Supplemental Digital Content is available in the text
Abstract
In Myanmar, hepatitis C virus (HCV) infection prevalence is 2%. A combination therapy of pegylated interferon alfa-2a and ribavirin (PEG-IFNa/RBV) is a standard treatment, but the effect of this antiviral therapy needs evaluation as to determine the efficacy and safety of dual PEG-IFNa/RBV therapy in treating patients infected with HCV in Myanmar.
This was a retrospective analysis of data from a single clinic exclusively for gastrointestinal diseases in Yangon, Myanmar. We assessed treatment responses at the defined time points and stratified by genotypes of HCV. We also determined incidences of adverse events (AEs). We investigated independent predictors of sustained virologic response (SVR) in the participants.
A total of 362 HCV-infected cases were included in this study. The majority were females (51.7%) with mean age of 47.12 years (±11.6) and noncirrhosis patients (82%). Rapid virologic response (RVR), early virologic response (EVR), end of treatment response (ETR), and SVR 24 weeks after completion of the dual treatment were 50.3% (178/362), 88% (314/357), 80.1% (286/357), and 85.6% (167/195), respectively. The most frequently reported AEs were nausea/anorexia (72.8%) and flu-like symptoms (62.4%). In multivariate analysis, 4 factors were independently associated with SVR; SVR to genotype 3 (odds ratio [OR] 2.4, 95% CI: 1.24–4.62), EVR (OR 0.54, 95% CI: 0.3–0.95), and duration of treatment (OR 1.52, 95% CI: 1.18–1.98). Study limitations were acknowledged.
The efficacy and safety of the dual therapy in treating HCV-infected patient in Myanmar was acceptable. We recommend a prospective randomized control trial looking at duration of therapy and rates of achieving SVR, which could significantly impact the care of HCV-infected patients in Myanmar and perhaps other countries as well.
INTRODUCTION
Hepatitis C is caused by the hepatitis C virus (HCV), a spherical, enveloped, and positive-strand ribonucleic acid (RNA) virus. By estimation, 2% to 3% (130–170 million) of the world's population are infected with HCV,1,2 and the higher prevalence (>2%) is reported in several countries including some countries in the Southeast Asia (2%, 1.7%–2.3%).3 Infection with HCV could end up with both acute and chronic hepatitis, leading to health consequences. Although acute infection rarely causes hepatic failure, it can develop into a chronic infection in 75% to 85% of cases.1,4 Among HCV-infected individuals, 20% to 30% eventually develop cirrhosis or hepatocellular carcinoma (HCC).5
The recommended treatment for patients with chronic hepatitis C has historically been a combination therapy of pegylated interferon (PEG-IFN) and ribavirin (RBV).6 The aim of this combination treatment is to achieve a sustained virologic response (SVR), defined as the absence of detectable HCV-RNA in the serum at 6 months after completion of therapy.7,8 Direct-acting antiviral agents (DAA) have been recently developed for HCV infection and are known to increase the response rate substantially.9 In many countries (including Myanmar in this case) a dual PEG interferon alfa-2a (PEG-IFNa) and RBV remain the standard of care for the years8 before newer DAA-containing regimens are approved and/or distributed at an affordable price. The dual therapy is also effective against all the genotypes of hepatitis viruses (pan-genotypic).2
Myanmar is a developing country situated in the Southeast Asia region and has recently emerged as a nation pursuing peaceful transition to democracy. In Myanmar, prevalence of HCV is 2%, and 24% of patients with HCC were infected with HCV.10 Therefore, a successful antiviral treatment would be important for the prevention of HCC and other health sequalae. There had been reports of achieving SVR by the combination therapy of PEG-IFNa/RBV in treating patients infected with HCV from other settings such as Japan,11 Iran,12 among others. The cure rate depends on several factors including the strain of virus (i.e., genotypes), type of treatment given,2 patient-related factors (i.e., treatment naive, noncirrhosis), and drug administration factors (i.e., dosing, regimen either PEG-IFNa/RBV or PEG-IFNb/RBV). Hence, country specific information on the efficacy of a combined PEG-IFNa/RBV would be a valuable contribution. Taken together, the objective of the present study was to determine the efficacy and safety of a dual PEG-IFNa/RBV therapy in treating patients infected with HCV in Myanmar.
MATERIALS AND METHODS
This was a single-center cohort study conducted in accordance with the ethical principles of the Declaration of Helsinki and of Good Clinical Practice. The combination of PEG-IFNa (Pegasys, Roche) 135 to 180 μg weekly plus RBV (Copegus, Roche) 800 to 1200 mg daily reflected the clinical practice in Myanmar at the time of this study. The protocol of this noninterventional study was approved by the institutional review committee of the Option Endoscopy Centre (OEC).
