Supplemental Digital Content is available in the text
Abstract
The aim of the study was to evaluate preoperative variables predictive of lethal morbidities in critically ill surgical patients at a national level.
There is no report of risk stratification for morbidities associated with mortality in critically ill patients with acute diffuse peritonitis (ADP).
We examined data from 16,930 patients operated during 2011 and 2012 in 1546 different hospitals for ADP identified in the National Clinical Database of Japan. We analyzed morbidities significantly associated with operative mortality. Based on 80% of the population, we calculated independent predictors for these morbidities. The risk factors were validated using the remaining 20%.
The operative mortality was 14.1%. Morbidity of any grade occurred in 40.2% of patients. Morbidities correlated with mortality, including septic shock, progressive renal insufficiency, prolonged ventilation >48 hours, systemic sepsis, central nervous system (CNS) morbidities, acute renal failure and pneumonia, and surgical site infection (SSI), were selected for risk models. A total of 18 to 29 preoperative variables were selected per morbidity and yielded excellent C-indices for each (septic shock: 0.851; progressive renal insufficiency: 0.878; prolonged ventilation >48 h: 0.849; systemic sepsis: 0.839; CNS morbidities: 0.848; acute renal failure: 0.868; pneumonia: 0.830; and SSI: 0.688).
We report the first risk stratification study on lethal morbidities in critically ill patients with ADP using a nationwide surgical database. These risk models will contribute to patient counseling and help predict which patients require more aggressive surgical and novel pharmacological interventions.
INTRODUCTION
Acute diffuse peritonitis (ADP) is defined as the uncontained spread of intraabdominal infection, rapidly proceeding beyond the source of infection into multiple (2–4) quadrants of the intraabdominal cavity.1 Most patients diagnosed with ADP are critically ill and therefore require emergency surgery, regardless of the source of infection.2–4 A high incidence of severe postoperative complications such as septic shock, pneumonia, and organ failure has resulted in a high mortality rate of approximately 30%, even in modern case series.4 Therefore, the identification of postoperative complications associated with mortality and their optimal treatment is necessary to improve outcomes. There have been risk models for mortality in critically ill patients. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score,5 Sequential Organ Failure Assessment score,6 and Mannheim Peritonitis Index7 have all been shown to be quite effective for predicting mortality in critically ill patients. However, there has been no risk model for the morbidity of critically ill patients using a nationwide database.
American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) risk models are powerful predictors of specific morbidities and mortality associated with gastrointestinal surgery.8–10 However, there has been no nationwide analysis of critically ill surgical patients. In one regional report, Turner et al11 showed that ACS-NSQIP criteria were associated with high APACHE II scores and poor outcomes in 340 surgical patients (mortality: 20.6%) treated in the intensive care unit of the University of Maryland Medical Center (Baltimore, MD). They found that APACHE II score predictions were consistent with ACS-NSQIP postoperative outcomes. This observation prompted us to hypothesize that ACS-NSQIP preoperative variables could be used to predict both postoperative morbidities and mortalities in ADP patients.
The National Clinical Database (NCD) in Japan, which commenced patient registration in January 2011, is a nationwide project linked to the surgical board certification system. 12,13 Submitting cases to the NCD is a prerequisite for all member institutions of both the Japan Surgical Society and Japanese Society of Gastroenterological Surgery, and only registered cases can be used for board certification. The NCD collaborates with the ACS-NSQIP10: they share the common goal of developing a standardized surgery database to achieve an improvement in treatment quality.14
Previously, we reported that patients with ADP are critically ill, most require emergency surgery, and their 30-day mortality and 90-day in-hospital mortality rates are 9% and 13.9%, respectively.15 In this study, we used data from 16,930 patients with ADP treated in 2011 and 2012 and registered with the NCD to create risk models for postoperative morbidities associated with mortality.
