the predictive ability for recanalization was better with one episode of “sudden drop.” Therefore, it would be interesting in future studies to investigate whether recanalization occurs at the exact time point with sudden drop in systolic BP after IVT, by continuous investigation such as TCD.
It is interesting that only a cutoff value of 20 mm Hg in systolic BP decrease was independently associated with recanalization, not less or more. This was in line with the recent observation that the decline in systolic BP of 20 mm Hg or greater with neurologic improvement may reflect recanalization.8 A retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) indicated that higher systolic BP levels at 2 to 24 hours after IVT had a linear association with sICH and a U-shaped association with mortality.16 There are also observational studies showed low initial diastolic BP and administration of intravenous antihypertensive medication were associated with unfavorable outcome (mRS 3–6),17,18 indicating a U-shaped association between BP and clinical outcome. Our finding may therefore provide a reference for effective control of elevated BP levels immediately following IVT. The absolute systolic BP decline from admission to 6/12/18/24 hours after thrombolysis was greater in patients with recanalization compared with patients without recanalization. This was similar with the findings in ischemic stroke patients after intraarterial thrombolysis.9 When thrombolysis succeeded to reopen the occluded vessel, systolic BP 6/12/18/24 hours after IVT declined significantly faster than when recanalization failed.
Monitoring of a “sudden drop” in systolic BP is straightforward and easily implemented. Compared with recent studies using neurological changes, defined as improvement of 4 or more points on the NIHSS score or an NIHSS score of 0 at 24 hours after IVT, with concurrent decline in systolic BP as a clinical sign of recanalization,8 our result is easier to apply in routine clinical practice. This phenomenon may help to identify thrombolysis patients with successful arterial recanlization. Additional need for aggressive treatment with intravenous fluids or rarely pressors may not need in these patients and repeat vessel imaging should be considered before bridging endovascular interventions.
Limitations include a retrospective design though we prospectively collected data using a stroke registry, and might have a potential risk of selection bias. Some severe stroke patients might be transferred to intensive care unit or receive surgical treatment next day so that they were unable to undergo follow-up scans within 24 hours. Second, we did not evaluate the BP and NIHSS score changes in patients without large vessel occlusion on pre-tPA treatment scan. Third, mental stress, bladder tonus, and other transient stimuli may modulate BP values and, therefore, measuring during the first 2 hours might not accurately reflect stroke alone. Fourth, we excluded patients treated with combined endovascular and rtPA therapy, future studies may monitor BP variation before and after recanalization. Fifth, the use of TOF-MRA is somewhat inaccurate for detecting vessel occlusion or stenosis.
We have demonstrated that the phenomenon of a “sudden drop” in systolic BP with 20 mm Hg or greater between 2 continuous measurements within the first 2 hours is independently associated with recanalization after IVT in patients with large vessel occlusion. Future prospective studies with much larger sample sizes are required to clarify our results.
Footnotes
Abbreviations: AOL = Arterial Occlusive Lesion, BP = blood pressure, DWI = diffusion-weighted imaging, HI = hemorrhagic infarction, IVT = intravenous thrombolysis, MRA = magnetic resonance angiography, mRS = modified Rankin Scale, NIHSS = National Institutes of Health Stroke Scale, PH = parenchymal hemorrhage, rtPA = recombinant tissue plasminogen activator, sICH = symptomatic intracranial hemorrhage.
Both SY and KL contributed equally to this work.
This work was supported by the Science Technology Department of Zhejiang Province (2013C03043-3), the National Natural Science Foundation of China (81171095 & 81471170). Dr Liebeskind: consultant/advisory board (modest)—Stryker and Covidien.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bath P, Chalmers J, Powers W, et al. International Society of Hypertension (ISH): statement on the management of blood pressure in acute stroke. J Hypertens 2003; 21:665–672. [DOI] [PubMed] [Google Scholar]
- 2.Christensen H, Meden P, Overgaard K, et al. The course of blood pressure in acute stroke is related to the severity of the neurological deficits. Acta Neurol Scand 2002; 106:142–147. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002; 33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 4.Butcher K, Christensen S, Parsons M, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke 2010; 41:72–77. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Mederos R, Ribo M, Rovira A, et al. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology 2008; 71:552–558. [DOI] [PubMed] [Google Scholar]
- 6.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 7.Ribo M, Alvarez-Sabin J, Montaner J, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke 2006; 37:1000–1004. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraja N, Warach S, Hsia AW, et al. Association between neurologic improvement with decline in blood pressure and recanalization in stroke. JAMA Neurol 2014; 71:1555–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattle HP, Kappeler L, Arnold M, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke 2005; 36:264–268. [DOI] [PubMed] [Google Scholar]
- 10.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864–870. [DOI] [PubMed] [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19:604–607. [DOI] [PubMed] [Google Scholar]
- 12.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947. [DOI] [PubMed] [Google Scholar]
- 13.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001; 32:438–441. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007; 25:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed N, Wahlgren N, Brainin M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 2009; 40:2442–2449. [DOI] [PubMed] [Google Scholar]
- 17.Hill MD, Buchan AM. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005; 172:1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsberg PJ, Soinne L, Roine RO, et al. Community-based thrombolytic therapy of acute ischemic stroke in Helsinki. Stroke 2003; 34:1443–1449. [DOI] [PubMed] [Google Scholar]