We retrospectively reviewed the medical records of HCV-infected patients in the OEC, a private clinic exclusively for gastrointestinal diseases in Yangon, Myanmar. Information for the current analysis was retrieved from the databank of OEC, which was obtained via a semistructured questionnaire conducted by healthcare staff; the collected information included socio-demographic characteristics, medical history, and clinical characteristics at the first visit to the clinic. Also, laboratory findings of the patients were recorded. The inclusion criteria in the present study were patients of any age and gender who attended the OEC having serologic evidence of chronic hepatitis C (anti-HCV antibody test and/or detectable HCV-RNA in plasma). Patients were not included in the present study if they were coinfected with HIV, pregnant women/lactating mothers, in the presence of decompensated cirrhosis or liver disease (i.e., autoimmune or drug-induced hepatitis), or with serious concurrent medical illnesses (e.g., severe cardiopulmonary disease, uncontrolled diabetes mellitus, or malignancy).
Ascertainment of HCV
For each patient, ascertainment of HCV was done by virological and serological tests. These included anti-HCV (ELISA), HCV-RNA (quantitative PCR) at each time point, HCV genotype (type-specific PCR), HBsAg, anti-HBs, anti-HBc, and anti-HIV (ELISA). For baseline information complete blood count, liver function test, renal function test, cardiopulmonary status, thyroid function test, immmunological test for connective tissue diseases, and serum iron status were assessed. The study period was from January 2008 to December 31, 2012.
Treatment Given
Drug Combination
Subcutaneous injection of PEG-IFNa 180 μg once weekly plus oral RBV 800 to 1200 mg daily (weight-based dosage) in divided dose (e.g., 400 mg twice a day for 800 mg).
Duration
Twenty four weeks treatment for patients with genotype GT2, GT3, or indeterminate GT (i.e., standard therapy) or 48 weeks for GT1 or GT6.
An escalating low-dose regimen, starting from PEG-IFNa 135 to 180 μg once weekly was given to those patients with compensated cirrhosis.
Follow-Up
All patients were followed for a further 24 weeks without additional treatment.
For those patients with anemia at baseline or during treatment, the dose of RBV was reduced to 200 mg. For those who had mild thrombocytopenia, the dose of PEG-IFNa was reduced to 135 mg. To those with severe form PEG-IFNa, dosing was further lowered to 90 mg.
Assessments for Efficacy
Treatment response at the defined time points was measured and categorized as:
Rapid virologic response (RVR): undetectable HCV-RNA (<50 IU/mL) at week 4.
Early virologic response (EVR): a decrease in viral titer of >2-log10 compared with base line (partial EVR) or negative results of HCV-RNA (<50 IU/mL) at week 12 of therapy (complete EVR).
End of treatment response (ETR): undetectable HCV-RNA (<50 IU/mL) at the end of treatment (i.e., 24 or 48 weeks).
SVR: undetectable HCV-RNA (<50 IU/mL) 24 weeks after completion of therapy.
Nonresponders: failing to clear HCV from serum at any time point during treatment.
Relapses: relapse either while on therapy (i.e., breakthrough) or after completion of the treatment.13
For safety assessments, incidences of adverse events (AEs) and serious AEs were recorded at each visit at the Out-Patient Department (OPD). In the present analysis, the primary outcome was SVR and the secondary outcome was treatment related AEs.
Statistical Analysis
Descriptive statistics were performed on all important variables. Continuous variables in this study were arranged into categorical variables, using the cut-off points. For the comparisons of those patients with and without SVR, Pearson Chi-square test or Fisher exact test was done as appropriate. To investigate the independent factor related to SVR, a multivariate analysis was performed through a stepwise logistic regression analysis. The final model was selected at P ≤ 0.05. Data analysis was done with STATA 12 (StataCorp, TX).
RESULTS
Study Population
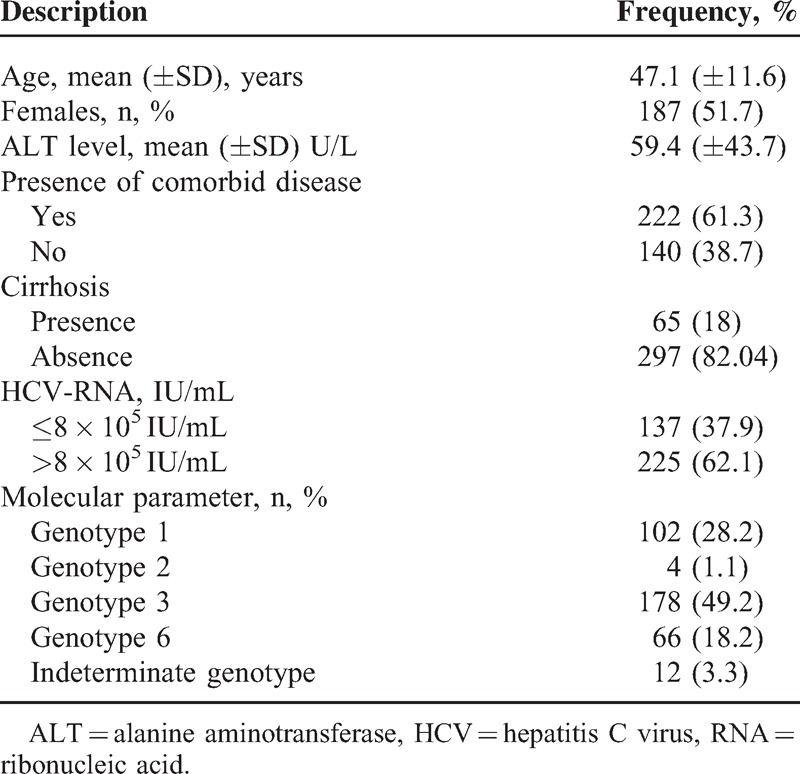
A total of 362 patients attended the OEC clinic met the inclusion criteria of the current study. The characteristics of participants are presented in Table 1. The majority were females (51.7%) with mean age of 47.12 years (±11.6) and noncirrhosis patients (82.04%). The most frequent participants were adults aged 40 to 60 years (145/362; 40.1%). The majority suffered from at least 1 comorbid disease (61.3%) and had viral load in HCV-RNA assay >800,000 IU/mL (62.1%). Of 222 patients with comorbid diseases, the common presentations were diabetes, hypertension, gall stones, and chronic hepatitis B. The most frequent HCV genotype among this cohort was HCV GT3 (178/362; 48.9%), followed by GT1 (28.2%). By age stratification, GT3 was also the highest proportion in all age groups and this was more pronounced in the 46–60 years age group (31.6%) (Supporting Table S1, http://links.lww.com/MD/A349).