METHODS
Patient Selection
The NCD is a nationwide project associated with the board certification system of surgery in Japan into which data from over 1,200,000 surgical cases treated at over 3500 hospitals are entered annually. We have created risk models of mortality for the 8 surgical procedures (esophagectomy, total gastrectomy, distal gastrectomy, right hemicolectomy, low anterior resection, hepatectomy, pancreaticoduodenectomy, and ADP) using NCD data sets, and the respective model was published separately,15–22 and the results were summarized as a review article.13 Thus, patient selection, preoperative and perioperative variables, and ethics consideration were quite consistent between the studies. The NCD continuously recruits individuals who approve these data, members of various departments in charge of cases, and data entry officers through a web-based data management system; thus, the traceability of the data is assured.12 In addition, the project constantly validates the consistency of these data by the inspection of randomly chosen institutions. Current laws, ordinances, and guidelines regarding the confidentiality of data are observed. Patients agree for their data to be included in research projects by using presumed consent with opt-out through the Web page and/or a notice of each hospital.20 The NCD project was approved on November 2010 by Japan Surgical Society Ethics Committee.
In this study, we focused on ADP in the Gastrointestinal Surgery section of the NCD. In the NCD, we identified 16,930 patients who underwent surgery for ADP in 2011 to 2012. Patients who declined to have their records entered in the NCD were excluded from our analysis. Records with missing data on patient age, sex, or status, 30 days after surgery were also excluded.
Preoperative and Perioperative Variables
The preoperative and perioperative variables used by the NCD are almost identical to those used by the ACS-NSQIP (http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_UserGuide.2012.pdf#search=’user+guide+for+the+2012+ACS+NSQIP). All variables, definitions, and inclusion criteria regarding the NCD are accessible to participating institutions on its website (http://www.ncd.or.jp/), which also features an E-learning system to instruct participants in how to input consistent data. The potential independent variables were previously described.13,15–22 These included patient demographics, preexisting comorbidities, preoperative laboratory values, and perioperative data (Table 1 ).
TABLE 1.
Preoperative Risk Profiles and Laboratory Data of the Study Population

Outcome Measures (Mortality and Postoperative Occurrences)
We calculated the 30-day mortality and operative mortality. The former was defined as death within 30 days of surgery, regardless of the patient's geographical location, even if the patient had been discharged from the hospital. The latter was defined as death within the index hospitalization period, regardless of the length of hospital stay (up to 90 days), as well as any death after discharge within 30 days of surgery.
The postoperative morbidities that occurred within 30 days of surgery included relaparotomy within 30 days of surgery; wound-related morbidities (superficial incisional surgical site infection [SSI], deep incisional SSI, organ/space SSI, wound disruption); respiratory morbidities (pneumonia, unplanned intubation, pulmonary embolism, ventilation >48 hours); urinary tract morbidities (progressive renal insufficiency, acute renal failure, urinary tract infection); central nervous system (CNS) morbidities (stroke/cerebrovascular accident [CVA], coma for <24 hours, peripheral nerve injury); cardiac morbidities (cardiac arrest, myocardial infarction); and other occurrences (bleeding 1–4 u or ≥5 u red blood cells, deep-vein thrombosis/thrombophlebitis, septic shock, severe sepsis, systemic inflammatory response syndrome [SIRS]).
Statistical Analysis
We used IBM SPSS Statistics for Windows (Version 20; IBM Corp, Armonk, NY) for data analysis. Univariate analysis of the data was performed using Fisher exact test, the unpaired Student t test, and the Mann–Whitney U test. Correlations between each morbidity and operative mortality and between respective morbidities were analyzed using the Pearson product–moment correlation.
Data were randomly assigned into2 subsets that were split 80/20: the first for model development and the second for validation. The 8 sets of logistic models (septic shock, systemic sepsis, progressive renal insufficiency, acute renal failure, ventilation >48 hours, pneumonia, CNS morbidities, and SSI) were constructed for dataset development using step-wise selection of the predictors with a probability (P) value for inclusion of 0.05. A “goodness-of-fit” test was performed to assess how well the model discriminated between patients with or without respective morbidities. Receiver operating characteristic (ROC) curves for respective morbidities were created for the validation dataset. A ROC curve is a plot of a test's true-positive rate (sensitivity) versus its false-positive rate (1−specificity).
RESULTS
Preoperative Risk Profiles and Laboratory Data of the Study Population
The demographic data and risk profile of 16,930 patients with ADP are shown in Table 1 . The patient population had a mean age of 64.9 ± 18.6 years (range: 0–106 years), and 60.5% (n = 10,248) were male. In this population, 37.7% arrived at hospital by ambulance, and 92.9% required emergency surgery. Their original disease and associated operative mortalities were acute peritonitis (15.1%), appendicitis (1%), gastroduodenal ulcer/perforation (9.5%), intestinal perforation (18.4%), intestinal obstruction (18.9%), cholecystitis/cholangitis (13.3%), and vascular insufficiency (31.2%). These proportions and mortalities are consistent with findings from 2011.15
An abbreviated risk profile for the study population is also shown in Table 1 . In brief, 58.4% of the patient population had an American Society of Anesthesiologists (ASA) classification of III–V, partial/total dependency for activities of daily living (ADL) was 41.2%, 0.5% of patients had body mass index (BMI) of >30 kg/m2, and 5.1% of patients had a weight loss of >10%. With regard to preexisting comorbidities, failure of various organs occurred in a percentage of patients, including ventilator dependence (3.8%), congestive heart failure (2.6%), and acute renal failure (4.4%). Signs of systemic sepsis were evident in 30.9% of patients. Blood transfusion was required in 4.1% of patients. An ASA classification of >IV and V and organ failure were associated with an operative mortality rate of >40%.
Postoperative Occurrences in Patients with ADP
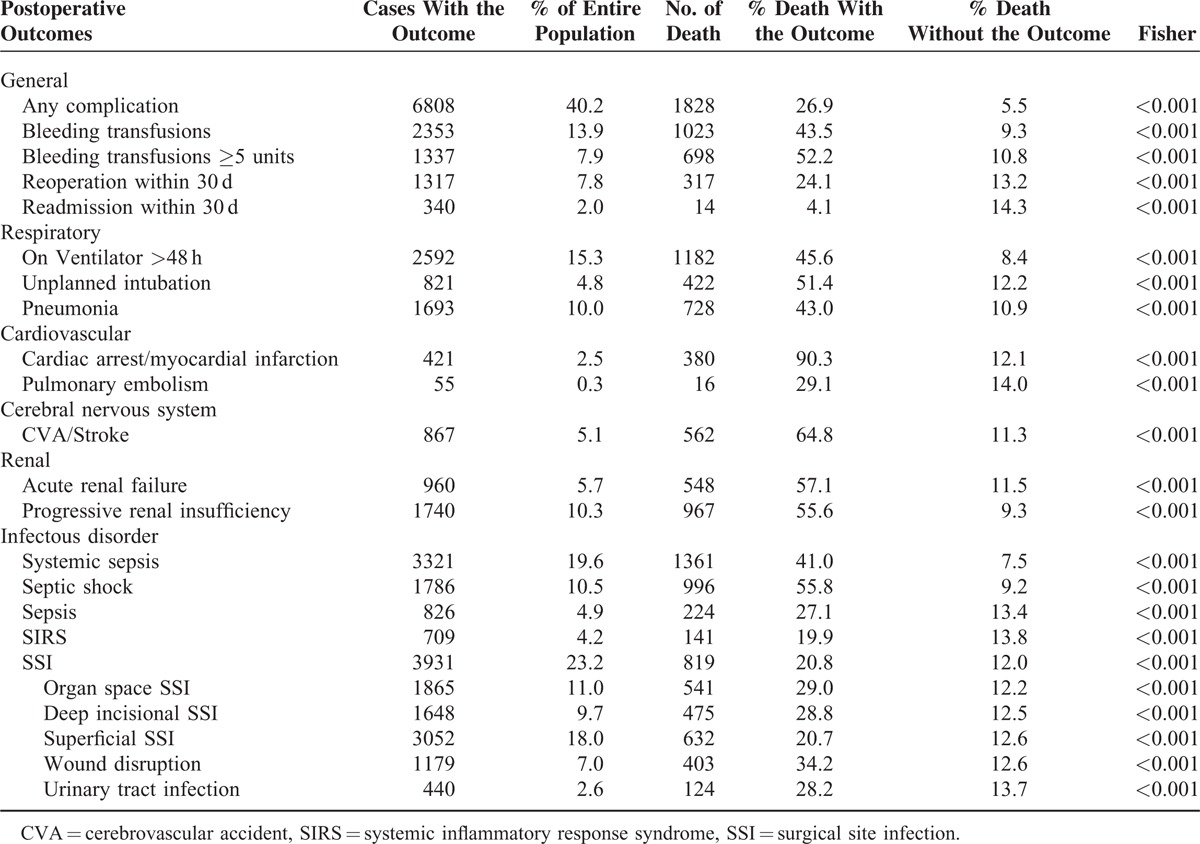
The 30-day mortality and operative mortality rates after surgery for ADP were 8.8% (1482) and 14.1% (2385), respectively. The incidences of various morbidities and percentage of consequent patient deaths are shown in Table 2. The postoperative morbidities that led to a high percentage of deaths (>40%) included transfusion (1–4 U: 43.5%; >5 U: 52.2%), prolonged ventilation (45.6%), unplanned intubation (51.4%), pneumonia (43%), cardiac and CNS morbidities (90.3% and 64.8%, respectively), acute renal failure (57.1%), progressive renal insufficiency (55.6%), any systemic sepsis (41%), and septic shock (55.8%). These morbidities occurred at a relatively high incidence (4.8%–15%) excepting cardiac morbidities (2.5%). SSI of any type, including organ space, deep incisional, and superficial incisional, occurred in 23.2% of patients and led to an operative mortality rate of 20.8%.
TABLE 1 (Continued).
Preoperative Risk Profiles and Laboratory Data of the Study Population

Correlation Between Postoperative Morbidities and Operative Mortality