TABLE 1.
Selected Baseline Characteristics of Participants (N = 362)
Efficacy Measured as Responses to Treatment
Figure 1 shows a flow diagram of disposition of patients treated with the dual therapy.
FIGURE 1.
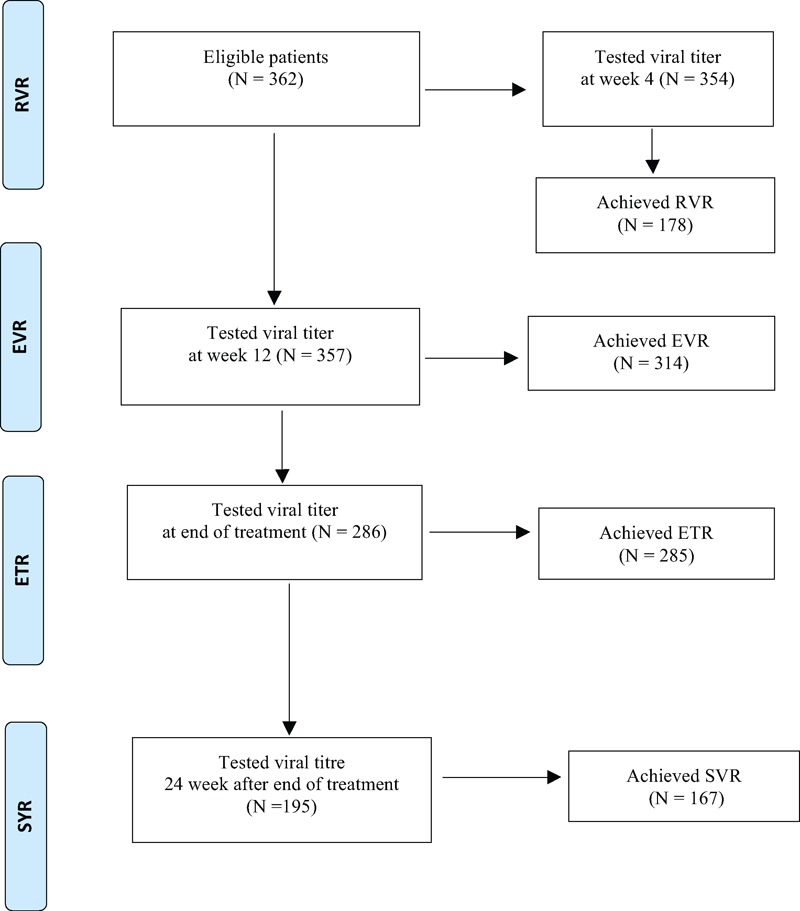
Flow diagram of the study, indicating virologic response rates during the treatment and follow-up periods.
A total of 354 patients were tested for quantitative viral titer at week 4. RVR was attained in 178 (50.3%); there was, however, no significant difference between those who attained RVR and non-RVR (178/354 vs 176/354, P = 0.93). In stratified analysis, the RVR rates were highest among patients with HCV-GT2 (3/4, 75%), followed by HCV-GT3 (99/178, 55.6%) (Supporting Table S2, http://links.lww.com/MD/A349).
The vast majority of patients who had tested qualitative HCV-RNA assay achieved EVR at 12 week (314/357, 88%). Most of them (286/357, 80.1%) were further assessed and all but 1 achieved ETR (285/286, 99.7%); this was more pronounced in HCV-GT3 (156/286, 54.5%). Based on the patients who attended the follow-up assessment, the majority achieved SVR (167/195, 85.6%) and a few patients got relapses (28/195, 14.4%). The remaining 91 patients (91/286, 31.8%) were unknown in their response state as they did not attend the follow-up assessment at the time of analysis; this was 8.4% (24) in GT1, 0.3% (1) in GT2, 16.1% (46) in GT3, 6.3% (18) in GT6, and 0.7% (2) in indeterminate GT.
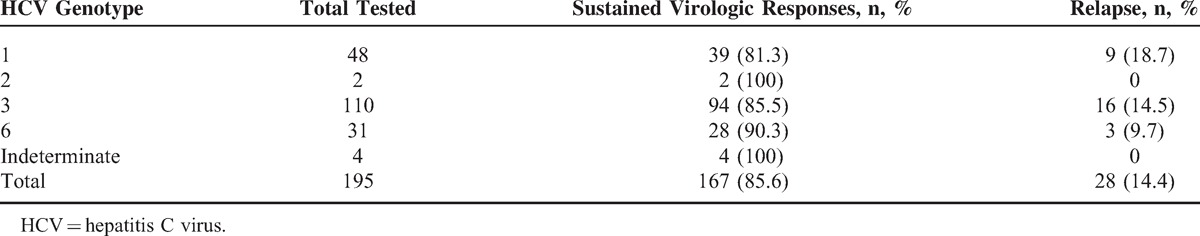
By stratification, among 195 tested, GT2 (2/2, 100%) and indeterminate GT (4/4, 1005) GT6 (28/31, 90.3%) had the highest attainment of SVR in response to dual treatment, while GT1 (39/48, 81.2%) and GT3 (94/110, 85.5%) were with the substantial response rates. For GT2 and GT6, it is to be noted that there were relatively very few cases included. Of 195 tested, relapse rate was highest in GT1 (18.8%, 9/195), followed by GT3 (14.6%, 16/195) (Table 2). By stratification, RVR, EVR, ETR, and SVR in GT6 were 54.5% (36/66), 84.8% (56/66), 97.9% (48/66), and 90.3% (28/66), respectively. Of 65 HCV patients with cirrhosis, 32 patients were tested 24 weeks after completion of the treatment. Of them, 21 (67.7%) achieved SVR.
TABLE 2.
Sustained Virologic Responses by Genotypes
Predictors of Viral Responses
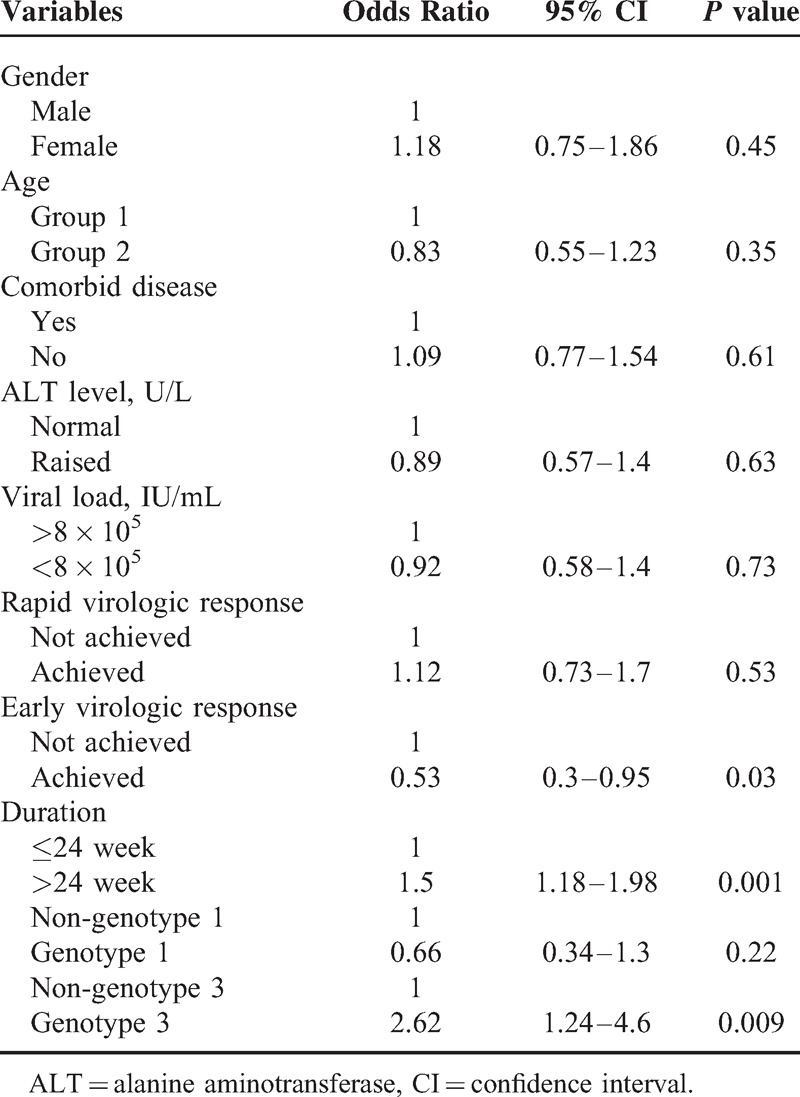
Univariate analysis showed HCV-GT3 (P = 0.008), HCV-GT1 (P = 0.003), EVR (P = 0.003), and duration of therapy (P < 0.001) were significant variables associated with SVR. In multivariate analysis, duration of therapy (odds ratio [OR] 1.52, 95%CI: 1.18–1.98), EVR (OR 0.53, 95%CI: 0.3–0.95), and patients infected with HCV-GT3 were independent predictor of SVR. Those infected with HCV-GT3 had 2 times increased likelihood of attaining an SVR than with other HCV genotypes (OR 2.4, 95%CI: 1.24–4.62) (Table 3).