Correlation between 30-day operative mortality rates and postoperative morbidities were analyzed using the Pearson product–moment correlation. The morbidities highly correlated with mortality (top 7) as well as SSI as the most representative complication of ADP were selected and are compared in Table 3. A better correlation with postoperative morbidities was found when operative rather than 30-day mortality was used. Among the postoperative morbidities, septic shock, progressive renal insufficiency, and ventilation >48 hours were highly correlated with each other (r >0.5). In contrast, SSI was only moderately correlated with systemic sepsis, and weakly correlated with ventilation >48 hours.
TABLE 2.
Postoperative Occurrences After ADP Surgery
Model Results and Performance
We developed risk models for postoperative morbidities with a relatively high incidence associated with high mortality (Table 4 ; Supplemental Table, http://links.lww.com/MD/A344, with 95% confidence intervals [CIs]). The postoperative morbidities selected correlated well with operative mortality. Septic shock, systemic sepsis (SIRS, sepsis, or septic shock), progressive renal insufficiency, acute renal failure, ventilation >48 hours, pneumonia, and CNS morbidities were selected, and SSI was also included as the most frequent morbidity.
TABLE 3.
Correlation Between Operative Mortality and Respective Postoperative Occurrences

TABLE 4.
Risk Models of Postoperative Occurrences After ADP Surgery

TABLE 4 (Continued).
Risk Models of Postoperative Occurrences After ADP Surgery

The logistic models of these morbidities with odds ratios are shown in Table 4 . The morbidities with a 95% CI showing statistical significance are shown in the Supplemental Table, http://links.lww.com/MD/A344. To evaluate the performance of the models, the C-index (a measure of model discrimination), which was the area under the ROC curve, was calculated for the validation sets (Figure 1). The C-indices and 95% CIs of each occurrence were 0.851 (0.841–0.860) for septic shock, 0.878 (0.870–0.887) for progressive renal insufficiency, 0.849 (0.841–0.858) for ventilation >48 hours, 0.848 (0.835–0.862) for CNS morbidities, 0.868 (0.856–0.880) for acute renal failure, 0.830 (0.819–0.840) for pneumonia, and 0.851 (0.841–0.860) for systemic sepsis. The C-index of SSI showed a weaker correlation (0.688 [0.677–0.698]) than other morbidities.
FIGURE 1.

Receiver operating characteristic (ROC) curves of each postoperative complication was shown with the C-indices and 95% CIs of each occurrence. ROC = receiver operating characteristic, CIs = confidence intervals.