TABLE 3.
Factors Independently Associated With Sustained Virologic Responses in the Multivariable Analysis
Adverse Events
Table 4 shows the incidence of AE among 354 HCV-infected patients seeking the dual treatment at the OEC. Of them, the most frequently reported AEs were nausea and anorexia (72.8%), followed by flu-like symptoms (62.4%). Some of these patients reported irritability and insomnia (41.9%) and a few got injection reactions (15.2%). In 74 (20.9%) and 69 patients (19.5%), dose reduction for PEG-IFNa and RBV were required, respectively.
TABLE 4.
Selected Adverse Events Among Patients Treated With the Combination Therapy (N = 354)

DISCUSSION
In clinical practice, any decision about a choice of therapy should weigh the balance between benefits and harms of the treatment. The present study has attempted to document the efficacy and safety profile of PEG-IFNa/RBV for the treatment of HCV-infected patients at a private specialist clinic in Myanmar.
Overall Efficacy
About 15% to 45% of infected persons spontaneously cleared the virus within 6 months of infection without treatment.1 Nevertheless, the majority of HCV-infected patients need effective antiviral treatment. The current study showed overall SVR > 80%. Comparable rates of SVR were found in 75% of patients in Pakistan.14 This was higher than the response rate in a Canadian study (54%).15 Host genetics,15 geographic variations,16 difference of body weight in race,17 and pretreatment conditions among study groups may account for the difference in response status. Our findings indicated that RVR was not a significant predictor of SVR, suggesting SVR was achieved without RVR in many cases. This was agreed with a study in Japan.5 Independent factors significantly associated with SVR levels in this study were similar to other studies such as GT318 and EVR.5,18
EVR was significantly associated with SVR. The week 12 “decision point” became widely adopted in clinical practice in the United States and Europe.11 A practical guide is to observe EVR nonresponses as a reliably predictor of when to discontinue in patients unlikely to respond.19
Genotype Specific Efficacy
Testing for HCV genotype is recommended to guide the selection of the most appropriate antiviral regimen.20 Genotyping is useful in epidemiological studies and in clinical management for predicting the likelihood of response and determining the optimal duration of therapy.21 The current study reported that the dual therapy gave a significantly better SVR rate in HCV-GT3. Comparable rate of SVR such as ≥80% in patients infected with GT3 were reported in other settings.16,22 In a serological study on HCV-infected patients in Myanmar, 75% (18/24) were with GT3.10 A concern was that there was a comparable SVR between GT1 (81.2%) and GT3 (85.5%). Relatively fewer samples in GT1 might be a possible reason why GT3 alone was a significant predictor in the current analysis.
GT6 is common in some Asian countries, including Southern China, Hong Kong and Myanmar. Studies have reported that GT6 was prevalent in Southern China and Hong Kong related to intravenous drug users (IVDU) that subsequently spread to the general population via blood transfusion.23,24 As the higher rate of SVR found in the current analysis highlighted that effective treatment to this special group (IVDU) and strict screening of HCV would further reduce the spread of HCV to the general population.
A faster progression of liver fibrosis in HCV-GT3 patients was reported in empirical studies;25 the better rate of SVR to the dual treatment in HCV-GT3 patients in the present study has therefore further clinical benefits from reduction of liver-related morbidity and mortality. For instance, 2 distant mechanisms of steatosis are postulated. In HCV-GT3-infected patients, steatosis is likely to be viral induced and associated with a direct cytopathic effect of HCV26 and have stronger negative effect on beta lipoprotein formation in hepatocyte. Thus, it is able to inhibit VLDL secretion from liver and to induce steatosis.27 Another explanation is that HCV-GT3 had significantly lower homeostatic model assessment (HOMA)-insulin resistance (IR) than other genotypes, and HOMA-IR was an independent predictor for the degree of fibrosis and the rate of fibrosis progression.28
Our findings could be confounded with the host factors such as interleukin 28 B (IL28B) and IR, albeit lack of these data in the patients profile. A cohort study on 75 HCV patients in Taiwan29 and a study on 264 HCV patients in Thailand30 as well as a meta-analysis of 46 individual studies31 assessed an impact of IL28B polymorphisms on the effect of PEG-IFNa/RBV treatment; single nucleotide polymorphisms (SNPs) of near IL28B (rs12979860 and rs8099917) were useful baseline predictors for virologic response in patients infected with GT129–31 and GT4 HCV.31 This implied that, for those nonresponses to treatment, identification of IL28B genotypes is necessary31 even before the treatment has begun.30 The impact of IL28 B on the dual treatment of HCV could be explained by the fact that HCV primarily induces IFN-Ks, which are a crucial driver of interferon-stimulated gene (ISG) induction and this cascade of events eventually result subsequent HCV eradication.32,33 The relationship between HCV and IR is complex and bidirectional, as described elsewhere.34 A cohort of 330 Taiwanese patients undertaken PEG-IFNa/RBV showed that those who had high HOMA-IR achieved significantly lower rate of SVR than those who had low IR (68.8% vs 82.1%, P = 0.008).35 A meta-analysis has documented that normal insulin sensitivity was about 3 times higher rate of SVR (OR 2.86, 95%CI: 1.97–4.16) than in patients with IR.36
Achieving SVR was diminished in HCV-infected patients with cirrhosis shown in our study, was also documented in a published review.7 Yet, this still has potential benefits to those patients who achieved SVR by preventing the development of oesophageal varices through a reduction of portal pressure.7
Adverse Events
The side effects of the dual therapy reported in the current study such as influenza-like symptoms, irritability, and injection site reactions were commonly observed in published studies.11,37 Interferons are signaling protein/cytokines in human immune systems and acting as modulator of immune function. Biosynthetic interferon-based drug (PEG-IFNa in this case) also shared the same biological properties. Hence, patients felt flu-like symptoms after initiation of the dual treatment including PEG-IFNa. The neuropsychiatric side effects of IFN such as depression/irritability were also reported. Depression and IFN relationship was hypothesized that IFN may induce proinflammatory cytokines (tumor necrosis factor [TNF], interleukin 1, and interleukin 6) to promote “sickness behavior” and may also have effects on the hypothalamic-pituitary-adrenal axis.38 Anemia less than 10 g/dL was reported in 18% of the patients who attained SVR to the dual therapy. RBV is taken up into erythrocytes and activated to RBV triphosphate, which is not hydrolyzed by erythrocytes. Therefore, the “trapped” drug within the erythrocyte could increase the levels greater than plasma concentrations. The associated depletion of red blood cell (RBC) adenosine triphosphate impairs antioxidant defence39 and induces a cascade of RBC damage.
In daily practice, patients’ understanding of the risks of AEs remains an important safeguard for the use of these drugs. As such, patients should be explained, before the beginning of therapy, about the expectations from experiencing AEs that could affect transiently on their quality of life.40
Study Limitations
Some limitations need to be acknowledged. Common to any retrospective and database study, accuracy of data is a concern. Potential confounding factors such as body weight,14 genetic polymorphism (e.g., IL 28B), and underlying clinical condition of the patients such as cirrhosis or IR could account for the SVR to the dual therapy. As some patients failed to attend the follow-up assessment 24 weeks after completion of the treatment, information bias is a concern. Such patients are the non-SVR worst case scenario or the SVR best case scenario. It is currently not clear as to which endpoint matters most. Hence, an interpretation of our results should lie in view of this bias. Age of patients in this study varied. But, this might not be an issue; an empirical study showed that response rates and safety profiles were comparable between adults and children.37 This was a single-private center study, involving a moderate to high socioeconomic status group. Their compliance to treatment and subsequent SVR rate were likely higher than in lower socioeconomic groups. Therefore, our findings are limited in generalizability. However, most of the HCV-infected patients preferred treatment at the specialist clinics in Myanmar, and this could reinforce our confidence in the efficacy estimation. The cost of treatment is a limitation to some patients. This indicates the needs for a cost effectiveness study. Nevertheless, findings of the present study suggest a routine clinical practice for HCV-infected patients in a country with limited resources.
Merits of the Outcome Measurement
The 24-week posttherapy determination of SVR as the primary end point in the current study is relevant because it is durable as well as clinically meaningful endpoint of successful therapy for HCV infection. Published meta-analyses reported that SVR achieving patients had 87% lower risk of developing liver decompensation (relative risk, 0.13; 95%CI 0.06–0.27)7 and 5-year survival among patients attaining SVR was comparable to that of the general population (standard mortality ratio, 1.4; 95% CI 0.3–2.5).41 Moreover, this combination antiviral treatment had lower risks of extra-hepatic outcomes pertinent to end-stage renal diseases (hazard ratio: 0.15; 95% CI: 0.07–0.31).42
The scientific basis of SVR applied in the present study as an outcome measure of the dual therapy may lie in the pathological process. The proinflammatory and homeostatic cytokines lymphotoxin a and b are members of the TNF superfamily. In mice models, it was found that activated, infiltrating immune cells secrete cytotoxic cytokines including TNFa, interferon gamma, and lymphotoxin a and b that cause tissue destruction, hepatocyte proliferation, cell death, and tissue remodeling. In such an environment, hepatocytes are susceptible to chromosomal aberrations leading to the development of HCC.43 Although exact mechanism is poorly understood, it has been suggested that if the inflammatory microenvironment could be attenuated by viral eradication (SVR in this case), this might lessen the occurrence of HCC.7
Implications
Studies had documented that SVR is associated with a long-term clearance of HCV infection, a virologic cure.44 The clearance of HCV from blood (i.e., at the attainment of SVR to the dual therapy) will also be beneficial in preventing end-stage chronic liver disease and its complications, and the subsequent eradication of HCV infection. This is important implication because HCV is the leading cause of liver transplantation in the developed countries, and the most common chronic blood-borne infection in the United States4 and elsewhere.