A total of 18 to 29 preoperative variables were selected as risk factors of each complication. Age, ASA classification, preoperative ventilation or pneumonia, acute renal failure, blood transfusion, and systemic sepsis, as well as selected preoperative laboratory values suggestive of severe infection and organ failure, were captured in the risk models as predictors of most of the complications.
DISCUSSION
We hypothesized that ACS-NSQIP preoperative variables could be used to predict both postoperative morbidities and mortalities in ADP patients. In total, 93% of 16,930 patients with ADP included in this study required emergency surgery, and the overall operative mortality was 14.1%. This was comparable with the findings of a previous analysis using NCD data from 2011,15 in which 93.1% of patients with ADP required emergency surgery, and the overall operative mortality was 8.8%. This suggests that there is a consistent population of critically ill surgical patients who require emergency surgery in Japan. By examining the data of a large number of patients with ADP, we were able to identify the postoperative complications associated with mortality and create risk models for each complication. Septic shock, progressive renal insufficiency, ventilation >48 hours and systemic sepsis were moderately correlated (r >0.36) with operative mortality, whereas CNS morbidities, acute renal failure, and pneumonia were weakly (0.2 < r ≤ 0.35) correlated with operative mortality. For these complications, risk models showed excellent C-indices (>0.830) in the validation dataset. To our knowledge, this is the first report to successfully show and validate using a large-scale dataset that the preoperative variables of the ACS-NSQIP can predict postoperative morbidities in critical ill patients.
The prediction of postoperative complications is essential to the decision-making process before surgery, and useful to identify patients eligible for participation in the evaluation of novel pharmacologic interventions23,24 or more aggressive surgical interventions. In the past, several scoring systems have been used to predict complications.25–31 ASA score is a useful predictor for mortality,25,26 but suffers from its reproducibility because of subjective parameters.26 APACHE II was developed in a mixed group of medical and surgical patients.27 It failed to predict the development of multiple organ failure syndrome or mortality with clinical utility in postoperative surgical patients.28 Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity has been studied as a possible surgical audit system29; however, it seems to overestimate mortality, particularly for the low risk group.30,31 A reliable model for predicting complications can only be based on the accurately recorded incidences of those complications. A comparison of the outcomes of patients with ADP registered with the NCD in 2011 with those registered in 2012 revealed that mortality and morbidities were highly correlated between these years (r = 0.9932; Supplemental Figure, http://links.lww.com/MD/A344). The thorough data retrieval system of the NCD and clinically clear entity of ADP made it possible to create successful risk models for these morbidities.
Severe sepsis/septic shock, defined as the presence of acute organ dysfunction in the context of infection, has a mortality rate of approximately 25% to 35%,32,33 but which can exceed 70%.34,35 Anaya and Nathens36 analyzed risk factors of severe sepsis in 11,202 patients using Washington State administrative hospital discharge data. They identified 11% with severe sepsis, which was present in 424 (62%) of the 686 decedents, and showed that source of infection, extent of peritonitis, increasing age, and preexisting organ dysfunction were independently associated with severe sepsis. Our findings on the mortality of patients with ADP were consistent with their study. The mortality of patients with ADP as a result of appendicitis was low (1%) compared with that associated with other causes such as intestinal/gastroduodenal perforation (18.4%/9.5%), vascular insufficiency (31.2%), and cholecystitis/cholangitis (13.3%). Regarding peritonitis, when it is localized within an abscess, the operative mortality rate of cases registered with the NCD was relatively low (4.6%; 254 deaths/5470 cases) compared with that of patients with ADP (14.1%). This study provides more reliable information on clinical variables and laboratory data compared with the findings of Anaya and Nathens.36 We were able to select significant variables to predict each complication, and discrimination and calibration using validation tests clearly showed the excellent performance of these models.