The trend of HCV treatment is moving toward the triple therapy. A review has reported that a triple combination therapy with PEG-IFNa, RBV, and a first-generation DAA, NS3/4A protease inhibitor, has shown limited efficacy in addressing nonresponsiveness to PEG-IFNa and RBV.45 Hemolytic anemia is a frequent AE in patients treated with RBV and this could be aggravated by the additional DAA (telaprevir or boceprevir) as a result of bone-marrow suppression. Follow-up studies with patients already achieved SVR to PEG-IFNa/RBV documented that such SVR is usually durable44 and associated with improvement in biomarkers of disease. There is a favorable long-term prognosis and lack of evidence of the progression of liver disease44 which may obviate the need for a protease inhibitor therapy. There is still a role for combination PEG-IFNa/RBV treatment in HCV-infected patients even in the triple therapy context.
CONCLUSION
The efficacy and safety of the dual therapy in treating HCV-infected patient particularly GT3 in Myanmar is comparable to other settings and at the acceptable level. We recommend a prospective randomized control trial looking at duration of therapy and rates of achieving SVR, which could significantly impact the care of HCV-infected patients in Myanmar and perhaps other countries as well.
Acknowledgments
The authors are grateful to the patients and the staff of the Option Endoscopy Centre (OEC) in Yangon, Myanmar. Also thank the reviewers and editors for the comments provided and helpful inputs to improve the manuscript and thankful to Prof James M Menke of the International Medical University in Malaysia for helping us in revising the manuscript. The views expressed are exclusively those of the authors.
Footnotes
Abbreviations: AE = adverse events, DAA = direct-acting antiviral agents, ETR = end of treatment response, EVR = early virologic response, GT1 = genotype 1, GT2 = genotype 2, GT3 = genotype 3, GT6 = genotype 6, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HOMA = homeostatic model assessment, IFNa = interferon alfa-2a, IR = insulin resistance, OEC = Option Endoscopy Centre, OR = odds ratio, PEG = pegylated, RBV = ribavirin, RVR = rapid virologic response, SVR = sustained virologic response, TNF = tumor necrosis factor.
CN and TS contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.CDC. Chapter 3: Infectious diseases related to travel. 2013. http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/hepatitis-c [January 12, 2015] [Google Scholar]
- 2.WHO. Hepatitis C. Fact sheet No.164. Updated April 2014. http://www.who.int/mediacentre/factsheets/fs164/en/ [January 12, 2015] [Google Scholar]
- 3.Hanafiah KM, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–1342. [DOI] [PubMed] [Google Scholar]
- 4.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005; 5:558–567. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa H, Iguchi E, Koshikawa Y, et al. The effect of pegylated interferon-alpha2b and ribavirin combination therapy for chronic hepatitis C infection in elderly patients. BMC Res Notes 2012; 5:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AASLD. Understanding and Implementing the AASLD's 2009 Practice Guidelines on the Diagnosis, Management, and Treatment of Hepatitis C: An Update. 2009. http://www.clinicaloptions.com/Hepatitis/Management%20Series/2009%20AASLD%20HCV%20Guidelines.aspx[January 12, 2015] [Google Scholar]
- 7.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52:889–890. [DOI] [PubMed] [Google Scholar]
- 8.Susser S, Herrmann E, Lange C, et al. Predictive value of interferon-lambda gene polymorphisms for treatment response in chronic hepatitis C. PLoS One 2014; 9:e112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh C, Heller T, Haynes-Williams V, et al. Long-term outcome of chronic hepatitis C after sustained virological response to interferon-based therapy. Aliment Pharmacol Ther 2013; 37:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai K, Win KM, Oo SS, et al. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol 2001; 39:1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuboki M, Iino S, Okuno T, et al. Peginterferon alpha-2a (40 KD) plus ribavirin for the treatment of chronic hepatitis C in Japanese patients. J Gastroenterol Hepatol 2007; 22:645–652. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Lawitz EJ, Shiffman M, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361:580–593. [DOI] [PubMed] [Google Scholar]
- 13.Yee HS, Chang MF, Pocha C, et al. Department of Veterans Affairs Hepatitis C Resource Center Program; National Hepatitis C Program Office. Update on the management and treatment of hepatitis C virus infection: recommendations from the department of veterans affairs hepatitis c resource center program and the national hepatitis c program office. Am J Gastroenterol 2012; 107:669–689. [DOI] [PubMed] [Google Scholar]
- 14.Aziz H, Raza A, Waheed Y, et al. Analysis of variables and interactions among variables associated with a sustained virological response to pegylated interferon alfa-2a plus ribavirin in hepatitis C virus genotype 3-infected patients. Int J Infect Dis 2012; 16:e597–e602. [DOI] [PubMed] [Google Scholar]
- 15.Pattullo V, Heathcote EJ, Wong DK. Superior response to pegylated interferon and ribavirin in Asians with chronic hepatitis C. Hepatol Int 2010; 4:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill U, Aziz H, Gill ML. Rapid virological response tailors the duration of treatment in hepatitis C virus genotype 3 patients treated with pegylated interferon alfa-2a and ribavirin in Pakistan. Int J Infect Dis 2013; 17:e1017–e1021. [DOI] [PubMed] [Google Scholar]
- 17.Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol 2009; 24:336–345. [DOI] [PubMed] [Google Scholar]
- 18.Bochud PY, Cai T, Overbeck K, et al. Swiss Hepatitis C Cohort Study Group. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–666. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay KL. Introduction to therapy of hepatitis C. Hepatology (Baltimore, MD) 2002; 36:S114–S120. [DOI] [PubMed] [Google Scholar]
- 20.AASLD. Recommendations for Testing, Managing, and Treating Hepatitis C. 2014. (http://www. aasld.org/publications/practice-guidelines-0#sthash.awnNL47N.dpuf [January 10, 2015] [Google Scholar]
- 21.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology (Baltimore, MD) 2009; 49:1335–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol 2005; 43:425–433. [DOI] [PubMed] [Google Scholar]
- 23.Rong X, Xu R, Xiong H, et al. Increased prevalence of hepatitis C virus subtype 6a in China: a comparison between 2004–2007 and 2008–2011. Arch Virol 2014; 159:3231–3237.Rong 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasitthankasem R, Vongpunsawad S, Siripon N, et al. Genotypic distribution of hepatitis C virus in Thailand and southeast Asia. PLoS One 2015; 10:e0126764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castera L, Hezode C, Roudot-Thoraval F, et al. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut 2004; 53:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castera L. Steatosis, insulin resistance and fibrosis progression in chronic hepatitis C. Minerva Gastroenterol Dietol 2006; 52:125–134. [PubMed] [Google Scholar]
- 27.Serfaty L, Andreani T, Giral P, et al. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol 2001; 34:428–434. [DOI] [PubMed] [Google Scholar]
- 28.Asselah T, Rubbia-Brandt L, Marcellin P, et al. Steatosis in chronic hepatitis C: why does it really matter? Gut 2006; 55:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen MY, Liu CH, Chen TC, et al. Value of interleukin-28B genetic polymorphism on retreatment outcomes of chronic hepatitis C genotype 1 relapsers by peginterferon alfa plus ribavirin. J Gastroenterol Hepatol 2014; 29:102–109. [DOI] [PubMed] [Google Scholar]
- 30.Thong VD, Wasitthankasem R, Tangkijvanich P, et al. Prevalence of tymine-adenine dinucleotide repeat, IL28B and IFNL4 in Thai population and correlation with spontaneous clearance and treatment outcome of hepatitis c infection. PLoS One 2015; 10:e0125400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia Z, Ding Y, Tian S, et al. Test of IL28B polymorphisms in chronic hepatitis C patients treated with PegIFN and ribavirin depends on HCV genotypes: results from a meta-analysis. PLoS One 2012; 7:e45698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe T, Sugauchi F, Tanaka Y, et al. Hepatitis C virus kinetics by administration of pegylated interferon-α in human and chimeric mice carrying human hepatocytes with variants of the IL28B gene. Gut 2013; 62:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura K, Watanabe T, Tanaka Y. Role of IL28B for chronic hepatitis C treatment toward personalized medicine. J Gastroenterol Hepatol 2014; 29:241–249. [DOI] [PubMed] [Google Scholar]
- 34.Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Ann Hepatol 2010; 9 Suppl:112–118. [PubMed] [Google Scholar]
- 35.Dai CY, Huang JF, Hsieh MY, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol 2009; 50:712–718. [DOI] [PubMed] [Google Scholar]
- 36.Eslam M, Aparcero R, Kawaguchi T, et al. Meta-analysis: insulin resistance and sustained virological response in hepatitis C. Aliment Pharmacol Ther 2011; 34:297–305. [DOI] [PubMed] [Google Scholar]
- 37.Jara P, Hierro L, de la Vega A, et al. Efficacy and safety of peginterferon-alpha2b and ribavirin combination therapy in children with chronic hepatitis C infection. Pediatr Infect Dis J 2008; 27:142–148. [DOI] [PubMed] [Google Scholar]
- 38.Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology 2000; 31:1207–1211. [DOI] [PubMed] [Google Scholar]
- 39.De Franceschi L, Fattovich G, Turrini F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology 2000; 31:997–1004. [DOI] [PubMed] [Google Scholar]
- 40.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982. [DOI] [PubMed] [Google Scholar]
- 41.Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004; 53:1504–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu YC, Ho HJ, Huang YT, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 2015; 64:495–503.doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 43.Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearlman BL, Ehleben C. Hepatitis C genotype 1 virus with low viral load and rapid virologic response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology 2014; 59:71–77. [DOI] [PubMed] [Google Scholar]
- 45.Saarrazin C, Hezode C, Zeuzem S, et al. Antiviral strategies in hepatitis C virus infection. J Hepatol 2012; 56 Suppl 1:S88–S100. [DOI] [PubMed] [Google Scholar]