It is interesting to note that the risk models for morbidities moderately associated with mortality (septic shock, any systemic sepsis, renal failure, acute renal failure, prolonged ventilation, pneumonia, and CNS morbidities) picked up similar variables as risk factors—age, ADL status, ASA classification, blood transfusions, and systemic sepsis—to those found to be risk factors of mortality in patients with ADP.15 Preoperative variables associated with organ dysfunction tended to be included as risk factors in most of the risk models: preoperative ventilation/pneumonia, acute renal failure, bleeding disorders, low white blood cell count, low albumin level, and elevation of blood urea nitrogen.15 High serum sodium levels, indicative of severe dehydration in patients, were also identified. In contrast, the risk model for SSI, which was poorly associated with mortality (r = 0.107), showed a relatively low C-index (0.688) compared with the other risk models. Risk factors such as pulmonary, renal, and cerebral disorders were not included in the risk model. The key part of these risk models is that variables that were not included as risk factors of mortality were picked up as predictors of morbidities leading to mortality. This will help to improve the postoperative management of patients with ADP.
There are several limitations to this study. First, although these risk models for morbidities effectively predicted their occurrence based on preoperative variables, the source of infection and degree of its control would affect mortality and morbidity. These intraoperative parameters will be evaluated in a future study. Second, in the NCD data-entry system, the final outcome of each morbidity, whether it improved, was unresolved, led to death, and was not recorded. It is not possible to relate each morbidity directly to mortality, although most fatal cases feature multiple organ failure at the end.
ADP is a clinically distinct entity requiring life-saving emergency surgery and intensive care. We created risk models for morbidities in critically ill patients with ADP, using variables recorded by the NCD comparable to those of the ACS-NSQIP, and these models performed well. These models could be formatted to feed information back to the NCD and can be expected to improve the quality of the surgical and postoperative care of patients with ADP.
Acknowledgments
The authors thank all data managers and hospitals participating in this NCD project for their great efforts in entering the data, and also thank the members and the working members of the JSGS database committee.
Footnotes
Abbreviations: ADL = activities of daily living, ADP = acute diffuse peritonitis, APACHE II = Acute Physiology and Chronic Health Evaluation II, ASA = American Society of Anesthesiologists, BMI = body mass index, CIs = confidence intervals, CNS = central nervous system, CVA = cerebrovascular accident, JSGS = Japanese Society of Gastroenterological Surgery, NCD = National Clinical Database, ROC = Receiver operating characteristic, SIRS = systemic inflammatory response syndrome, SSI = surgical site infection.
This study was partially supported by a research grant from Ministry of Health, Labour and Welfare.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Seiler CA, Brugger L, Forssmann U, et al. Conservative surgical treatment of diffuse peritonitis. Surgery 2000; 127:178–184. [DOI] [PubMed] [Google Scholar]
- 2.Ordonez CA, Puyana JC. Management of peritonitis in the critically ill patient. Surg Clin North Am 2006; 86:1323–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blot S, De Waele JJ. Critical issues in the clinical management of complicated intra-abdominal infections. Drugs 2005; 65:1611–1620. [DOI] [PubMed] [Google Scholar]
- 4.Pieracci FM, Barie PS. Management of severe sepsis of abdominal origin. Scand J Surg 2007; 96:184–196. [DOI] [PubMed] [Google Scholar]
- 5.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829. [PubMed] [Google Scholar]
- 6.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710. [DOI] [PubMed] [Google Scholar]
- 7.Linder MM, Wacha H, Feldmann U, et al. The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis. Chirurg 1987; 58:84–92. [PubMed] [Google Scholar]
- 8.Huffman KM, Cohen ME, Ko CY, et al. A Comprehensive Evaluation of Statistical Reliability in ACS NSQIP Profiling Models. Ann Surg 2014. [DOI] [PubMed] [Google Scholar]
- 9.Raval MV, Cohen ME, Ingraham AM, et al. Improving American College of Surgeons National Surgical Quality Improvement Program risk adjustment: incorporation of a novel procedure risk score. J Am Coll Surg 2010; 211:715–723. [DOI] [PubMed] [Google Scholar]
- 10.Hall BL, Hamilton BH, Richards K, et al. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg 2009; 250:363–376. [DOI] [PubMed] [Google Scholar]
- 11.Turner PL, Ilano AG, Zhu Y, et al. ACS-NSQIP criteria are associated with APACHE severity and outcomes in critically ill surgical patients. J Am Coll Surg 2011; 212:287–294. [DOI] [PubMed] [Google Scholar]
- 12.Miyata H, Gotoh M, Hashimoto H, et al. Challenges and prospects of a clinical database linked to the board certification system. Sur Today 2014; 44:1991–1999. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh M, Miyata H, Hashimoto H, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viehl CT, Kraus R, Zurcher M, et al. The Acute Physiology and Chronic Health Evaluation II score is helpful in predicting the need of relaparotomies in patients with secondary peritonitis of colorectal origin. Swiss Med Wkly 2012; 142:w13640. [DOI] [PubMed] [Google Scholar]
- 15.Nakagoe T, Miyata H, Gotoh M, et al. Surgical risk model for acute diffuse peritonitis based on a Japanese nationwide database: an initial report on the surgical and 30-day mortality. Surg Today 2014. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014; 260:259–266. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg 2014; 260:1034–1039. [DOI] [PubMed] [Google Scholar]
- 18.Kurita N, Miyata H, Gotoh M, et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese patients collected using a nationwide web-based data entry system. Ann Surg 2015. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Miyata H, Gotoh M, et al. Risk model for right hemicolectomy based on 19,070 Japanese patients in the National Clinical Database. J Gastroenterol 2014; 49:1047–1055. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 2014; 57:1075–1081. [DOI] [PubMed] [Google Scholar]
- 21.Kenjo A, Miyata H, Gotoh M, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 2014; 218:412–422. [DOI] [PubMed] [Google Scholar]
- 22.Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014; 259:773–780. [DOI] [PubMed] [Google Scholar]
- 23.Artigas A, Niederman MS, Torres A, et al. What is next in sepsis: current trials in sepsis. Expert Rev Anti Infect Ther 2012; 10:859–862. [DOI] [PubMed] [Google Scholar]
- 24.Hutchins NA, Unsinger J, Hotchkiss RS, et al. The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol Med 2014; 20:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacanti CJ, VanHouten RJ, Hill RC. A statistical analysis of the relationship of physical status to postoperative mortality in 68,388 cases. Anesth Analg 1970; 49:564–566. [PubMed] [Google Scholar]
- 26.Prause G, Ratzenhofer-Comenda B, Pierer G, et al. Can ASA grade or Goldman's cardiac risk index predict peri-operative mortality? A study of 16,227 patients. Anaesthesia 1997; 52:203–206. [DOI] [PubMed] [Google Scholar]
- 27.Jones DR, Copeland GP, de Cossart L. Comparison of POSSUM with APACHE II for prediction of outcome from a surgical high-dependency unit. Br J Surg 1992; 79:1293–1296. [DOI] [PubMed] [Google Scholar]
- 28.Cerra FB, Negro F, Abrams J. APACHE II score does not predict multiple organ failure or mortality in postoperative surgical patients. Arch Surg 1990; 125:519–522. [DOI] [PubMed] [Google Scholar]
- 29.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 1991; 78:355–360. [DOI] [PubMed] [Google Scholar]
- 30.Whiteley MS, Prytherch DR, Higgins B, et al. An evaluation of the POSSUM surgical scoring system. Br J Surg 1996; 83:812–815. [DOI] [PubMed] [Google Scholar]
- 31.Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg 1998; 85:1217–1220. [DOI] [PubMed] [Google Scholar]
- 32.Barie PS, Hydo LJ, Shou J, et al. Efficacy and safety of drotrecogin alfa (activated) for the therapy of surgical patients with severe sepsis. Surg Infect 2006; 7 Suppl 2:S77–S80. [DOI] [PubMed] [Google Scholar]
- 33.Barie PS, Vogel SB, Dellinger EP, et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Cefepime Intra-abdominal Infection Study Group. Arch Surg 1997; 132:1294–1302. [DOI] [PubMed] [Google Scholar]
- 34.Farthmann EH, Schoffel U. Principles and limitations of operative management of intraabdominal infections. World J Surg 1990; 14:210–217. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Sabrido JL, Tallado JM, Christou NV, et al. Treatment of severe intra-abdominal sepsis and/or necrotic foci by an ’open-abdomen’ approach. Arch Surg 1988; 123:152–156. [DOI] [PubMed] [Google Scholar]
- 36.Anaya DA, Nathens AB. Risk factors for severe sepsis in secondary peritonitis. Surg Infect 2003; 4:355–362. [DOI] [PubMed] [Google Scholar]


